Abstract
Background and objectives
Porphyromonas gingivalis (P. gingivalis) is regarded as a significant contributor in the pathogenesis of periodontitis and certain systemic diseases, including atherosclerosis. P. gingivalis occasionally translocates from periodontal pockets into the circulation, where it adheres to red blood cells (RBCs). This may protect the bacterium from contact with circulating phagocytes without affecting its viability.
Materials and methods
In this in vitro study, we investigated whether human peripheral blood neutrophils from 10 subjects with localized aggressive periodontitis (LAgP) and ten healthy controls release the pro-inflammatory cytokines interleukin (IL)-6, tumor necrosis factor α (TNF-α), the chemokine (C-X-C motif) ligand 8 (CXCL8; also known as IL-8) and chemokine (C-C motif) ligand 2 (CCL2; also known as monocyte chemotactic protein (MCP)-1), and intracellular reactive oxygen species (ROS) in response to challenge with P. gingivalis. In addition, the impact of RBC interaction with P. gingivalis was investigated. The actions of resolvin E1 (RvE1), a known regulator of P. gingivalis induced neutrophil responses, on the cytokine and ROS responses elicited by P. gingivalis in cultures of neutrophils were investigated.
Results
Upon stimulation with P. gingivalis, neutrophils from subjects with LAgP and healthy controls released similar quantities of IL-6, TNF-α, CXCL8, CCL2 and intracellular ROS. The presence of RBCs amplified the release of IL-6, TNF-α and CCL2 statistically significant in both groups, but reduced the generation of ROS in the group of healthy controls, and showed a similar tendency in the group of subjects with LAgP. RvE1 had no impact on the production of intracellular ROS, TNF-α, IL-6, CXCL8 and CCL2 by neutrophils from either group, but tended to reduce the generation of ROS in subjects with LAgP in absence of RBCs.
Conclusions
Our data support that binding to RBCs protects P. gingivalis from ROS and concomitantly enhances neutrophil release of pro-inflammatory cytokines providing a selective advantage for P. gingivalis growth.
Keywords: Aggressive periodontitis, neutrophils, Porphyromonas gingivalis, red blood cells, resolvin, cytokines
Introduction
Neutrophils constitute an essential arm in the defense against bacteria by virtue of their production of proteases, antimicrobial peptides, and formation of neutrophil extracellular traps (NETs) (1–2). Neutrophils also phagocytize bacteria and bacterial products, which activate the protein kinase-C, mitogen-activated protein kinase cascades and the nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase enzyme complex to generate superoxide anion radicals and other reactive oxygen species (ROS) (3). Together with proteases and antimicrobial peptides, these products destroy the phagocytosed bacteria intracellularly (3). Neutrophils have been shown to secrete cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 with potent autocrine actions – albeit being produced in very low quantities compared to those produced by mononuclear cells, when stimulated with lipopolysaccharide (LPS) from the periodontal pathogen Porphyromonas gingivalis (P. gingivalis) (4). In the context of P. gingivalis physiology, induction of continued neutrophil mediated inflammation and tissue destruction favors the growth and survival of P. gingivalis, an assaccharolytic organism that uses amino acids from tissue breakdown for energy (5).
TNF-α, IL-1β and bacterial LPS stimulate the production of chemokine (C-X-C motif) ligand 8 (CXCL8; also known as IL-8) by macrophages and epithelial cells (1). CXCL8 is a strong chemoattractant for neutrophils, and prevents neutrophils from undergoing apoptosis (1, 5–6). Another chemoattractant, (C-C motif) ligand 2 (CCL2; also known as monocyte chemoattractant protein (MCP)-1) secreted by monocytes, macrophages and dendritic cells also induces chemotaxis of neutrophils and monocytes (1, 5–6). Notably, both CXCL8 and CCL2 may be produced by neutrophils themselves (1, 5–6). Attracted by chemokines, neutrophils are readily recruited to sites of bacterial infection and inflammation, such as in periodontitis (6).
Periodontitis is a prevalent inflammatory disease induced by bacteria, including P. gingivalis, growing in biofilms on tooth surfaces adjacent to the gingiva (7–8). Periodontitis manifests as breakdown of tooth supporting tissues with deepening and ulceration of periodontal pockets through which bacteria may gain access to the blood stream (7–11) as the result of daily procedures such as chewing and tooth brushing (9–13). A severe form of periodontitis, designated localized aggressive periodontitis (LAgP) (7, 14), seen in younger children and adolescents appears to be most prevalent in African-American populations (2.6 %) followed by Hispanics and Whites, 0.5% and 0.06% respectively, in the United States (15), and is characterized by early onset and rapid loss of attachment (14), as well as by the presence of a hyper-active phenotype of neutrophils with enhances pro-inflammatory activity (14, 16–17). Notably, the majority of leukocytes, i.e. ≥95%, within the inflamed periodontal pockets characteristic for periodontitis are neutrophils (18).
Resolution of inflammation is an active process mediated by endogenous lipid agonists, including lipoxins, resolvins and maresins (19–20). Resolvin E1 (RvE1) is biosynthesized by aspirin-modified cyclooxygenase-2 from the precursor essential ω-3 polyunsaturated fatty acid eicosapentaenoic acid derived from the diet (19). RvE1 prevents inflammatory tissue damage and osteoclast-mediated bone resorption, which facilitates the return to homeostasis in the periodontium (21–22). Binding of RvE1 to leukotriene B4 receptor 1 (BLT-1) on neutrophils leads to attenuation of TNF-α-mediated nuclear factor-κB cell (NF- κB) activation in neutrophils (23–24), and inhibition of chemotaxis and leukocyte infiltration (25). Furthermore, RvE1 has been shown to dampen O2− release from LAgP neutrophils (22) and to induce clearance of P. gingivalis in periodontitis (21–22). It remains to be clarified, whether RBCs modify the RvE1 inhibition of release of pro-inflammatory cytokines, chemokines and intracellular ROS by neutrophils during stimulation with P. gingivalis.
