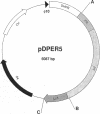
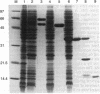
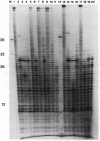
Abstract
Two constituent protein domains of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase were expressed separately and purified to homogeneity. The N-terminal domain (p51) behaves as a monomeric protein exhibiting salt-sensitive DNA polymerase activity. The C-terminal domain (p15) on its own has no detectable RNase H activity. However, the combination of both isolated p51 and p15 in vitro leads to reconstitution of RNase H activity on a defined substrate. These results demonstrate that domains of HIV-1 reverse transcriptase are functionally interdependent to a much higher degree than in the case of reverse transcriptase from Moloney murine leukemia virus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Champoux J. J., Gilboa E., Baltimore D. Mechanism of RNA primer removal by the RNase H activity of avian myeloblastosis virus reverse transcriptase. J Virol. 1984 Mar;49(3):686–691. doi: 10.1128/jvi.49.3.686-691.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Dierks P., Parsons J. T., Faras A. J. RNase H hydrolysis of the 5' terminus of the avian sarcoma virus genome during reverse transcription. Nature. 1978 Mar 9;272(5649):181–184. doi: 10.1038/272181a0. [DOI] [PubMed] [Google Scholar]
- Dudding L. R., Harington A., Mizrahi V. Endoribonucleolytic cleavage of RNA: oligodeoxynucleotide hybrids by the ribonuclease H activity of HIV-1 reverse transcriptase. Biochem Biophys Res Commun. 1990 Feb 28;167(1):244–250. doi: 10.1016/0006-291x(90)91757-j. [DOI] [PubMed] [Google Scholar]
- Ferris A. L., Hizi A., Showalter S. D., Pichuantes S., Babe L., Craik C. S., Hughes S. H. Immunologic and proteolytic analysis of HIV-1 reverse transcriptase structure. Virology. 1990 Apr;175(2):456–464. doi: 10.1016/0042-6822(90)90430-y. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Mitra S. W., Goff S., Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979 Sep;18(1):93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Graves M. C., Meidel M. C., Pan Y. C., Manneberg M., Lahm H. W., Grüninger-Leitch F. Identification of a human immunodeficiency virus-1 protease cleavage site within the 66,000 Dalton subunit of reverse transcriptase. Biochem Biophys Res Commun. 1990 Apr 16;168(1):30–36. doi: 10.1016/0006-291x(90)91670-n. [DOI] [PubMed] [Google Scholar]
- Hansen J., Schulze T., Mellert W., Moelling K. Identification and characterization of HIV-specific RNase H by monoclonal antibody. EMBO J. 1988 Jan;7(1):239–243. doi: 10.1002/j.1460-2075.1988.tb02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizi A., Barber A., Hughes S. H. Effects of small insertions on the RNA-dependent DNA polymerase activity of HIV-1 reverse transcriptase. Virology. 1989 May;170(1):326–329. doi: 10.1016/0042-6822(89)90389-9. [DOI] [PubMed] [Google Scholar]
- Hizi A., Hughes S. H., Shaharabany M. Mutational analysis of the ribonuclease H activity of human immunodeficiency virus 1 reverse transcriptase. Virology. 1990 Apr;175(2):575–580. doi: 10.1016/0042-6822(90)90444-v. [DOI] [PubMed] [Google Scholar]
- Hizi A., McGill C., Hughes S. H. Expression of soluble, enzymatically active, human immunodeficiency virus reverse transcriptase in Escherichia coli and analysis of mutants. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1218–1222. doi: 10.1073/pnas.85.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostomsky Z., Appelt K., Ogden R. C. High-level expression of self-processed HIV-1 protease in Escherichia coli using a synthetic gene. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1056–1063. doi: 10.1016/0006-291x(89)91350-8. [DOI] [PubMed] [Google Scholar]
- Johnson M. S., McClure M. A., Feng D. F., Gray J., Doolittle R. F. Computer analysis of retroviral pol genes: assignment of enzymatic functions to specific sequences and homologies with nonviral enzymes. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7648–7652. doi: 10.1073/pnas.83.20.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotewicz M. L., Sampson C. M., D'Alessio J. M., Gerard G. F. Isolation of cloned Moloney murine leukemia virus reverse transcriptase lacking ribonuclease H activity. Nucleic Acids Res. 1988 Jan 11;16(1):265–277. doi: 10.1093/nar/16.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug M. S., Berger S. L. Ribonuclease H activities associated with viral reverse transcriptases are endonucleases. Proc Natl Acad Sci U S A. 1989 May;86(10):3539–3543. doi: 10.1073/pnas.86.10.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Ingraham R. H., Skoog M. T., Wimmer E., Pallai P. V., Carter C. A. Activity of purified biosynthetic proteinase of human immunodeficiency virus on natural substrates and synthetic peptides. Proc Natl Acad Sci U S A. 1989 Feb;86(3):807–811. doi: 10.1073/pnas.86.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B., Purifoy D., Powell K., Darby G. AIDS virus reverse transcriptase defined by high level expression in Escherichia coli. EMBO J. 1987 Oct;6(10):3133–3137. doi: 10.1002/j.1460-2075.1987.tb02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grice S. F., Ette R., Mills J., Mous J. Comparison of the human immunodeficiency virus type 1 and 2 proteases by hybrid gene construction and trans-complementation. J Biol Chem. 1989 Sep 5;264(25):14902–14908. [PubMed] [Google Scholar]
