Abstract
Objectives
Early life adversity has been shown to be associated with cardiovascular disease and mortality in later life, but little is known about the mechanisms that underlie this association. Prenatal undernutrition, a severe early life stressor, is associated with double the risk of coronary heart disease and increased blood pressure responses to psychological stress. In the present study, we tested the hypothesis that prenatal undernutrition induces alterations in the autonomic nervous system, which may increase the risk of developing heart disease.
Methods
We studied autonomic function in 740 men and women (aged 58 years, SD 0.9 years) who were members of the Dutch famine birth cohort. We compared those exposed to famine during early (n=64), mid (n=107) or late gestation (n=127) to those unexposed to famine in utero (n=442). Participants underwent a series of three psychological stressors (Stroop, mirror-tracing and speech) whilst their blood pressure and heart rate were recorded continuously.
Results
Data had sufficient quality in 602 participants for derivation of autonomic function indices by spectral analysis. The stress protocol led to significant sample-level changes in SBP, HR and all cardiovascular control measures (all p < .001). None of the autonomic function parameters, at rest or in response to stress, differed significantly (all p > 0.050) according to prenatal famine exposure.
Conclusion
Prenatal undernutrition was not associated with autonomic function in late adulthood. We conclude that altered autonomic function does not seem to explain our previous findings of increased CHD risk among those exposed to famine prenatally.
Keywords: prenatal undernutrition, autonomic function, heart disease, psychological stress, famine
Introduction
Early life adversity has been associated with hypertension, cardiovascular disease and mortality. Both prenatal and postnatal exposures to a variety of physically and/or emotionally stressful environmental factors, including malnutrition, trauma, abuse and neglect have been linked to increased risks of developing hypertension and heart disease in later life (1–4). The mechanisms that underlie these associations remain poorly understood.
Poor nutritional circumstances during the earliest stages of gestation constitute a severe early life stressor. A lack of essential nutritional building blocks for development of the fetus, presumably combined with high levels of psychological stress, have been shown to be detrimental to mental and physical health in later life by several studies that have followed people exposed to famines in early life (5). Results of studies on the effects of perinatal undernutrition are also often strikingly similar to results of studies on effects of perinatal stress (6).
We have shown previously that men and women who were undernourished in early gestation had a twofold increase of coronary artery disease at age 50 years and increased blood pressure responses to psychological stress at age 58 (1, 7). Moreover, at age 63 years, women who had been exposed to famine in early gestation were more likely to have died from cardiovascular disease than their unexposed contemporaries (8).
Experimental evidence suggests that prenatal undernutrition may program autonomic nervous system function, leading to perturbations in cardiovascular control that predispose to cardiovascular disease. Restricted maternal nutrition in early gestation in sheep has been shown to alter autonomic function, but specific effects depended on the amount reduction of food intake and sex. A 15% reduction in global maternal intake was shown to induce lower blood pressure in the offspring and a downward resetting of baroreflex control (9). However, a 50% reduction was shown to shift the baroreflex setpoint upwards and in another study induced increased epinephrine levels in response to stress, but only in female offspring (10, 11). Offspring of rats that were undernourished during the entire period of gestation and lactation showed raised catecholamine levels, an increase in catecholamine receptors and diminished cardiac beta-adrenergic responsiveness (12, 13). A review of studies of rats that were exposed to protein malnutrition in early life showed several effects on cardiovascular control systems, including increased sympathetic and decreased parasympathetic nervous system activity (14). There is also some evidence in humans that poor maternal nutritional status may impact sympathetic nervous system activity in the offspring, although the direction of the association is opposite to that demonstrated in most animal work. Children whose mothers had low vitamin B12 status during pregnancy, a vitamin that is crucial to neuronal development, showed lower resting cardiac sympathetic activity as demonstrated by reduced heart rate variability (15).
In the present study, we tested the hypothesis that human maternal exposure to famine during gestation leads to increased sympathetic nervous system activity. We specifically expected those exposed to famine in early gestation to show altered autonomic function as this group was previously shown to display more CHD and increased blood pressure stress responsiveness (1, 7). To test this hypothesis, we investigated autonomic function in people who were exposed to famine in early, mid or late gestation and in people who were not exposed to the Dutch famine during gestation. Parameters at rest and in response to psychological stress were measured.
