Abstract
Purpose
Accurate identification of the source of a detectable serum PSA in the postprostatectomy setting is a major challenge amongst the urologic community. The aim of this study was to assess positivity rates of imaging examinations performed in patients with early PSA rise after prostatectomy; and to summarize the management strategies adopted in this clinical scenario.
Methods
IRB-approved retrospective study of 142 post-prostatectomy patients with PSA rise up to 1 ng/mL who underwent evaluation with combination of multiparametric pelvic MRI +/− whole-body or bone MRI, bone scintigraphy (BS), CT chest-abdomen-pelvis (CT-CAP), FDG-PET/ CT and/or NaF-PET/CT at a single tertiary cancer center. Imaging results were summarized per modality and compared to pathology findings.
Results
Pelvic MRI was positive in 15/142 (11%) patients (14 patients with local recurrence in the surgical bed and 1 patient with pelvic osseous metastases). Ten of these 15 patients underwent additional imaging exams; none revealed positive findings. Of the 127 patients with negative pelvic MRI, 54 (43%) underwent additional imaging exams; only 1/54 had positive findings (false positive T8 lesion on BS and FDG-PET/CT-biopsy negative for cancer). 12/16 patients with positive imaging findings and 75/126 (60%) patients with negative imaging received treatment (radiation, hormones and/or chemotherapy).
Conclusion
conventional imaging identified sites of disease, almost always in the form of local recurrence, in a minority of patients with early PSA rise post-prostatectomy.
Keywords: Bone scan, CT, MRI, PET, prostate cancer, recurrence
INTRODUCTION
Radical prostatectomy is an established option for the definitive treatment of clinically significant prostate cancer. Despite the adequate oncologic control and low complication rates achieved with the current surgical approaches, a percentage of patients are faced with disease recurrence, usually manifesting first as detectable serum prostate-specific antigen (PSA) levels. Accurate identification of the source of a rising PSA in the post-prostatectomy setting is currently a major challenge amongst the urologic oncology community. Current guidelines recommend the consideration of salvage radiotherapy (SRT) to the prostate bed in patients with biochemical recurrence after prostatectomy [1–3]. Several reports have found that SRT is more effective in achieving durable PSA suppression when treating patients with lower PSA levels, and thus SRT is being increasingly administered to patients with the initial manifestation of a rising detectable PSA [4]. Despite this, the likelihood of maintaining an undetectable PSA at two years after such treatment is often less than 40%, especially among high-risk patients with elevated Gleason scores [5; 6]. A number of factors may contribute to the failure of SRT, one of which is the presence of undetected metastatic sites of disease outside of the pelvic radiation treatment field.
Conventional imaging modalities for the detection of post-prostatectomy recurrence include magnetic resonance imaging (MRI), bone scintigraphy (BS), computed tomography (CT) and positron-emission tomography (PET). A combination of these is typically applied, and there are many reports documenting the use of these techniques, in particular MRI, for the detection of recurrent disease [7–13]. However, all of these studies include patients with relatively high PSA levels, although some do document less optimal performance in patients with lower PSA values. It is clear that better techniques for disease localization in patients with early PSA rise are desirable, and several experimental approaches are undergoing initial evaluation for use in this context. Before implementing new strategies, better understanding of the baseline scenario with the current standard of care tools is necessary for benchmarking. To date, no studies have reported on the use of conventional imaging specifically for the assessment of early postprostatectomy PSA rise. Thus, the purpose of this study was to assess the positivity rates of imaging examinations performed in patients with early PSA rise (≤1.0 ng/mL) after radical prostatectomy; and to summarize the management strategies (e.g. biopsies, treatments) adopted in parallel with imaging in this patient population.
METHODS
Patients
The Institutional Review Board approved this retrospective study and issued a waiver of informed consent. Inclusion criteria were: (i) Radical prostatectomy with subsequent serum PSA drop to undetectable levels; (ii) Imaging performed for the evaluation of early PSA rise ≤ 1 ng/mL between October 2014 and August 2015. 285 patients fulfilled these inclusion criteria. Patients with prior pelvic radiation and/or hormone therapy within 90 days prior to imaging (n=143) were excluded. Thus, the final study population consisted of 142 patients. Clinical and demographic characteristics of the patient population are presented in Table 1.
