Abstract
Background
Thoracic aortic aneurysms (TAAs) are common, but experimental TAA models are limited and the role of interleukin-1β (IL-1β) is undetermined.
Methods and Results
IL-1β protein was measured in human TAAs and control aortas, and IL-1β protein was increased ≈20-fold in human TAAs. To develop an experimental model of TAAs, 8- to 10-week-old male C57Bl/6 mice (wild type [WT]) underwent thoracotomy with application of periadventitial elastase (WT TAA) or saline (WT control; n=30 per group). Elastase treatment to thoracic aortas resulted in progressive dilation until day 14 with maximal dilation of 99.6±24.7% compared with 14.4±8.2% for WT saline control (P<0.0001). WT TAAs demonstrated elastin fragmentation, smooth muscle cell loss, macrophage infiltration, and increased IL-1β expression. Next, TAAs were induced in mice deficient of IL-1β (IL-1β knockout) or IL-1 receptor (IL-1R knockout; n=10 each). Genetic deletion of IL-1β and IL-1R significantly decreased thoracic aortic dilation (IL-1β knockout=54.2±16.8% and IL-1R knockout=62.6±17.2% versus WT TAA=104.7±23.8%; P<0.001for both). IL-1β knockout and IL-1R knockout aortas demonstrated preserved elastin and smooth muscle cells with fewer inflammatory cells. Correspondingly, IL-1β and IL-1R knockout aortas had decreased inflammatory cytokine and matrix metalloproteinase 9 expression. Separately, WT mice pretreated with either IL-1R antagonist anakinra (100 mg/kg per day) or vehicle alone (control) underwent elastase treatment. Pretreatment of WT mice with anakinra attenuated TAA formation (control: 99.2±15.5% versus anakinra: 68.3±19.2%; P<0.005). Finally, to investigate treatment of small TAAs, WT mice were treated with anakinra 3 days after TAA induction. Anakinra treatment in WT mice with small TAAs reduced aortic dilation on day 14 (control treatment: 89.1±18.6% versus anakinra treatment: 59.7±25.7%; P=0.01).
Conclusions
Periadventitial application of elastase to murine thoracic aortas reproducibly produced aneurysms with molecular and histological features consistent with TAA disease. Genetic and pharmacological inhibition of IL-1β decreased TAA formation and progression, indicating that IL-1β may be a potential target for TAA treatment.
Keywords: aneurysm, animal models of human disease, inflammation, interleukin-1, interleukin-1 receptor antagonist protein
The descending thoracic aorta is involved in 30% to 40% of patients with thoracic aortic aneurysms (TAAs).1,2 TAAs tend to progress in size over time, increasing the risk of aortic rupture.3 TAA rupture is lethal, and elective repair carries significant morbidity. Thus, medical therapies that could slow aneurysm progression and prevent the need for surgery are needed.4 Presently, there are no specific medical therapies to treat or slow the progression of TAAs, largely because of an incomplete understanding of TAA pathogenesis.
Most of the scientific knowledge on the pathogenesis of TAAs is derived from experimental models using the abdominal aorta.5 Although the thoracic and abdominal aortas share multiple similarities, regional heterogeneity exists within the aorta, and the thoracic aorta differs from the abdominal aorta in structure and embryological origin.6,7 The thoracic aorta has increased elastin and collagen content, as well as an increased elastin:collagen ratio compared with the abdominal aorta. The thoracic aorta also has a more prevalent vasa vasorum than the abdominal aorta, resulting in less vessel wall ischemia. Finally, the embryological origin of cells within the thoracic aorta is heterogeneous and regionally distinct from the abdominal aorta.5 Based on these structural and developmental differences, a reliable model for TAAs is needed to investigate pathogenesis and potential treatment options for TAAs.
Although the thoracic aorta and abdominal aorta are regionally heterogeneous, aneurysms in both locations involve chronic inflammatory conditions, with extensive degradation of extracellular matrix and smooth muscle cell (SMC) loss leading to aortic wall weakening and dilation.8,9 Targeting inflammatory aspects of TAAs may provide insight into TAA formation and treatment. Interleukin-1β (IL-1β) is one of the key upstream mediators of inflammation and has previously been demonstrated to be critical in experimental abdominal aortic aneurysm (AAA) formation.10,11 IL-1β is upregulated in TAAs and functions by creating a cycle of inflammation through binding its receptor (IL-1R), releasing multiple proinflammatory cytokines, including more IL-1β.10,12 However, as of yet, there is no direct evidence that IL-1β contributes to the development of TAAs.
The purpose of this study was to develop a novel model of TAAs with reproducible, robust (>50% than baseline) aortic dilation and test the hypothesis that IL-1β plays a critical role in TAA formation and either genetic or pharmacological inhibition of IL-1β can decrease TAA formation and progression.
Methods
Animal protocols were approved by the University of Virginia Institutional Animal Care and Use Committee (No. 3634).
Elastase TAA Model
A novel method for TAAs was evaluated in 8- to 12-week-old male C57Bl/6 (wild-type [WT] TAA) mice after orotracheal intubation and thoracic aortic exposure through a left thoracotomy incision (detailed in the online-only Data Supplement). Technical aspects were adapted from the Ikonomidis’ topical calcium chloride (CaCl2) model.13 The overlying pleura was opened, and a 5 mm×1 mm sponge soaked with undiluted purified porcine elastase (Sigma Aldrich, St. Louis, MO) was applied for 5.5 minutes. Afterward, the left lung was re-expanded, and the chest was closed in layers. Mice were extubated and allowed to recover. At the time of aortic harvest at days 3, 7, 14, 21, or 28, mice were reintubated, and the thoracotomy was opened. The aorta was dissected free, and maximal aortic diameter was measured using photomicrometry while the animal was alive (n=5 per group per time point). The aorta was then harvested for further analysis.