Methods
The Dutch famine
The Dutch famine was a 5-month period of severe food shortage during the last winter of World War II, affecting the cities in the western Netherlands most. The famine resulted from the failure of the operation Market Garden, aimed at liberating the western and northern part of the Netherlands combined with a banning of all food transports by the German occupational forces. The famine began quite suddenly in late November 1944 and ended in early May 1945 after the liberation by the Allied forces. Food rations decreased to as low as 400-800 calories per day. Although it was a humanitarian disaster, the Dutch famine presents a unique opportunity to directly examine the effects of a short but severe period of maternal undernutrition on the health of the offspring in later life.
Study population
The Dutch famine birth cohort consists of 2414 term singletons, born in the Wilhelmina Gasthuis in Amsterdam, the Netherlands. All singleton babies who were born alive between 1 November 1943 and 28 February 1947 after a pregnancy duration of at least 259 days were included (16). Of the 2414 included, 160 babies had not been registered at birth and were lost to follow up. A further 328 people had died, 213 had emigrated, 157 did not allow their address to be given to the researchers, 125 were not traceable to a current address and 8 had requested removal of their address, leaving an eligible cohort of 1423. Of this group of 1423, a total of 740 people (age range 55.9 to 60.8 years) visited the clinic (52%) between September 2002 and October 2004. This cohort study has resulted in a series of publications and results specific to the blood pressure responses to stress have been published previously.(1,7,8) The present paper focuses primarily on the heart rate derived measures of autonomic nervous system functioning. The local Medical Ethics Committee approved the study, which was carried out in accordance with the Declaration of Helsinki. All participants gave written informed consent.
Exposure to famine
We defined famine exposure according to the daily official food-rations for adults. In addition to the official rations, food from other sources, such as church organizations, central kitchens, and the “black market,” was also available and the people may have had access to up to double the rationed amount at the peak of the famine. The rations do, however, adequately reflect the fluctuation of food availability during the famine (17). We considered a person prenatally exposed to famine if the average daily ration for adults during any 13–week period of gestation was less than 1000 calories. Therefore, people born between 7 January 1945 and 8 December 1945 were considered exposed prenatally. We defined periods of 16 weeks each to differentiate between those who were exposed in late gestation (born between 7 January and 28 April 1945), mid gestation (born between 29 April and 18 August 1945) and in early gestation (born between 19 August and 8 December 1945). Cohort members born before 7 January 1945 and those born after 8 December 1945 (conceived after famine) were considered unexposed to famine in utero and acted as control groups.
Study parameters and stress protocol
The medical birth records provided information about the mother, the course of pregnancy and the size of the baby at birth (16). During a one-day clinic visit, trained study nurses carried out all measurements, conducted interviews and performed a standardized stress protocol. Height was measured twice using a fixed or portable stadiometer and weight was measured twice using Seca and portable Tefal scales. Body mass index (BMI) was calculated as weight (kg)/height (m2) from the averages of the two height and weight measures. Socio-economic status (SES) was defined according to the International Socio-Economic Index (ISEI)-92, which is based on the participant’s or their partner’s occupation, whichever has the higher status. Values on the ISEI-92 range from 16 (low status) to 87. Participants were asked about smoking and use of medication. The presence of coronary heart disease (CHD) was defined as the presence of one or more of the following: angina pectoris according to the Rose/WHO questionnaire; Q waves on the electrocardiogram (Minnesota codes 1-1 or 1-2); or a history of coronary revascularization (angioplasty or bypass surgery).
Stress testing was performed in the afternoon on the clinic day, about an hour after participants had eaten a light lunch. The protocol started with a 20-minute baseline period, followed by three 5-minute psychological stress tests (Stroop, mirror tracing, and speech); the inter-task interval was 6 min. The final task, the speech, was followed by a 30-min recovery period during which participants sat quietly and were allowed to read something. The Stroop test was a single trial computerized color–word conflict challenge. After a short introduction, participants were allowed to practice until they fully understood the requirements of the task. Errors and exceeding the response time limit of 5 s triggered a short auditory beep. In mirror tracing, a star had to be traced that could only be seen in mirror image (Lafayette Instruments Corp, Lafayette, IN, USA). Every divergence from the line of the star induced a short beep. In the speech test, participants were told to imagine being accused of pick-pocketing and instructed to give a 3-minute defense of the accusation, which was videotaped. They were given 2 min to prepare their defense. Participants were told that the number of repetitions, eloquence, and persuasiveness of their performance would be marked by a team of communication-experts and psychologists. We continuously recorded heart rate (HR) and blood pressure (BP) using a Finometer or a Portapres Model-2 (Finapres Medical Systems, Amsterdam the Netherlands) during the entire stress protocol.
