Abstract
Aim:
Biological disease-modifying antirheumatic drugs (bDMARDs) are used to treat rheumatoid arthritis (RA) with adalimumab and etanercept the most used bDMARDs in Brazil. This open prospective cohort study evaluated their effectiveness and safety among RA patients in the Brazilian Public Health System given their costs.
Methods:
The Clinical Disease Activity Index was primarily used to assess their effectiveness after 6 and 12 months of follow-up. The Health Assessment Questionnaire and EuroQol-5D were also used.
Results:
A total of 266 RA patients started treatment with adalimumab or etanercept. Adalimumab was the most widely used bDMARD (70%). In total, 46% achieved remission or low-disease activity at 12 months with no difference in effectiveness between them (p = 0.306). bDMARDs were more effective in patients who had better functionality at treatment onset and had spent longer in education.
Conclusion:
This real-world study demonstrated that adalimumab and etanercept are equal alternatives for RA treatment and both were well tolerated.
Keywords: : adalimumab, Brazil, cohort study, effectiveness, etanercept, rheumatoid arthritis
Rheumatoid arthritis (RA) is a systemic, chronic and progressive inflammatory disease which affects the synovial membrane of joints, and which may lead to bone and cartilage destruction [1]. It is one of the more common autoimmune disorders estimated to affect between 0.3 and 1% of the worlds' population [2,3]. In Brazil, a multicenter study found adult RA prevalence between 0.2 and 1% of the population [4], with a further Brazilian study performed in Montes Claros (Minas Gerais) finding a prevalence of 0.46% [5].
Treatment of RA includes NSAIDs, systemic or intra-articular glucocorticoids, conventional synthetic (sDMARDs) and biological (bDMARDs) disease-modifying antirheumatic drugs. Despite being effective in alleviating the symptoms of RA, bDMARDs are typically indicated for patients with persistent disease activity despite sDMARDs in view of their expense [6–8].
In Brazil, all citizens are entitled to universal and equal access to services directed toward the promotion, protection and recovery of health [9]. Consequently the State must, indirectly, by way of public policies, and directly, by the Public Health System (SUS), provide complete treatment, including pharmaceutical care for patients with RA [10].
The bDMARDs for the treatment of patients with RA were included in the Specialized Pharmaceutical Assistance Component (CEAF) of SUS from 2002 onwards, initially with infliximab. Adalimumab and etanercept were included from 2006 onwards [11,12]. Access to these expensive medicines in CEAF depends on compliance with the Clinical Protocols and Therapeutic Guidelines published by the Ministry of Health; otherwise 100% patient copayment for the medicines [13]. Requests for access to these high-cost medicines are checked by each State Department of Health or those contracted to them such as the SUS Collaborating Centre – Health Technology Assessment & Excellence at the College of Pharmacy, Federal University of Minas Gerais.
Adalimumab and etanercept are the most used bDMARDs in Brazil [14]. However, information about their comparative effectiveness and safety in routine clinical practice need to be ascertained as the first step in assessing their value in the context of scare resources. Consequently, the objective of this study was to evaluate the comparative effectiveness of adalimumab and etanercept in routine clinical practice in Brazil through an open prospective cohort of patients with RA, approved for their use within the SUS. This is important given the diversity of patients attending specialist centers in Brazil.
Methods
The study population comprised patients diagnosed with RA, classified according to the American College of Rheumatology (ACR) criteria, who were treated with bDMARDs by SUS. The date of first dispensation was defined as the first day of inclusion in the cohort since patients need to have their prescription approved by the State Department of Health before they can receive any bDMARD. The cohort was initiated in March 2011, and patients were followed up at 6 and 12 months.
A standardized research form was developed documenting the medicines used, comorbidities, the disease activity composite index, patients' functionality and an assessment of their quality of life. The research forms were piloted to ascertain and address particular problems such as the wording of the questions, ordering and questionnaire layout. The patient interviews were subsequently performed face to face in SUS pharmacies at three time points. The interviews were conducted by Pharmacy postgraduate students of Federal University of Minas Gerais who had received training from rheumatologists in all pertinent aspects of the management of patients with RA. The first interview was conducted at the first dispensing of treatment for RA, the second interview at 6 months from the first interview and third interview at 6 months following the second interview.
At baseline, the sociodemographic features were collected. The Clinical Disease Activity Index (CDAI), the Health Assessment Questionnaire (HAQ) and the EuroQol-5D (EQ-5D) were also assessed at baseline, and subsequently at 6 and 12 months. The CDAI is a clinical index of disease activity which evaluates painful and swollen joints, as well as assessing disease activity by the patient and physician. The clinical index range is 0–76, with the following classification system: remission ≤2.8; low-disease activity ≤10; moderate-disease activity ≤22; and high-disease activity >22 [15]. The HAQ assesses the patient's functionality through a self-administered questionnaire containing 20 questions related to the difficulty in performing daily activities [16]. The EQ-5D was also used as it is a generic indicator of the patient's health condition through assessing five dimensions: mobility, personal care, usual activities, pain/discomfort and anxiety/depression, and additionally a visual analog scale of their health condition [17,18].