In the control group, similarly aged WT mice underwent left thoracotomy, pleural dissection, and sponge application with saline alone (WT control). Aortic dilation was similarly evaluated over time. Aortas were collected for immunohistochemistry from elastin fibers, smooth muscle α-actin (SMαA), Mac2, and IL-1β. Histology was quantitatively graded by an experienced blinded reviewer and compared with χ2 test (detailed in the online-only Data Supplement). Of the 60 mice used to compare WT TAAs with WT controls, operative mortality was 8.3% (5 of 60 mice). Causes of death included damage to the lung during initial dissection leading to persistent pneumothorax (n=2), oversedation (n=1), empyema (n=1), and exsanguination during harvest (n=1).
IL-1β in Human TAA
Human TAA tissue was obtained from patients undergoing open descending thoracic aneurysm repair (n=12). Patient characteristics are shown in the Table. Control thoracic aortic tissue was obtained from organ transplantation donors with healthy aortas (n=5). Tissue collection was approved by the University of Virginia Institutional Review Board for Health Sciences Research (No. 13178) with informed consent. Thoracic aortic protein was isolated from human tissue, and IL-1β protein content was determined with ELISA. Further evaluation was performed using immunohistochemistry and confocal microscopy to detect IL-1β in human thoracic aortas (detailed in the online-only Data Supplement).
Table.
Characteristics of Patients With Thoracic Aortic Aneurysms
| Age (mean±SD), y | 70.6±9.3 |
| Sex | 54% women (n=7/13) |
| Aortic diameter, cm | 6.7±0.6 |
| Comorbidities | |
| Hypertension | 77% (n=10/13) |
| Peripheral artery disease | 62% (n=8/13) |
| Smoking | 54% (n=7/13) |
| Coronary artery disease | 38% (n=5/13) |
| COPD | 23% (n=4/13) |
| Connective tissue disorder | 8% (n=1/13) |
COPD indicates chronic obstructive pulmonary disease.
Genetic Deletion of IL-1β and IL-1R
Eight- to 12-week-old mice with genetic deletion of IL-1β or IL-1R on a C57/Bl6 background (IL-1β knockout and IL-1R knockout) underwent the elastase TAA model as above. Mice were acquired from Dr Gary Owens at the University of Virginia and bred onto a C57/Bl6 background in-house.14 IL-1β knockout and IL-1R knockout mice were used as 2 approaches to disrupt IL-1β signaling and compared with WT TAA (n=10/group). Aortic dilation was measured 14 days after elastase exposure, and aortas were collected for protein analysis (n=7/group) and histology (n=3/group). Aortas were stained for markers of aortic wall structure (SMαA for SMCs and Verhoeff–Van Gieson for elastin fibers) and inflammatory cells (Mac2 for macrophages and Ly6B for neutrophils). Additional mice in each group underwent the TAA model with aortic harvest on day 7 for inflammatory cytokine protein array and gelatin zymography to evaluate matrix metalloproteinases (n=5/group, detailed in online-only Data Supplement).
In Vitro z-VAD Treatment
Confocal immunohistochemistry was performed on day 14 WT TAA and WT control samples to evaluate IL-1β, caspase-1, and the Nod-like receptor, pyrin domain containing-3 (NLRP3 or NALP3) inflammasome protein. Separately, in vitro experiments were performed on 3 groups of explanted WT mouse thoracic aortas exposed to the following: (1) vehicle alone, (2) 10% elastase solution, and (3) 10% elastase solution with the pan-caspase inhibitor z-VAD (InvivoGen, San Diego, CA [50 μmol/L]) treatment for 2 hours before and after elastase treatment (n=6 thoracic aortas/group). After 24 hours, IL-1β protein and gene expression was measured in the supernatant (detailed in online-only Data Supplement).
IL-1R Antagonism
Pharmacological antagonism of IL-1R was evaluated with anakinra (Kineret; Biovitrum, Stockholm, Sweden), a commercially available recombinant human IL-1R antagonist.15 Anakinra does not affect heart rate or blood pressure.16 Because anakinra’s half-life is 4 to 6 hours, osmotic pumps were placed subcutaneously to deliver a steady dose of anakinra without subjecting mice to multiple intraperitoneal injections (pump placement detailed in online-only Data Supplement).
As a prevention strategy, 100 mg/kg per day of anakinra was given 3 days before TAA induction in WT mice. WT mice with osmotic pumps filled with vehicle alone were used as controls. To evaluate anakinra for small aneurysm treatment, anakinra delivery was started 3 days after TAA induction and compared with WT control mice with vehicle exposure. Aortic dilation was measured for all groups at day 14, and aortas were collected.
