Abstract
Pluripotent stem cells, both human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSC), provide an important resource to produce specialized cells such as osteogenic cells for therapeutic applications such as repair or replacement of injured, diseased or damaged bone. hESCs and iPSCs can also be used to better define basic cellular and genetic mechanisms that regulate the earliest stages of human bone development. However, current strategies to mediate osteogenic differentiation of hESC and iPSC are typically limited by the use of xenogeneic components such as fetal bovine serum (FBS) that make defining specific agents that mediate human osteogenesis difficult. Runt-related transcription factor 2 (RUNX2) is a key regulator required for osteogenic differentiation. Here, we used a RUNX2-YFP reporter system to characterize the novel ability of fibrinogen to mediate human osteogenic development from hESC and iPSC in defined (serum-free) conditions. These studies demonstrate that fibrinogen mediates significant osteo-induction potential. Specifically, fibrinogen binds to the surface integrin (α9β1) to mediate RUNX2 gene expression through the SMAD1/5/8 signaling pathway. Additional studies characterize the fibrinogen-induced hESC/iPSC-derived osteogenic cells to demonstrate these osteogenic cells retain the capacity to express typical mature osteoblastic markers. Together, these studies define a novel fibrinogen-α9β1-SMAD1/5/8-RUNX2 signaling axis can efficiently induce osteogenic differentiation from hESCs and iPSCs.
Keywords: Human embryonic stem cells, Human induced pluripotent stem cells, Yellow fluorescent protein, SMAD pathway, Fibrinogen, α9β1, RUNX2, Osteoblast
Introduction
Human embryonic cells (hESC) and induced pluripotent stem cells (iPSC) have previously been used to generate osteoblasts for potential therapeutic applications such as bone repair or replacement of injured, diseased, or damaged bone [1–4]. However, these strategies typically utilize poorly defined serum-based differentiation conditions. While use of fetal bovine serum (FBS) does lead to development of mesenchymal stromal cells and osteogenic cells, the specific signaling pathways that mediate this development remain poorly defined [2]. Also, use of xenogeneic serum may limit reproducibility of differentiation strategies and clinical applications of these cells [3]. Identification of osteogenic cells derived from hESCs and iPSCs is typically based on cell surface antigen phenotype that can overlap with other cell populations. Therefore, we aimed to use our recently described RUNX2 reporter system engineered in hESCs (hESC-RUNX2-YFP cells) [4] to characterize more defined, serum-free conditions that mediate development of early human osteogenic cells.
Runt-related transcription factor 2 (RUNX2) is known to be a critical and early regulator of osteogenic development [5–7]. Runx2−/− knockout in mice results in complete depletion of bone formation [8, 9]. Mutations in Runx2 result in skeletal defects such as cleidocranial dysplasia (CCD) [10] and prevents chondrocytic hypertrophy and osteoblastic maturation [8, 10]. Notably, Runx2−/− murine calvarial cells can differentiate into chondrocytes or adipocytes but not into osteoblasts in an osteogenic-inducing microenvironment [11]. Studies in mice have described Runx2 as regulator of osteoblast development by mediating expression of key osteogenic genes, including collagen I (Col1a1), Alkaline Phosphatase (Alpl), Osteocalcin (Bglap), Osterix and Dlx [8]. Recently, our group reported a novel reporter system utilizing RUNX2 P1 promotor to drive expression of yellow fluorescent protein (YFP) to better identify osteogenic progenitor cells derived from human pluripotent stem cells [4]. The osteogenic characteristics of differentiated cells isolated based on the RUNX2-YFP reporter system were demonstrated in vitro and in vivo [4]. These current studies aim to use these hESC-RUNX2-YFP reporter cells to identify defined conditions that mediate development of human osteogenic progenitor cells. We initially tested known morphogen signaling molecules such as Wnt/β-catenin, TGFβ/BMP, FGF and rapamycin that are well characterized to induce bone development [12–15]. Next, we used this model to identify a novel fibrinogen-SMAD 1/5/8-RUNX2 signaling axis that has not been previously characterized to promote osteogenic development.
Methods and Materials
Cell Cultures and Induction of Osteogenic Differentiation
hESC line H9 (WiCell, Madison, WI) and umbilical cord blood-derived iPSC (UiPSC produced previously by our group) [16] were maintained as undifferentiated cells as previously described [16]. hESC-RUNX2-YFP cells used to report osteogenic differentiation were previously described [4].
Prior to the osteogenic differentiation, pluripotent stem cells were adapted to passage as single cells using TrypLE (Thermo Fisher Scientific, Waltham, Massachusetts, U.S. www.thermofisher.com) for 10 passages as previously described [17]. For osteogenic differentiation, the undifferentiated TrypLE adopted hESC were dissociated into single cells by TrypLE, and replated on 0.1% gelatin pre-coated culture plate in Essential 8™ Media (E8) (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.). For differentiation, the following day, E8 media was replaced with osteogenic media (basal media (1% P/S, 1% MEM-NEAA, 2 mM L-Glutamine in α-MEM (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.), 10% Knockout Serum replacer (KOSR)), 50 ug/ml ascorbic acid, 10 mM β–glycerophosphate, 100 nM dexamethasone supplemented with or without osteogenic mediators: BMP2 (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.) and/or FGF9 (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.) and/or rapamycin (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.) and/or Wnt3a (Creative Biomart, Shirley, New York, U.S. http://www.creativebiomart.net/) and/or human fibrinogen (Sigma-Aldrich, St. Louis, Missouri, www.sigmaaldrich.com) for 5 days. Differentiation after 5 days was continue with osteogenic media without osteogenic mediators till day 28 from the start of differentiation. Differentiated cells were cultured in osteogenic media only for three passages. Media was changed every 3 days. Cells were cultured at 37°C in 5% CO2 at 95% humidity. Concurrently, cells kept in basal media supplemented with KOSR and in osteogenic media supplemented with 10% FBS were taken as negative and positive controls respectively.
Flow Cytometric Analysis, Fluorescent Imaging and Cell Sorting
Single cell suspension of differentiated cells was prepared as previously described [4], and evaluated for YFP fluorescence expression and surface proteins using the fluorescent-activated cell-sorting facility (FACSCalibur, BD, San Jose, California, U.S. www.bdbiosciences.com). Flow cytometry data was analysed with FlowJo software (Tree Star, Ashland, Oregon, U.S. www.manta.com). Immunofluorescence staining was performed as described previously [18].
For isolation of α9β1 positive and negative population of undifferentiated hESC-RUNX2-YFP, indirect magnetic labelling and separation with Anti-PE Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany, www.miltenyibiotec.com) were used according to manufacturer’s guidance. Their purity of sorted cells was determined by flow cytometry. The antibodies used in this study were listed in Supporting Information Table S2.
Real-Time Reverse-Transcription Polymerase Chain Reaction Analysis
Total RNA was extracted using Qiagen RNeasy Mini Kit (Qiagen, Valencia, California, U.S. https://www.qiagen.com/us/) and 2 ug of RNA was reverse transcribed into cDNA using SuperScript II Reverse Transcriptase (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.), based on the manufacturer’s instructions. Quantitative real-time reverse-transcription polymerase chain reactions (qRT-PCRs) were performed using 150 ng cDNA product with the SYBR Green PCR Master Mix (Qiagen, Valencia, California, U.S. https://www.qiagen.com) in a 25 ul PCR assay volume per reaction according to the recommended conditions as previously described [4]. The osteogenic genes to be amplified and the PCR conditions were listed in Supporting Information Table S1. The level of the target genes were correlated to the standard concentrations and normalized by the levels of GAPDH as an endogenous reference.