Table 1.
Summary statistics of the patients’ clinical characteristics
| Clinical Characteristic | Summary Statistics | |
|---|---|---|
| Age | Median | 64 years |
| Range | 46–71 years | |
| PSA (ng/mL) | Median | 0.21 |
| IQR | 0.15–0.36 | |
| PSA doubling time (months) | Median | 6.5 |
| Range | 0–37.9 | |
| Radical Prostatectomy | Gleason Score | |
| 3+3 | 8 (6%) | |
| 3+ | 53 (37%) | |
| 4+ | 51 (36%) | |
| ≥4+4 | 28 (20%) | |
| N/A | 2 (1%) | |
| Pathologic stage | 99 (70%) | |
| ≤pT2 | 43 (30%) | |
| pT3a | 71 (50%) | |
| ≥pT3b | 28 (20%) | |
| Surgical Margins | ||
| Negative | 79 (56%) | |
| Positive | 61 (43%) | |
| N/A | 2 (1%) | |
| Interval prostatectomy to Imaging (days) | Median | 933 days |
| Range | 59–5572 days | |
PSA: prostate specific antigen; IQR: inter-quantile range
Imaging
The use of MRI, bone scintigraphy, CT and PET is not standardized at our institution with regards to the optimal timing for performing them or the preferred imaging modality. In general, patients undergo a combination of these studies to assess for local recurrence (i.e prostatectomy bed) and distant disease. mp-MRI was performed using a standard acquisition protocol (see supplementary methods) which includes axial T1-weighted, multiplanar T2-weighted, diffusion-weighted (DW) and contrast-enhanced sequences. Whole body bone scintigraphy was performed approximately 3 hours following the IV injection of 25 mCi MDP-99mTc. 18FFluorodeoxyglucose (FDG) PET/CT was acquired approximately 60 min following the IV injection of 12 mCi FDG and 18F-sodium fluoride (NaF) PET/CT was acquired approximately 60 min following the IV injection of 10 mCi NaF.
The imaging reports issued as part of standard clinical care were reviewed in consensus by 3 urologic oncology fellows. At our institution, all radiology reports indicate the radiologist’s subjective level of certainty for the presence of recurrent disease using a lexicon scale as previously reported [14; 15]. This Lexicon has been in place for all radiologic examinations (except breast imaging) since 2009 and consists of a 5-point scale ranging from 1: recurrence unlikely (<10% probability) to 5: findings consistent with recurrence (>90% probability).
Statistical Analysis
For analysis, imaging findings were dichotomized as positive (score of 4–5) versus negative (scores of 1–3) according to our institutional lexicon. The proportion and nature of positive imaging findings for each imaging modality were described using summary statistics. The clinical strategies adopted within 1 month of the first imaging study performed are also reported using a similar approach. When biopsies were performed, the official pathology report was reviewed and compared to the imaging findings reported. Odds ratio with 95% CI is reported for the association between serum PSA levels and positive imaging findings.