The CDAI was subsequently used to assess the effectiveness of both bDMARDs by examining changes in the index value between baseline, 6- and 12-month follow-up. The bDMARDs were considered effective when the patient achieved remission or low-disease activity, and considered ineffective when there was still moderate- or high-disease activity at 12 months. The association between sociodemographic and clinical characteristics, with disease activity measured by the CDAI, was also investigated. Frequency distributions were compiled for the sociodemographic variables and the mean and standard deviation (SD) was used for clinical variables. Normality was assessed using the Kolmogorov–Smirnov test and all measures are normally distributed [19]. Normally distributed continuous variables were compared using Student’s t-test, and Pearson’s Chi-square was used for categorical variables. The paired Student t-test was established to evaluate the differences between the averages of the measurement of the disease activity (CDAI) within the three interviews. Pearson’s Chi-square was applied for the univariate analysis to evaluate the association of effectiveness measured by the CDAI with the sociodemographic (gender, education, marital status and race) and clinical variables (type of drug, EQ-5D and HAQ). Logistic regression was applied in the multivariate analysis of the variables that presented a p-value <0.20 during the univariate analysis. The Statistical Package for Social Sciences Software version 19.0, was used (IBM, IL, USA).
The study was approved by the Research Ethics Committee of the Federal University of Minas Gerais (COEP-UFMG) under No 0069.0.203.000 -11.
Results
Participants
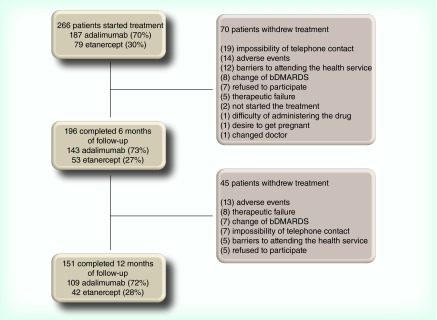
A total of 266 patients started treatment with adalimumab or etanercept, of whom 196 and 151 completed 6 and 12 months of follow-up, respectively. The reasons for withdraw included the impossibility of telephone contact, adverse events, barriers to attending the health service and treatment failure (Figure 1).
Figure 1. . Follow-up and withdraw of rheumatoid arthritis patients at 6 and 12 months.
bDMARD: Biological disease-modifying antirheumatic drug.
The mean age of patients was 54.4 years (SD: ± 14.7) and the mean disease duration was 10.3 years (SD: ± 88.6). Additionally, 88% of the patients were female, 46% white and 59% married. The most widely used bDMARD was adalimumab (70%), with etanercept used by 30%.
There were no statistically significant differences between the patient cohorts who used etanercept and adalimumab with regards to the baseline variables, except for duration of the disease, prior exposure to bDMARDs and CDAI (Table 1).
Table 1. . Baseline of rheumatoid arthritis patients treated with adalimumab and etanercept.
| Characteristic | Total (n = 266) | Adalimumab (n = 187) | Etanercept (n = 79) | p-value† |
|---|---|---|---|---|
| Age, mean ± SD (years) |
54.4 ± 14.7 |
54.5 ± 14.9 |
54.06 ± 14.3 |
0.833 |
| Duration of the disease, average ± SD (years) |
10.3 ± 8.6 |
9.6 ± 7.8 |
11.97 ± 10.2 |
0.043* |
| Women, n (%) |
233 (88) |
166 (89) |
67 (85) |
0.371 |
| Race, n (%): | ||||
| – White | 122 (46) | 85 (46) | 37 (47) | 0.958 |
| – Brown | 103 (39) | 72 (39) | 31 (39) | |
| – Black |
32 (12) |
23 (12) |
9 (11) |
|
| Marital status, n (%): | 0.424 | |||
| – Married | 157 (59) | 110 (59) | 47 (60) | |
| – Single |
61 (23) |
40 (21) |
21 (27) |
|
| Education, n (%): | 0.527 | |||
| – ≤8 years | 93 (35) | 65 (35) | 28 (35) | |
| – >8 years |
170 (64) |
119 (64) |
51 (65) |
|
| Current drugs, n (%): | ||||
| – Methotrexate | 123 (46) | 84 (45) | 39 (49) | 0.506 |
| – Leflunomide | 110 (41) | 81 (43) | 29 (37) | 0.317 |
| – sDMARD ≥1 | 202 (76) | 140 (75) | 62 (79) | 0.529 |
| – Corticosteroid | 208 (78) | 148 (79) | 60 (76) | 0.564 |
| – NSAIDs |
93 (35) |
66 (35) |
27 (34) |
0.861 |
| Previous drugs, n (%): | ||||
| – sDMARD | 257 (97) | 179 (96) | 78 (99) | 0.214 |
| – bDMARD |
38 (14) |
19 (10) |
19 (24) |
0.003* |
| Clinical measurements (mean ± SD): | ||||
| – CDAI | 25.1 ± 15.1 | 23.79 ± 14.5 | 28.2 ± 16.0 | 0.028* |
| – HAQ | 1.4 ± 0.7 | 1.4 ± 0.7 | 1.5 ± 0.7 | 0.235 |
| – EQ-5D | 0.6 ±0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.529 |
†p-value for etanercept × adalimumab.
*p < 0.05.
bDMARD: Biological disease-modifying antirheumatic drug; CDAI: Cinical Disease Activity Index; EQ-5D: EuroQol-5D; HAQ: Health assessment questionnaire; SD: Standard deviation; sDMARD: Synthetic disease-modifying antirheumatic drug.