Characterization of Osteogenic Phenotypes
To detect the mineral deposition in the matrix of the differentiated cells, Von Kossa staining of the differentiated cells was performed as reported previously [4]. The images were acquired randomly in each field and analyzed using software AxioVision LE (Carl Zeiss, Thornwood, New York, U.S. www.zeiss.com).
Immuno-Blotting and Immuno-Precipitation
Cell extracts were lysed with lysis buffer containing 2 × 50 ml, 0.025M Tris, 0.15 M Sodium Chloride, 0.001 M EDTA, 1% NP-40, 5% glycerol, pH 7.4 (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.). The sample was centrifuged and the supernatant was collected. Samples were kept for 10 minutes at 90°C and then electrophoresed on 4–20% Mini-PROTEAN® TGX™ Precast Gels (Bio-Rad, Hercules, California, U.S. www.bio-rad.com/) and transferred onto 0.45 Micron, 7.96 cm × 10.5 cm Nitrocellulose Membranes (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.). The membrane was blocked for 1 hour with 5% nonfat dry milk in PBS with tween (0.1% Tween 20) (PBST) and probed with the primary antibody (1:1000 in 5% nonfat dry milk in PBST, overnight at 4°C). Blots were then incubated with peroxidase-conjugated secondary antibody (1:5000 in 5% non-fat dry milk in PBST, 1 hour at RT), and were developed by chemiluminescence SuperSignal® West Dura Extended Duration Substrate (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.). Immunoprecipitation (IP) was performed as previously reported [19] according to manufacturer’s guidance (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.; Catalog number: 26146). Band intensities were quantified using ImageJ software and normalized to β-Actin. The antibodies used in this study were listed in Supporting Information Table S2.
Statistical Analysis
Each experiment was repeated biologically three times independently, if not stated otherwise. As noted in the figure legends, “n” stands for the number of biologically independent experiments. Results were presented as mean ± SEM. Statistical analysis was performed using Microsoft Excel. One-way or two-way ANOVA was used for multiple comparisons. p values was calculated by one-tailed Student’s t test, and the significant difference was defined by p < .05 or .01 or .001 as indicated in figures.
Results
Standardized Conditions to Mediate Osteogenic Differentiation of Human Pluripotent Stem Cells
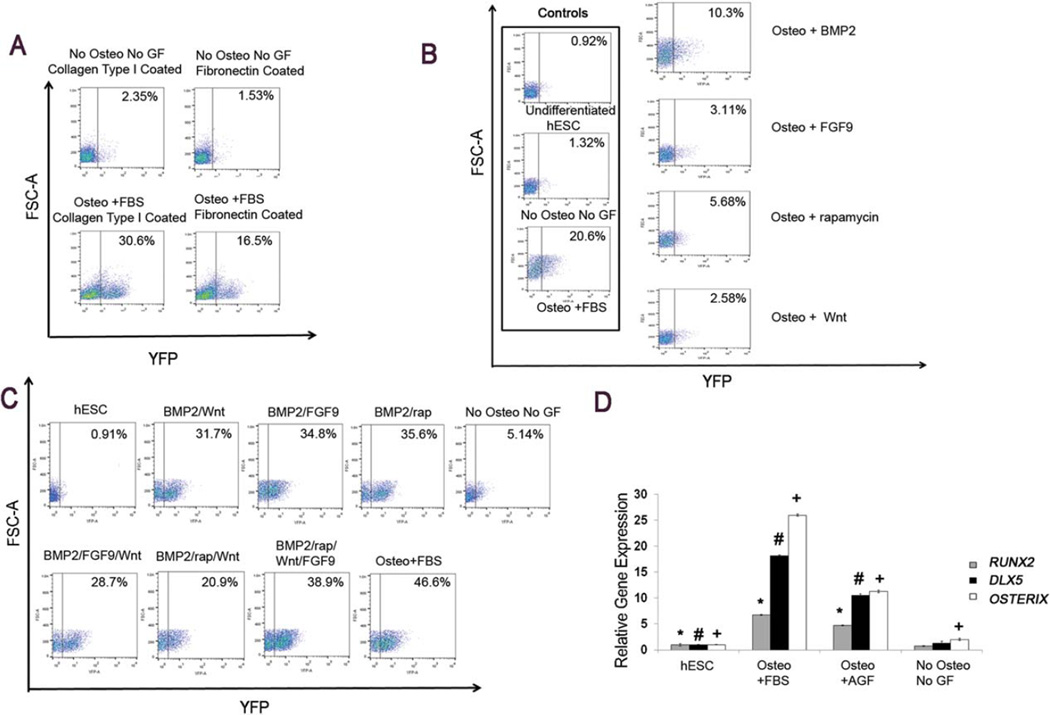
Collagen type I [4] and fibronectin [1] have both been previously demonstrated to support osteogenic differentiation of hESC and iPSC. To optimize a defined in vitro microenvironment for osteogenic induction, we utilized our previously described hESC-RUNX2-YFP cells [4]. These previous studies demonstrate these hESC-RUNX2-YFP cells faithfully report osteogenic cell development via a RUNX2 promoter driving YFP expression [4]. Here, we first wanted to compare the collagen type I and fibronectin as matrix. Collagen type I was found to promote significantly higher osteogenic differentiation as measured by YFP (as a measure for RUNX2) expression, compared to fibronectin in the presence of osteogenic media supplemented with 10% FBS (Fig. 1A).
Figure 1.
Optimization of osteogenic differentiation of hESC-RUNX2-YFP cells by known signaling pathways mediators. (A): Flow cytometric analysis showing the effect of collagen type I (0.1% v/v) and fibronectin (0.4% v/v) coated substrate on osteogenic differentiation. hESC-RUNX2-YFP seeded on collagen type I and fibronectin-coated tissue culture plastic and treated with osteogenic media supplemented with 10% FBS (lower panel) or basal media with 10% KOSR (upper panel; negative control). Osteogenic differentiation was quantified by RUNX-YFP expression. (B): Flow cytometric analysis showing the individual osteogenic effect of known signaling pathway mediators (BMP2 (100 ng/ml), Wnt3a (0.5 ug/ml), FGF9 (40 ng/ml), and rapamycin (4 nM)) on hESC-RUNX2-YFP. To analyze the individual effect of known signaling mediator, hESC-RUNX2-YFP cells were treated with osteogenic media supplemented with 10% KOSR and known signaling pathway mediator (BMP2 or Wnt3a or FGF9 or rapamycin). (C): Flow cytometric analysis of hESC-RUNX2-YFP cell differentiation in different combination of growth factors (BMP2 and/or Wnt3a and/or FGF9 and/or rapamycin). Osteogenic differentiation was quantified by RUNX-YFP expression. (D): qRT- PCR of osteogenic genes in an optimized in vitro conditions (combined use all growth factors (AGF: BMP2 + Wnt3a + FGF9 + rapamycin). Error bars indicate the mean ± SEM (n = 3); * = p < .05 versus RUNX2 gene of undifferentiated hESC, # = p < .05 versus DLX5 gene of undifferentiated hESC, + = p < .05 versus OSTERIX gene of undifferentiated hESC. (B, C, and D): Undifferentiated hESC-RUNX2-YFP and hESC-RUNX2-YFP differentiated in basal media with 10% KOSR only were taken as a negative control. hESC-RUNX2-YFP cells differentiated in osteogenic media supplemented with 10% FBS was taken as positive control. (A–D): All analyses were done at Day 6 and done in triplicate. hESCs that stably express the RUNX2-YFP reporter system (RUNX2 P1 promoter to drive expression of yellow fluorescent protein (YFP)) (hESC-RUNX2-YFP cells) were used in all experiments. Abbreviations: AGF: all growth factors; FBS: fetal bovine serum; GF: growth factors; hESC: undifferentiated human embryonic stem cells; Osteo: osteogenic media; YFP: yellow fluorescent protein.