RESULTS
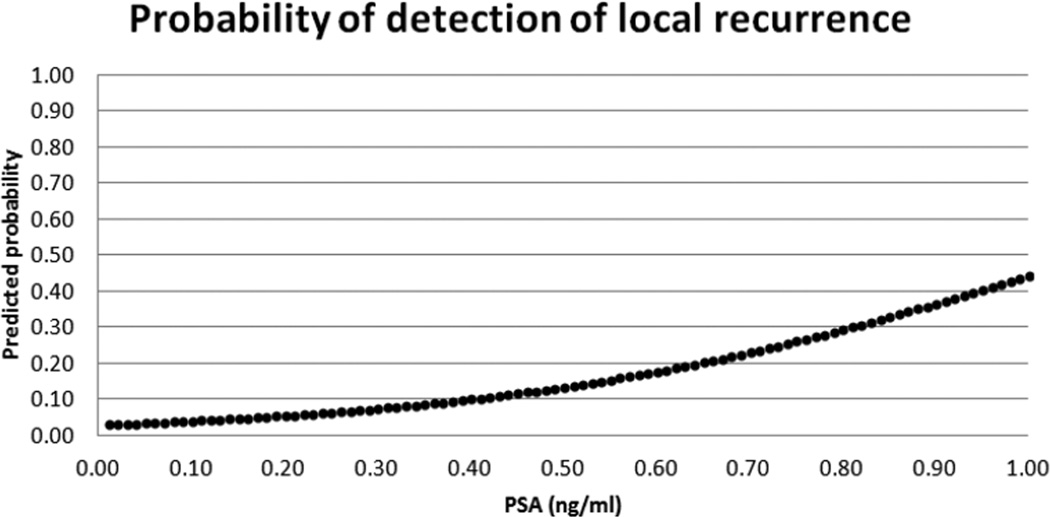
The median PSA at the time of imaging was 0.21 ng/mL (interquartile range 0.15–0.36 ng/mL). There was a significant association between higher PSA levels and positive imaging findings (Odds Ratio: 26.6 (95% CI: 3.1–230.6); p=0.0029) (Figure 1). Radical prostatectomy Gleason Score was 3+3 in 8 patients; 3+4 in 53 patients; 4+3 in 51 patients; ≥4+4 in 28 patients and not available in 2 patients. There was extracapsular extension at prostatectomy in 99/142 patients and seminal vesicle invasion in 28/142 patients. The median interval between radical prostatectomy and imaging was 933 days (range 59–5572 days).
Figure 1.
Logistic regression depiction of the probability of detection of local recurrence according to serum PSA level
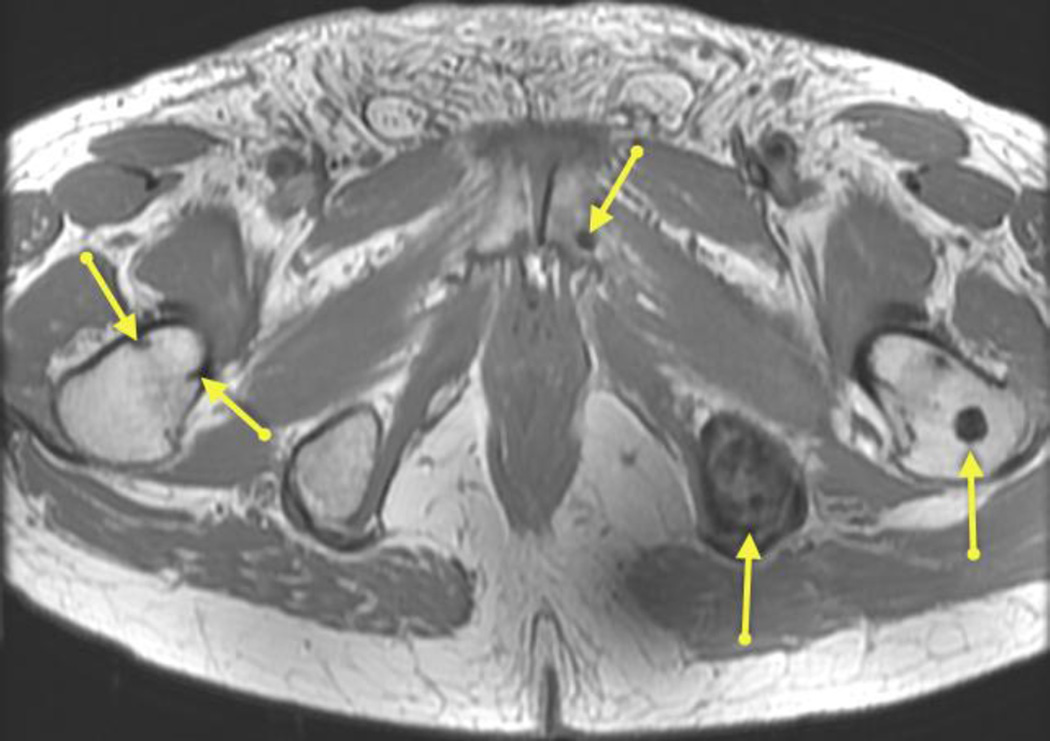
All 142 patients underwent at least one imaging exam. Pelvic MRI was performed in all patients; and was positive in 15/142 (11%) patients (in 14 patients it showed local recurrence in the prostatectomy bed and in 1 patient it showed a pelvic osseous metastasis [Figure 2]). None of these patients had abnormal lymph nodes on pelvic MRI. The median PSA of patients with positive pelvic MRI findings was 0.18 ng/mL (range 0.07–0.9); and their median time from prostatectomy to imaging was 983 days (range 152–4288 days). Prostatectomy Gleason score was 3+4 in 6/15 patients, 4+3 in 6/15 patients, ≥4+4 in 2/15 patients and unknown in 1/15 patient. 13/15 patients had extracapsular extension; 5/15 patients had seminal vesicle invasion and 7/15 patients had positive surgical margins on the radical prostatectomy specimen. Ten of these 15 patients underwent additional imaging exams; none revealed local recurrence in the prostatectomy bed or additional positive findings. Prostatectomy bed biopsy was performed in 5 of the 14 patients with local disease identified on MRI; pathologic evaluation was positive for recurrence in 4/5 of these patients. Of the 15 patients with positive imaging findings on pelvic MRI, 12 received treatment (3/12 received salvage radiation, 8/12 received combined salvage radiation and androgen deprivation therapy and 1 received systemic chemotherapy), 1 patient declined treatment, in 1 patient salvage radiation was considered but withheld due to toxicity risk in view of coexisting inflammatory bowel disease; and 1 patient was lost to follow-up.
Figure 2.