Follow-up at 6 & 12 months
At baseline, 78% patients were using corticosteroids, 35% were using NSAIDs and 76% were using sDMARDs. During follow-up, the frequency of concomitant therapy dropped, except for NSAIDs. There were no statistically significant differences between adalimumab and etanercept regarding the use of concomitant drugs at 6 and 12 months, except for methotrexate concomitant use (p < 0.05) (Table 2).
Table 2. . Use of therapeutic drugs by patients with rheumatoid arthritis at baseline and 6 and 12 months.
|
Concomitant drug |
6 months (n = 196) |
12 months (n = 151) |
||||||
|---|---|---|---|---|---|---|---|---|
| Total, n (%) | Adalimumab, n = 143 (%) | Etanercept, n = 53 (%) | Valor p-value | Total, n (%) | Adalimumab, n = 109 (%) | Etanercept, n = 42 (%) | p-value | |
| Corticosteroid |
133 (68) |
96 (67) |
37 (70) |
0.721 |
103 (68) |
75 (70) |
28 (67) |
0.800 |
| NSAID |
70 (36) |
49 (34) |
21 (40) |
0.487 |
52 (34) |
40 (37) |
12 (29) |
0.346 |
| sDMARD |
130 (66) |
91 (64) |
39 (74) |
0.191 |
100 (66) |
69 (63) |
31 (74) |
0.221 |
| Methotrexate |
69 (35) |
42 (29) |
27 (51) |
0.005* |
58 (38) |
34 (31) |
24 (57) |
0.003* |
| Leflunomide | 60 (31) | 49 (34) | 11 (21) | 0.068 | 41 (27) | 33 (30) | 8 (19) | 0.165 |
*p < 0.05.
sDMARD: Synthetic disease-modifying antirheumatic drug.
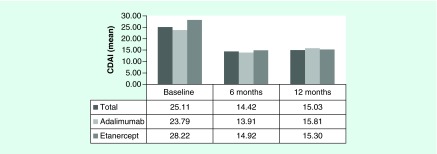
The mean CDAI values at baseline and following 6 and 12 months of drug use were: 25.1 (SD: ± 15.1), 14.4 (SD: ± 12.8) and 15.0 (SD: ± 14.2), respectively. Statistically significant differences were observed for the average CDAI values between the baseline and 6 months (p < 0.001) and the baseline and 12 months (p < 0.001) These data were normally distributed using the Kolmogorov–Smirnorv test.
The mean CDAI value at 6 months was 13.9 (SD: ±12.6) and 15.8 (SD: ± 13.2) for adalimumab and etanercept, respectively (p = 0.357). At 12 months, the mean CDAI value was 14.9 (SD: ± 12.97) for adalimumab and 15.3 (SD: ± 17.1) for etanercepet (p = 0.883) (Figure 2).
Figure 2. . Mean Clinical Disease Activity Index effectiveness of adalimumab and etanercept during a 1 year of follow-up.
CDAI: Clinical Disease Activity Index.
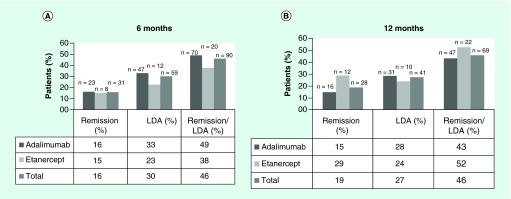
Taking the two bDMARDs together, the number of patients achieving clinical remission were 31 (16%) at 6 months and 28 (19%) at 12 months. Furthermore, 59 (30%) and 41 (27%) patients achieved low-disease activity at 6 and 12 months, respectively. Overall, the bDMARDs were effective for a total of 90 (46%) and 69 (46%) patients who achieved remission or low-disease activity at 6 and 12 months, respectively. The bDMARDs were classified as not effective for the remaining patients. No statistically significant differences in effectiveness were observed between adalimumab and etanercept at 6 (p = 0.162) and 12 months (p = 0.306) (Figure 3).
Figure 3. . Patients who achieved remission and low disease-activity of adalimumab and etanercept at 6 and 12 months.
LDA: Low-disease activity.
At 12 months, 269 adverse events were reported by 108 (71.5%) patients. The most common adverse events were application site reactions (19.9%), headaches (19.2%), nausea (17.9%) and alopecia (15.9). A number of cases of infection were observed, including 19 upper respiratory infections, 15 urinary tract infections, six fungal infections and three cases of pneumonia at 12 months. The frequency distribution of most adverse events remained approximately constant at 6 and 12 months (Table 3).
Table 3. . Adverse events reported by patients with rheumatoid arthritis at 6 and 12 months.
