Abstract
Brubaker and colleagues describe translation of two distinct mammalian proteins, MAVS and miniMAVS, from a single bicistronic mRNA. Interestingly, miniMAVS antagonizes the ability of full length MAVS to stimulate type I interferon production during viral infection. The authors also find evidence that several additional innate immune effectors may use bicistronic mRNAs to encode multiple protein variants, suggesting that non-canonical translation may be a common feature of innate immune regulation.
Generating diverse proteins to carry out complex biological processes is an important challenge met by different means in the various kingdoms of life. Bacteria use the operon model to generate multiple proteins from serial open reading frames (ORFs) encoded by a single mRNA transcript. On the other hand, viruses are evil geniuses who use a myriad of mechanism to generate a complex proteome from a comparatively small transcriptome. In contrast the vast majority of the eukaryotic transcripts are thought to be monocistronic (i.e. one mRNA produces a single protein) although alternative splicing can also generate several matched mRNA-protein isoforms from a single primary unspliced transcript. For most eukaryotic mRNAs the process of translation begins when the initiation complex assembles on the 5′-terminal cap of the message. The small ribosomal subunit scans the 5′ untranslated region (5′UTR) of the message until finding the first AUG codon. The large subunit then joins the complex and peptide synthesis begins. However in the some cases ribosomes bypass the first initiation codon in a process termed leaky scanning. In these instances the ribosome instead recognizes a downstream initiation codon, resulting in the production of a “truncated” protein isoform. In this issue Brubaker et al. provide a fascinating example of how leaky scanning generates two isoforms of the antiviral protein MAVS, which have surprisingly different roles in the innate immune response (Brubaker, 2014).
MAVS is a critical signaling protein in the type I interferon pathway downstream of RIG-I and MDA5. Brubaker et al. show that expression of the full-length 72kDa MAVS protein (FL MAVS) is also accompanied by expression of a truncated 50kDa counterpart, aptly named miniMAVS. FL MAVS and miniMAVS are both transcribed from the same coding region and are not the products of alternative splicing. While several mechanisms could explain the usage of the alternative start codon, (Kozak, 2002) Brubaker et al. demonstrate that leaky scanning leads to miniMAVS expression. Ribosomes translating the MAVS mRNA can skip the first AUG they find because of its weak translational context (i.e weak Kozak sequence), initiating translation at the FL MAVS AUG; essentially leaky scanning results in translation of FL MAVS. Interestingly, sometimes the ribosome recognizes the weak first AUG, resulting in translation of a cryptic upstream open reading frame (uORF) that bypasses the FL MAVS start site. The big surprise was that translation of this uORF terminates shortly after skipping the FL MAVS AUG start site, and that the ribosome re-initiates scanning afterwards, proceeding until it finds the AUG of miniMAVS over 400 bp downstream. Thus, classical scanning followed by re-initiation results in expression of miniMAVS. Placing strong artificial start sites between the full-length and miniMAVS initiation sites severely attenuated miniMAVS translation. Conversely strengthening the Kozak sequence of the uORF promoted the skipping of the FL MAVS start site, resulting in more efficient miniMAVS translation. Elegant mutational and frame shift studies complimented by ribosomal profiling further confirmed a role for leaky scanning in miniMAVS translation. Importantly, the endogenous 5′ untranslated region (5′UTR) was sufficient for the translation of both FL MAVS and miniMAVS. Therefore, the context of a start site greatly influences alternative translation of the MAVS mRNA.
The authors further go on to examine the roles of these two alternatively translated proteins during viral infections. MAVS has a well-established role in antiviral immunity. While TLRs detect viral nucleic acids in the extracellular and endsosomal compartment, cytosolic viral RNA is sensed by RIG-I or MDA5, which signal through MAVS. MAVS then initiates a signaling cascade that stimulates transcription of type I interferons. As expected, overexpression of FL MAVS artificially triggers signaling, resulting in secretion of IFN-β. Surprisingly, when FL MAVS and miniMAVS were co-expressed, IFN-β production was severely reduced. Consequently, FL MAVS restricted viral replication while the presence of miniMAVS attenuated FL MAVS responses. Using a panel of start site mutants representing strong and weak translational contexts, the authors establish that miniMAVS counteracts the FL MAVS signaling cascade. Mechanistically, miniMAVS does not directly bind to MAVS but rather interacts with at least two downstream adaptors TRAF2 and TRAF6, although the mechanism whereby these interactions inhibit MAVS signaling remains to be fully elucidated. miniMAVS essentially serves as an inhibitor of FL MAVS signaling, presumably to limit excessive type I interferon production which could be detrimental to the host once infection is cleared. In line with this concept, FL MAVS levels decrease during infection, while miniMAVS levels remain stable or rise. It is also interesting to note that both FL MAVS and miniMAVS can promote type I interferon independent cell death, the physiologic role of which remains unclear. Therefore, while some functions are antagonistic, others may be shared between FL MAVS and miniMAVS. Another interesting observation is that miniMAVS does not bind FL MAVS directly to form prion-like filaments as has been reported during FL MAVS signaling (Kagan, 2012). In addition to mitochondria, FL MAVS localizes to mitochondria-associated ER membrane (MAM) and peroxisomes (Kagan, 2012). Given the findings reported here, it can be speculated that FL MAVS and miniMAVS may utilize different cellular compartments as signaling platforms during viral infections.