Autonomic function measures
The cardiovascular stress response is underpinned by influences of the autonomic nervous system on the heart and vasculature, which is dually innervated by the sympathetic and parasympathetic nervous system. The actions of these systems are partially separable in the frequency domain, with distinct low- and high-frequency bands associated with HR and BP variability (18). Estimates of spectral power in these bands yields indices relating to autonomic cardiovascular control. The cardiovascular parameters we used included low frequency (LF) systolic blood pressure (SBP), which is mainly a measure of sympathetic activation, and high frequency (HF) heart period (HP), which is mainly a measure of parasympathetic activation, and the ratio of LF/HF HP, which is often used as a measure of sympathovagal balance.
Signal processing
Data were interpreted using Beatscope 1.1 (Finapres Medical Systems, Amsterdam, Netherlands). Blood pressure data, sampled at 200Hz (Finometer) or 100Hz (Portapres), were extracted using Beatscope and imported into MATLAB (The Mathworks, Natick, MA, United States). Three observers, using an automated HP (the interval between adjacent heart beats) rejection algorithm as a guide, edited the data to remove artifacts and occasional ectopic beats (19). SBP and HR were extracted and HP and SBP variability were estimated in two frequency bands: LF (0.04-0.15 Hz) and HF (0.15-0.4 Hz). Cardiovascular parameters were derived from these signals using spectral analysis, applying a standard Fourier-based algorithm.
Statistical methods
In line with previous studies in this cohort, we compared those exposed to famine in late, mid or early gestation to those unexposed to famine during gestation. The latter group combines those born before the famine and those conceived after the famine, who did not differ on any of the outcomes. For all parameters, average values during the five-minute rest and stress periods were obtained. We used linear and logistic regression to compare the maternal, birth and adult characteristics of people exposed to famine during different periods of gestation to unexposed people (adjusted for sex) (Table 1). To test differences in stress responses between exposure-groups, we used repeated measures analyses (linear mixed models, SPSS version 21), applying an unstructured covariance matrix to analyze cardiovascular responses to the stress protocol (stress measure on the three different stress tests minus baseline measure). We also computed an average stress reactivity measure (mean of the three stress tasks minus baseline) and investigated differences between exposed and unexposed groups using linear regression analysis. In all these models, we adjusted for sex and for baseline level of the tested cardiovascular variable. Additionally, to investigate whether effects of prenatal famine exposure differed according to genders, we added an interaction term (sex*exposure) to the repeated measures models investigating autonomic nervous system activity.
Table 1. Maternal, birth and adult characteristics of study participants.
| Exposure to famine during gestation | ||||||
|---|---|---|---|---|---|---|
| Late | Mid | Early | Unexposed | All (SD) | N | |
| N included | 111 | 91 | 52 | 348 | 602 | |
| Men (%) | 48 | 43 | 46 | 50 | 48 | 602 |
| Maternal & birth characteristics | ||||||
| Maternal weight end of pregnancy (kg) | 62.9 a | 62.7 a | 69.4 | 68.1 | 66.4 (8.8) | 533 |
| Birth weight (g) | 3202 a | 3207 a | 3483 | 3443 | 3366 (463) | 602 |
| Adult characteristics | ||||||
| Socio-economic status b | 53 a | 51 | 46 | 49 | 50 (14) | 598 |
| Body mass index (kg/m2) | 28.2 | 28.1 | 28.5 | 28.8 | 28.6 (4.6) | 602 |
| Current smoker (%) | 23 | 24 | 25 | 20 | 22 | 601 |
| Antihypertensive medication (%) | 18 | 29 | 10 a | 25 | 23 | 602 |
| Anti-diabetic medication (%) | 5 | 9 | 8 | 6 | 7 | 600 |
| Coronary heart disease (%) | 6 | 4 | 10 | 7 | 7 | 594 |
| Baseline cardiovascular measures | ||||||
| SBP (mmHg) | 128.6 | 128.4 | 124.1 | 128.6 | 128.2 (20.4) | 601 |
| HR (bpm) | 72.9 | 73.5 | 75.8 | 74.6 | 74.2 (10.5) | 601 |
| LF-SBP (mmHg2) c | 11.1 | 11.7 | 11.6 | 11.4 | 11.6 (2.1) | 601 |
| HF-HP (ms2) c | 116.2 | 117.5 | 93.6 | 114.1 | 113.0 (2.7) | 601 |
| LF/HF ratio c | 2.2 | 2.0 | 2.2 | 2.3 | 2.2 (2.3) | 601 |
Data are given as means and standard deviation (or geometric means and standard deviations), except where given as percentages;
p<0.05 compared to unexposed, adjusted for sex, based on linear/logistic regression;
Socio-economic index according to ISEI-92 (range 16 to 87); SBP = systolic blood pressure; HR = heart rate; LF = low frequency; HF = high frequency; HP = heart period.