P. gingivalis is a Gram-negative, anaerobic rod with a variety of virulence factors, including LPS, capsular polysaccharide, fimbriae and gingipains (12). P. gingivalis can disengage bacterial clearance mechanisms by promoting cross-talk between toll-like receptor (TLR)-2 and C5a receptors (C5aR) in neutrophils (26). More specifically, P. gingivalis induces proteasomal degradation of myeloid differentiation primary response protein 88 (MyD88), and thereby suppresses MyD88 mediated antimicrobial activity (26). Moreover, C5aR–TLR-2 crosstalk inhibits the phagocytosis of P. gingivalis and bystander bacteria and stimulates a robust inflammatory response (26). P. gingivalis itself produces superoxide dismutase, thiol peroxidase, and rubrerythrin, which, due to their antioxidant properties, makes P. gingivalis resistant to oxidative burst killing by neutrophils (27–30). P. gingivalis may spread systemically both in plasma (31) and bound to dendritic cells (14–15, 32–33) or red blood cells (RBCs) (34). The latter requires that C3b and, to a lesser extent, iC3b, deposited on the bacterium bind to complement receptor 1 (CR1) on RBCs (35–38).
We have previously shown that binding of P. gingivalis to RBCs restricts phagocytosis of the bacterium by monocytes and neutrophils (34), and we now hypothesize that RBCs may also impact P. gingivalis-stimulated release of pro-inflammatory cytokines and production of intracellular ROS by neutrophils. Since RvE1 is known to normalize excessive extracellular production of ROS by LAgP neutrophils, we hypothesize that RvE1 may also act on neutrophils in the presence of RBCs. These hypotheses were tested by P. gingivalis-induced in vitro release of pro-inflammatory cytokines, chemokines and production of ROS in neutrophils from healthy donors and subjects with LAgP.
Materials and methods
Donors
Ten patients diagnosed with LAgP, 1 male and 9 females with a mean age of 37.6 years, range: 29–46 years, were recruited at The Forsyth Institute (Cambridge, MA) September 5th to 25th 2013. The patients were diagnosed clinically as subjects presenting severe, early-onset bone loss around first molars and incisor teeth (39), by a licensed periodontist in the Center for Clinical and Translational Research at The Forsyth Institute. Ten healthy donors, a male and 9 females with a mean age of 46.6 years (range 32–67 years), who were recruited in the same period at The Forsyth Institute served as controls.
The LAgP group consisted of 6 African-Americans, 3 Caucasians and 1 Hispanic, whereas the healthy group consisted of 4 African-Americans, 5 Caucasians and 1 Asian.
Ethics
All donors gave informed written consent prior to blood donation. The study and the consent form were approved by the Institutional Review Board at The Forsyth Institute (Protocol #11-05).
Blood collection
Seventy-two mL of peripheral venous blood were drawn from the median cubital vein, after topical disinfection with an alcohol wipe, into six even 15 mL heparinized tubes from each donor. The collected blood was kept at room temperature in the dark for one hour before separation.
Preparation of bacteria
P. gingivalis A7436 was grown anaerobically at 37°C on trypticase soy broth (TSB) agar plates containing 1 µg haemin ml−1, 1 µg menadione ml−1, 20% defimbrinated sheep blood and 1.5% agar solid growth media and then transferred into a sterile tube containing Wilkins-Chalgren broth (#CM0643B, Thermo Fisher Scientific Inc., Waltham, MA). The tube was incubated anaerobically at 37°C for 18 hours. Bacteria were pelleted by centrifugation (10,000 × g) at 4°C and washed twice in phosphate buffered saline (PBS). After discarding the supernatant, 2 mL PBS was added, and the bacteria were then counted and adjusted to a final concentration of 2×108 bacteria/mL, using spectrophotometrical OD600 of 1.0=~ 109 bacteria per mL.
Labeling of P. gingivalis
P. gingivalis was labeled with 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) (#1378384, Molecular Probes Inc., Eugene, OR) for positive selection during flow cytometry. CFSE labels intact cells, including bacteria, by coupling to intracellular lysine residues and other amine sources. Due to this covalent coupling reaction, fluorescent CFSE is retained within cells and once incorporated within cells the CFSE does not transfer to adjacent cells, nor is there any surface alteration. CFSE was dissolved in PBS to a final concentration of 10 µM and incubated for 15 minutes at 4°C with the bacteria. Subsequently, the bacteria were pelleted by centrifugation (10,000 × g), resuspended in PBS and re-incubated at 4°C for 30 minutes to ensure complete modification of the CFSE probe. After incubation, bacteria were again pelleted by centrifugation. The supernatant was discarded and the bacteria resuspended in pooled serum from human, male, blood group AB donors, USA origin, sterile-filtered (#H4522, Sigma-Aldrich, Saint Louis, MO), henceforward referred to as “AB serum”. The CFSE-labeled bacteria were placed in the dark at 4°C for opsonization until added to cells.
Isolation and labeling of neutrophils
Neutrophils were isolated by gradient centrifugation of heparinized blood using Histopaque®-1007 and -1119 (Sigma-Aldrich Co., MO) at 150× g. The neutrophils that appeared in a distinct band well above the band of mononuclear cells were collected by gentle pipetting and subjected to isotonic lysis of contaminating RBCs for 5 minutes, washed twice in Ca2+/Mg2+-free PBS and resuspended in the same buffer. Cell preparations were ≥99% neutrophils with ≥95% viability as determined by trypan blue exclusion. APC-conjugated monoclonal anti-CD15 (#551376, BD Bioscience, San Jose, CA) was added to the neutrophils at a final concentration of 0.3 µg/mL to allow positive selection during flow cytometry. Dihydroethidium (DHE) (#D1168, Molecular Probes Inc., Eugene, OR) was added at a finale concentration of 5 µg/mL to detect ROS during flow cytometry formed by the partial reduction of oxygen. The neutrophils were then incubated in the dark at 37°C for 30 minutes, and afterwards washed twice in PBS and resuspended in media containing RPMI-LG 2 mM L-glutamine with 10% fetal bovine serum, 10 mM HEPES buffer, 1 mM sodium pyruvate, 4500 mg/L glucose, and 1500 mg/L sodium bicarbonate. By direct counting under a light microscope, the neutrophils were adjusted to a final concentration of 25×106 cells/mL and kept at 37°C in the dark until incubation with bacteria. Supernatants for chemokine and cytokine analyses were collected after incubation of neutrophils with P. gingivalis, but prior to ROS measurements. As negative control, we used anti-CD15 labeled and DHE loaded neutrophils, which were incubated under similar conditions for 1 h without any P. gingivalis.