Axial T1-weighted image of a 75 year old man with PSA 0.71 9 years after radical prostatectomy (Gleason 4+3; positive extracapsular extension; no seminla vesicle invasion) showing multiple pelvic bone metastases (arrows).
Of the 127 patients with negative pelvic MRI, 54 (43%) underwent additional imaging exams; which were positive in 1/28 BS (thoracic spine lesion, outside the field of view of prostate MRI, biopsy negative for malignancy), 0/18 CT chest-abdomen-pelvis (CAP), 0/14 MRI (whole body or abdominal or spine), 1/4 FDG PET (same patient with false positive T8 lesion on BS) and 0/10 NaF PET. Of the 126 patients with negative findings on imaging, 75 (60%) were treated with salvage radiation with or without androgen deprivation therapy, 46 (37%) were managed with observation. Five patients with negative imaging were lost to follow-up. The median PSA of patients with negative imaging findings was 0.14 ng/mL (range <0.05–0.93).
DISCUSSION
In this study we document that conventional imaging studies have a low yield for localizing sites of recurrent disease in patients with early PSA rise after radical prostatectomy. Local recurrence in the prostatectomy bed was identified in 14/142 patients, while distant disease (pelvic osseous metastasis), was only detected in 1/142 patients. Despite this, the majority of patients with negative imaging went on to have empirical salvage radiation treatment, alone or in combination with androgen deprivation therapy.
This finding is relevant as it highlights the fact that salvage RT is often done empirically without clear evidence of potential sites of disease recurrence. Although several studies have found associations between improved outcomes and lower PSA levels at the time of SRT [16] [17] [18], SRT failure rates are as high as 40% have also been reported [19].
Several factors have been implicated in this relatively poor performance, one of the most intuitive being the inclusion of patients with extra-pelvic metastatic disease that is not detected with conventional imaging techniques, and remains untreated after SRT due to its location outside of the pelvic template used for SRT. It is postulated that if such patients could be identified prior to treatment, the increased risk for radiation-related morbidities and the health care costs incurred for expensive courses of SRT which would ultimately prove ineffective would be avoided. At the same time, applying SRT to patients with disease localized to the pelvis would ultimately improve the local control rates.