Results
Study population
Of the 740 people who visited the clinic, 19 participants did not complete the stress protocol due to technical or logistical problems. Of the remaining 721 participants who completed the stress protocol, the blood pressure and heart rate data of 119 participants could not be used in the spectral analyses due to one of the following reasons: significant arrhythmia (including atrial fibrillation, atrio-ventricular block or premature atrial beats), a signal of insufficient technical quality or an otherwise compromised signal (due to coughing, persistent talking or moving despite study instructions). This left 602 participants with data suitable for spectral analysis.
The birth weights of people participating in the study (mean, 3363 g) did not differ significantly (p = 0.41) from birth weights of eligible people not included (mean, 3343 g). Birth weights of those included (mean, 3366 g) also did not differ (p = 0.70) from birth weights of those excluded (mean, 3350 g) from the spectral analyses. Percentages of people excluded did not differ between the exposed and unexposed groups (19.7% excluded from those born before the famine, 11.9 % exposed in late gestation, 15.0% exposed in mid gestation, 16.1% exposed in early gestation and 18.3% conceived after the famine, p = 0.39). Of the 602 people included in the spectral analyses, 254 (42%) had been exposed to famine in utero.
Famine exposure, maternal, neonatal and adult characteristics
Table 1 shows that exposure in late and mid gestation was associated with a 5.2 and 5.4 kg lower maternal weight at the end of gestation (p < 0.001) and a 238 and 228 gram lower birth weight respectively (p < 0.001, adjusted for sex). People who were exposed to famine in early gestation were less likely to be taking anti-hypertensive medication (odds ratio = 0.3, p = 0.017) and people exposed to famine in late gestation had a higher socio-economic status (4 points, p = 0.011).
Stress responses
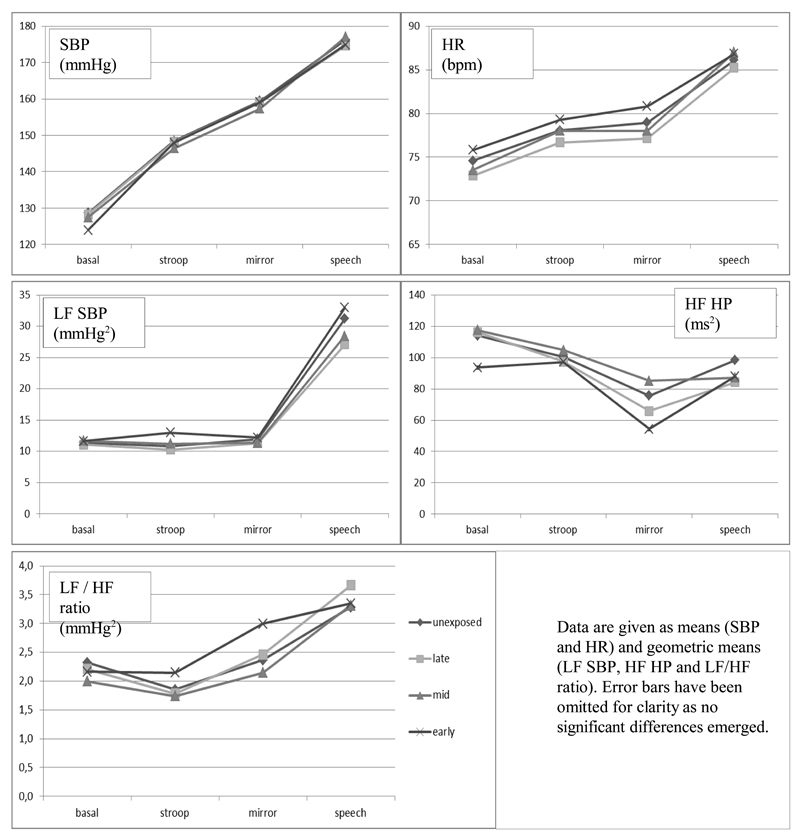
The stress protocol in general significantly perturbed SBP, HR and all cardiovascular control measures (all p < .001, see Fig.1). Mean SBP response was significantly associated with mean HR, LF SBP, HF HP and LF/HF responses. None of the cardiovascular stress response profiles were associated with birth weight (all p > 0.34, adjusted for sex) or maternal weight at the end of gestation (all p > 0.21), with the exception of HR response which showed a trending negative association with maternal weight (p = 0.056). Mean SBP and HR stress response profiles were higher in men (p < .001 and p = 0.020 respectively), lower in smokers (p < .001 and p = 0.004) and lower in those with higher BMI (p = 0.028 and p < 0.001 respectively). Mean LF SBP and LF/HF stress profiles were lower in smokers (p = 0.023 and p = 0.054) and LF/HF profile was lower in those using anti-hypertensive medication (p = 0.029). HF HP stress profile was higher in smokers (p = 0.017). Stress profiles were not different for those with and those without CHD (all p > 0.05).