Isolation and labeling of RBCs
After gradient centrifugation, RBCs were harvested from the pellet of the tubes used for neutrophil isolation. After two washes, the RBCs were counted under a light microscope. Phosphatidylethanolamine (PE)-conjugated monoclonal anti-human CD235a (Glycophorin A) (#349105, Biolegend®, San Diego, CA) was added to a final concentration of 0.5 µg per million cells in 100 µL to allow positive selection during flow cytometry. RBCs were incubated on ice for 15 minutes in the dark and then washed twice in PBS, pelleted and resuspended in AB serum (#H4522, Sigma-Aldrich, Saint Louis, MO) to a final concentration of 4.5×108 RBCs/mL to mimic leukocytosis, where ratios of RBCs per leukocyte are estimated to be the lowest. RBCs were kept at 4°C in the dark until incubation with bacteria. As the interaction with P. gingivalis is mediated via complement receptor 1, PE-conjugated anti-CD235a does not affect the interaction (34).
Stimulation of neutrophils with P. gingivalis
5×105 labeled neutrophils in 20 µL were added to 345 µL Roswell Park Memorial Institute (RPMI)-LG medium with 2 mM L-glutamine, 10% fetal bovine serum, 10 mM HEPES, 1 mM sodium pyruvate, 4500 mg/L glucose, and 1500 mg/L sodium bicarbonate supplemented with AB serum to a final concentration 20% (vol/vol). 3.6×107 labeled RBCs in 80 µL was then added. RvE1 (336.5 Mw ≈ 297.2 µM stock solution) was dissolved in RPMI in darkness, sonicated for 5 seconds and added to the samples at a final concentration of 10nM. Each culture was infected with 1×107 labeled P. gingivalis in 50 µL, and incubated at 37°C in the dark for 1 hour to mimic the physiologic conditions and duration of a transient bacteremia (9). Cells were pelleted by centrifugation, and 100 µL of the supernatant transferred to new vials and stored at −80°C until cytokine analysis.
Measurement of cytokines in culture supernatant
Levels of TNF-α, IL-6 and CXCL8 in culture supernatants were determined using the Human High Sensitivity Cytokine Magnetic Bead panel (HSCYTMAG-60-SK-04, #2360198, EMD Millipore, Billerica, MA) with a sensitivity range of 0.11 – 8.17 pg/mL, and CCL2 in the supernatants was determined using the Human Cytokine Magnetic Bead panel (HCYTOMAG-60K-01, #2360199, EMD Millipore) with a lower limit of detection at 1.9 pg/mL, according to the manufacturer’s protocol. In brief, magnetic beads pre-coated with capture antibodies were incubated for 2 h at room temperature with supernatants in 96-well plates. Following 3 washes with washing buffer, the beads were incubated with biotinylated detection antibodies for 1 h at room temperature with gentle shaking. Streptavidin conjugated with R-phycoerythrin (streptavidin-RPE) was added to the beads, incubated for 60 min at room temperature with shaking, washed 3 times, and resuspended in sheath fluid (#342003, BD Biosciences). The beads were analyzed on a Bio-Plex 200 instrument (Bio-Rad Laboratories Inc., Hercules, CA) using Bio-Plex Manager version 6.1 (Bio-Rad Laboratories Inc., Hercules, CA).
Measurement of ROS in neutrophils stimulated with P. gingivalis
Intracellular ROS formed by the partial reduction of oxygen in APC-conjugated anti-CD15-labeled neutrophils stimulated with P. gingivalis was detected with DHE present during the stimulation at a final concentration of 5 µg/mL and measured flow cytometrically at an excitation wavelength of 500–530 nm after identification of neutrophils on the basis of labeling with APC- anti-CD15. Samples without RBCs were resuspended in 500 µL PBS on ice, whereas samples containing RBCs were resuspended in 2500 µL PBS on ice to reduce cell density before reading samples on the flow cytometer. All samples were filtered through BD Falcon™ 35 µm nylon mesh cell strainers (BD Biosciences) to reduce cell clumping and debris prior to data acquisition using a FACSAria flow cytometer (BD Biosciences). Data were analyzed using FlowJo vX.0.6 software (FlowJo LLC, Ashland, OR) for PC. ROS production was tested in 6 healthy donors and 6 subjects with LAgP.
Statistics
Paired one-sided t tests were performed to assess differences in ROS production by unstimulated neutrophils and neutrophils stimulated with P. gingivalis and to analyze the impact of RBCs and RvE1 on ROS production by neutrophils. Multiple t tests using Sidak-Bonferroni correction for multiple comparisons were performed for analysis of cytokines. All statistical analyses were performed using GraphPad Prism 6.0b for Mac (GraphPad Software Inc., La Jolla, CA). P-values <0.05 were considered significant.
Results
Cytokine release by neutrophils from healthy donors and LAgP-patients in P. gingivalis stimulated cultures is increased by RBCs
Neutrophils isolated from either subjects with LAgP or healthy controls failed to release cytokine levels above the stated lower detection limits of the assays. By contrast, stimulation of the neutrophils with P. gingivalis for 1 hour resulted in significant release of TNF-α, IL-6, CXCL8, and CCL2 (Fig. 1A–D). We observed no differences in cytokine release by neutrophils derived from healthy donors compared with that of neutrophils from subjects with LAgP.