|
Adverse events |
6 months (n = 196) |
12 months (n = 151) |
||||
|---|---|---|---|---|---|---|
| Total n (%) | Adalimumab, n (%) | Etanercept, n (%) | Total n (%) | Adalimumab, n (%) | Etanercept, n (%) | |
| Application site reaction |
50 (26) |
33 (23) |
17 (32) |
30 (20) |
18 (17) |
12 (29) |
| Headache |
39 (20) |
31 (22) |
8 (15) |
29 (19) |
24 (22) |
5 (12) |
| Nausea |
32 (16) |
26 (18) |
6 (11) |
27 (18) |
21 (19) |
6 (14) |
| Alopecia |
29 (15) |
23 (16) |
6 (11) |
24 (16) |
19 (17) |
5 (12) |
| Upper respiratory infection |
15 (8) |
9 (6) |
6 (11) |
19 (13) |
13 (12) |
6 (14) |
| Influenza |
30 (15) |
21 (15) |
9 (17) |
17 (11) |
10 (9) |
7 (17) |
| Hypertension |
22 (11) |
20 (14) |
2 (4) |
16 (11) |
12 (11) |
4 (10) |
| Urinary tract infection |
24 (12) |
17 (12) |
7 (13) |
15 (10) |
12 (11) |
3 (7) |
| Pruritus |
29 (15) |
22 (15) |
7 (13) |
14 (9) |
9 (8) |
5 (12) |
| Asthenia |
23 (12) |
16 (11) |
7 (13) |
14 (9) |
9 (8) |
5 (12) |
| Rash |
21 (11) |
19 (13) |
2 (4) |
10 (7) |
9 (8) |
1 (2) |
| Migraine |
5 (3) |
3 (2) |
2 (4) |
8 (5) |
4 (4) |
4 (10) |
| Fever |
8 (4) |
6 (4) |
2 (4) |
8 (5) |
6 (6) |
2 (5) |
| Fungal infection | 4 (2) | 1 (1) | 3 (6) | 6 (4) | 5 (5) | 1 (2) |
Predictors of effectiveness of bDMARDs measured by the CDAI
Analyzing the association between effectiveness (CDAI) at 12 months with sociodemographic and clinical baseline variables identified a statistically significant difference in education status. Biological DMARDs were more effective at 12 months in patients who had spent a longer time in education (>8 years). They were also more effective in patients which presented better functionality (HAQ <1) than patients who presented with poor functionality (HAQ >2). Sex, race, marital status, type of drug (corticosteroids, NSAIDs, sDMARD and previous bDMARDs), the patient's age, duration of disease and quality of life did not prove to be predictors of effectiveness (Table 4).
Table 4. . Predictive baseline characteristics of effectiveness response at 12 months.
|
Baseline characteristics |
n |
Effective, n (%) |
Not effective, n (%) |
Univariate (p-value) |
Multivariate |
||
|---|---|---|---|---|---|---|---|
| OR | p-value | 95% CI | |||||
| Sex: | |||||||
| – Female | 134 | 58 (43) | 76 (57) | 0.095 | |||
| – Male |
17 |
11 (65) |
6 (35) |
|
|
|
|
| Race: | |||||||
| – White | 64 | 31 (48) | 33 (52) | 0.611 | |||
| – Brown | 57 | 25 (44) | 32 (56) | ||||
| – Black |
22 |
11 (50) |
11 (50) |
|
|
|
|
| Education: | |||||||
| – ≤8 years | 51 | 16 (31) | 35 (69) | 0.012* | 1.000 (Ref) | ||
| – >8 years |
100 |
53 (53) |
47 (47) |
|
2.087 |
0.049* |
1.002–4.346 |
| Age (years): | |||||||
| – ≤50 | 52 | 25 (48) | 27 (52) | 0.670 | |||
| – >50 |
99 |
44 (44) |
55 (56) |
|
|
|
|
| Period of disease: | |||||||
| – ≤3 years | 29 | 14 (48) | 15 (52) | 0.756 | |||
| – >3 years |
122 |
55 (45) |
67 (55) |
|
|
|
|
| Prior bDMARD: | |||||||
| – Yes | 22 | 9 (41) | 13 (59) | 0.626 | |||
| – No |
129 |
60 (47) |
69 (53) |
|
|
|
|
| sDMARD: | |||||||
| – None | 37 | 15 (41) | 22 (59) | 0.469 | |||
| – More than one |
114 |
54 (47) |
60 (53) |
|
|
|
|
| Corticosteroids: | |||||||
| – Yes | 120 | 52 (43) | 68 (57) | 0.252 | |||
| – No |
31 |
17 (55) |
14 (45) |
|
|
|
|
| NSAID: | |||||||
| – Yes | 56 | 22 (39) | 34 (61) | 0.225 | |||
| – No |
95 |
47 (50) |
48 (50) |
|
|
|
|
| HAQ: | |||||||
| – >2 | 27 | 7 (26) | 20 (74) | 0.016* | 1.000 (Ref) | ||
| – >1–2 | 87 | 39 (45) | 48 (55) | 1.903 | 0.114 | 0.856–4.227 | |
| – ≤1 |
37 |
23 (62) |
14 (38) |
|
3.807 |
0.019* |
1.249–11.602 |
| EQ-5D: | |||||||
| – ≤0.6 | 94 | 40 (43) | 54 (57) | 0.320 | |||
| – >0.6 | 57 | 29 (51) | 28 (49) | ||||
*p < 0.05.
bDMARD: Biological disease-modifying antirheumatic drug; Eq-5D: EuroQol-5D; HAQ: Health assessment questionnaire; NR: Not reported; OR: Odds ratio; sDMARD: Synthetic disease-modifying antirheumatic drug.
Discussion
Both bDMARDs, adalimumab and etanercept, reduced disease activity as measured by the CDAI at 6 and 12 months. However, no statistically significant difference (p < 0.05) was observed between them for remission and low-disease activity at 6 and 12 months. Both bDMARDs were well tolerated and effective in almost half of the patients, who achieved the target of remission or low-disease activity according to the CDAI (Figure 3). bDMARDs were more effective in patients who presented with better functionality (HAQ <1) at treatment onset, and had spent a longer time in education (>8 years) (Table 4).