Fig. 1.
Cardiovascular and cardiovascular control measures during psychological stress according to exposure to prenatal famine.
Famine exposure and cardiovascular measures
Baseline measures of SBP, HR, LF SBP, HF HP and the ratio of LF / HF HP did not differ according to famine exposure (Table 1). Figure 1 shows the cardiovascular stress response profiles over time according to exposure group. Mean SBP stress response profile was borderline significantly higher in those exposed to famine in early gestation (B = 4.1 mmHg [-0.2 to 8.4], p = .065; 3.9 [-0.3 to 8.2, p = 0.073, adjusted for sex]) (Table 2). Mean stress response profiles of HR, LF SBP, HF HP and LF/HF ratio did not differ according to prenatal famine exposure (Table 2). Adjusting for potential confounding variables (maternal weight, birth weight, socio-economic status, smoking, BMI) did not change these results. Excluding participants with type 2 diabetes, hypertension and/or CHD (leaving n=420 for analysis) also did not change the results. Also, there were no significant sex*exposure interactions. Analysis of the average stress reactivity across tasks did not show any differences between those exposed to the famine in early, mid or late gestation and those prenatally unexposed (all p <= 0.129).
Table 2. Effect sizes for associations between prenatal famine exposure and cardiovascular measures in response to stress.
| Exposure to famine during gestation | ||||||
|---|---|---|---|---|---|---|
| Late (B [95%CI]) | p-value | Mid (B [95%CI]) | p-value | Early (B [95%CI]) | p-value | |
| SBP (mmHg) | 0.39 [-2.81 to 3.58] | 0.81 | -0.20 [-3.58 to 3.19] | 0.91 | 3.93 [-0.31 to 8.18] | 0.069 |
| HR (bpm) | -0.01 [-1.12 to 1.10] | 0.99 | 0.44 [-0.76 to 1.65] | 0.47 | 0.17 [-1.34 to 1.68] | 0.82 |
| LF-SBP (mmHg2) | -0.97 [-3.51 to 1.56] | 0.45 | -1.59 [-4.34 to 1.16] | 0.26 | -0.46 [-3.89 to 2.97] | 0.79 |
| HF-HP (ms2) | -20.2 [-50.0 to 9.7] | 0.19 | 6.0 [-26.4 to 38.3] | 0.72 | 17.3 [-22.7 to 22.1] | 0.98 |
| LF/HF ratio | 0.12 [-0.28 to 0.52] | 0.57 | -0.06 [-0.49 to 0.38] | 0.79 | 0.34 [-0.20 to 0.88] | 0.22 |
Data are given as B [95% confidence intervals] with p-value from repeated mixed measures analysis comparing mean stress reactivity profiles for those exposed to famine during gestation compared to those unexposed during gestation; models were adjusted for sex and baseline cardiovascular activity; CI = confidence interval; SBP = systolic blood pressure; HR = heart rate; LF = low frequency; HF = high frequency; HP = heart period.