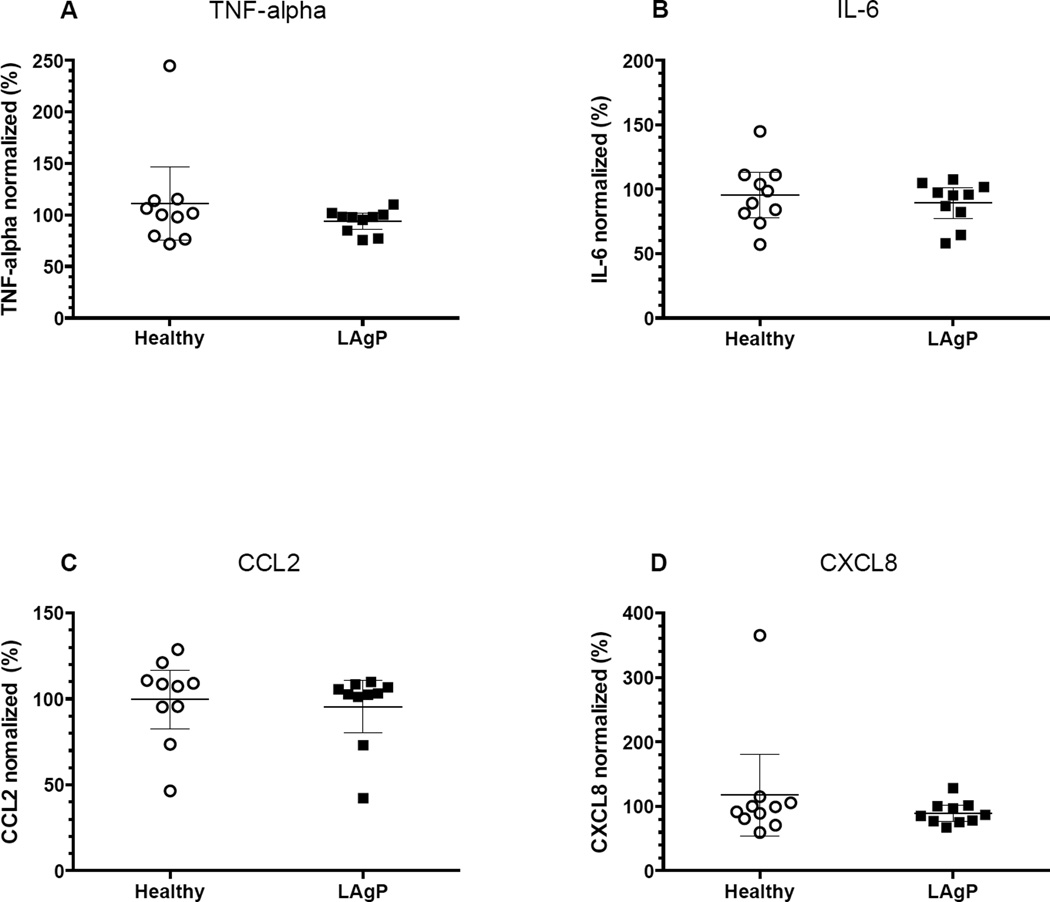
Fig. 1. A–D: P. gingivalis–induced neutrophil cytokine and chemokine responses.
5×105 neutrophils, from either healthy controls (n=10) or subjects with localized aggressive periodontitis (LAgP) (n=10), suspended in RPMI-medium containing 20% (v/v) AB serum, were stimulated with 1×107 P. gingivalis for 1 hour at 37°C in presence (■) or absence (○) of 3.6×107 autologous red blood cells (RBCs). Culture supernatants were thereafter assessed for content of A) TNF-α, B) IL-6, C) CCL2 and D) CXCL8. The presence of RBCs bound to neutrophils significantly increased release of TNFα, IL-6 and CCL2. There were no differences in the response of LAgP and healthy control neutrophils under any conditions. The lower limits of detection provided by the manufacturer were A) 0.16, B) 0.11, C) 1.9 and D) 0.13 as indicated with a dotted line. ***p<0.0001.
To determine the impact of RBCs on the release of TNF-α, IL-6, CXCL8, and CCL2, neutrophils were stimulated with P. gingivalis in presence and absence of autologous RBCs. Notably, IL-6, TNF-α, and CCL2 levels all increased significantly upon addition of RBCs (p<0.0001), whereas no statistically significant differences were observed for CXCL8 (Fig. 1A–D). RBCs did not affect cytokine release by otherwise unstimulated neutrophils.
P. gingivalis-induced intracellular ROS production by neutrophils is inhibited by RBCs
Stimulation of isolated neutrophils with P. gingivalis for 1 hour in the presence of normal human AB serum resulted in marked intracellular production of ROS (Fig. 2). This production did not differ between neutrophils derived from patients with LAgP and those derived from healthy donors (Fig. 2).
Fig. 2. P. gingivalis–induced generation of reactive oxygen species (ROS) in neutrophils.
Dihydroethidium (DHE)-labeled 5×105 neutrophils (PMN), from either healthy controls (n=6) (○) or subjects with localized aggressive periodontitis (LAgP) (n=6) (■) were suspended in media containing 20% (v/v) AB serum and stimulated with 1×107 Porphyromonas gingivalis (P.g.) for 1 hour at 37°C. ROS is expressed as median fluorescence intensity of individual donors, in i) unstimulated, non-labelled neutrophils, ii) unstimulated DHE-labeled neutrophils and iii) DHE-labeled neutrophils stimulated with P.g. for 1 hour. Mean and 95% confidence intervals are shown (*p-values <0.05).