We next analyzed different signaling pathways to mediate human osteogenic differentiation in defined serum-free conditions using BMP2, FGF9, rapamycin, and Wnt3a, all previously characterized to play a role in osteogenesis in other developmental systems [12–15, 20, 21]. Use of single factors alone only lead to relatively low RUNX2-YFP expression (Fig. 1B).
Other studies have shown that BMP2 targets RUNX2 gene and is crucial for osteogenic development [22]. Therefore, BMP2 was tested in different combination with other growth factors (Wnt3a, FGF9 and/or rapamycin) (Fig. 1C). Overall, we found that combinations of these factors all lead to significant increases in RUNX2-YFP expression without a significant difference in RUNX2-YFP expression between different combinations of BMP with other single or pairs of factors (p > .05). However, combined use of all four factors together (BMP2, Wnt3a, FGF9 and rapamycin) resulted in maximal and more consistent increase in RUNX2-YFP expression compared to the different pairs of growth factors with BMP2 (BMP2 and/or Wnt3a and/or FGF9 and/or rapamycin) (Fig. 1C). Therefore, we selected application of all four factors together as an optimized serum-free condition to mediate osteogenic development from human pluripotent stem cells.
Gene expression analysis demonstrated up-regulation of RUNX2, OSTERIX and DLX5 expression in cells differentiated in osteogenic media supplemented with all four factors together (BMP2, Wnt3a, FGF9 and rapamycin) compared to undifferentiated hESC-RUNX2-YFP. As expected, up-regulation of RUNX2, OSTERIX, and DLX5 was observed in cells differentiated in osteogenic media supplemented with 10% FBS as a positive control (Fig. 1D).
Fibrinogen Promotes hESC Differentiation into Osteogenic Cells
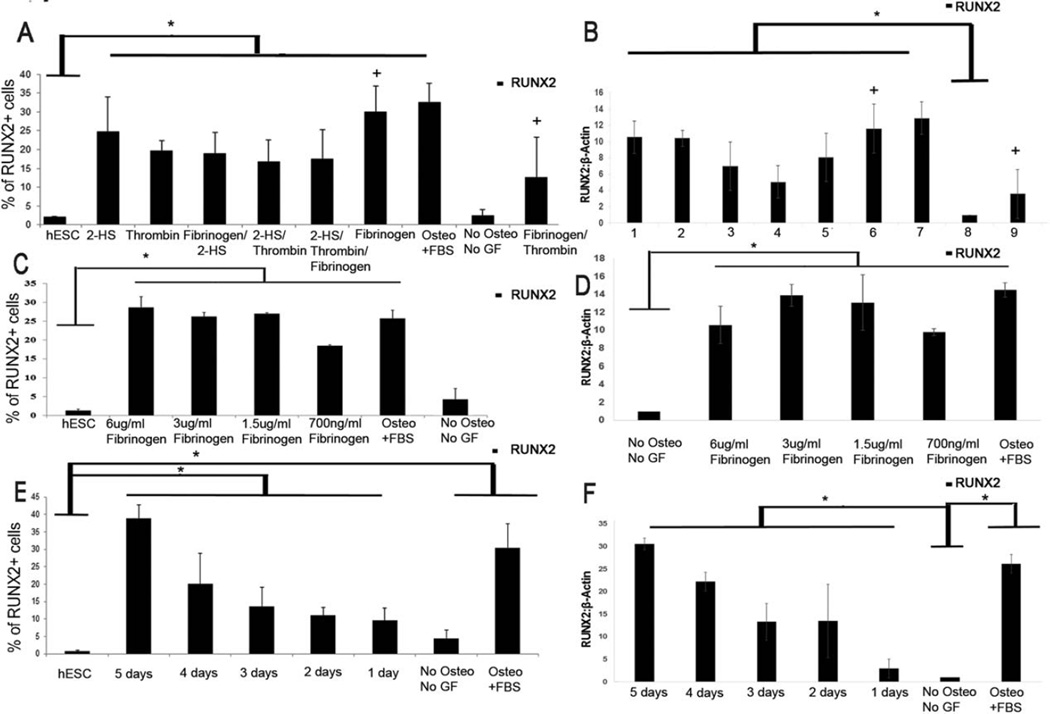
To further define signaling pathways that promote early human osteogenesis, we next screened serum components that could potentially induce osteogenic differentiation of hESC-RUNX2-YFP cells. Previously, it has been reported that fibrinogen [23, 24], α-2-HS glycoprotein (2-HS) [25] and thrombin [26] play an important role in osteogenesis and bone mineralization in vitro. Therefore, these serum proteins were tested in the hESC-RUNX2-YFP reporter system individually and in combinations (Fig. 2A). Notably, fibrinogen (1.5 ug/ml) induced significant osteogenic differentiation (RUNX2-YFP expression), more than 2-HS (0.25 ug/ml) or thrombin (1.5 ug/ml) when each were applied individually. As expected, adding thrombin to degrade fibrinogen reduces the RUNX2-YFP expression (Fig. 2A). RUNX2 expression in these studies was confirmed through immunoblot (Fig. 2B).
Figure 2.
Optimization of dose and temporal effect of fibrinogen: (A): Flow cytometric analysis showing the mean RUNX2-YFP expression by individual and the different combination of different blood proteins (fibrinogen (1.5 ug/ml) and/or thrombin (1.5 ug/ml) and/or 2-HS (0.25 ug/ml)) on hESC-RUNX-YFP cells. + = p< .05 versus Sample “Fibrinogen/Thrombin”. Undifferentiated hESC-RUNX2-YFP and hESC-RUNX2-YFP differentiated in basal media with 10% KOSR only were taken as a negative control. (B): Immunoblot showing the quantitative analysis of RUNX2 expression by thrombin, 2-HS, and fibrinogen. Lanes: 1: osteogenic media+ α 2-HS glycoprotein; 2: osteogenic media+ thrombin; 3: osteogenic media+ fibrinogen+ α 2-HS glycoprotein; 4: osteogenic media+ thrombin+α 2-HS glycoprotein; 5: osteogenic media+ fibrinogen+ α 2-HS glycoprotein+ thrombin; 6: osteogenic media+ fibrinogen; 7: osteogenic media+ FBS; 8: no osteogenic media no growth factors; 9: osteogenic media+ fibrinogen+ thrombin. (A and B): hESC-RUNX2-YFP cells were treated with osteogenic media supplemented with 10% KOSR and individual and/or different combination of different bone proteins (fibrinogen (1.5 ug/ml) and/or thrombin (1.5 ug/ml) and/or 2-HS (0.25 ug/ml)). + = p< .05 versus Sample “9” (C): Mean RUNX2-YFP expression by different doses of fibrinogen. (D): Immunoblot showing the mean expression of RUNX2 by different concentrations of fibrinogen. (C and D): hESC-RUNX2-YFP cells were treated with osteogenic media supplemented with 10% KOSR and different concentration of fibrinogen (6 ug/ml or 3 ug/ml or 1.5 ug/ml or 0.7 ug/ml). (E): Flow cytometric analysis showing the mean RUNX2-YFP expression by different fibrinogen time course treatment on hESC-RUNX2-YFP. (F): Immunoblot showing the quantitative analysis of RUNX2 expression by temporal application of fibrinogen treatment. (E and F): hESC-RUNX2-YFP cells were treated with osteogenic media supplemented with 10% KOSR and 1.5ug/ml of fibrinogen for 5 or 4 or 3 or 2 or 1 days respectively. (A, C and E): Osteogenic effect was quantified by RUNX-YFP expression. Error bars indicate the mean ± SEM (n = 3); * = p< .01 versus Sample “hESC”. Undifferentiated hESC-RUNX2-YFP and hESC-RUNX2-YFP differentiated in basal media with 10% KOSR only were taken as a negative control. (B, D and F): β-Actin was taken as a loading control. Error bars indicate the mean +/− SEM (n = 3); * = p < .05 versus Sample “No Osteo No GF”. (A–F): hESC-RUNX2-YFP cells differentiated in osteogenic media supplemented with 10% FBS was taken as positive control. All analyses were done at Day 6. Abbreviations: FBS: fetal bovine serum; GF: growth factors; hESC: undifferentiated human embryonic stem cells; Osteo: osteogenic media; YFP: yellow fluorescent protein.