Discussion
In the present study, we did not find evidence for an association between prenatal famine exposure and autonomic function during rest or in response to psychological stress. Measures of sympathetic function and parasympathetic function did not differ between those unexposed and those exposed to famine during late, mid or early gestation.
Previous results from the Dutch famine birth cohort studies showed that people exposed to famine during early gestation have an increased risk of coronary heart disease, increased blood pressure responsiveness to stress and higher female cardiovascular mortality (1, 7, 8). Perinatal malnutrition in sheep and rats has been shown to result in programming of autonomous nervous system activity and subsequent hypertension, providing a potential mechanism explaining the link between poor early life nutrition and increased heart disease risk (7–13). We set out to test the hypothesis that prenatal famine exposure results in altered autonomic function and specifically higher sympathetic activation, but the results from spectral analyses in the present study do not support this hypothesis. Those exposed to famine in early gestation had an increased SBP response to stress. This increase of 4 mmHg was similar in size as we showed before, but not statistically significant (p=0.07), which is probably due the smaller sample size (602 compared to 721 in the older study) (7). However, we did not find differences in indices of sympathetic and parasympathetic function during rest or in response to stress between people exposed or unexposed prenatally to famine
Our findings do not match those from the mentioned animal experimental studies, which may be explained by the fact that the majority of these studies did not follow the animal into older age as we did with the people prenatally exposed to famine. However, our study also had a number of limitations that may explain the lack of associations between prenatal undernutrition and autonomic function. We studied potential effects of prenatal famine exposure in a group of subjects who are well into middle age. Aging brings greater autonomic instability and the function of autonomic cardiovascular control mechanisms may no longer be relevant if age-related vascular disease has developed to a point where arteries no longer respond adequately to neurological control. However, further analysis of our data following exclusion of all patients with established hypertension, diabetes and cardiovascular disease did not expose significant findings masked by the inclusion of subjects with established disease.
We found a substantial burden of cardiac arrhythmia in our population. Combined with some poor signals due to technical issues with the equipment, this led to the data from 119 people out of the original database of 721 people being unsuitable for analysis. However, there were no statistically significant differences in the proportions of participants excluded from the spectral analyses between the exposed and unexposed groups arguing against an effect of selective exclusion on our ability to find differences between these groups.
It should also be noted that our method for deriving the autonomic measures relied upon spectral analyses of the time intervals between pulse arrival times at the finger. Although there have been publications that support this method, validating measures derived in this manner against those derived using ECG, ECG recording with a high sample rate (1000 Hz) would, in retrospect, have been a preferred method for estimating heart rate variability measures (20). However, we believe that it is unlikely that use of this gold-standard approach would have yielded positive findings, given favorable comparisons between the two methods and the fact that we did not find associations between famine exposure and autonomic measures of borderline significance or in measures that did not depend on pulse timing (data not shown).
A final explanation may be that of selective participation of more healthy subjects in our study. Compared to a round of data collection performed in 1994-1996, people with type 2 diabetes, cardiovascular disease, high cholesterol levels and high blood pressure were less likely to participate in the present study (data not shown). In addition, we found higher mortality among women exposed to famine in early gestation up to age 63 years (8). Participants in the present study exposed to famine in early gestation were less likely to use antihypertensive medication suggesting that they may be a somewhat healthier group in this respect. Although adjusting for use of antihypertensive medication did not alter the results, we cannot completely rule out an effect of selective participation.