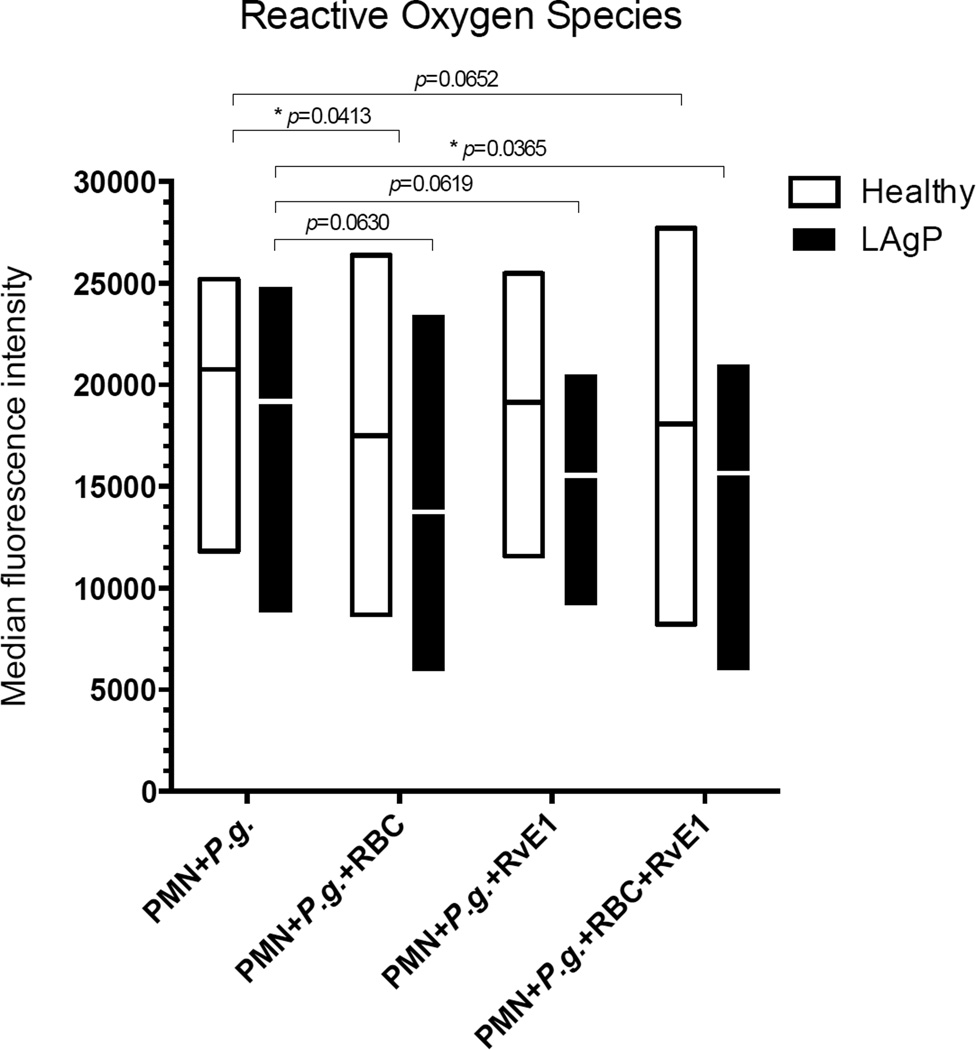
To examine the influence of RBCs on intracellular ROS production, neutrophils were stimulated with P. gingivalis in absence and presence of RBCs (Fig. 3). RBCs caused a significant reduction in ROS production, amounting to 18 % in healthy donors and 26 % in subjects with LAgP (Fig. 3). Like RBCs, RvE1 reduced ROS production, however, not with statistical significance (p=0.630). We asked the question whether RvE1 had a further impact on RBC inhibition of ROS production by neutrophils, but no such additive actions were observed.
Fig. 3. Red blood cells (RBC) and resolvin E1 (RvE1) reduce P. gingivalis–induced generation of reactive oxygen species (ROS) in neutrophils.
Dihydroethidium (DHE)-labeled 5×105 neutrophils (PMN), from either healthy controls (n=6) (□) or subjects with localized aggressive periodontitis (LAgP) (n=6) (■) were suspended media containing 20% (v/v) AB serum and stimulated with 1×107 Porphyromonas gingivalis (P.g.) for 1 hour at 37°C in absence or presence of 3.6×107 autologous red blood cells (RBCs). All experiments were performed in triplicate. Half of the cultures received RvE1 (final concentration: 10nM). Each box represents the intracellular content of reactive oxygen species (ROS) of either healthy controls or patients with LAgP. Boxes indicate minimum and maximum with line at the mean. ROS are expressed as median fluorescence intensity (*p-values <0.05). RvE1 reduces intracellular ROS as do RBC; however, the additive effect is statistically significant only in subjects with LAgP (p<0.05), although there is a clear trend in controls as well (p=0.0652).
RvE1 in P. gingivalis-stimulated cultures with RBCs
To examine whether RvE1 acts on P. gingivalis-induced cytokine release by neutrophils in presence of RBCs, RvE1 was added to cultures of isolated neutrophils with RBCs (Fig. 4A–D). RvE1 had no impact on the release of any pro-inflammatory cytokines or chemokines in the presence of RBCs (Fig. 4A–D).
Fig. 4. A–D: Relative effect of resolvin E1 (RvE1) on P. gingivalis–induced neutrophil cytokine and chemokine responses.
5×105 neutrophils from either healthy controls (n=10) (○) or subjects with localized aggressive periodontitis (LAgP) (n=10) (■), suspended in RPMI-medium containing 20% (v/v) AB serum were stimulated with 1×107 P. gingivalis for 1 hour at 37°C in presence of 3.6×107 autologous red blood cells (RBCs). Half of the cultures received RvE1 (final concentration: 10nM). Culture supernatants were assessed for content of A) TNF-α, B) IL-6, C) CCL2 and D) CXCL8. The data points represents mean ± 95% confidence intervals of the cytokine levels observed in presence of RvE1 normalized to those observed in absence of RvE1 (=100%). No statistically significant differences (p-values >0.05) from 100% were observed.
Discussion
Bacteria-induced cytokine production and release by neutrophils has until recently been a matter of few investigations (4–6). We examined cytokine release by neutrophils after stimulation with P. gingivalis, and compared the responses of neutrophils derived from healthy donors with those derived from subjects with LAgP. Moreover, we examined whether the presence of RBCs had any influence on cytokine responses or generation of intracellular ROS. Neutrophils from healthy donors and subjects with LAgP responded similarly to P. gingivalis with release of TNF-α, IL-6, CCL2 and, to a lesser extent, CXCL8. It should be noted that cytokines were measured in culture supernatants after diffusion away from the neutrophils had occurred. The local concentrations in the vicinity of the neutrophils, where e.g. autocrine stimulation occurs, may have been considerably higher. There were no obvious differences between healthy and LAgP cytokine release in response to P. gingivalis. Although the groups were not matched by race and some differences in disease prevalence exist between ethnic groups (15), there is no evidence that the disease course is different. We found no differences between races in either LAgP or controls.