- Levin J. G., Crouch R. J., Post K., Hu S. C., McKelvin D., Zweig M., Court D. L., Gerwin B. I. Functional organization of the murine leukemia virus reverse transcriptase: characterization of a bacterially expressed AKR DNA polymerase deficient in RNase H activity. J Virol. 1988 Nov;62(11):4376–4380. doi: 10.1128/jvi.62.11.4376-4380.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoote M. M., Coligan J. E., Folks T. M., Fauci A. S., Martin M. A., Venkatesan S. Structural characterization of reverse transcriptase and endonuclease polypeptides of the acquired immunodeficiency syndrome retrovirus. J Virol. 1986 Nov;60(2):771–775. doi: 10.1128/jvi.60.2.771-775.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. M., Aitken A., Bradley C., Darby G. K., Larder B. A., Powell K. L., Purifoy D. J., Tisdale M., Stammers D. K. HIV-1 reverse transcriptase: crystallization and analysis of domain structure by limited proteolysis. Biochemistry. 1988 Dec 13;27(25):8884–8889. doi: 10.1021/bi00425a002. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mizrahi V. Analysis of the ribonuclease H activity of HIV-1 reverse transcriptase using RNA.DNA hybrid substrates derived from the gag region of HIV-1. Biochemistry. 1989 Nov 14;28(23):9088–9094. doi: 10.1021/bi00449a020. [DOI] [PubMed] [Google Scholar]
- Mizrahi V., Lazarus G. M., Miles L. M., Meyers C. A., Debouck C. Recombinant HIV-1 reverse transcriptase: purification, primary structure, and polymerase/ribonuclease H activities. Arch Biochem Biophys. 1989 Sep;273(2):347–358. doi: 10.1016/0003-9861(89)90493-1. [DOI] [PubMed] [Google Scholar]
- Mous J., Heimer E. P., Le Grice S. F. Processing protease and reverse transcriptase from human immunodeficiency virus type I polyprotein in Escherichia coli. J Virol. 1988 Apr;62(4):1433–1436. doi: 10.1128/jvi.62.4.1433-1436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B., Restle T., Weiss S., Gautel M., Sczakiel G., Goody R. S. Co-expression of the subunits of the heterodimer of HIV-1 reverse transcriptase in Escherichia coli. J Biol Chem. 1989 Aug 25;264(24):13975–13978. [PubMed] [Google Scholar]
- Omer C. A., Faras A. J. Mechanism of release of the avian rotavirus tRNATrp primer molecule from viral DNA by ribonuclease H during reverse transcription. Cell. 1982 Oct;30(3):797–805. doi: 10.1016/0092-8674(82)90284-7. [DOI] [PubMed] [Google Scholar]
- Oyama F., Kikuchi R., Crouch R. J., Uchida T. Intrinsic properties of reverse transcriptase in reverse transcription. Associated RNase H is essentially regarded as an endonuclease. J Biol Chem. 1989 Nov 5;264(31):18808–18817. [PubMed] [Google Scholar]
- Prasad V. R., Goff S. P. Linker insertion mutagenesis of the human immunodeficiency virus reverse transcriptase expressed in bacteria: definition of the minimal polymerase domain. Proc Natl Acad Sci U S A. 1989 May;86(9):3104–3108. doi: 10.1073/pnas.86.9.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Repaske R., Hartley J. W., Kavlick M. F., O'Neill R. R., Austin J. B. Inhibition of RNase H activity and viral replication by single mutations in the 3' region of Moloney murine leukemia virus reverse transcriptase. J Virol. 1989 Mar;63(3):1460–1464. doi: 10.1128/jvi.63.3.1460-1464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick R., Omer C. A., Faras A. J. Involvement of retrovirus reverse transcriptase-associated RNase H in the initiation of strong-stop (+) DNA synthesis and the generation of the long terminal repeat. J Virol. 1984 Sep;51(3):813–821. doi: 10.1128/jvi.51.3.813-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz O., Cromme F. V., Grüninger-Leitch F., Le Grice S. F. Point mutations in conserved amino acid residues within the C-terminal domain of HIV-1 reverse transcriptase specifically repress RNase H function. FEBS Lett. 1989 Nov 6;257(2):311–314. doi: 10.1016/0014-5793(89)81559-5. [DOI] [PubMed] [Google Scholar]
- Schatz O., Mous J., Le Grice S. F. HIV-1 RT-associated ribonuclease H displays both endonuclease and 3'----5' exonuclease activity. EMBO J. 1990 Apr;9(4):1171–1176. doi: 10.1002/j.1460-2075.1990.tb08224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. K., Cywinski A., Taylor J. M. Specificity of initiation of plus-strand DNA by Rous sarcoma virus. J Virol. 1984 Nov;52(2):314–319. doi: 10.1128/jvi.52.2.314-319.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanese N., Goff S. P. Domain structure of the Moloney murine leukemia virus reverse transcriptase: mutational analysis and separate expression of the DNA polymerase and RNase H activities. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1777–1781. doi: 10.1073/pnas.85.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Tisdale M., Ertl P., Larder B. A., Purifoy D. J., Darby G., Powell K. L. Characterization of human immunodeficiency virus type 1 reverse transcriptase by using monoclonal antibodies: role of the C terminus in antibody reactivity and enzyme function. J Virol. 1988 Oct;62(10):3662–3667. doi: 10.1128/jvi.62.10.3662-3667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilchek M., Miron T. Polymers coupled to agarose as stable and high capacity spacers. Methods Enzymol. 1974;34:72–76. doi: 10.1016/s0076-6879(74)34008-6. [DOI] [PubMed] [Google Scholar]
- di Marzo Veronese F., Copeland T. D., DeVico A. L., Rahman R., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of highly immunogenic p66/p51 as the reverse transcriptase of HTLV-III/LAV. Science. 1986 Mar 14;231(4743):1289–1291. doi: 10.1126/science.2418504. [DOI] [PubMed] [Google Scholar]