Our study also had a number of strengths. We measured cardiovascular activity in a large study group using a highly standardized protocol. Autonomic functioning was measured during rest as well as in response to an extensive stress protocol. Generally, differences in cardiovascular function are more likely to become apparent when the system is stressed. The stress protocol significantly perturbed blood pressure and heart rate and sympathetic activity increased in response to stress, while parasympathetic activity decreased, as expected. The finding that LF SBP, as an indicator of sympathetic activity, diminished marginally in response to the Stroop task and only slightly increased in response to the mirror task but increased substantially in response to the speech task corresponds with data published previously (21). Furthermore, as reported previously, cardiovascular stress responses and measures of autonomic function differed according to sex, smoking status, use of anti-hypertensive medication and BMI (22, 23). A final strength is that the Dutch famine birth cohort study is a unique counterpart for animal models that study the effect of prenatal undernutrition during different stages of gestation. Despite these strengths, we were unable to show an effect of prenatal famine exposure on autonomic function. This could mean that this effect does not exist, that there could be an effect acting in early life to cause disease that is no longer apparent by late adulthood, or that it is a very small effect that is difficult to detect. In any case, our findings suggest that prenatal programming of systems other than the autonomic nervous system is likely to underpin the effects of prenatal undernutrition on risk of later cardiovascular disease. Potential candidates include an altered myocardial structure, endothelial dysfunction or increased oxidative stress (24). Furthermore, we have shown previously that increased dyslipidemia, type 2 diabetes, altered blood coagulation and a preference for fatty foods are features of those exposed to famine in early gestation, as well as more obesity in women and more symptoms of depression in men, which may all contribute to the development of heart disease (25).
In conclusion, we did not find evidence that prenatal undernutrition alters autonomic functioning in late adulthood. Therefore, fetal programming of the autonomic nervous system does not offer an explanation for our previous findings of increased heart disease and increased cardiovascular mortality after prenatal famine exposure.
Acknowledgments
Sources of funding: Medical Research Council (UK), Netherlands Heart Foundation (grant number 2001B087), the European Community FP7 HEALTH, Project 279281 (BRAINAGE) and Horizon2020, Project 633595 (DynaHealth).
Abbreviations
- BMI
body mass index
- HR
heart rate
- BP
blood pressure
- HP
heart period
- SBP
systolic blood pressure
- LF
low frequency
- HF
high frequency
Footnotes
Conflicts of interest: The authors declare no conflicts of interest;
References
- 1.Painter RC, de Rooij SR, Roseboom TJ, Bossuyt PMM, Simmers TA, Osmond C, Barker DJP, Bleker OP. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. AJCN. 2006;84:322–7. doi: 10.1093/ajcn/84.1.322. [DOI] [PubMed] [Google Scholar]
- 2.Alastalo H, Raikkonen K, Pesonen AK, Osmond C, Barker DJ, Kajantie E, Heinonen K, Forsen TJ, Eriksson JG. Cardiovascular health of Finnish war evacuees 60 years later. Ann Med. 2009;41:66–72. doi: 10.1080/07853890802301983. [DOI] [PubMed] [Google Scholar]
- 3.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American journal of preventive medicine. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 4.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–6. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 5.Lumey LH, Stein AD, Susser E. Prenatal famine and adult health. Annu Rev Public Health. 2011;32:237–62. doi: 10.1146/annurev-publhealth-031210-101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monk C, Georgieff MK, Osterholm EA. Research review: maternal prenatal distress and poor nutrition - mutually influencing risk factors affecting infant neurocognitive development. Journal of child psychology and psychiatry, and allied disciplines. 2013;54:115–30. doi: 10.1111/jcpp.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Painter RC, de Rooij SR, Bossuyt PM, Phillips DI, Osmond C, Barker DJ, Bleker OP, Roseboom TJ. Blood pressure response to psychological stressors in adults after prenatal exposure to the Dutch famine. J Hypertens. 2006;24:1771–8. doi: 10.1097/01.hjh.0000242401.45591.e7. [DOI] [PubMed] [Google Scholar]