No previous studies have addressed cytokine release and generation of intracellular ROS by neutrophils stimulated with potentially viable P. gingivalis, or the influence of RBCs on these processes. However, neutrophils derived from LAgP patients have previously been shown to both generate significantly higher levels of extracellular superoxide compared to healthy controls, and to release excessive amounts of superoxide upon stimulation with ceruloplasmin (40–41). In this study, we focused on the intracellular ROS production designated to kill phagocytosed bacteria in neutrophils.
We have previously shown that P. gingivalis binds to RBCs in a CR1-dependent manner restricted the phagocytosis of P. gingivalis by neutrophils and monocytes (34). Subsequently, Brekke and colleagues showed that the Gram-negative bacteria Escherichia coli and Neisseria meningitides, both known to cause sepsis in humans, also bind to RBCs in a CR1-dependent and LPS-independent manner (31). Notably, 80–90% of the bacteria in their study were found associated with RBCs rather than in planktonic form in plasma (31). They demonstrated that blockade of CR1 increased phagocytosis of E. coli, and E. coli-induced intracellular ROS production, indicating that binding of bacteria to RBCs via CR1 protects the bacteria from being phagocytized and killed (31). Our finding here that the presence of RBCs restricts P. gingivalis-induced ROS production supports this notion. In a physiological context it would seem appropriate that RBCs limit activation of circulating neutrophils and their subsequent release of cytotoxic substances, that may cause damage to endothelial cells (42), as we have previously proposed in relation to RBC-mediated inhibition of immune complex-induced ROS production by neutrophils (35, 43).
We expected that RBCs would also restrict bacteria-induced cytokine release by neutrophils. However, we observed that addition of RBCs resulted in an increase of TNF-α, IL-6, and CCL2 levels in neutrophil cultures (Fig.1A–C). The simplest explanation for this is that lysis of RBCs by hemolysin derived from P. gingivalis causes the release of endogenous danger signals that activate cytokine production by neutrophils. Hemolysin is a virulence factor of P. gingivalis, by means of which it provides itself with heme that is essential for its survival (44). The stimulated cytokine production may promote host tissue degradation by neutrophils, which directly aids the survival of the bacterium by enriching the environment with the bacterium’s main source of energy, amino acids (8). Neutrophil mediated inflammation and tissue injury manifests in the periodontium as degradation of collagen by matrix metalloproteinases of neutrophil origin resulting in an abundance of collagen peptides (6–7, 21). P. gingivalis expresses an array of gingipains, trypsin like proteases that degrade the peptides into essential amino acids. This large reservoir of free amino acids promotes the overgrowth of P. gingivalis in the periodontal pocket providing more inflammation and more gingipains (26). These observations may provide a plausible explanation for the observed overgrowth of P. gingivalis (6–7, 21) occurring after deep pocket formation (45).
In the periodontium, P. gingivalis enhanced levels of IL-6 and TNF-α may up-regulate osteoclastic activity via RANK/RANKL/OPG pathways (46). Moreover, these cytokines may be spilled over from the periodontium to the circulation (47) and promote synthesis of acute-phase proteins such as C-reactive protein (CRP), serum amyloid P protein and pentraxins (48–51). The acute-phase proteins may promote further inflammation. This is thought to be important in atherogenesis through low-grade inflammation (52–53) where periodontitis contributes to systemic inflammation thereby further contributing to the atherogenic potential of P. gingivalis (54–55). Prior publications report the impact of RvE1 on extracellular ROS and other neutrophil functions (21–22, 40). We show that RvE1 likewise tended to reduce intracellular ROS production by neutrophils derived from subjects with LAgP in absence of RBCs, which mimics the conditions of the crevicular fluid where only sparse RBCs are present. However, in presence of RBCs, as in the circulation, Rve1 did not enhance the inhibitory effect of RBCs on ROS production. In healthy controls and subjects with LAgP, RvE1 had no significant influence on TNF-α and IL-6 released by neutrophils in presence of RBCs (Fig. 4A–B). Moreover, RvE1 did not influence the release of CCL2 and CXCL8 by neutrophils in presence of RBCs (Fig. 4C–D).
Another interesting observation is the differences between intracellular ROS measurements and cytokine release in the subjects with LAgP. It is clear from several earlier reports that superoxide released extracellularly measured by cytochrome c reduction is markedly elevated in LAgP (14, 16–17). However, intracellular P. gingivalis-induced ROS production did not differ between healthy donors and subjects with LAgP, and was suppressed by RBCs, as well as by RvE1. At the methodological level, these findings are consistent as DHE measures intracellular ROS, presumably from mitochondrial or other intracellular sources, and it is not sensitive to NADPH oxidase activity, which resides in the plasma membrane. Physiologically, reduced intracellular ROS enhances P. gingivalis intracellular survival, while extracellular superoxide enhances tissue damage and degradation providing energy for P. gingivalis.
In conclusion, neutrophils readily release pro-inflammatory cytokines and chemokines, and produce intracellular ROS upon stimulation with P. gingivalis. RBCs restrict ROS production, but enhance release of pro-inflammatory cytokines by neutrophils from both healthy controls and LAgP subjects. These data suggest that P. gingivalis is able to control neutrophil functions to its advantage causing extracellular tissue damage and reducing intracellular oxidative killing.
Acknowledgments
The authors thank Danielle Stephens, Department of Applied Oral Sciences, Center for Periodontology, The Forsyth Institute, Cambridge, MA for performing the cytokine analysis, and Mads Emil Bjørn, Institute for Inflammation Research, Centor for Rheumatology and Spine Disease, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark for valuable input on the analyses of ROS. This study was supported by grants from the Danish Dental Association and the Ottilia and Christian Brorsons Travel Grant for Young Scientists and USPHS grants DE015566 and DE25020 from the National Institute of Dental and Craniofacial Research, USA.
Footnotes
Disclosure: The authors declare that there are no conflicts of interest in this study.