Recent systematic reviews of randomized clinical trials and cohort studies which assessed the efficacy and effectiveness of the bDMARDs also reported no differences between adalimumab and etanercept with outcomes measured with either the disease activity score (DAS 28); European League Against Rheumatism (EULAR) scores; ACR 20, 50, 70; CDAI remission or simplified disease activity index [20–23]. However, other systematic reviews which evaluated efficacy in randomized clinical trials with ACR 20, 50 and 70 reported that etanercept was more effective than adalimumab [24–26], except one study that suggested adalimumab was more effective than etanercept [27].
Published clinical trial studies with etanercept have shown 46.2% efficacy (remission and low-disease activity) at 24 weeks as measured by the CDAI [3,28], with similar findings seen in our study. Other clinical trials with etanercept have also shown remission of 8.5 and 39% for etanercept at 24 weeks and 3 years, respectively [28,29]. Observational studies have reported similar effectiveness at 24 weeks to that seen in our study for etanercept [30], and similar CDAI remission (18%) to our study when patients with RA were treated with adalimumab or etanercept for 12 months [31–33]. However, other studies have documented greater remission as measured by the CDAI for treatment with etanercept (35%) at 3 years and for adalimumab (27%) at 12 weeks [29,34].
The effectiveness of adalimumab decreased in our study, which was probably due to the production of autoantibodies that has been reported in other studies [35,36]. However, it was not possible to analyze the production of autoantibodies with the data obtained in this cohort study. On the other hand, the increased effectiveness of etanercept should be treated with caution because a higher proportion of patients withdrawing from treatment between 6 and 12 months had higher CDAIs. Consequently, the patients who presented a lower level of disease activity remained in the study, impacting on comparisons of effectiveness.
Cohort studies have reported that sex, age, duration of disease, the number of prior sDMARDs and concurrent nonsteroidal anti-inflammatory use at baseline do not influence the response to treatment, and similar results were observed in this study. Others studies using HAQ as a prognostic indicator of effectiveness have shown that better functionality at treatment onset is associated with a greater response to treatment [37–39]. This was also shown in our study beyond the observation that the bDMARDs were more effective at 12 months in patients who had spent a longer time in education (>8 years).
Both adalimumab and etanercept were well tolerated by patients in this cohort study. Application site reaction, headache, nausea and alopecia were the most common adverse events, similar to those described in other studies [40,41]. A number of cases of infection were observed. As mentioned, these included 19 upper respiratory infections, 15 urinary tract infections, six fungal infections and three cases of pneumonia at 12 months (Table 3). Infections should be a major cause for concern among the adverse reactions, because there is evidence that the potential for patients to experiences serious infections tends to increase with bDMARDs [42]. This will be monitored closely in further studies with this cohort population.
Overall, half of the patients in this study did not achieve the target with bDMARDs. In this situation various international bodies, including the EULAR, the American College Rheumatology and the Clinical Protocol and Therapeutic Guidelines for RA in Brazil, recommend replacement of current bDMARDs [43–45].
However, in current clinical practice in Brazil, there are a difficulties with continuous pharmacotherapeutic monitoring and with access to medicines under SUS (i.e., only infliximab, etanercept and adalimumab were provided by the SUS until the end of 2013), which may be a possible explanation for the maintenance of current bDMARDs in our study even in those patients who have not achieved their treatment target. In such cases, before the replacement of current bDMARDs, additional pharmacotherapeutic monitoring was encouraged to identify the reasons for treatment failure or lack of effectiveness and adverse events as part of a ‘treat-to-target’ strategy. The ‘treat-to-target’ is defined as a treatment strategy in which the clinician treats the patient aggressively, adopting as a target either remission or low-disease activity. This strategy enables the physician and the patient to discuss and adopt therapeutic changes within the required period of time [46,47]. Studies have reported that this strategy has become increasingly important in clinical practice to improve remission rates [48–50]. Other professionals, such as nurses and pharmacists, could also act together with rheumatologists and consider patient choices in order to facilitate the implementation of a ‘treat-to-target’ strategy [51,52]. We will be investigating this in the future.
Limitations
We are aware that this study was conducted during the daily dispensing of medicines within the SUS and some biases could not be controlled. The patients were not randomized, there was no control group and treatment was administered in accordance with the rheumatologist’s prescriptions. The study was also performed under real-life conditions (i.e., without a control group), thus differences were observed in the number of participants among the groups, with the group on etanercept smaller than adalimumab. In addition, there was also no routine data collection of autoantibodies (RF, ACPAs) nor routine collection of laboratory data such as ESR or CRP. This though reflects reality in real-life studies undertaken with SUS patients in Brazil.
We are also aware of the relatively small number of patients enrolled into this real-world study. However we believe this study is important in order to supplement the results of clinical trials, as it demonstrates the effectiveness of the bDMARDs in routine clinical practice within a Brazilian population. The routine use of a CDAI measure is practical and objective as it does not require laboratory data for its calculation. Moreover, it has presented good to moderate correlations with other clinical indicators of disease activity (DAS 28, EULAR and ACR) [15,53–56]. Consequently, we believe our findings are robust.