- 8.van Abeelen AF, Veenendaal MV, Painter RC, de Rooij SR, Dijkgraaf MG, Bossuyt PM, Elias SG, Grobbee DE, Uiterwaal CS, Roseboom TJ. Survival effects of prenatal famine exposure. The American journal of clinical nutrition. 2012;95:179–83. doi: 10.3945/ajcn.111.022038. [DOI] [PubMed] [Google Scholar]
- 9.Hawkins P, Steyn C, Ozaki T, Saito T, Noakes DE, Hanson MA. Effect of maternal undernutrition in early gestation on ovine fetal blood pressure and cardiovascular reflexes. AmJ Physiol RegulIntegrComp Physiol. 2000;279:R340–R8. doi: 10.1152/ajpregu.2000.279.1.R340. [DOI] [PubMed] [Google Scholar]
- 10.Gardner DS, Pearce S, Dandrea J, Walker R, Ramsay MM, Stephenson T, Symonds ME. Peri-implantation undernutrition programs blunted angiotensin II evoked baroreflex responses in young adult sheep. Hypertension. 2004;43:1290–6. doi: 10.1161/01.HYP.0000126991.67203.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poore KR, Boullin JP, Cleal JK, Newman JP, Noakes DE, Hanson MA, Green LR. Sex- and age-specific effects of nutrition in early gestation and early postnatal life on hypothalamo-pituitary-adrenal axis and sympathoadrenal function in adult sheep. J Physiol. 2010;588:2219–37. doi: 10.1113/jphysiol.2010.187682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petry CJ, Dorling MW, Wang CL, Pawlak DB, Ozanne SE. Catecholamine levels and receptor expression in low protein rat offspring. DiabetMed. 2000;17:848–53. doi: 10.1046/j.1464-5491.2000.00392.x. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Twinn DS, Ekizoglou S, Wayman A, Petry CJ, Ozanne SE. Maternal low-protein diet programs cardiac beta-adrenergic response and signaling in 3-mo-old male offspring. AmJPhysiol RegulIntegrComp Physiol. 2006;291:R429–R36. doi: 10.1152/ajpregu.00608.2005. [DOI] [PubMed] [Google Scholar]
- 14.Silva FC, de Menezes RC, Chianca DA., Jr The implication of protein malnutrition on cardiovascular control systems in rats. Frontiers in physiology. 2015;6:246. doi: 10.3389/fphys.2015.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sucharita S, Dwarkanath P, Thomas T, Srinivasan K, Kurpad AV, Vaz M. Low maternal vitamin B12 status during pregnancy is associated with reduced heart rate variability indices in young children. Maternal & child nutrition. 2014;10:226–33. doi: 10.1111/j.1740-8709.2012.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravelli ACJ, van der Meulen JHP, Michels RPJ, Osmond C, Barker DJP, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–7. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 17.Trienekens G. Tussen ons volk en de honger ('Between our people and famine') Utrecht: Matrijs; 1985. [Google Scholar]
- 18.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 19.Berntson GG, Quigley KS, Jang JF, Boysen ST. An approach to artifact identification: application to heart period data. Psychophysiology. 1990;27:586–98. doi: 10.1111/j.1469-8986.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 20.McKinley PS, Shapiro PA, Bagiella E, Myers MM, De Meersman RE, Grant I, Sloan RP. Deriving heart period variability from blood pressure waveforms. Journal of applied physiology (Bethesda, Md 1985) 2003;95:1431–8. doi: 10.1152/japplphysiol.01110.2002. [DOI] [PubMed] [Google Scholar]
- 21.Jones A, Beda A, Ward AM, Osmond C, Phillips DI, Moore VM, Simpson DM. Size at birth and autonomic function during psychological stress. Hypertension. 2007;49:548–55. doi: 10.1161/01.HYP.0000257196.13485.9b. [DOI] [PubMed] [Google Scholar]
- 22.de Rooij SR. Blunted cardiovascular and cortisol reactivity to acute psychological stress: a summary of results from the Dutch Famine Birth Cohort Study. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2013;90:21–7. doi: 10.1016/j.ijpsycho.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Ginty AT, Jones A, Carroll D, Roseboom TJ, Phillips AC, Painter R, de Rooij SR. Neuroendocrine and cardiovascular reactions to acute psychological stress are attenuated in smokers. Psychoneuroendocrinology. 2014;48:87–97. doi: 10.1016/j.psyneuen.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Thornburg KL. The programming of cardiovascular disease. J Dev Orig Health Dis. 2015;6:366–76. doi: 10.1017/S2040174415001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roseboom TJ, Painter RC, van Abeelen AF, Veenendaal MV, de Rooij SR. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70:141–5. doi: 10.1016/j.maturitas.2011.06.017. [DOI] [PubMed] [Google Scholar]