Statistical Analysis
Maximal aortic dilation was calculated by (maximal aortic diameter–internal control diameter)/maximal aortic diameter×100%. The internal control diameter was a section of the thoracic aorta distal to elastase exposure and above the diaphragm where the overlying pleura was not exposed. Using this internal control accounted for natural growth of the aorta, as well as changes in blood pressure because of blood loss after aortic dissection. All diameters were measured from the same photo while the animal was still alive to minimize error because of changes from systolic and diastolic blood pressure. This method allows reproducible aortic measurements with minimal interobserver variability.11 Statistical analysis was performed using GraphPad Prism (GraphPad, San Diego, CA). Values are reported as mean± SD. Mann–Whitney test was used to determine differences between 2 groups. For multiple groups, nonparametric Kruskal–Wallis test was used with the Dunn multiple comparison test to detect differences between individual groups with α=0.05. Similar analysis was performed based on maximal aortic diameter alone and demonstrated concordant results (Figure I in the online-only Data Supplement).
Results
Periadventitial Elastase Treatment of the Thoracic Aorta Results in Formation of TAAs
Application of periaortic elastase led to significant increases in aortic dilation compared with saline at days 3, 7, 14, 21, and 28 after exposure (Figure 1A). Maximal aortic dilation occurred at day 14 (WT TAA: 99.6±24.7% versus WT control: 14.4±8.2%; P<0.0001). WT TAAs had fragmented and thinned elastin fibers, less SMαA staining, increased Mac2 expression, and increased IL-1β expression (Figure 1B). Together, elastase application resulted in reproducible thoracic aortic dilation associated with aortic structural breakdown and markers of inflammation consistent with that seen in human disease.
Figure 1.
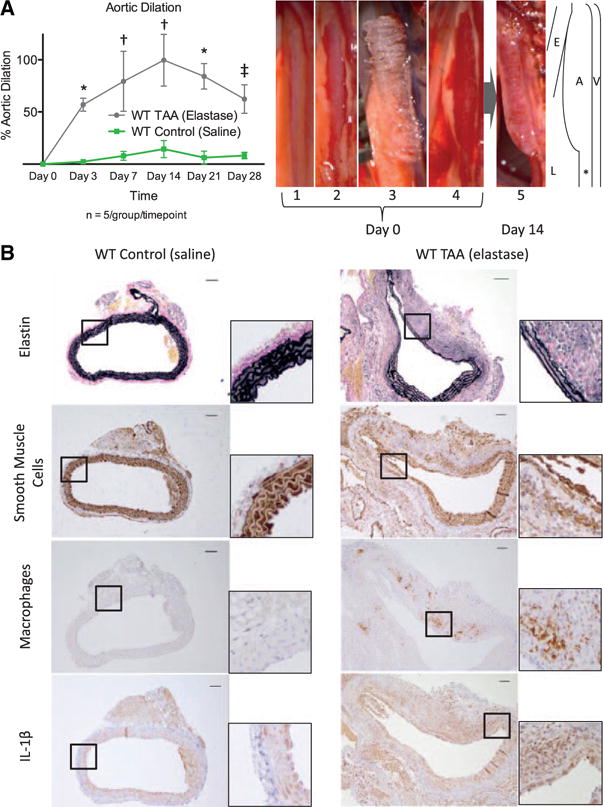
A, Aortic dilation over time for wild-type (WT) mice exposed to elastase (gray) and saline (green). *P<0.001, †P<0.0001, ‡P<0.01. Sample photographs of the thoracic aortic aneurysm (TAA) model showing (1) initial exposure of the thoracic aorta through a left thoracotomy, (2) dissection of the pleura, (3) application of an elastase soaked sponge, and (4) sponge removal. For aortic harvest (5), the thoracotomy was reopened, and thoracic aorta was dissected free and measured. Illustration to the right demonstrates relative association of aorta (A) with esophagus (E), lung (L), and hemiazygos vein (V). The internal control section of unexposed aorta is marked with *. B, Sample cross-sections of WT control and WT TAAs on day 14 staining for elastin, smooth muscle cells, macrophages, and interleukin-1β (IL-1β). Scale bar, 50 μm.
IL-1β Is Upregulated in Human TAAs
IL-1β protein levels in human TAAs were ≈20-fold more than normal thoracic aortas (P<0.001; Figure 2A). Accordingly, human TAAs demonstrated increased IL-1β staining (Figure 2B). Using confocal immunohistochemistry, IL-1β colocalized with cells expressing Mac2, as well as SMαA (Figure 3), suggesting that both macrophages and SMCs are sources of IL-1β in human TAAs. IL-1R colocalized primarily with SMαA+ cells.
Figure 2.
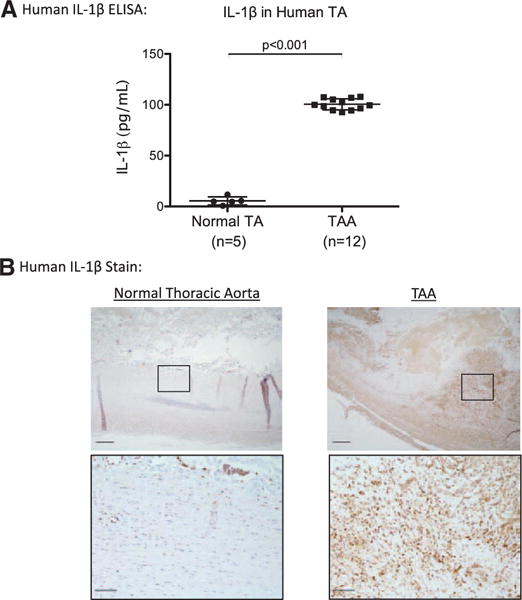
A, Human thoracic aortic aneurysms (TAAs) had significantly increased amounts of interleukin-1β (IL-1β) protein compared with normal thoracic aorta (TA) by ELISA. B, Correspondingly, TAAs had increased staining for IL-1β compared with normal TAs. IL-1β antibody is shown in brown. Scale bar, 200 μm.