Ribosomal profiling has previously demonstrated the use of alternative start sites to generate extended or truncated forms or proteins (Ingolia et al., 2011). Using human monocytic cells, the authors reiterate this finding and suggest that several other antiviral genes such as IFIH1, MX2, IFITM2, and TRIM25 could be alternatively translated. This study adds to the growing list of mammalian messages that are alternatively translated suggesting that eukaryotes may utilize bicistronic mRNAs much more commonly than previously appreciated (Cocka and Bates, 2012; Gray et al., 1999; Ingolia et al., 2011; Shinohara et al., 2008; Yin et al., 2002). Moreover, some of these alternatively translated protein variants function in the immune system where timing of initiation and resolution of a response is critical (Cocka and Bates, 2012; Shinohara et al., 2008).
One question raised by these studies is how cells achieve an appropriate balance between FL MAVS and miniMAVS expression. Too much FL MAVS could lead to unchecked inflammation, while elevated miniMAVS levels may leave cells vulnerable to infection. A possible answer might be found in the critical role of the uORF in miniMAVS translation. Several uORFs have been shown to promote translation of downstream reading frames during periods of cell stress, such as viral infection. Kinases that phosphorylate the eIF2α translation initiation factor are known to stimulate uORF-directed translation. Intriguingly the interferon response induces expression of the eIF2a kinase PKR. In addition PKR is activated by cytosolic viral RNA, which also triggers the RIG-I/MAVS signaling pathway. Therefore, it is tempting to speculate that FL MAVS signaling, through interferon-dependent PKR activation, eventually results in the preferential translation of miniMAVS over FL MAVS, functioning as a negative feedback loop. The finding that similar leaky scanning events occur on multiple innate immune sensors and effectors suggest that such a system may be integral to restoring homeostasis upon virus clearance.
Figure 1. Full length (FL) MAVS and miniMAVS are produced from a single bicistronic message.
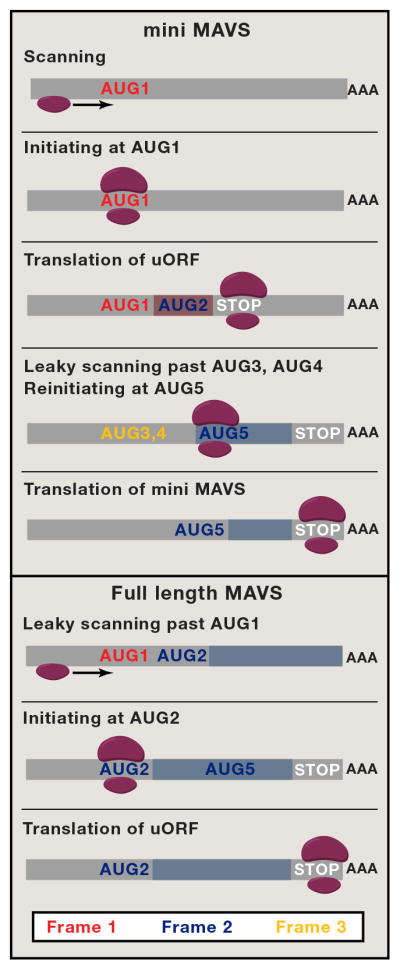
FL MAVS and miniMAVS are produced by a complex web of interlaced leaky scanning events. Re-initiation after translation of uORF leads to production of the truncated miniMAVS, which inhibits the signal transduction pathway initiated by FL MAVS during viral infection. This inhibition appears to act as a negative feedback loop to limit MAVS signaling.
References
- Bubaker, et al. 2014 [Google Scholar]
- Cocka LJ, Bates P. Identification of Alternatively Translated Tetherin Isoforms with Differing Antiviral and Signaling Activities. PLoS Pathog. 2012;8:e1002931. doi: 10.1371/journal.ppat.1002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TA, Saitoh S, Nicholls RD. An imprinted, mammalian bicistronic transcript encodes two independent proteins. Proc Natl Acad Sci USa. 1999;96:5616–5621. doi: 10.1073/pnas.96.10.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Lareau LF, Weissman JS. Ribosome Profiling of Mouse Embryonic Stem Cells Reveals the Complexityand Dynamics of Mammalian Proteomes. Cell. 2011;147:789–802. doi: 10.1016/j.cell.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC. Signaling Organelles of the Innate Immune System. Cell. 2012;151:1168–1178. doi: 10.1016/j.cell.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Pushing the limits of the scanning mechanism for initiation of translation. Gene. 2002;299:1–34. doi: 10.1016/S0378-1119(02)01056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci USa. 2008;105:7235–7239. doi: 10.1073/pnas.0802301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Stephen CW, Luciani MG, Fåhraeus R. p53 stability and activity is regulated by Mdm2-mediated induction of alternative p53 translation products. Nat Cell Biol. 2002;4:462–467. doi: 10.1038/ncb801. [DOI] [PubMed] [Google Scholar]