Temporal and Dose Effect of Fibrinogen
Since this fibrinogen-mediated osteogenesis in hESC has not been previously reported, we aimed to optimize the dose and temporal effect of fibrinogen for maximum osteogenic differentiation. Dose response studies of fibrinogen-treated hESC-RUNX2-YFP cells demonstrated no significant difference at any dose greater than 1.5ug/ml (Fig. 2C). Expression of RUNX2 was again confirmed with immunoblot (Fig. 2D). The concentration of 1.5 ug/ml of fibrinogen was selected for further experiments.
To determine if different durations of fibrinogen application could affect osteogenic induction; hESC-RUNX2-YFP cells were treated with fibrinogen for 1–5 days. While these studies demonstrated increases of RUNX2-YFP after only 1 day of fibrinogen treatment, there was a notable increase in expression over the 5 day time course (Fig. 2E). Expression of RUNX2 expression confirmed with immunoblot (Fig. 2F). Next, hESC-RUNX2-YFP cells were differentiated for up to 21 days in osteogenic media supplemented with fibrinogen. During this extended time course, we did not find a significant difference in RUNX2-YFP expression between 5 days, 14 days or 21 days of fibrinogen treatment (p > 0.05) (Fig. 3A). Therefore, we selected 5 days of fibrinogen exposure as an optimal differentiation time point in subsequent studies.
Figure 3.
Optimization of extended time course of fibrinogen and comparison of fibrinogen with known signaling pathways mediators: (A): Flow cytometric analysis showing the extended time course of fibrinogen. hESC-RUNX2-YFP cells were treated with osteogenic media supplemented with 10% KOSR and 1.5 ug/ml of fibrinogen for 5, 14, and 21 days. Analyses were done at Day 21. Error bars indicate the mean ± SEM (n = 3); * = p < .05 versus Sample “hESC”. (B): Quantitative gene analysis showing osteogenic gene expression. hESC-RUNX2-YFP cells were treated with osteogenic media supplemented with 10% KOSR and 1.5ug/ml of fibrinogen for 5 days. Error bars indicate the mean ± SEM (n = 3); * = p < .01 versus RUNX2 gene of undifferentiated hESC, # = p < .001 versus DLX5 gene of undifferentiated hESC, + = p < .01 versus OSTERIX gene of undifferentiated hESC. (C): Flow cytometric analysis showing the osteogenic effect of fibrinogen and AGF (BMP + Wnt3a + FGF9 and rapamycin). hESC-RUNX2-YFP cells were treated with osteogenic media supplemented with 10% KOSR and 1.5 ug/ml of fibrinogen and/or AGF for 5 days. Osteogenic differentiation was quantified by RUNX-YFP expression. Error bars indicate the mean ± SEM (n = 3); * = p < .01 versus Sample “hESC”. (A, B and C): Undifferentiated hESC-RUNX2-YFP and hESC-RUNX2-YFP differentiated in basal media with 10% KOSR only were taken as a negative control. hESC-RUNX2-YFP differentiated in osteogenic media supplemented with 10% FBS was taken as positive control. (B and C): All analyses were done at Day 6. Abbreviations: AGF: all growth factors; D: Days; Fib: fibrinogen; FBS: fetal bovine serum; GF: Growth Factors; hESC: undifferentiated human embryonic stem cells; Osteo: Osteogenic Media.
To confirm the expression of other osteogenic genes, qRT-PCR analysis demonstrated the marked increases in RUNX2, OSTERIX, and DLX5 expression in cells differentiated in optimized condition (osteogenic media supplemented with 1.5 ug/ml of fibrinogen applied for 5 days) (Fig. 3B). This gene expression was comparable to use of 10% FBS as a positive control (Fig. 3B).
Next we compared our two serum-free systems, i.e. cells differentiated in osteogenic media supplemented with fibrinogen (sample: Fibrinogen) and cells differentiated in osteogenic media supplemented with known signaling agents (BMP2 + Wnt3a+ FGF9+ rapamycin) (sample: AGF). Interestingly, we did not find any significant difference between these two serum-free systems (Fig. 3C).
Fibrinogen Binds to α9β1 Expressed on hESC-RUNX2-YFP Cells
We next sought to determine the receptor(s) on hESCs that may mediate fibrinogen activity. Here we tested for the expression of integrins and receptors (β1, β2, β3, α4, CD49b, CD11, αvβ1, αvβ3, α5β1, αx, α9β1, and CD49e) know to be expressed on undifferentiated hESC or know to bind fibrinogen on other cell lines [27–34]. We found significant expression of β1, α4, αvβ3, α9β1, CD49b, and CD49e on undifferentiated hESC-RUNX2-YFP cells and iPSCs (Fig. 4A).
Figure 4.
Fibrinogen interaction with integrins/receptors on hESC: (A): Flow cytometric analysis showing the mean expression of fibrinogen attachment and expression of different integrins/receptors on hESC-RUNX2-YFP, iPSC and HUVEC cells. Samples were treated with APC conjugated fibrinogen or respective integrin/receptors antibody for 1 hour. Error bars indicate the mean ± SEM (n = 3); * = p < .05 versus Sample “HUVEC”; # = p < .05 versus Sample “hESC”. (B): Mean expression of integrins/receptors on hESC with or without fibrinogen prior treatment for 1 hour. Data was analyzed by flow cytometry. Error bars indicate the mean ± SEM (n = 3); * = p < .05 versus samples staining with no prior treatment of fibrinogen; Samples “No Prior Fibrinogen Treatment”. (C): Quantitative flow analysis of APC-conjugated fibrinogen attachment with hESC-RUNX2-YFP before and after the monoclonal—non conjugated α9β1 antibody treatment for 1 hour. Error bars indicate the mean ± SEM (n = 3); * = p < .05 versus Sample “APC-Fibrinogen only”. (A–C): Samples treated with respective isotype antibody were taken as negative control. (D): Immunoprecipitation showing the qualitative analysis of fibrinogen-α9β1 interaction. Interaction was analysed in hESC with or without fibrinogen treatment. Abbreviations: hESC: undifferentiated human embryonic stem cells; HUVEC: human umblical vein cell; iPSC: induced pluripotent stem cells; 1: first treatment; 2: second treatment.
To define which integrins may bind to fibrinogen and potentially mediate osteogenic development, flow cytometric analysis was done to quantify the effect on specific integrin expression with or without fibrinogen pre-treatment. The rationale of the fibrinogen pre-treatment was to determine if fibrinogen blocks integrin-specific antibody binding. Treatment with fibrinogen for one hour before the respective integrin staining resulted in significant reduction only for α9β1 antibody binding (p <.05). However, no other integrin staining was reduced by fibrinogen treatment (Fig. 4B). To confirm the interaction of α9β1 with fibrinogen, we also analyzed the effect of pre-α9β1 antibody treatment on fibrinogen binding. Pre-treatment with anti-α9β1 antibody for one hour before the treatment of APC-conjugated fibrinogen resulted in significant reduction in fibrinogen attachment (p < .05) (Fig. 4C). Interaction between α9β1 and fibrinogen was further confirmed by co-immunoprecipitation (Fig. 4D).