Conclusion
Only half of the patients achieved the treatment target of remission or low-disease activity with either adalimumab or etanercept. No statistically significant differences were observed between them. The remaining patients should have their therapeutic options reviewed over these 12 months. Both adalimumab and etanercept were well tolerated. In addition, bDMARDs were more effective in patients who had spent a longer time in education (>8 years) and presented better functionality at treatment onset as measured by the HAQ.
In view of the high cost of the bDMARDs to SUS and, consequently, to society, versus sDMARDs continuous pharmacotherapeutic monitoring should be performed by a multidisciplinary team. This could achieve better results, assuring the quality of use of the bDMARDs. Further studies should focus on important issues like adherence and costs, especially factors that might affect persistence as this will appreciably impact on the long-term effectiveness and costs of medicines to treat this chronic condition.
Executive summary.
In total, 266 rheumatoid arthritis patients started treatment with adalimumab or etanercept, of whom 196 and 151 completed 6 and 12 months of follow-up, respectively. The most widely used biological disease-modifying antirheumatic drug was adalimumab (70%), with etanercept used by 30% of patients.
The percentage of patients achieving remission or low-disease activity was 46%, with no difference in effectiveness between adalimumab and etanercept (p = 0.306).
Patients who had not achieved the treatment target of disease remission or low-disease activity remained in treatment at 12 months. They should have their therapeutic options regularly reviewed.
Both adalimumab and etanercept were well tolerated.
Overall, the biological disease-modifying antirheumatic drugs were more effective in patients who had better disease functionality (health assessment questionnaire <1) at treatment onset, and had spent a longer time in education (>8 years).
Additional pharmacotherapeutic monitoring should be encouraged to identify the reasons for treatment failure or lack of effectiveness and adverse events as part of a ‘treat-to-target’ strategy.
Footnotes
Financial & competing interests disclosure
AM Kakehasi has received educational grants from Abbvie, AstraZeneca, BMS, Roche, Eli-Lilly, Pfizer, Roche, Janssen, Novartis and Sanofi. This work was supported by National Council for Scientific and Technological Development, Brazil (grant number 471819/2013-1) and Fapemig, the Minas Gerais State Research Foundation, Brazil (grant number PPM-0015-15). The write-up was in part supported by a Newton Advanced Fellowship awarded to AA Guerra Jr by the Academy of Medical Sciences, through the UK Government's Newton Fund program. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest
- 1.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 2.WHO: Chronic rheumatic conditions. www.who.int/chp/topics/rheumatic/en/
- 3.Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a synopsis. Am. J. Manag. Care. 2014;20(7. Suppl.):S128–S135. [PubMed] [Google Scholar]
- 4.Marques-Neto J, Gonçalves E, Langen L, Cunha M, Radominski S, Oliveira S. Multicentric study of the prevalence of adult rheumatoid arthritis in Brazilian population samples. Rev. Bras. Reumatol. 1993;33:169–173. [Google Scholar]
- 5.Senna ER, De Barros AL, Silva EO, et al. Prevalence of rheumatic diseases in Brazil: a study using the COPCORD approach. J. Rheumatol. 2004;31:594–597. [PubMed] [Google Scholar]
- 6.da Mota LM, Cruz BA, Brenol CV, et al. 2012 Brazilian Society of Rheumatology Consensus for the treatment of rheumatoid arthritis. Rev. Bras. Reumatol. 2012;52:152–174. [PubMed] [Google Scholar]
- 7.Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann. Rheum. 2010;69:964–975. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64:625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.BRASIL. 1988. www.planalto.gov.br/ccivil_03/constituicao/constituicaocompilado.htm Senado Federal. Constituição da República Fedeativa do Brasil. Brasil.
- 10.Acurcio FA. Medicamentos: políticas, assistência farmacêutica, farmacoepidemiologia e farmacoeconomia. Belo Horizonte: Coopmed. 2013:13–70. [Google Scholar]
- 11.BRASIL. 2002. www.saudedireta.com.br/docsupload/1340498699do_a05_01.pdf Ministério da Saúde. Secretaria de Atenção à Saúde. Portaria n. 865, de 25 de novembro de 2002. Aprova o Protocolo Clínico e Diretrizes Terapêuticas da Artrite Reumatoide. Brasil.
- 12.BRASIL. 2006. http://bvsms.saude.gov.br/bvs/saudelegis/sctie/2006/prt0066_01_11_2006_comp.html Ministério da Saúde. Secretaria de Atenção à Saúde. Portaria n. 66, de 1 de novembro de 2006. Aprova o Protocolo Clínico e Diretrizes Terapêuticas da Artrite Reumatoide. Brasil.
- 13.Costa JO, Almeida-Brasil CC, Godman B, et al. Implementation of clinical guidelines in Brazil: should academic detailing be used? J. Pharm. Health Services Res. 2016;7(2):105–115. [Google Scholar]
- 14.BRASIL. 2016. www2.datasus.gov.br/DATASUS/index.php?area=02 Ministério da Saúde. Departamento de Informática do SUS - DATASUS. Informações em saúde (TABNET). Assistência a Saúde. Produção ambulatorial. DATASUS.