Figure 3.
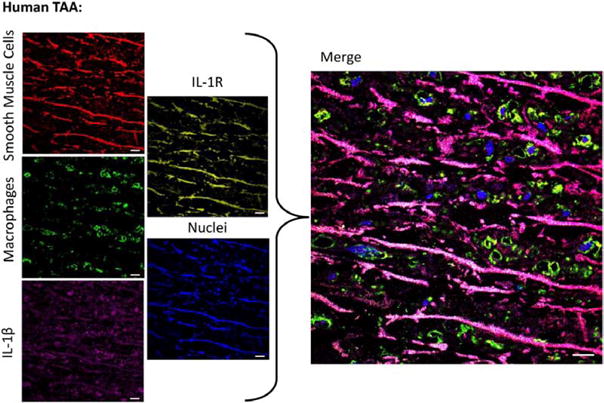
Confocal fluorescence immunohistochemistry demonstrates interleukin-1β (IL-1β; purple) colocalizing with smooth muscle cell markers (red) and macrophage markers (green). Interleukin-1 receptor (IL-1R; yellow) colocalizes primarily with smooth muscle α-actin+cells. Nuclei (DAPI) are shown in blue. Scale bar, 10 μm. TAA indicates thoracic aortic aneurysm.
IL-1β Is Required for TAA Formation
To test the hypothesis that IL-1β is critical in TAA formation, IL-1β knockout and IL-1R knockout mice were examined with the elastase TAA model. IL-1β knockout and IL-1R knockout mice had ≈50% and ≈40% reduction in aortic dilation, respectively, compared with WT mice (IL-1β knockout: 54.2±16.8% and IL-1R knockout: 62.6±17.2% versus WT TAA: 104.7±23.8%; both P<0.001 versus WT; Figure 4A). Aortic dilations in IL-1β knockout and IL-1R knockout mice were similar (P=0.29). In contrast to WT TAA, IL-1β and IL-1R knockout aortas had subjectively less elastin fragmentation, Mac2 staining, and neutrophil staining, with preserved concentric SMαA staining (Figure 4B).
Figure 4.
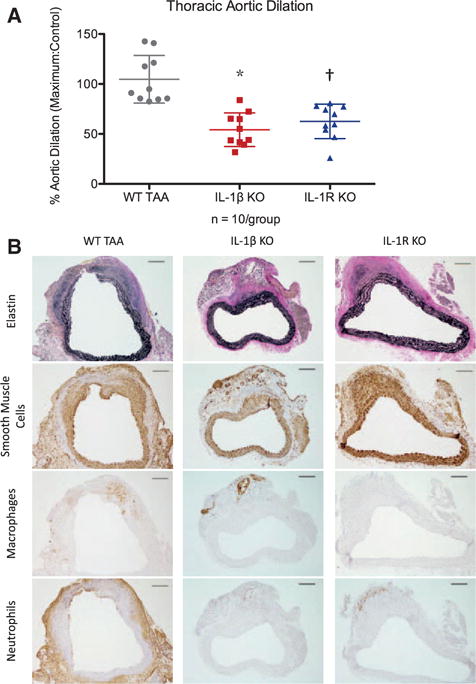
A, Thoracic aortic dilations in interleukin-1β (IL-1β) knockout (KO) and IL-1 receptor (IL-1R) KO mice exposed to elastase were significantly less than wild-type (WT) thoracic aortic aneurysm (TAA) mice. *P<0.0001, †P<0.001. B, By immunohistochemistry, representative samples demonstrated increased staining of elastin fibers (black) and smooth muscle cells (brown) in IL-1β KO and IL-1R KO mice compared with WT TAAs. IL-1β KO and IL-1R KO aortas also had decreased macrophage staining and neutrophil staining (brown). Scale bar, 100 μm.
To better define which proinflammatory cytokines and proteases contribute to TAAs, cytokine array and zymographic analyses were performed 7 days after elastase exposure. IL-1β and IL-1R knockout aortas had decreased proinflammatory cytokines in protein extracts, including tumor necrosis factor-α, interferon-γ, IL-1α, interleukin-6, interleukin-17, and interleukin-23 (Figure 5A). By gelatin zymography, levels of both pro- and active forms of matrix metalloproteinase 9 were decreased in IL-1β knockout and IL-1R knockout aortas compared with WT TAAs (Figure 5B).
Figure 5.