Fibrinogen Induces RUNX2 Expression through BMP-SMAD Pathway
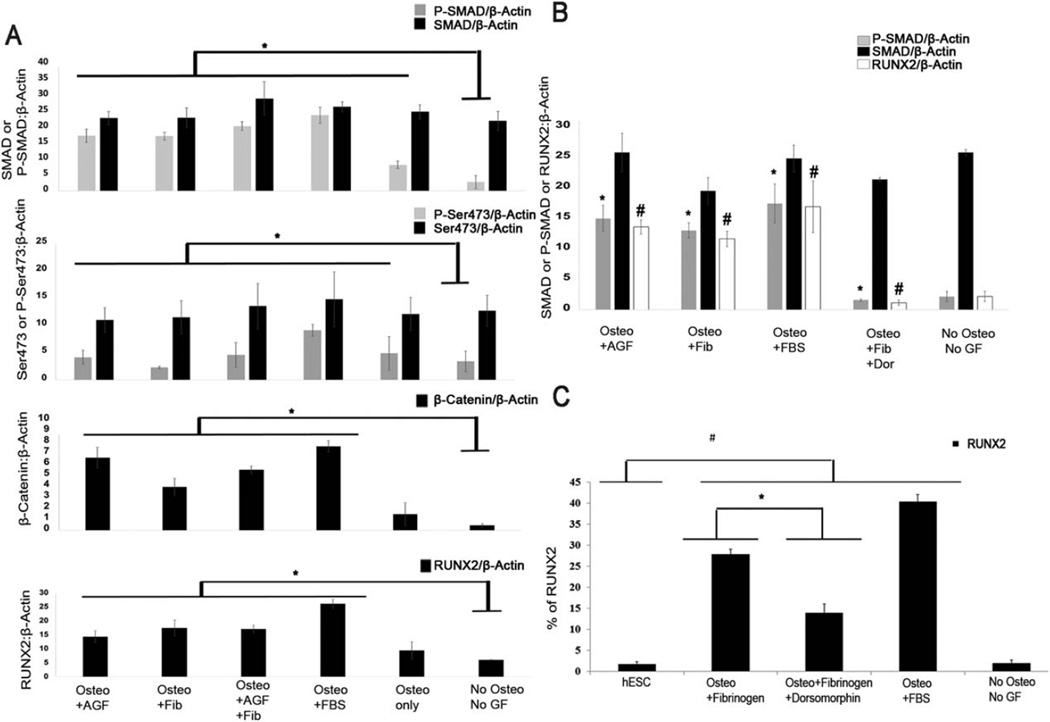
Once we characterized the fibrinogen—α9β1 interaction, we next aimed to define the down-stream signaling pathway that mediates the fibrinogen-α9β1-RUNX2 axis. Therefore, to identify the downstream pathway involved in fibrinogen-induced osteogenic differentiation, we analyzed the SMAD signaling pathways that are well established to mediate osteogenesis [22]. Our results demonstrated the activation of the SMAD 1/5/8 pathway in fibrinogen-stimulated osteogenic differentiation (Fig. 5A). We also found the higher expression of Wnt pathway (total endogenous β-Catenin) induced by fibrinogen treatment compared to cells with no fibrinogen treatment (Fig. 5A). Interestingly, we did not find strong activation of AKT pathway by fibrinogen treatment (Fig. 5A).
Figure 5.
Activation of signaling pathways mediated by fibrinogen: (A): Immunoblot analysis showing the quantitative expression of SMAD, Akt, Wnt pathways and RUNX2 expression mediated by fibrinogen. For differentiation, hESC-RUNX2-YFP cells were treated with osteogenic media supplemented with 10% KOSR and 1.5 ug/ml of fibrinogen and/or AGF (BMP + Wnt3a + FGF9 and rapamycin) for 5 days. hESC-RUNX2-YFP differentiated in basal media with 10% KOSR only or osteogenic media supplemented with 10% KOSR were taken as a negative control. hESC-RUNX2-YFP differentiated in osteogenic media supplemented with 10% FBS was taken as positive control. (B): Immunoblot analysis showing inhibition of SMAD 1/5/8 down-stream pathway by 1 uM of dorsomorphin. For differentiation, hESC-RUNX2-YFP cells were treated with osteogenic media supplemented with 10% KOSR and 1.5 ug/ml of fibrinogen or AGF or 1.5 ug/ml of fibrinogen + 1uM of dorsomorphin for 5 days. hESC-RUNX2-YFP differentiated in basal media with 10% KOSR only was taken as a negative control. hESC-RUNX2-YFP differentiated in osteogenic media supplemented with 10% FBS was taken as positive control. (A and B): β-Actin was taken as a loading control. (C): Flow cytometric analysis showing the mean expression of RUNX2-YFP expression effected by SMAD 1/5/8 pathways inhibition in hESC-RUNX2-YFP. Undifferentiated hESC-RUNX2-YFP and hESC-RUNX2-YFP differentiated in basal media with 10% KOSR only were taken as a negative control. hESC-RUNX2-YFP differentiated in osteogenic media supplemented with 10% FBS was taken as positive control. (A–C): Error bars indicate the mean ± SEM (n = 3). (A): * = p < .05 versus P-SMAD or P-SER473 or β-Catenin or RUNX2 of Sample “No Osteo No GF”. (B): * = p < .05 versus P-SMAD of Sample “Osteo + Fib + Dor”, # = p < .05 versus RUNX2 of Sample “Osteo + Fib + Dor”. (C): * = p < .001 versus Sample “Osteo +Fibrinogen”; # = p < .001 versus Sample “hESC”. (A–C): All analyses were done at Day 6. Abbreviations: AGF: all growth factors; Dor: dorsomorphin; FBS: fetal bovine serum; Fib: fibrinogen; GF: growth factors; hESC: undifferentiated human embryonic stem cells; Osteo: osteogenic media.
To confirm the role of SMAD 1/5/8 signaling to mediate the fibrinogen-induced RUNX2 expression, we treated cells with this signaling pathway with dorsomorphin, known to specifically inhibit SMAD1/5/8 signaling [35]. As expected, dorsomorphin treatment inhibited SMAD 1/5/8 phosphorylation. Moreover, dorsomorphin also inhibited the downstream RUNX2 expression (Fig. 5B). Results were confirmed and quantified by flow cytometry (Fig. 5C).
We next sought to determine if the osteogenic effect of fibrinogen was α9β1-specific. Here, undifferentiated hESC-RUNX2-YFP cells were sorted for α9β1-positive and α9β1-negative cell populations (Supporting Information Fig. S1B). α9β1-negative undifferentiated hESC-RUNX2-YFP cells were treated with osteo media supplemented with fibrinogen for 5 days to analyze the RUNX2 expression and P-SMAD 1/5/8 activation (Supporting Information Fig. S1D). Fibrinogen treatment of α9β1-negative hESC-RUNX2-YFP cells demonstrated significantly lower expression of P-SMAD 1/5/8 and RUNX2 compared to α9β1-positive cells (Supporting Information Fig. S1D). To confirm the SMAD 1/5/8-RUNX2 axis, we inhibited the SMAD 1/5/8 pathway by dorsomorphin in α9β1-negative cells which resulted in almost complete inhibition of RUNX2 expression (Supporting Information Fig. S1D). We also found that the treatment of FBS was not able to mediate significantly higher expression of RUNX2 in α9β1 negative—SMAD pathway inhibited cells (Supporting Information Fig. S1D). α9β1-positive hESC-RUNX2-YFP cells were treated with osteo media supplemented with fibrinogen and used as positive controls for these studies. Notably, the α9β1-negative cells do demonstrate some increase in P-SMAD. Therefore, an α9β1-independent activation of RUNX2 expression is also mediated by fibrinogen. Indeed, the antibody blocking studies also demonstrate only partial inhibition of fibrinogen binding to hESCs with anti- α9β1 treatment. Therefore, while this fibrinogen-α9β1-SMAD 1/5/8-RUNX2 axis plays a key role in mediating these effects, it is likely not the only pathway activated in this system.