Prior reports have hinted at the relatively limited use of bone scans and CT in the post-prostatectomy setting, even though these studies often evaluated patients with much higher PSA levels than the ones reported in our study as they were not specifically focusing on the setting of early biochemical recurrence. In a study by Moreira et al, bone scans were positive in <5% of post-prostatectomy patients with a PSA ≤4.9 ng/mL [20]. Dotan et al reported bone scan positivity in 4% of post-prostatectomy patients with PSA <10 ng/mL (median 8.4 ng/mL) [21]. A study evaluating the CT appearance of 22 cases of pathologically confirmed local recurrence after radical prostatectomy, positive findings on CT were found in only 8/22 patients (36%), all of which had recurrent masses ≥ 2cm. [22]. Serum PSA was not reported in that study. MRI is considered the imaging modality of choice for the evaluation of local recurrence. In one study, for detecting recurrence in the prostate bed after RP, the area under the curve for a combined T2-weighted and contrast-enhanced approaches were 0.86 and 0.88 for two independent readers [13]. Other groups have also reported a high sensitivity of MRI to identify local recurrence after prostatectomy; the median PSA levels of patients included in those studies are substantially higher than the PSA levels in patients with early PSA rise reported in this manuscript [7; 23; 24]. Our patient population is more comparable to 11 patients with suspected post-RP recurrence and a mean PSA of 0.24±0.13 ng/mL reported by Rischke et al. [12], all of whom had a negative prostate MRI as part of their pre-SRT workup. We did not find any imaging evidence of metastatic lymphadenopathy in our patient population, but others have reported their presence in other cohorts. Kitajima et al. have investigated the use of MRI to identify local recurrences and lymph node metastases in a retrospective series of 115 patients with recurrent prostate cancer using histology as a reference standard [9]. A subset of 70 patients (median PSA 2.5 ng/mL) was studied by contrast-enhanced and diffusion-weighted MRI acquired on 1.5 T and 3 T scanners. The sensitivity of MRI for local recurrence and pelvic lymph node metastases was 89% and 64%, respectively [9]. Hernandez et al evaluated 70 patients with a median PSA of 0.38 (range 0.00–8.05), and identified 7 (10%) of patients with nodal metastases [25]. There was no pathologic correlation for the nodal findings, and it is unclear if patients receiving ADT at the time of imaging were included in the analysis.
In addition to its use for local staging of PCa, MRI is considered the most sensitive conventional imaging modality for the detection of bone marrow involvement by PCa. Despite this, technical factors have limited its use to single anatomical areas (e.g., pelvis or a specific bone). More recently, technological advances have led to the feasibility of examining multiple anatomical stations using MRI in under one hour. Although the state-of-the-art equipment and technical expertise for acquisition and interpretation of whole-body MRI (WB-MRI) surveys is not yet universally available, its value is being increasingly identified in the literature [26–28]. However, specifically in the context of early PSA rise after prostatectomy, none of the whole-body MRIs performed in our study yielded a positive finding.
PET/CT has also been evaluated in the context of post-prostatectomy recurrence. Although FDG PET has limited usefulness in the assessment of localized newly-diagnosed prostate cancer, Schoder et al reported a serum PSA of 2.4 ng/mL as best cutoff for the detection of local recurrence or metastatic disease after prostatectomy by FDG PET [29]. Other PET tracers have also been investigated. The diagnostic accuracy of choline PET/CT in recurrent prostate cancer has been summarized in two meta-analyses [30; 31]. Umbehr et al. analyzed the results of 12 studies including 1,095 patients and found a pooled sensitivity of 85% on a patient basis, but the mean PSA level in these studies was 7.9 ng/mL [31]. Evangelista et al. analyzed 19 studies including 1,555 patients. They report a pooled sensitivity/specificity for local recurrence and lymph node metastases of 75%/82% and 100%/82%, respectively. However, the authors also emphasize that the frequency of positive choline PET/CT is low (≤ 20%) if the PSA is less than 1.0 ng/mL or if the PSA doubling time is more than three months [32; 33].
PET imaging using the leucine analog 18F-FACBC (1-amino-3-fluorine 18-fluorocyclobutane-1-carboxylic acid) has been studied in both primary and recurrent PCa [34; 35]. FACBC PET/CT was found to perform significantly better than SPECT with the prostate-specific membrane antigen (PSMA) antibody 111In-capromab for detection of local recurrence and extraprostatic disease in a group of 93 patients with biochemical recurrence after primary therapy [36]. The median PSA in this study was 4.0 ng/mL and therefore markedly higher than the target population in our study. In a study of 28 patients with biochemical recurrence after primary therapy, FACBC PET/CT visualized prostate cancer with higher contrast than 11C-choline PET/CT [35]. Mean PSA in this study was 2.9 ng/ml (range of 0.2–14.6), again markedly higher than the patients included in the current study.