Authorship
C. Damgaard participated in designing the study, performed the experiments, analyzed data and wrote the paper.
A. Kantarci participated in designing the study and gave valuable criticism of the manuscript.
P. Holmstrup participated in designing the study and gave valuable criticism of the manuscript.
H. Hasturk recruited the participants and gave valuable criticism of the manuscript.
C. H. Nielsen participated in designing the study and writing the manuscript.
T. E. Van Dyke participated in designing the study and writing the manuscript.
References
- 1.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv Immunol. 1999;73:369–509. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 3.Chapple ILC, Matthews JB, Wright HJ, Scott AE, Griffiths HR, Grant MM. Ascorbate and α-tocopherol differentially modulate reactive oxygen species generation by neutrophils in response to FcγR and TLR agonists. Innate Immun. 2012;19:152–159. doi: 10.1177/1753425912455207. [DOI] [PubMed] [Google Scholar]
- 4.Sugita N, Kimura A, Matsuki Y, Yamamoto T, Yoshie H, Hara K. Activation of transcription factors and IL-8 expression in neutrophils stimulated with lipopolysaccharide from Porphyromonas gingivalis. Inflammation. 1998;22:253–267. doi: 10.1023/a:1022344031223. [DOI] [PubMed] [Google Scholar]
- 5.Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993;178:2207–2211. doi: 10.1084/jem.178.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajishengallis G, Chavakis T, Hajishengallis E, Lambris JD. Neutrophil homeostasis and inflammation: novel paradigms from studying periodontitis. J Leukoc Bio. 2014 Dec 29; doi: 10.1189/jlb.3VMR1014-468R. pii: jlb.3VMR1014-468R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartold PM, Van Dyke TE. Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol 2000. 2013;62:203–217. doi: 10.1111/j.1600-0757.2012.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damgaard C, Holmstrup P, Van Dyke TE, Nielsen CH. The complement system and its role in the pathogenesis of periodontitis: current concepts. J Periodontal Res. 2014 Jul 5; doi: 10.1111/jre.12209. 2014. [DOI] [PubMed] [Google Scholar]
- 9.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33:401–407. doi: 10.1111/j.1600-051X.2006.00924.x. 2006. [DOI] [PubMed] [Google Scholar]
- 10.Kozarov EV, Dorn BR, Shelburne CE, Dunn WA, Jr, Progulske-Fox A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol. 2005;25:e17–e18. doi: 10.1161/01.ATV.0000155018.67835.1a. [DOI] [PubMed] [Google Scholar]
- 11.Gaetti-Jardim E, Jr, Marcelino SL, Feitosa AC, Romito GA, Avila-Campos MJ. Quantitative detection of periodontopathic bacteria in atherosclerotic plaques from coronary arteries. J Med Microbiol. 2009;58:1568–1575. doi: 10.1099/jmm.0.013383-0. [DOI] [PubMed] [Google Scholar]
- 12.Horliana AC, Chambrone L, Foz AM, Artese HP, Rabelo Mde S, Pannuti CM, et al. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: a systematic review. PLoS One. 2014;9:e98271. doi: 10.1371/journal.pone.0098271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomás I, Diz P, Tobías A, Scully C, Donos N. Periodontal health status and bacteraemia from oral activities: systematic review/meta-analysis. J Clin Periodontol. 2012;39:213–228. doi: 10.1111/j.1600-051X.2011.01784.x. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni C, Kinane DF. Host response in aggressive periodontitis. Periodontol 2000. 2014;65:79–91. doi: 10.1111/prd.12017. [DOI] [PubMed] [Google Scholar]
- 15.Albandar JM, Tinoco EM. Global epidemiology of periodontal diseases in children and young persons. Periodontol 2000. 2002;29:153–176. doi: 10.1034/j.1600-0757.2002.290108.x. [DOI] [PubMed] [Google Scholar]
- 16.Meng H, Xu L, Li Q, Han J, Zhao Y. Determinants of host susceptibility in aggressive periodontitis. Periodontol 2000. 2007;43:133–159. doi: 10.1111/j.1600-0757.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- 17.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 18.Delima AJ, Van Dyke TE. Origin and function of the cellular components in gingival crevice fluid. Periodontol 2000. 2003;31:55–76. doi: 10.1034/j.1600-0757.2003.03105.x. [DOI] [PubMed] [Google Scholar]
- 19.Freire MO, Van Dyke TE. Natural resolution of inflammation. Periodontol 2000. 2013;63:149–164. doi: 10.1111/prd.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;610:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, et al. RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 22.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 23.Keinan D, Leigh NJ, Nelson JW, Oleo LD, Baker OJ. Understanding resolvin signaling pathways to improve oral health. Int J Mol Sci. 2013;14:5501–5518. doi: 10.3390/ijms14035501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samson M, Edinger AL, Stordeur P, Rucker J, Verhasselt V, Sharron M, et al. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur J Immunol. 1998;28:1689–1700. doi: 10.1002/(SICI)1521-4141(199805)28:05<1689::AID-IMMU1689>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 26.Maekawa T, Krauss JL, Abe T, Jotwani R, Triantafilou M, Triantafilou K, et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 2014;15:768–778. doi: 10.1016/j.chom.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bostanci N, Belibasakis GN. Porphyromonas gingivalis: an invasive and evasive opporturnistic oral pathogen. FEMS Microbiol Lett. 2012;333:1–9. doi: 10.1111/j.1574-6968.2012.02579.x. [DOI] [PubMed] [Google Scholar]
- 28.Sztukowska M, Bugno M, Potempa J, Travis J, Kurtz DM., Jr Role of rubrerythrin in the oxidative stress response of Porphyromonas gingivalis. Mol Microbiol. 2002;44:479–488. doi: 10.1046/j.1365-2958.2002.02892.x. [DOI] [PubMed] [Google Scholar]
- 29.Mydel P, Takahashi Y, Yumoto H, Sztukowska M, Kubica M, Gibson FC, 3rd, et al. Roles of the host oxidative immune responses and bacterial antioxidant rubrerythrin during Porphyromonas gingivalis infection. PLoS Pathogen. 2006;2:e76. doi: 10.1371/journal.ppat.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kikuchi Y, Ohara N, Sato K, Yoshimura M, Yukitake H, Sakai E, et al. Novel stationary-phase-upregulated protein of Porphyromonas gingivalis influences production of superoxide dismutase, thiol peroxidase and thioredoxin. Microbiol. 2005;151:841–853. doi: 10.1099/mic.0.27589-0. [DOI] [PubMed] [Google Scholar]