Characterization of hESC Derived Osteogenic Cells for Osteogenic Phenotype
With this characterization of a novel fibrinogen-α9β1-SMAD 1/5/8-RUNX2 axis, we next analyzed the ability of fibrinogen-mediated hESC-RUNX2-YFP derived osteogenic cells to express mature osteoblastic markers. Therefore, hESC-RUNX2-YFP-derived osteogenic cells were characterized for mature osteogenic markers at the protein level at day 28 from the start of differentiation. hESC-RUNX2-YFP -derived osteogenic cells induced by fibrinogen demonstrated OSTEOPONTIN (Fig. 6A) Moreover, OSTEOCALCIN, RUNX2, and OSTERIX were also produced at protein level as previously shown (Fig. 6B). Cells were also analyzed for the expression of pluripotency markers (OCT4, SOX2, and NANOG) to confirm their differentiation (Fig. 6C). Furthermore, OCT4 was also found to be downregulated at protein level (Fig. 6D). Fibrinogen-induced osteogenic cells in serum-free conditions are also able to produce calcium, as confirmed with Von Kossa staining (Fig. 6E).
Figure 6.
Characterization of fibrinogen-mediated osteogenic cells derived from hESC: (A): Immunofluorescence staining of OSTEOPONTIN staining in hESC-RUNX2-YFP differentiated in osteogenic media supplemented with 10% KOSR and 1.5 ug/ml of fibrinogen. 1.5 ug/ml of fibrinogen was applied for 5 days only. Undifferentiated hESC-RUNX2-YFP served as negative control. Saos2 cells and hESC-RUNX2-YFP cells differentiated in osteogenic media supplemented with 10% FBS served as positive controls. Samples without primary antibody (1°) was taken as staining control. Yellow scale bar represents 50 µm (left lane) and 20 µm (middle and right lane). (B): Immunoblot analysis showing the mean expression of osteoblastic markers in hESC-RUNX2-YFP derived osteogenic cells differentiated in osteogenic conditions as indicated. Saos2 cells, human bone marrow derived MSC and hESC-RUNX2-YFP cells differentiated in osteogenic media supplemented with 10% FBS served as positive controls. hESC-RUNX2-YFP cells differentiated in basal media supplemented with 10% KOSR was taken as negative control (NC). β-Actin was taken as a loading control. Error bars indicate the mean ± SEM (n = 3). * = p < .05 versus OSTEOCALCIN of Sample “NC”. # = p < .01 versus RUNX2 of Sample “NC”, ** = p < .05 versus OSTERIX of Sample “NC”. (C): qRT-PCR showing of pluripotency genes in hESC-RUNX2-YFP derived osteogenic cells differentiated in osteogenic conditions as indicated. Undifferentiated hESC-RUNX2-YFP cells were taken as positive control. hESC-RUNX2-YFP cells differentiated in osteogenic media supplemented with 10% FBS and in basal media with 10% KOSR served as negative control. Three technical replicates of same samples were analysed. Error bars indicate the mean ± SD. (D): Images showing the immunofluorescence staining of OCT 4 in hESC-RUNX2-YFP derived osteogenic cells differentiated in osteogenic conditions as indicated. Undifferentiated hESC-RUNX-YFP cells were taken as positive control. hESC-RUNX-YFP cells differentiated in osteogenic media supplemented with 10% FBS was taken as negative control. (E): Von Kossa staining showing the calcification in osteogenic cells derived from hESC-RUNX2-YFP differentiated using conditions as indicated. hESC-RUNX-YFP differentiated in osteogenic media supplemented with 10% FBS and Saos2 cells were taken as positive controls. hESC-RUNX-YFP differentiated in basal media supplemented with 10% KOSR was taken as negative control. (D and E): Scale bar represents 50 µm. (A–E): All analyses were done at Day 28. Abbreviations: Ab: antibody; Fib: fibrinogen; FBS: fetal bovine serum; GF: growth factors; hESC: undifferentiated human embryonic stem cells; MSC: mesenchymal stem cells; NC: negative control; Osteo: osteogenic media; OP: osteopontin; Saos2: sarcoma osteogenic cell.
Finally, we wanted to demonstrate fibrinogen-mediated osteogenic development from human iPSC. Previously optimized conditions of fibrinogen resulted in a similar osteogenic effect in UiPSC (umbilical cord blood-derived iPSC produced previously by our group) [16]. UiPSC were able to efficiently differentiate into osteogenic lineages mediated by fibrinogen. Cells were characterized at mRNA (Fig. 7A) and protein level (Fig. 7B) to confirm the expression of osteogenic genes and markers respectively. Fibrinogen mediated iPSC-derived osteogenic cells were also able to stain positively for Von Kossa staining (Fig. 7C).
Figure 7.
Characterization of fibrinogen-mediated osteogenic cells derived from iPSC: (A): qRT-PCR of osteogenic genes and pluripotency genes in iPSC derived osteogenic cells differentiated in osteogenic media supplemented with 10% KOSR and 1.5 ug/ml of fibrinogen or AGF (BMP + Wnt3a + FGF9 and rapamycin). iPSC differentiated in osteogenic media supplemented with 10% FBS was taken as positive control. iPSC differentiated in basal media supplemented with 10% KOSR and undifferentiated iPSC were taken as negative controls. Error bars = mean ± SEM (n = 3); ++ = p < .05 versus OCT 4 gene of undifferentiated iPSC, * = p < .05 versus RUNX2 gene of undifferentiated iPSC, # = p < .05 versus DLX5 gene of undifferentiated iPSC, + = p < .05 versus OSTERIX gene of undifferentiated iPSC. Analysis was done at day 6. (B): Immunofluorescence staining of OSTEOCALCIN (green) and OSTEOPONTIN (red) in iPSC-derived osteogenic cells differentiated in osteogenic conditions as indicated. Undifferentiated iPSC served as negative control. iPSC differentiated in osteogenic media supplemented with 10% FBS served as positive controls. Samples without primary antibody (1°) were used as staining control. (C): Von Kossa staining showing the calcification in iPSC derived osteogenic cells differentiated in osteogenic conditions as indicated. iPSC differentiated in osteogenic media supplemented with 10% FBS and Saos2 cells were taken as positive controls. iPSC differentiated in basal media supplemented with 10% KOSR was taken as negative control. (B and C): Scale bar represents 50 µm. Analyses were done at Day 28. Abbreviations: Ab: antibody; AGF: all growth factors; Fib: fibrinogen; FBS: fetal bovine serum; GF: growth factors; iPSC: undifferentiated induced pluripotent stem cells; Osteo: osteogenic media; OC: osteocalcin; OP: osteopontin; Saos2: sarcoma osteogenic cell.
Discussion
Pluripotent stem cells provide an excellent model to study signaling pathways mediating early human development, including into bone cells [16, 36]. However, previous strategies have typically utilized FBS-containing conditions, making it difficult to identify of distinct cellular mechanisms that mediate this early human osteogenic development [2]. Here, we used hESC-RUNX2-YFP cellular reporter system to identify defined serum-free conditions that mediate early human osteogenic development. In addition to combinations of known growth factors, we found that fibrinogen alone could mediate osteogenic differentiation. Previously, osteogenic potential of fibrinogen has been reported in MSCs [24, 37]. However, this fibrinogen-mediated differentiation of hESC and iPSC towards the osteogenic lineage and fibrinogen-mediated downstream pathway during osteogenic induction has not been reported previously. Here, we demonstrated that fibrinogen binds to α9β1which leads to SMAD 1/5/8 phosphorylation and RUNX2 expression.