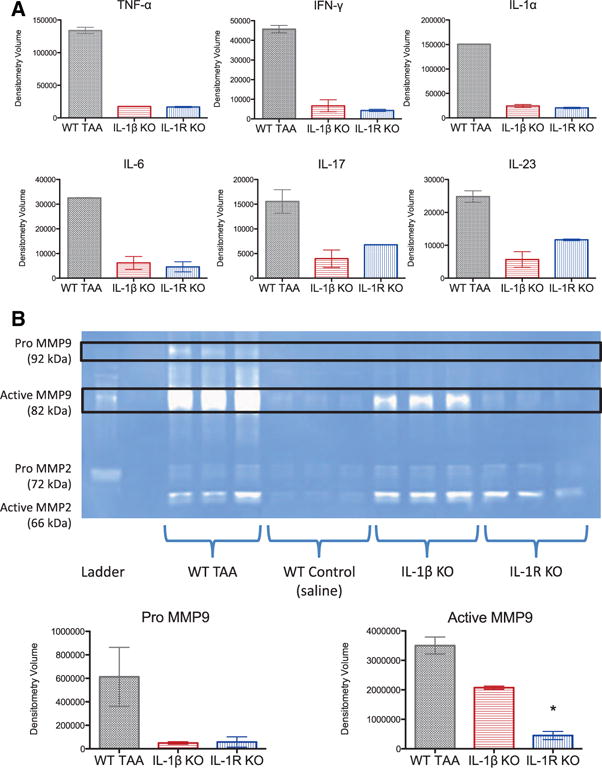
A, On day 7, interleukin (IL)-1β knockout (KO) and IL-1 receptor (IL-1R) KO aortas had decreased inflammatory cytokines determined by cytokine protein array with decreased levels of tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), IL-1α, IL-6, IL-17, and IL-23. B, By gelatin zymography, pro- and active forms of matrix metalloproteinase (MMP) 9 were decreased in thoracic aortas of IL-1β KO and IL-1R KO mice at day 7 (expressed as white bands on a blue background). MMP2 was detected in IL-1β KO and IL-1R KO aortas and did not differ from wild-type (WT) thoracic aortic aneurysms (TAAs). *P<0.01 versus WT TAA.
IL-1β Production by Thoracic Aorta Is Related to Caspase
In day 14 WT TAAs, caspase-1 and NLRP3 were detected in cells expressing IL-1β by confocal immunohistochemistry (Figure 6A). To further evaluate a potential role for caspase in TAA IL-1β production, explanted thoracic aortic tissue was treated with the pan-caspase inhibitor z-VAD before and after elastase exposure in vitro (Figure 6B). IL-1β protein and gene expression was significantly decreased in the supernatant of thoracic aortic tissue exposed to z-VAD, indicating that caspase may contribute to IL-1β production by thoracic aortic cells (Figure 6C).
Figure 6.
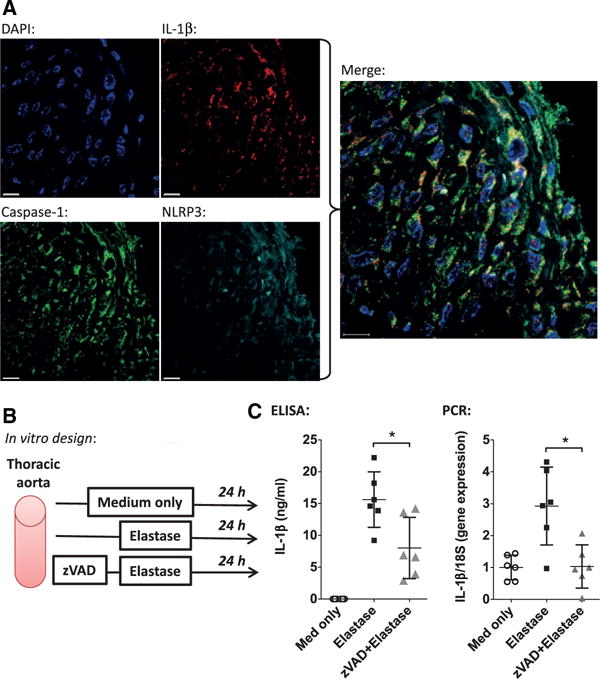
A, Confocal fluorescence immunohistochemistry of wild-type thoracic aortic aneurysm at day 14 demonstrating colocalization of cells (blue) expressing interleukin-1β (IL-1β; red), caspase-1 (green) and Nod-like receptor, pyrin domain containing-3 (NLRP3) inflammasome (teal). Scale bar, 10 μm. B, Experimental design with explanted thoracic aortas exposed to medium alone, elastase, or elastase with caspase inhibitor z-VAD pretreatment (50 μmol/L for 2 hours). C, ELISA and polymerase chain reaction (PCR) demonstrating significantly decreased IL-1β protein and gene expression after z-VAD treatment. *P<0.05.
Pharmacological IL-1R Antagonism Attenuates TAA Formation
Pharmacological inhibition of IL-1β during TAA formation was evaluated using recombinant human IL-1R antagonist, anakinra, which functioned as a competitive inhibitor of IL-1β binding. WT mice were treated with anakinra 100 mg/kg per day or with vehicle alone (control) starting 3 days before TAA induction with elastase (Figure 7A). Anakinra pretreatment was associated with a 30% reduction in aortic dilation (vehicle: 99.2±15.5% versus anakinra: 68.3±19.2%; P<0.005; Figure 7B). Anakinra application also correlated with preservation of elastin and SMC markers and decreased macrophage and neutrophil staining (Figure 7C).
Figure 7.