- 15.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin. Exp. Rheumatol. 2005;23(Suppl. 39):S100–S108. [PubMed] [Google Scholar]
- 16.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: a review of its history, issues, progress, and documentation. J. Rheumatol. 2003;30:100–108. [PubMed] [Google Scholar]
- 17.EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Pol. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 18.Santos M, Cintra MA, Monteiro AL, et al. Brazilian valuation of EQ-5D-3L health states: results from a saturation study. Med. Decis. Making. 2015;36(2):253–263. doi: 10.1177/0272989X15613521. [DOI] [PubMed] [Google Scholar]
- 19.Conover WJ. Practical Nonparametric Statistics. John Wiley & Sons; NY, USA: 1971. [Google Scholar]
- 20.Gartlehner G, Hansen RA, Jonas BL, Thieda P, Lohr KN. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. J. Rheumatol. 2006;33:2398–2408. [PubMed] [Google Scholar]; • Good systematic review of randomized controlled trials on the efficacy and safety of biological disease-modifying antirheumatic drugs.
- 21.Hochberg MC, Tracy JK, Hawkins-Holt M, Flores RH. Comparison of the efficacy of the tumour necrosis factor alpha blocking agents adalimumab, etanercept, and infliximab when added to methotrexate in patients with active rheumatoid arthritis. Ann. Rheum. Dis. 2003;62(Suppl. 2):ii13–ii16. doi: 10.1136/ard.62.suppl_2.ii13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donahue KE, Gartlehner G, Jonas DE, et al. Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis. Ann. Intern. Med. 2008;148:124–134. doi: 10.7326/0003-4819-148-2-200801150-00192. [DOI] [PubMed] [Google Scholar]
- 23.Dos Santos JB, Costa JO, Oliveira Junior HA, et al. What is the best biological for arthritis rheumatoid? A systematic review of effectiveness. World J. Rheumatol. 2015;5(2):108–126. [Google Scholar]; • Good systematic review of observational studies on the effectiveness of biological disease-modifying antirheumatic drugs.
- 24.Tvete IF, Natvig B, Gåsemyr J, Meland N, Røine M, Klemp M. Comparing effects of biologic agents in treating patients with rheumatoid arthritis: a multiple treatment comparison regression analysis. PLoS ONE. 2015;10(9):e0137258. doi: 10.1371/journal.pone.0137258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turkstra E, Ng SK, Scuffham PA. A mixed treatment comparison of the short-term efficacy of biologic disease modifying anti-rheumatic drugs in established rheumatoid arthritis. Curr. Med. Res. Opin. 2011;27(10):1885–1897. doi: 10.1185/03007995.2011.608655. [DOI] [PubMed] [Google Scholar]
- 26.Mandema JW, Salinger DH, Baumgartner SW, Gibbs MA. A dose-response meta-analysis for quantifying relative efficacy of biologics in rheumatoid arthritis. Clin. Pharmacol. Ther. 2011;90(6):828–835. doi: 10.1038/clpt.2011.256. [DOI] [PubMed] [Google Scholar]
- 27.Devine EB, Alfonso-Cristancho R, Sullivan SD. Effectiveness of biologic therapies for rheumatoid arthritis: an indirect comparisons approach. Pharmacotherapy. 2011;31(1):39–51. doi: 10.1592/phco.31.1.39. [DOI] [PubMed] [Google Scholar]
- 28.Hobbs K, Deodhar A, Wang B, et al. Randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of etanercept in patients with moderately active rheumatoid arthritis despite DMARD therapy. Springerplus. 2015;4:113. doi: 10.1186/s40064-015-0895-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannon GW, Wang BC, Park GS, Koenig A, Collier DH, Keystone EC. Remission in rheumatoid arthritis patients treated with etanercept monotherapy: clinical practice and clinical trial experience. Clin. Exp. Rheumatol. 2013;31(6):919–925. [PubMed] [Google Scholar]
- 30.Martin WJ, Shim M, Paulus HE, et al. Older age at rheumatoid arthritis onset and comorbidities correlate with less health assessment questionnaire-disability index and clinical disease activity index response to etanercept in the RADIUS 2 registry. J. Clin. Rheumatol. 2014;20(6):301–305. doi: 10.1097/RHU.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canhão H, Rodrigues AM, Mourão AF, et al. Comparative effectiveness and predictors of response to tumour necrosis factor inhibitor therapies in rheumatoid arthritis. Rheumatology (Oxford) 2012;51:2020–2026. doi: 10.1093/rheumatology/kes184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenberg JD, Reed G, Decktor D, et al. A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registry. Ann. Rheum. Dis. 2012;71:1134–1142. doi: 10.1136/annrheumdis-2011-150573. [DOI] [PubMed] [Google Scholar]
- 33.Hirabara S, Takahashi N, Fukaya N, et al. Clinical efficacy of abatacept, tocilizumab, and etanercept in Japanese rheumatoid arthritis patients with inadequate response to anti-TNF monoclonal antibodies. Clin. Rheumatol. 2014;33(9):1247–1254. doi: 10.1007/s10067-014-2711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burmester GR, Ferraccioli G, Flipo RM, et al. Clinical remission and/or minimal disease activity in patients receiving adalimumab treatment in a multinational, open-label, twelve-week study. Arthritis Rheum. 2008;59(1):32–41. doi: 10.1002/art.23247. [DOI] [PubMed] [Google Scholar]
- 35.Bartelds GM, Krieckaert CL, Nurmohamed MT, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305(14):1460–1468. doi: 10.1001/jama.2011.406. [DOI] [PubMed] [Google Scholar]