A recent study included a subpopulation of 12 patients with a PSA of less than 0.2 ng/ml undergoing 68Ga-HBED-CC-PSMA PET/CT in the setting of biochemical recurrence of prostate cancer. The population was heterogeneous including a combination of post-prostatectomy and post-radiation patients. PSMA PET/CT was positive in 13 cases (48%). This positivity rate is markedly higher than reported for any other imaging modality. While these results are highly promising, the study did not perform systematic pathologic verification of the positive imaging findings, nor did it describe the location of the positive findings to determine if recurrence was due to pelvic vs extra-pelvic sites of disease. Furthermore, the analysis included patients with castration-resistant prostate cancer receiving ADT and patients that had received radiotherapy prior to the PET scan.
Our study had several limitations. Most importantly, it is retrospective, and since there were no standardized indications for imaging there is heterogeneity with regards to the timing, frequency and choice of imaging modalities applied. Also, pathologic confirmation of positive findings was not performed in all patients. While it is certainly a limitation, this reflects actual practice where biopsy confirmation, although desirable, is not always possible or feasible for management decision-making. In addition, although 4 out of 5 biopsies performed in patients with MR detected local recurrences were positive, the biopsy was negative in 1 patient. This highlights the fact that specificity is also important, although it must also be acknowledged that small recurrences may be difficult to target with biopsy and therefore a negative biopsy does not completely rule out the presence of local recurrence. Finally, some patients in our study had an undetectable PSA at the time of MRI. Typically, these patients have been assessed for suspicious symptoms and/or a detectable PSA at an outside laboratory, however repeat PSA at our institution closer to the time of the MRI is undetectable. None of these patients had positive imaging findings.
In summary, our study documents the suboptimal performance of standard of care imaging techniques for localizing sites of disease in patients with early PSA rise after prostatectomy. The findings highlight the need for more accurate assessment methods in this patient population and provide baseline data relevant to the design of prospective trials evaluating the incremental value of novel techniques used to be used in this scenario. A recently published study suggests a diagnostic technology capable of identifying whether men with biochemical recurrence after radical prostatectomy have localized versus metastatic disease would be a cost-effective alternative to current standard work-up. The results support additional investment in development and validation of such a diagnostic [37].
Supplementary Material
Highlights.
In patients with early PSA rise (<1 ng/mL) after prostatectomy:
-
-
Pelvic MRI was positive in 15/142 (11%) patients
-
-
14/15 patients had local recurrence and 1/15 had osseous metastases
-
-
No patient with a negative pelvic MRI had metastases on other imaging exams
Acknowledgments
This project was supported in part by NIH grant P30 CA008748
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Freedland SJ, Rumble RB, Finelli A, et al. Adjuvant and salvage radiotherapy after prostatectomy: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2014;32:3892–3898. doi: 10.1200/JCO.2014.58.8525. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190:441–449. doi: 10.1016/j.juro.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 4.Fossati N, Karnes RJ, Cozzarini C, et al. Assessing the Optimal Timing for Early Salvage Radiation Therapy in Patients with Prostate-specific Antigen Rise After Radical Prostatectomy. Eur Urol. 2016;69:728–733. doi: 10.1016/j.eururo.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Perez BA, Koontz BF. Radiotherapy before and after radical prostatectomy for high-risk and locally advanced prostate cancer. Urol Oncol. 2015;33:226–234. doi: 10.1016/j.urolonc.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 6.van der Poel HG, Tillier C, de Blok W, Acar C, van Muilekom EH. Salvage radiotherapy after robot-assisted laparoscopic radical prostatectomy. Urology. 2013;82:834–838. doi: 10.1016/j.urology.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 7.Cirillo S, Petracchini M, Scotti L, et al. Endorectal magnetic resonance imaging at 1.5 Tesla to assess local recurrence following radical prostatectomy using T2-weighted and contrastenhanced imaging. Eur Radiol. 2009;19:761–769. doi: 10.1007/s00330-008-1174-8. [DOI] [PubMed] [Google Scholar]