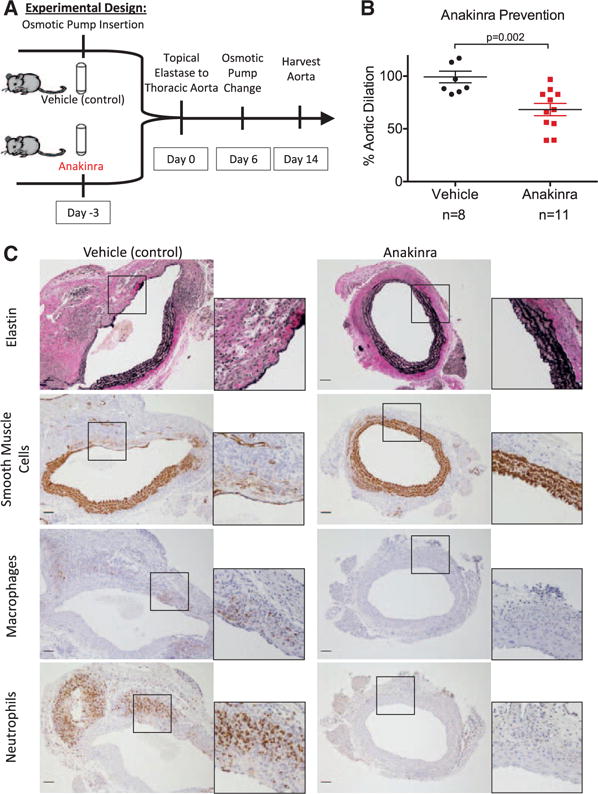
A, Experimental design for thoracic aortic aneurysm prevention in wild-type mice with anakinra. B, Anakinra pretreatment significantly decreased aortic dilation compared with pretreatment with vehicle only. C, Anakinra-pretreated mice had increased elastin and smooth muscle cell staining, along with decreased macrophage and neutrophil staining. Scale bar, 50 μm.
IL-1R Antagonism Slows Growth of Small TAAs
To test whether anakinra could inhibit small TAA growth, WT mice were given anakinra or vehicle solution 3 days after elastase TAA initiation (Figure 8A). By 3 days, small TAAs (defined as aortic dilation >50%) existed with aortic dilation of 56.9±6.2% in WT mice (Figure 1A). WT mice treated with anakinra had significantly reduced aortic dilation at day 14 compared with control treatment with vehicle alone (anakinra treatment: 59.7±25.7% versus vehicle treatment: 89.1±18.6%; P=0.01; Figure 8B). Anakinra treatment was associated with preserved elastin fibers and aortic structure (Figure 8C).
Figure 8.
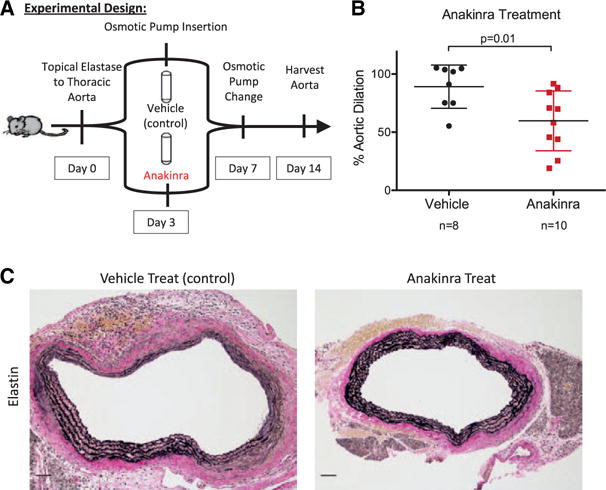
A, Experimental design for treatment of small thoracic aortic aneurysms (TAAs) with anakinra delivered 3 days after TAA induction. B, Anakinra treatment significantly decreased thoracic aortic dilation. C, Looking at aortic structure, anakinra treatment was associated with increased concentric rings of elastin staining. Scale bar, 50 μm.
Discussion
Periaortic elastase application to the thoracic aorta reproducibly generated robust TAAs with SMC loss, elastin degradation, inflammatory cell infiltration, and increased inflammatory cytokines. IL-1β was increased in both human and experimental TAAs, and inhibition of IL-1β, either through genetic deletion or pharmacological antagonism, decreased experimental TAA formation. Importantly, delayed antagonism of IL-1β limited TAA growth, suggesting a fundamental role for IL-1β during both formation and progression of TAAs.
Population studies suggest that TAA incidence is steadily increasing.17 However, the pathogenesis of TAAs remains incompletely understood. Other experimental models of TAAs exist, but these models have not been able to consistently generate aortic dilations >50%. The proposed model described here was adapted from Ikonomidis’ topical CaCl2 model.13 The elastase TAA model differs in 3 ways: (1) aorta was exposed to topical elastase for 5.5 minutes compared with topical CaCl2 exposure for 15 minutes, (2) aortic dilation occurred in 2 weeks compared with 16 weeks in CaCl2 model, and (3) aortic dilation was consistently ≈100%. This novel elastase TAA model generated large aneurysms in a shorter time frame.
