Abstract
Neoadjuvant chemotherapy (NACT) for locally advanced breast cancer (LABC), apart from increasing breast conservation rates, also provides an opportunity to assess tumour response to chemotherapy, with Pathological Complete Response (pCR) described as an independent prognostic factor and a surrogate marker for better outcome and survival. Our primary aim was to identify clinical and pathological factors associated with pCR following NACT in patients with LABC treated at our institution. Our secondary aim was to analyze the impact of pCR and associated factors on disease free survival (DFS) and overall survival (OS). A retrospective analysis of LABC patients treated with NACT between Jun 2011 and Dec 2013. Clinical and histological variables were analyzed for association with pCR (no invasive or in situ carcinoma in breast or axillary lymph nodes). Kaplan-Meier curves and Cox regression model was used for survival analysis. All values were twosided, and statistical significance was defined as p < 0.05. 240 patients were included. The median tumor size was 6 cm, with T4 disease in 49.8 %. 45 % of tumors were of low grade (G1 + G2) and 53.8 % of high grade (G3). Estrogen Receptor (ER) was positive in 70.8 %, progesterone receptor (PR) in 53.3 % and Her2 in 38.8 %. The preferred NACT regimen was sequential anthracycline and taxane and 88.8 % of patients received this regimen. Of 93 potential Her2 Positive patients, only 23 received trastuzumab. Overall 23.2 % patients had pCR. At median follow up of 21 months (range, 3–42), 16.3 % of patients had recurrent disease, and 6.7 % had died. High tumor grade (p = 0.04), PR negative status (p < 0.01) and trastuzumab treatment (p = 0.01) were significant predictors of pCR in univariate analysis. On multivariate analysis PR negativity (OR 3.2, 95 % CI = 1.6 to 6.04, p = 0.001) and Trastuzumab use (OR 0.24, 95 % CI = 0.1 to 0.6, p = 0.004) were significant. Patients with pCR had positive associations with survival (p < .02,OS& .02,DFS) and interestingly PR positivity had positive association with DFS (p = 0.02) in Kaplan-Meier curves. On Cox regression, PR positivity (HR = 0.3, p < 0.01) and pCR (HR = 0.2, p < 0.01) correlated with DFS, though not with early OS. for the PR positive patients were paradoxical. Though less likely to have pCR (15 %, vs 32 % if PR negative), they had better DFS (p = 0.02), and achieving pCR had no survival benefit in this group. In contrast, PR negative patients, irrespective of ER status, had a high pCR rate, and achieving pCR had survival advantage (p < 0.05,DFS& p < 0.02,OS). PR negative patients without pCR had the worst DFS (p < 0.01) among all. High grade and Trastuzumab treatment as predictors of pCR, and pCR as a surrogate marker for survival are well recognized, and are supported by our findings. In present cohort, PR negativity showed prognostic importance independent of ER status. However these results were derived from sub-group, post-hoc analysis of data from a pre-existing cohort, without ‘a-priori’ hypothesis for survival analysis in relation to PR. These “hypothesis generating” results need confirmation by a well-designed prospective cohort or a randomized trial.
Keywords: Breast cancer, Neoadjuvant chemotherapy, Pathological complete response, Predictors, Progesterone receptor, Impact on survival
Background
Neoadjuvant chemotherapy (NACT) is the standard of care for the management of locally advanced breast cancer (LABC) and is also frequently used to downsize early-stage disease to reduce tumor volume in patients with high tumor to breast size ratio who are keen to go ahead with breast conserving surgery (BCS) [1, 5]. The preoperative setting is increasingly recognized as an in vivo human model, when the efficacy of different chemotherapeutic strategies may be explored. It presents a short, preoperative ‘window of opportunity’ to look for evidence of drug resistance, when there might be an option to switch to a different group of chemotherapeutic agents [2], although this has not yet entered standard protocols.
Some patients respond better to chemotherapy compared to others, and pathological complete response (pCR) after NACT is increasingly used as a measure of response. There is accumulating evidence for the importance of pCR in predicting clinical outcomes, with patients who achieve pCR after NACT doing better than those who do not. In addition to being a surrogate marker for better outcome and survival, pCR is also described as an independent prognostic factor [3]. It is clinically useful to have the ability to predict response before starting therapy, as this would allow an individualized plan of management [4], and several studies have attempted to define predictive factors, with varied results.
The aim of this study was to identify clinical and pathologic factors associated with pCR in patients with LABC treated with NACT at our institution. Our secondary aim was to analyze the impact of pCR and associated factors on disease free survival (DFS) and early over-all survival (OS) in this cohort.