- 8.Eiber M, Holzapfel K, Ganter C, et al. Whole-body MRI including diffusion-weighted imaging (DWI) for patients with recurring prostate cancer: technical feasibility and assessment of lesion conspicuity in DWI. J Magn Reson Imaging. 2011;33:1160–1170. doi: 10.1002/jmri.22542. [DOI] [PubMed] [Google Scholar]
- 9.Kitajima K, Murphy RC, Nathan MA, et al. Detection of recurrent prostate cancer after radical prostatectomy: comparison of 11C-choline PET/CT with pelvic multiparametric MR imaging with endorectal coil. J Nucl Med. 2014;55:223–232. doi: 10.2967/jnumed.113.123018. [DOI] [PubMed] [Google Scholar]
- 10.Linder BJ, Kawashima A, Woodrum DA, et al. Early localization of recurrent prostate cancer after prostatectomy by endorectal coil magnetic resonance imaging. Can J Urol. 2014;21:7283–7289. [PubMed] [Google Scholar]
- 11.Muller BG, Kaushal A, Sankineni S, et al. Multiparametric magnetic resonance imaging-transrectal ultrasound fusion-assisted biopsy for the diagnosis of local recurrence after radical prostatectomy. Urol Oncol. 2015;33:425, e421–e426. doi: 10.1016/j.urolonc.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rischke HC, Schafer AO, Nestle U, et al. Detection of local recurrent prostate cancer after radical prostatectomy in terms of salvage radiotherapy using dynamic contrast enhanced-MRI without endorectal coil. Radiat Oncol. 2015;7:185. doi: 10.1186/1748-717X-7-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wassberg C, Akin O, Vargas HA, Shukla-Dave A, Zhang J, Hricak H. The incremental value of contrast-enhanced MRI in the detection of biopsy-proven local recurrence of prostate cancer after radical prostatectomy: effect of reader experience. AJR Am J Roentgenol. 2012;199:360–366. doi: 10.2214/AJR.11.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wibmer A, Vargas HA, Sosa R, Zheng J, Moskowitz C, Hricak H. Value of a standardized lexicon for reporting levels of diagnostic certainty in prostate MRI. AJR Am J Roentgenol. 2014;203:W651–W657. doi: 10.2214/AJR.14.12654. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz LH, Panicek DM, Berk AR, Li Y, Hricak H. Improving communication of diagnostic radiology findings through structured reporting. Radiology. 2011;260:174–181. doi: 10.1148/radiol.11101913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King CR. The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int J Radiat Oncol Biol Phys. 2012;84:104–111. doi: 10.1016/j.ijrobp.2011.10.069. [DOI] [PubMed] [Google Scholar]
- 17.Trabulsi EJ, Valicenti RK, Hanlon AL, et al. A multi-institutional matched-control analysis of adjuvant and salvage postoperative radiation therapy for pT3-4N0 prostate cancer. Urology. 2008;72:1298–1302. doi: 10.1016/j.urology.2008.05.057. discussion 1302–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ost P, De Troyer B, Fonteyne V, Oosterlinck W, De Meerleer G. A matched control analysis of adjuvant and salvage high-dose postoperative intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:1316–1322. doi: 10.1016/j.ijrobp.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 19.Goenka A, Magsanoc JM, Pei X, et al. Long-term outcomes after high-dose postprostatectomy salvage radiation treatment. Int J Radiat Oncol Biol Phys. 2012;84:112–118. doi: 10.1016/j.ijrobp.2011.10.077. [DOI] [PubMed] [Google Scholar]