Genetic deletion of IL-1β or IL-1R was associated with a significant reduction in thoracic aortic dilation and preservation of aortic structure. TAAs in mice with IL-1β or IL-1R deletion had less macrophage staining, inflammatory cytokines, and matrix metalloproteinase 9 levels. Together, the global reduction of inflammatory markers suggests that IL-1β signaling is one of the major inciting events in inflammation.18 Although IL-1β and inflammation are critical in TAA formation, the origin of the inflammation during TAA formation is not fully understood. Decreased IL-1β secretion by thoracic aortic cells in vitro with z-VAD suggests a critical upstream role for caspase in TAA IL-1β production. Z-VAD is a potent inhibitor of caspase, which cleaves inactive pro–IL-1β to its active form.19 Caspase activation occurs as part of the inflammasome, a large multiprotein complex containing the NLRP3 protein responsible for caspase activation.20 The NLRP3 inflammasome is known to play a role in multiple inflammatory conditions and is increased in TAAs.21 The presence of NLRP3 and caspase in experimental TAA sections, along with the decrease in IL-1β production with caspase inhibition, suggests that caspase and the NLRP3 inflammasome are potential alternative targets upstream of IL-1β production that may be useful for further investigation of TAA treatment.
Downstream of IL-1β activation, pharmacological inhibition of IL-1R with anakinra resulted in decreased TAA formation similar to IL-1β and IL-1R knockout mice. Fortunately, anakinra is approved for human use for treatment of rheumatoid arthritis and gout. Long-term daily use in humans is safe with infrequent adverse effects (most common side effects being injection site reaction and upper respiratory tract infections).22 There are multiple other pharmacological agents that target IL-1β signaling, including direct antibodies toward IL-1β, direct antibodies to IL-1R, and recombinant IL-1R inhibitors. Although IL-1β signaling was once thought to be limited to autoimmune and chronic inflammatory disorders, the role of IL-1β in vascular disease is becoming an area of active research. For example, canakinumab, a monoclonal antibody to IL-1β, is currently under investigation in a large phase 3 trial for prevention of recurrent myocardial infarction.23
Although aortic heterogeneity underscores the importance for a reproducible, robust model of TAAs that involves the thoracic aorta, the critical roles of inflammation and IL-1β are similar in both experimental TAA and AAA formation. Prior study from our laboratory has highlighted the importance of IL-1β in experimental AAAs using an elastase perfusion model of the infrarenal abdominal aorta.11 Importantly, that study demonstrated that increasing doses of anakinra provided a stepwise decrease in IL-1β protein and abdominal aortic dilation, indicating an appropriate dose response. Although the mechanics and genetic origins of TAAs and AAAs differ, inflammation likely plays a crucial role in aneurysm formation regardless of location. The influence of inflammation in aortic breakdown and dilation may be a valuable target for aortic aneurysm treatment with IL-1β inhibition.
The present study has limitations. First, experimental models cannot fully model human disease. In contrast to the elastase TAA model with aortic dilation occurring in 2 weeks after one application of elastase, human TAA formation is multifactorial and evolves over years. However, a model that requires months to years to evaluate treatment options is not useful for investigational purposes. The proposed model demonstrates similar aortic wall degradation, cytokine profile, and dilation to human TAA disease in 2 weeks. Second, unlike human TAAs, experimental TAAs decreased in size from 2 to 4 weeks after elastase treatment. This healing period is a focus of active investigation to understand potential mechanisms of aortic repair after injury. A third limitation was the subjective method of histological grading. Entire aortic cross sections were reviewed in blinded fashion and graded according to subjective scale in an effort to decrease selection bias and counting errors frequently included in more quantitative measures. Fourth, the choice of statistical analyses affected measured significance. For our study, groups were compared with nonparametric statistics, which is a more conservative approach than parametric studies and may not have detected significant differences between small groups. Although the cytokine and matrix metalloproteinase analyses did not demonstrate significant differences by nonparametric statistics, the results should not be considered equivocal and indicated a trend toward decreased inflammation with IL-1β and IL-1R knockout animals. Fifth, the dose of anakinra tested was 100-fold greater than the dose approved for human use. Because anakinra is recombinant human IL-1R antagonist, an increased dose may be required to have an effect on mouse IL-1β signaling. Finally, anakinra treatment in small TAAs did not regress TAA size but rather arrested further dilation (day 3 WT TAA: 57% versus day 14 anakinra-treated TAA: 60%; P=0.82). Because human descending TAAs naturally continue to dilate at a rate of 0.12 to 0.29 cm/y, prevention of further dilation in humans with small TAAs may prevent aortic rupture and need for subsequent repair.24
The present study highlights the role of IL-1β in human TAAs and proposes a novel model of TAA disease with reproducible, large TAAs. In experimental TAAs, inhibition of IL-1β through genetic deletion or pharmacological antagonism decreased TAA formation and progression. IL-1β signaling should be considered a potential target for TAA treatment with further consideration given to investigation in humans with small TAAs.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health 5K08HL098560 (Dr Ailawadi) and RO1 HL081629 (Dr Upchurch) grants. In addition, this project was supported by Award Number T32HL007849 from the National Heart, Lung, and Blood Institute (NHLBI; Dr Johnston, PI: Irving L. Kron, MD) and by the Thoracic Surgery Foundation for Research and Education (TSFRE) Research Grant (PI: Dr Ailawadi). The content is solely the responsibility of the authors and does not necessarily represent the views of the NHLBI or the TSFRE.
Footnotes
Presented at the 2013 American Heart Association meeting in Dallas, TX, November 16–20, 2013.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.113.006800/-/DC1.
Disclosures
None.