BMP2-mediated SMAD 1/5/8 activation is known to induce osteoblastic differentiation of MSC and plays an important role in bone healing [38, 39]. Additionally, Kumar and coworkers reported that BMP2-mediated SMAD/1/5/8 activation requires additional activation of Wnt signaling pathways to mediate significant osteogenic induction [21]. Similarly, we found that BMP2 required additional growth factors (Wnt, FGF9 and rapamycin) to mediate significant osteogenic differentiation from hESC-RUNX2-YFP cells. Human serum [40], platelet-rich plasma and human platelet lysate[41] have all been shown to induce osteogenic differentiation of MSCs. Additionally, several studies also demonstrate that the components of human plasma i.e., fibrinogen [23, 24], 2-HS [25] and thrombin [26] stimulate osteoblast differentiation. We used our recently reported hESC-RUNX2-YFP reporter system to identify the osteogenic potential of these human proteins [4]. Interestingly and somewhat unexpectedly, fibrinogen alone was found to have significant osteo-induction potential. Moreover, different combinations of fibrinogen with thrombin and 2-HS resulted in a reduction of the osteogenic effect of fibrinogen. A possible explanation for this could be that the other proteins could be competing with fibrinogen for ligand interactions. Moreover, thrombin cleaves fibrinogen and releases fibrinopeptides which could inhibit the fibrinogen effect (Fig. 2A, 2B) [42].
Previous studies demonstrate α9β1 integrin can mediate cross-talk between human hematopoietic stem and osteoblastic progenitor cells in the bone marrow stem cell niche and plays an important role in osteoblast-specific Runx2 expression in vivo [43]. Furthermore, the osteoblastic cells adhesive interaction in endosteal stem cell niche depends on α9β1 [43]. Additionally, α9β1 is also responsible for signal transduction between osteoblast and hematopoietic stem cells in endosteal stem cell niche [43]. While a previous report did not find α9β1 interacts with fibrinogen [44], as we characterized here (Fig. 4D), this previous study used α9-transfected human embryonic kidney cell line 293 (which express β1) and the human colon carcinoma cell line SW480 cell lines which are markedly different from our experimental model. Additionally, we also found that fibrinogen—α9β1 complex induces endogenous SMAD 1/5/8 pathway activation which mediates RUNX2 expression (Fig. 5A, 5B; Supporting Information Fig. S1D). We analyzed SMAD 1/5/8 pathway because the SMAD 1/5/8-RUNX2 axis in osteogenic differentiation is well established [20, 22]. Furthermore, our study demonstrates novel mechanistic insight of fibrinogen- α9β1- SMAD 1/5/8-RUNX2 axis to mediate osteogenic differentiation of human pluripotent stem cells. We also found that fibrinogen—α9β1 complex is not the sole pathway for the induction of SMAD 1/5/8 pathway and osteogenic differentiation. While fibrinogen was found to mediate significantly lower induction of SMAD 1/5/8-RUNX2 axis in α9β1-negative cells (Supporting Information Fig. S1D), some activation of SMAD pathway in α9β1-negative hESC-RUNX2-YFP cells by the treatment of fibrinogen could be due to the interaction of fibrinogen with other integrins or receptors on the cells surface. Interestingly, we also found that the treatment of α9β1 negative—SMAD pathway inhibited—hESC-RUNX2-YFP cells with osteogenic media supplemented with FBS resulted in significantly lower expression of RUNX2 (Supporting Information Fig. S1D). FBS is known to activate several other pathways [2]. Therefore, the possible explanation for the RUNX2 expression in α9β1 negative— SMAD pathway inhibited—hESC-RUNX2-YFP cells by FBS could be due to other non-SMAD pathways. Overall, these studies still demonstrate that fibrinogen-α9β1-SMAD 1/5/8 axis plays an important role in RUNX2 expression.
As more hESC and iPSC-derived cells move into clinical trials, better defining and standardizing culture conditions to mediate efficient differentiation into defined cell lineages has become of greater interest [45]. While additional studies are necessary to analyze the potential of serum-free, fibrinogen-mediated osteogenic cells from hESC/iPSC to form a bone cells suitable for fracture repair in vivo, the characterization of this defined pathway may facilitate clinical translation. Additionally, these studies open new avenues to better understand basic mechanisms that control the coordinated regulation of transcription factors and proteins involved in human osteogenic development.
Supplementary Material
Significance Statement.
These studies use a novel human pluripotent stem cell-based RUNX2 reporter system to better define signaling pathways that mediate early human bone development. Specifically, these studies demonstrate fibrinogen (a component of human serum) stimulates a signaling pathway that leads to bone production. These studies better define serum-free conditions that mediate bone development and will help better produce stem cell-derived cells for bone repair and regeneration.
Acknowledgments
We graciously thank Drs. Jennifer Westendorf and Andre J. van Wijnen (Mayo Clinic) for part of the RUNX2 vector. With thank Dr. Conrado Aparicio and Mat Anglos for helpful review of the manuscript. This work is supported by NIH/NIDCR grants DE022556 (D.S.K.) and R90 DE023058 (F.K.K.).
Dr. Kaufman serves as a consultant and receives research funding from Fate Therapeutics for work unrelated to these studies.
Footnotes
Author Contributions
F.K. and D.K.: Study design. F.K. and J.E.: Data acquisition. F.K., J.E., L.Z., and D.K.: Data analysis. F.K. and D.K.: Data analysis and interpretation. F.K.: Drafting manuscript. D.K. and F.K., takes responsibility for the integrity of the data analysis: Approval final version of manuscript.
Disclosure of Conflicts of Interest
There are no other conflicts of interest.
See www.StemCells.com for supporting information available online.