- 20.Moreira DM, Cooperberg MR. Predicting bone scan positivity after biochemical recurrence following radical prostatectomy in both hormone-naive men and patients receiving androgen-deprivation therapy: results from the SEARCH database. 2014;17:91–96. doi: 10.1038/pcan.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dotan ZA, Bianco FJ, Rabbani F, et al. Pattern of Prostate-Specific Antigen (PSA) Failure Dictates the Probability of a Positive Bone Scan in Patients With an Increasing PSA After Radical Prostatectomy. Journal of Clinical Oncology. 2005;23:1962–1968. doi: 10.1200/JCO.2005.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer S, Gorich J, Gottfried HW, et al. Sensitivity of computed tomography in detecting local recurrence of prostatic carcinoma following radical prostatectomy. Br J Radiol. 1997;70:995–999. doi: 10.1259/bjr.70.838.9404201. [DOI] [PubMed] [Google Scholar]
- 23.Giannarini G, Nguyen DP, Thalmann GN, Thoeny HC. Diffusion-weighted magnetic resonance imaging detects local recurrence after radical prostatectomy: initial experience. Eur Urol. 2012;61:616–620. doi: 10.1016/j.eururo.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Cha D, Kim CK, Park SY, Park JJ, Park BK. Evaluation of suspected soft tissue lesion in the prostate bed after radical prostatectomy using 3T multiparametric magnetic resonance imaging. Magn Reson Imaging. 2015;33:407–412. doi: 10.1016/j.mri.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez D, Salas D, Gimenez D, et al. Pelvic MRI findings in relapsed prostate cancer after radical prostatectomy. Radiat Oncol. 2015;10:262. doi: 10.1186/s13014-015-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jambor I, Kuisma A, Ramadan S, et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol. 2015 doi: 10.3109/0284186X.2015.1027411. 10.3109/0284186x.2015.1027411:1-9. [DOI] [PubMed] [Google Scholar]
- 27.Minamimoto R, Loening A, Jamali M, et al. Prospective Comparison of 99mTc MDP Scintigraphy, Combined 18F-NaF and 18F-FDG PET/CT and Whole-Body MRI in Patients with Breast and Prostate Cancers. J Nucl Med. 2015 doi: 10.2967/jnumed.115.162610. 10.2967/jnumed.115.162610. [DOI] [PubMed] [Google Scholar]
- 28.Pasoglou V, Michoux N, Peeters F, et al. Whole-body 3D T1-weighted MR imaging in patients with prostate cancer: feasibility and evaluation in screening for metastatic disease. Radiology. 2015;275:155–166. doi: 10.1148/radiol.14141242. [DOI] [PubMed] [Google Scholar]
- 29.Schöder H, Herrmann K, Gönen M, et al. 2-[18F]Fluoro-2-Deoxyglucose Positron Emission Tomography for the Detection of Disease in Patients with Prostate-Specific Antigen Relapse after Radical Prostatectomy. Clinical Cancer Research. 2005;11:4761–4769. doi: 10.1158/1078-0432.CCR-05-0249. [DOI] [PubMed] [Google Scholar]
- 30.Evangelista L, Zattoni F, Guttilla A, Saladini G, Colletti PM, Rubello D. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med. 2013;38:305–314. doi: 10.1097/RLU.0b013e3182867f3c. [DOI] [PubMed] [Google Scholar]
- 31.Umbehr MH, Muntener M, Hany T, Sulser T, Bachmann LM. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. European urology. 2013;64:106–117. doi: 10.1016/j.eururo.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Picchio M, Briganti A, Fanti S, et al. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. European urology. 2011;59:51–60. doi: 10.1016/j.eururo.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Giovacchini G, Picchio M, Scattoni V, et al. PSA doubling time for prediction of [(11)C]choline PET/CT findings in prostate cancer patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2010;37:1106–1116. doi: 10.1007/s00259-010-1403-7. [DOI] [PubMed] [Google Scholar]
- 34.Schuster DM, Votaw JR, Nieh PT, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med. 2007;48:56–63. [PubMed] [Google Scholar]
- 35.Nanni C, Schiavina R, Boschi S, et al. Comparison of 18F-FACBC and 11C-choline PET/CT in patients with radically treated prostate cancer and biochemical relapse: preliminary results. Eur J Nucl Med Mol Imaging. 2013;40(Suppl 1):S11–S17. doi: 10.1007/s00259-013-2373-3. [DOI] [PubMed] [Google Scholar]
- 36.Schuster DM, Nieh PT, Jani AB, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014;191:1446–1453. doi: 10.1016/j.juro.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barocas DA, Bensink ME, Berry K, et al. Economic evaluation of diagnostic localization following biochemical prostate cancer recurrence. Int J Technol Assess Health Care. 2014;30:345–353. doi: 10.1017/S0266462314000476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.