References
- 1.Bickerstaff LK, Pairolero PC, Hollier LH, Melton LJ, Van Peenen HJ, Cherry KJ, Joyce JW, Lie JT. Thoracic aortic aneurysms: a population-based study. Surgery. 1982;92:1103–1108. [PubMed] [Google Scholar]
- 2.Johansson G, Markström U, Swedenborg J. Ruptured thoracic aortic aneurysms: a study of incidence and mortality rates. J Vasc Surg. 1995;21:985–988. doi: 10.1016/s0741-5214(95)70227-x. [DOI] [PubMed] [Google Scholar]
- 3.Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, Elefteriades JA. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–27. doi: 10.1016/s0003-4975(01)03236-2. discussion 27. [DOI] [PubMed] [Google Scholar]
- 4.Bavaria JE, Appoo JJ, Makaroun MS, Verter J, Yu ZF, Mitchell RS, Gore TAG Investigators Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007;133:369–377. doi: 10.1016/j.jtcvs.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 5.Wassef M, Upchurch GR, Jr, Kuivaniemi H, Thompson RW, Tilson MD., III Challenges and opportunities in abdominal aortic aneurysm research. J Vasc Surg. 2007;45:192–198. doi: 10.1016/j.jvs.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Ruddy JM, Jones JA, Spinale FG, Ikonomidis JS. Regional heterogeneity within the aorta: relevance to aneurysm disease. J Thorac Cardiovasc Surg. 2008;136:1123–1130. doi: 10.1016/j.jtcvs.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ailawadi G, Knipp BS, Lu G, Roelofs KJ, Ford JW, Hannawa KK, Bishop K, Thanaporn P, Henke PK, Stanley JC, Upchurch GR., Jr A nonintrinsic regional basis for increased infrarenal aortic MMP-9 expression and activity. J Vasc Surg. 2003;37:1059–1066. doi: 10.1067/mva.2003.163. [DOI] [PubMed] [Google Scholar]
- 8.MacSweeney ST, Powell JT, Greenhalgh RM. Pathogenesis of abdominal aortic aneurysm. Br J Surg. 1994;81:935–941. doi: 10.1002/bjs.1800810704. [DOI] [PubMed] [Google Scholar]
- 9.Theruvath TP, Jones JA, Ikonomidis JS. Matrix metalloproteinases and descending aortic aneurysms: parity, disparity, and switch. J Card Surg. 2012;27:81–90. doi: 10.1111/j.1540-8191.2011.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 11.Johnston WF, Salmon M, Su G, Lu G, Stone ML, Zhao Y, Owens GK, Upchurch GR, Jr, Ailawadi G. Genetic and pharmacologic disruption of interleukin-1beta signaling inhibits experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2013;33:294–304. doi: 10.1161/ATVBAHA.112.300432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Absi TS, Sundt TM, III, Tung WS, Moon M, Lee JK, Damiano RR, Jr, Thompson RW. Altered patterns of gene expression distinguishing ascending aortic aneurysms from abdominal aortic aneurysms: complementary DNA expression profiling in the molecular characterization of aortic disease. J Thorac Cardiovasc Surg. 2003;126:344–357. doi: 10.1016/s0022-5223(02)73576-9. discission 357. [DOI] [PubMed] [Google Scholar]
- 13.Ikonomidis JS, Gibson WC, Gardner J, Sweterlitsch S, Thompson RP, Mukherjee R, Spinale FG. A murine model of thoracic aortic aneurysms. J Surg Res. 2003;115:157–163. doi: 10.1016/s0022-4804(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 14.Alexander MR, Murgai M, Moehle CW, Owens GK. Interleukin-1ß modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-κB-dependent mechanisms. Physiol Genomics. 2012;44:417–429. doi: 10.1152/physiolgenomics.00160.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter DB, Deibel MR, Jr, Dunn CJ, Tomich CS, Laborde AL, Slightom JL, Berger AE, Bienkowski MJ, Sun FF, McEwan RN. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990;344:633–638. doi: 10.1038/344633a0. [DOI] [PubMed] [Google Scholar]
- 16.Ikonomidis I, Lekakis JP, Nikolaou M, Paraskevaidis I, Andreadou I, Kaplanoglou T, Katsimbri P, Skarantavos G, Soucacos PN, Kremastinos DT. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation. 2008;117:2662–2669. doi: 10.1161/CIRCULATIONAHA.107.731877. [DOI] [PubMed] [Google Scholar]
- 17.Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–2618. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 18.Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. IL-1a and IL-1ß recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 19.Slee EA, Zhu H, Chow SC, MacFarlane M, Nicholson DW, Cohen GM. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD. FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem J. 1996;315(Pt 1):21–24. doi: 10.1042/bj3150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 21.Wu D, Albini P, Xu G, Xie W, Ren P, Zhang L, Palmero L, Coselli J, Shen Y, LeMaire S. Nlrp3 inflammasome is upregulated in thoracic aortic aneurysms and dissections. J Surg Res. 2012;172:280. [Google Scholar]
- 22.Fleischmann RM, Tesser J, Schiff MH, Schechtman J, Burmester GR, Bennett R, Modafferi D, Zhou L, Bell D, Appleton B. Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1006–1012. doi: 10.1136/ard.2005.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: Rationale and design of the canakinumab anti-inflammatory thrombosis outcomes study (cantos) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Coady MA, Rizzo JA, Hammond GL, Kopf GS, Elefteriades JA. Surgical intervention criteria for thoracic aortic aneurysms: a study of growth rates and complications. Ann Thorac Surg. 1999;67:1922–1926. doi: 10.1016/s0003-4975(99)00431-2. discussion 1953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