References
- 1.Arpornmaeklong P, Wang Z, Pressler MJ, et al. Expansion and characterization of human embryonic stem cell-derived osteoblast-like cells. Cell Reprogram. 2010;12:377–389. doi: 10.1089/cell.2009.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanke K, Masaki H, Saito T, et al. Stepwise differentiation of pluripotent stem cells into osteoblasts using four small molecules under serum-free and feeder-free conditions. Stem Cell Reports. 2014;2:751–760. doi: 10.1016/j.stemcr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng Y, Bocker MT, Holm J, et al. Human fibroblast matrices bio-assembled under macromolecular crowding support stable propagation of human embryonic stem cells. J Tissue Eng Regen Med. 2012;6:e74–e86. doi: 10.1002/term.1560. [DOI] [PubMed] [Google Scholar]
- 4.Zou L, Kidwai FK, Kopher RA, et al. Use of RUNX2 expression to identify osteogenic progenitor cells derived from human embryonic stem cells. Stem Cell Reports. 2015;4:190–198. doi: 10.1016/j.stemcr.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komori T. Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol. 2010;658:43–49. doi: 10.1007/978-1-4419-1050-9_5. [DOI] [PubMed] [Google Scholar]
- 6.Maruyama Z, Yoshida CA, Furuichi T, et al. Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Dev Dyn. 2007;236:1876–1890. doi: 10.1002/dvdy.21187. [DOI] [PubMed] [Google Scholar]
- 7.Sudhakar S, Li Y, Katz MS, et al. Translational regulation is a control point in RUNX2/Cbfa1 gene expression. Biochem Biophys Res Commun. 2001;289:616–622. doi: 10.1006/bbrc.2001.6033. [DOI] [PubMed] [Google Scholar]
- 8.Dalle Carbonare L, Innamorati G, Valenti MT, et al. Transcription factor Runx2 and its application to bone tissue engineering. Stem Cell Rev. 2012;8:891–897. doi: 10.1007/s12015-011-9337-4. [DOI] [PubMed] [Google Scholar]
- 9.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 10.Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi H, Gao YH, Ueta C, et al. Multilineage differentiation of Cbfa1-deficient calvarial cells in vitro. Biochem Biophys Res Commun. 2000;273:630–636. doi: 10.1006/bbrc.2000.2981. [DOI] [PubMed] [Google Scholar]
- 12.Lee KW, Yook JY, Son MY, et al. Rapamycin promotes the osteoblastic differentiation of human embryonic stem cells by blocking the mTOR pathway and stimulating the BMP/smad pathway. Stem Cells Dev. 2010;19:557–568. doi: 10.1089/scd.2009.0147. [DOI] [PubMed] [Google Scholar]
- 13.Behr B, Leucht P, Longaker MT, et al. Fgf-9 is required for angiogenesis and osteogenesis in long bone repair. Proc Natl Acad Sci U S A. 2010;107:11853–11858. doi: 10.1073/pnas.1003317107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrison KR, Shemilt I, Donell S, et al. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev. 2010;6:CD006950. doi: 10.1002/14651858.CD006950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JH, Liu X, Wang J, et al. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther Adv Musculoskelet Dis. 2013;5:13–31. doi: 10.1177/1759720X12466608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopher RA, Penchev VR, Islam MS, et al. Human embryonic stem cell-derived CD34+ cells function as MSC progenitor cells. Bone. 2010;47:718–728. doi: 10.1016/j.bone.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng JH, Heng JCD, Loh YH, et al. Transcriptional and epigenetic regulations of embryonic stem cells. Mutat Res. 2008;647:52–58. doi: 10.1016/j.mrfmmm.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson JG. Immunofluorescence staining. In: Coligan John E., editor. Curr Protoc Immunol. Unit 21.3. Chapter21. 2002. pp. 1–6. [DOI] [PubMed] [Google Scholar]
- 19.Selth LA, Close P, Svejstrup JQ, et al. Studying RNA-protein interactions in vivo by RNA immunoprecipitation. Methods Mol Biol. 2011;791:253–264. doi: 10.1007/978-1-61779-316-5_19. [DOI] [PubMed] [Google Scholar]
- 20.Dragoo JL, Choi JY, Lieberman JR, et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 21.Kumar J, Swanberg M, McGuigan F, et al. LRP4 association to bone properties and fracture and interaction with genes in the Wnt- and BMP signaling pathways. Bone. 2011;49:343–348. doi: 10.1016/j.bone.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Rosen V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009;20:475–480. doi: 10.1016/j.cytogfr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Karp JM, Sarraf F, Shoichet MS, et al. Fibrin-filled scaffolds for bone-tissue engineering: An in vivo study. J Biomed Mater Res A. 2004;71:162–171. doi: 10.1002/jbm.a.30147. [DOI] [PubMed] [Google Scholar]
- 24.Linsley C, Wu B, Tawil B, et al. The effect of fibrinogen, collagen type I, and fibronectin on mesenchymal stem cell growth and differentiation into osteoblasts. Tissue Eng Part A. 2013;19:1416–1423. doi: 10.1089/ten.TEA.2012.0523. [DOI] [PubMed] [Google Scholar]
- 25.Szweras M, Liu D, Partridge EA, et al. alpha 2-HS glycoprotein/fetuin, a transforming growth factor-beta/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J Biol Chem. 2002;277:19991–19997. doi: 10.1074/jbc.M112234200. [DOI] [PubMed] [Google Scholar]
- 26.Han B, Woodell-May J, Ponticiello M, et al. The effect of thrombin activation of platelet-rich plasma on demineralized bone matrix osteoinductivity. J Bone Joint Surg Am. 2009;91:1459–1470. doi: 10.2106/JBJS.H.00246. [DOI] [PubMed] [Google Scholar]
- 27.Prowse ABJ, Doran MR, Cooper-White JJ, et al. Long term culture of human embryonic stem cells on recombinant vitronectin in ascorbate free media. Biomaterials. 2010;31:8281–8288. doi: 10.1016/j.biomaterials.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 28.Yang JT, Rayburn H, Hynes RO, et al. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Diacovo TG, Grenache DG, et al. The α Integrin subunit-deficient mouse. A multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmits R, Kündig TM, Baker DM, et al. LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J Exp Med. 1996;183:1415–1426. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gailit J, Clark RA. Studies in vitro on the role of alpha v and beta 1 integrins in the adhesion of human dermal fibroblasts to provisional matrix proteins fibronectin, vitronectin, and fibrinogen. J Invest Dermatol. 1996;106:102–108. doi: 10.1111/1523-1747.ep12328177. [DOI] [PubMed] [Google Scholar]
- 32.Yang JT, Rayburn H, Hynes RO, et al. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 33.Tronik-Le Roux D, Roullot V, Poujol C, et al. Thrombasthenic mice generated by replacement of the integrin alpha(IIb) gene: Demonstration that transcriptional activation of this megakaryocytic locus precedes lineage commitment. Blood. 2000;96:1399–1408. [PubMed] [Google Scholar]
- 34.Bazigou E, Xie S, Chen C, et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu PB, Beppu H, Kawai N, et al. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280:24443–24450. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- 36.Mahmood A, Napoli C, Aldahmash A, et al. In vitro differentiation and maturation of human embryonic stem cell into multipotent cells. Stem Cells Int. 2011;2011:735420. doi: 10.4061/2011/735420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim BS, Kim HJ, Choi JG, et al. The effects of fibrinogen concentration on fibrin/atelocollagen composite gel: An in vitro and in vivo study in rabbit calvarial bone defect. Clin Oral Implants Res. 2015;26:1302–1308. doi: 10.1111/clr.12455. [DOI] [PubMed] [Google Scholar]
- 38.Polak SJ, Levengood SKL, Wheeler MB, et al. Analysis of the roles of microporosity and BMP-2 on multiple measures of bone regeneration and healing in calcium phosphate scaffolds. Acta Biomater. 2011;7:1760–1771. doi: 10.1016/j.actbio.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 39.Luzi E, Marini F, Sala SC, et al. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Miner Res. 2008;23:287–295. doi: 10.1359/jbmr.071011. [DOI] [PubMed] [Google Scholar]
- 40.Aldahmash A, Haack-Sørensen M, Al-Nbaheen M, et al. Human serum is as efficient as fetal bovine serum in supporting proliferation and differentiation of human multipotent stromal (mesenchymal) stem cells in vitro and in vivo. Stem Cell Rev. 2011;7:860–868. doi: 10.1007/s12015-011-9274-2. [DOI] [PubMed] [Google Scholar]
- 41.Kawase T, Okuda K, Saito Y, et al. Platelet-rich plasma provides nucleus for mineralization in cultures of partially differentiated periodontal ligament cells. In Vitro Cell Dev Biol Anim. 2005;41:171–176. doi: 10.1290/0502013.1. [DOI] [PubMed] [Google Scholar]
- 42.Binnie CG, Lord ST. The fibrinogen sequences that interact with thrombin. Blood. 1993;81:3186–3192. [PubMed] [Google Scholar]
- 43.Schreiber TD, Steinl C, Essl M, et al. The integrin alpha9beta1 on hematopoietic stem and progenitor cells: Involvement in cell adhesion, proliferation and differentiation. Haematologica. 2009;94:1493–1501. doi: 10.3324/haematol.2009.006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokosaki Y, Palmer EL, Prieto AL, et al. The integrin alpha 9 beta 1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J Biol Chem. 1994;269:26691–26696. [PubMed] [Google Scholar]
- 45.Kimbrel EA, Lanza R. Current status of pluripotent stem cells: moving the first therapies to the clinic. Nat Rev Drug Discov. 2015;14:681–692. doi: 10.1038/nrd4738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.