- 31.Brekke OL, Hellerud BC, Christiansen D, Fure H, Castellheim A, Nielsen EW, et al. Neisseria meningitidis and Escherichia coli are protected from leukocyte phagocytosis by binding to erythrocyte complement receptor 1 in human blood. Mol Immunol. 2011;48:2159–2169. doi: 10.1016/j.molimm.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117:3118–3125. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hujoel PP, White BA, Garcia RI, Listgarten MA. The dentogingival epithelial surface area revisited. J Periodontal Res. 2001;36:48–55. doi: 10.1034/j.1600-0765.2001.00011.x. [DOI] [PubMed] [Google Scholar]
- 34.Belstrøm D, Holmstrup P, Damgaard C, Borch TS, Skjodt MO, Bendtzen K, et al. The atherogenic bacterium Porphyromonas gingivalis evades circulating phagocytes by adhering to erythrocytes. Infect Immun. 2011;79:1559–1565. doi: 10.1128/IAI.01036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen CH, Antonsen S, Matthiesen SH, Leslie RG. The roles of complement receptors type 1 (CR1, CD35) and type 3 (CR3, CD11b/CD18) in the regulation of the immune complex-elicited respiratory burst of polymorphonuclear leukocytes in whole blood. Eur J Immunol. 1997;27:2914–2919. doi: 10.1002/eji.1830271125. [DOI] [PubMed] [Google Scholar]
- 36.Fearon DT. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980;152:20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chevalier J, Kazatchkine MD. Distribution in clusters of complement receptor one (CR1) on human erythrocytes. J Immunol. 1989;142:7016–7029. [PubMed] [Google Scholar]
- 38.Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity. 2012;37:199–207. doi: 10.1016/j.immuni.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Gronert K, Kantarci A, Levy BD, Clish CB, Odparlik S, Hasturk H, et al. A molecular defect in intracellular lipid signaling in human neutrophils in localized aggressive periodontal tissue damage. J Immunol. 2004;172:1856–1861. doi: 10.4049/jimmunol.172.3.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwata T, Kantarci A, Yagi M, Jackson T, Hasturk H, Kurihara H, et al. Ceruloplasmin induces polymorphonuclear leukocyte priming in localized aggressive periodontitis. J Periodontol. 2009;80:1300–1306. doi: 10.1902/jop.2009.090092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fligiel SE, Ward PA, Johnson KJ, Till GO. Evidence for at role of hydroxyl radical in immune-complex-induced vasculitis. Am J Pathol. 1984;115(3):375–382. [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen CH, Rasmussen JM, Voss A, Junker P, Leslie RG. Diminished ability of erythrocytes from patients with systemic lupus erythematosus to limit opsonized immune complex deposition on leukocytes and activation of granulocytes. Arthritis Rheum. 1998;41(4):613–622. doi: 10.1002/1529-0131(199804)41:4<613::AID-ART8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 44.Deshpande RG, Khan MB. Purification and charaxterization of hemolysin from Porphyromonas gingivalis A7436. FEMS Microbiol Lett. 1999;176(2):387–394. doi: 10.1111/j.1574-6968.1999.tb13688.x. [DOI] [PubMed] [Google Scholar]
- 45.Tanner AC, Kent R, Kanasi E, Lu SC, Paster BJ, Sonis ST, et al. Clinical characteristics and microbiota of progressing slight chronic periodontitis in adults. J Clin Periodontol. 2007;34:917–930. doi: 10.1111/j.1600-051X.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 46.Preshaw PM, Taylor JJ. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J Clin Periodontol. 2011;38:S60–S84. doi: 10.1111/j.1600-051X.2010.01671.x. [DOI] [PubMed] [Google Scholar]
- 47.Forner L, Nielsen CH, Bendtzen K, Larsen T, Holmstrup P. Increased plasma levels of IL-6 in bacteremic periodontitis patients after scaling. J Clin Periodontol. 2006;33:724–729. doi: 10.1111/j.1600-051X.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- 48.Short LL, Zoellner H, Hunter N. Association of amyloid P protein with pathology in periodontal tissues. J Oral Pathol Med. 1994;23:354–357. doi: 10.1111/j.1600-0714.1994.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 49.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35:277–290. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 50.Lakshmanen R, Jayakumar ND, Sankari M, Padmalatha O, Varghese S. Estimation of pentraxin-3 levels in the gingival tissues of chronic and aggressive periodontitis participants – an in vivo study. J Periodontol. 2014;85:290–297. doi: 10.1902/jop.2013.120718. [DOI] [PubMed] [Google Scholar]
- 51.Inforzato A, Doni A, Barajon I, Leone R, Garlanda C, Bottazzi B, et al. PTX3 as a paradigm for the interaction of pentraxins with the complement system. Semin Immuno. 2013;25:79–85. doi: 10.1016/j.smim.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Yoshii S, Tsuboi S, Morita I, Takami Y, Adachi K, Inukai J, et al. Temporal association of elevated C-reactive protein and periodontal disease in men. J Periodontol. 2009;80:734–739. doi: 10.1902/jop.2009.080537. [DOI] [PubMed] [Google Scholar]
- 53.Wu T, Trevisan M, Genco RJ, Falkner KL, Dorn JP, Sempos CT. Examination of the relation between periodontal health status and cardiovascular risk factors: serum total and high density lipoprotein cholesterol, C-reactive protein, and plasma fibrinogen. Am J Epidemiol. 2000;151:273–282. doi: 10.1093/oxfordjournals.aje.a010203. [DOI] [PubMed] [Google Scholar]
- 54.Tonetti MS, Van Dyke TE. Periodontitis and atherosclerotic caridovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 2013;84:24–29. doi: 10.1902/jop.2013.1340019. [DOI] [PubMed] [Google Scholar]
- 55.Belstrøm D, Damgaard C, Nielsen CH, Holmstrup P. Does a causal relation between cardiovascular disease and periodontitis exist? Microbes Infect. 2012;14:411–418. doi: 10.1016/j.micinf.2011.12.004. [DOI] [PubMed] [Google Scholar]