- 36.Maneiro JR, Salgado E, Gomez-Reino JJ. Immunogenicity of monoclonal antibodies against tumor necrosis factor used in chronic immune-mediated Inflammatory conditions: systematic review and meta-analysis. JAMA Intern Med. 2013;173(15):1416–1428. doi: 10.1001/jamainternmed.2013.7430. [DOI] [PubMed] [Google Scholar]
- 37.Hyrich KL, Watson KD, Silman AJ, Symmons DP. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2006;45(12):1558–1565. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 38.Karlsson JA, Kristensen LE, Kapetanovic MC, Gülfe A, Saxne T, Geborek P. Treatment response to a second or third TNF-inhibitor in RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology (Oxford) 2008;47(4):507–513. doi: 10.1093/rheumatology/ken034. [DOI] [PubMed] [Google Scholar]
- 39.Kristensen LE, Kapetanovic MC, Gülfe A, Söderlin M, Saxne T, Geborek P. Predictors of response to anti-TNF therapy according to ACR and EULAR criteria in patients with established RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology. 2008;47(4):495–499. doi: 10.1093/rheumatology/ken002. [DOI] [PubMed] [Google Scholar]
- 40.van de Putte LBA, Atkins C, Malaise M, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann. Rheum. Dis. 2004;63(5):508–516. doi: 10.1136/ard.2003.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dore RK, Mathews S, Schechtman J, et al. The immunogenicity, safety, and efficacy of etanercept liquid administered once weekly in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2007;25(1):40–46. [PubMed] [Google Scholar]
- 42.Faleiro LR, Araújo LHR, Varavallo MA. A Terapia anti-TNF-α na Artrite Reumatoide. Semina Cienc. Biol. Saude. 2011;32:77–94. [Google Scholar]
- 43.American College Rheumatology Subcommittee On Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 Update. Arthritis Rheum. 2002;46(2):328–346. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 44.Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann. Rheum. Dis. 2013;73(3):492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.BRASIL. 2015. http://bvsms.saude.gov.br/bvs/saudelegis/sas/2015/prt0996_30_09_2015.html Ministério da Saúde. Secretaria de Atenção à Saúde. Portaria SAS/MS nº 996, de 30 de setembro de 2015. Aprova o Protocolo Clínico e Diretrizes Terapêuticas da Artrite Reumatoide. Brasil.; • Discusses clinical protocols and therapeutic guidelines regarding medicines that are available within Public Health System for patients with rheumatoid arthritis.
- 46.Solomon DH, Bitton A, Katz JN, et al. Review: treat to target in rheumatoid arthritis: fact, fiction, or hypothesis? Arthritis Rheumatol. 2014;66(4):775–782. doi: 10.1002/art.38323. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Good paper emphasizing that the clinician in ‘treat-to-target’ adopts as a target either remission or low-disease activity.
- 47.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann. Rheum. Dis. 2010;69:631–637. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Good paper emphasizing that multidisciplinary teams could act together with rheumatologists and consider patient choices in order to facilitate the implementation of a ‘treat-to-target’ strategy.
- 48.Jurgens MS, Welsing PM, Jacobs JW. Overview and analysis of treat-to-target trials in rheumatoid arthritis reporting on remission. Clin. Exp. Rheumatol. 2012;30(4, Suppl. 73):S56–S63. [PubMed] [Google Scholar]
- 49.Schoels M, Knevel R, Aletaha D, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search. Ann. Rheum. Dis. 2010;69(4):638–643. doi: 10.1136/ard.2009.123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vermeer M, Kievit W, Kuper HH, et al. Treating to the target of remission in early rheumatoid arthritis is cost-effective: results of the DREAM registry. BMC Musculoskelet. Disord. 2013;14:350. doi: 10.1186/1471-2474-14-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solomon DH, Bitton A, Fraenkel L, Brown E, Tsao P, Katz JN. Roles of nurse practitioners and physician assistants in rheumatology practices in the US. Arthritis Care Res. 2014;66(7):1108–1113. doi: 10.1002/acr.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gromnica-Ihle E, Rink M. Treat-to-target from the patient perspective. Z. Rheumatol. 2011;70(8):678–684. doi: 10.1007/s00393-011-0773-y. [DOI] [PubMed] [Google Scholar]
- 53.Greenberg JD, Harrold LR, Bentley MJ, Kremer J, Reed G, Strand V. Evaluation of composite measures of treatment response without acute-phase reactants in patients with rheumatoid arthritis. Rheumatology (Oxford) 2009;48(6):686–690. doi: 10.1093/rheumatology/kep054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gunaydın R, Karatepe AG, Kaya T. The performance of the clinical disease activity index in patients with rheumatoid arthritis. Turk. Romatoloji. Dergisi. 2006;21:45–48. [Google Scholar]
- 55.Klarenbeek NB, Koevoets R, van der Heijde DM, et al. Association with joint damage and physical functioning of nine composite indices and the 2011 ACR/EULAR remission criteria in rheumatoid arthritis. Ann. Rheum. Dis. 2011;70(10):1815–1821. doi: 10.1136/ard.2010.149260. [DOI] [PubMed] [Google Scholar]
- 56.Singh H, Kumar H, Handa R, Talapatra P, Ray S, Gupta V. Use of clinical disease activity index score for assessment of disease activity in rheumatoid arthritis patients: an Indian experience. Arthritis. 2011;2011:146398. doi: 10.1155/2011/146398. [DOI] [PMC free article] [PubMed] [Google Scholar]