Materials and Methods
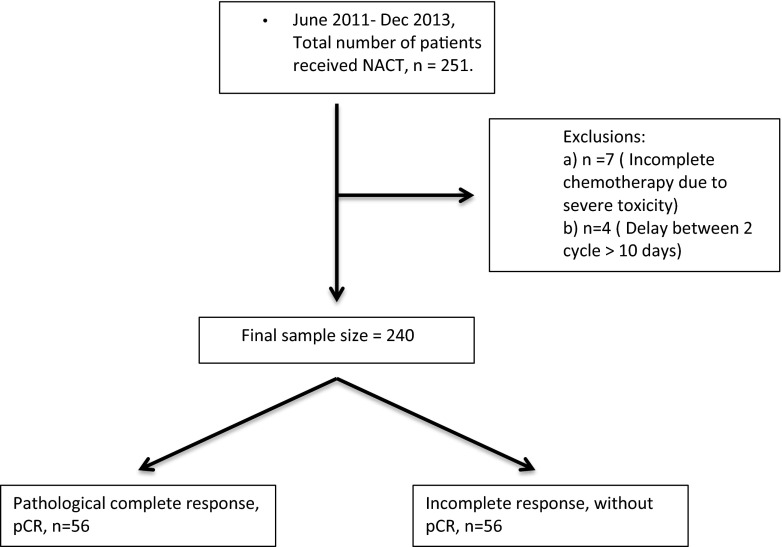
This was a retrospective analysis of data for a consecutive series of patients. According to the institutional treatment algorithm, NACT was advised for patients presenting with inoperable disease, patients who preferred breast conservation but presented with large tumors, or patients with multiple fixed or matted lymph nodes, whatever the size of their breast lesion. 251 consecutive patients were assessed for inclusion into this study, of which 11 patients were excluded from analysis as they did not fit the inclusion criteria (See Consort diagram, Fig. 1). After exclusions, the study cohort consisted of 240 patients, who received NACT in this institution between June 2011 and December 2013.
Fig. 1.
Consort diagram
All patients had core biopsy to establish the pathologic diagnosis, grade, estrogen receptor (ER), progesterone receptor (PR), and Her2 status. Grade was defined by Modified Bloom-Richardson grading. Tissue sections of 4-μm thickness were stained for ER, PR, HER-2 neu (Her 2) using Ventana BenchMark XT automated staining system. Immunohistochemistry for ER was performed using CONFIRM anti-ER (SP1) ready to use (RTU), rabbit monoclonal antibody that recognizes human estrogen receptor alpha. CONFIRM anti-Progesterone Receptor (PR) (1E2) rabbit monoclonal antibody (RTU) that detects the A and B forms of human progesterone receptor was used to determine PgR expression and PATHWAY anti-HER-2/neu (4B5) rabbit monoclonal primary antibody was used for HER2 immunohistochemistry. ER, PR, and HER2 results were interpreted according to the current American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines [6–8]. Tumors with greater than 1 % stained cells were considered to have positive receptor status. All patients had Her2 status assessed by IHC. Patients with equivocal Her2 IHC and without amplification on Fluorscence in situ Hybridization (FISH) were considered negative. pCR was determined by microscopic examination of the excised tumor and lymph nodes after completion of therapy, and defined as the complete absence of invasive or ductal carcinoma in situ (DICS) components in the breast and axillary lymph nodes (ypT0 ypN0), as per institutional protocol.
All patients received 6 cycles of chemotherapy at 3-week intervals prior to surgery. Surgery was planned 4 weeks after completion of chemotherapy for all patients, with a delay of 1 week if there were significant features of chemo toxicity at the end of the third week. Patients underwent either modified radical mastectomy or breast conserving surgery with or without reconstruction, with axillary clearance. All patients subsequently received radiotherapy (40 GY in 15 fraction to whole breast with additional cavity boost if following BCS), 6 weeks after surgery. All patients who were either ER and/or PR positive were prescribed adjuvant endocrine treatment for 5 years, starting concurrently with radiotherapy.
Statistical Analysis
The associations between the clinico-pathological characteristics and pathological response after NACT were examined using the chi-square and Fisher exact test for categorical variable and Student-t test for quantitative variables in univariate analysis. Variables that were significant in the univariate analysis (grade, PR status, use of trastuzumab) were included in the multiple binary logistic regression models as independent variables. Although the type of chemotherapy was not significant in univariate analysis, it was also included in the multivariate model as an independent variable as it was a main exposure. The dependent variable was pCR. Disease free survival (DFS) was defined as the interval between the dates of diagnosis and the first documented disease relapse. Overall survival (OS) was defined as the interval between the dates of diagnosis and the last follow up or death documented in the hospital management system. Kaplan-Meier curves were generated to evaluate the DFS and OS. Cox regression model was used to calculate the hazard ratio and significance score. All values were two-sided, and statistical significance was defined as p < 0.05. SPSS version 20.0 software was used for all statistical analyses.
Results
Over the period Jun 2011-Dec 2013, 240 patients completed 6 cycles of chemotherapy with treatment delay of <10 days and were therefore included for analysis. Patient and tumor characteristics are shown in Table 1. The median age at the time of diagnosis was 48 years (range: 24–71 Yrs.). The median Body Mass Index (BMI) and tumor size were 25.8 Kg/m2 (range 15.64–39.51 Kg/m2) and 6 cm (range 1–15 cm) respectively. Median follow-up was 21 months (range 3–42 months) with 5 % of patients being lost to follow up.
Table 1.
Demographics and clinico-pathological characteristics
| Variables | Number of patients | Value |
|---|---|---|
| Age (median, range) | 240 | 48 Years (25–71) |
| BMI (median, range) | 240 | 25.8 Kg/m2 (15.6–39.5) |
| Tumor size (median, range) | 240 | 6.00 cm (1–15 cm) |
| Morphology (%) | ||
| IDC | 231 | 96.3 % |
| ILC | 8 | 3.3 % |
| Others | 1 | 0.4 % |
| Grade (%) | ||
| Low grade(G1 + G2) | 108 | 45 % |
| High grade(G3) | 129 | 53.8 % |
| Missing data | 3 | 1.2 % |
| Type of surgery (%) | ||
| MRM | 140 | 58.3 % |
| MRM + Reconstruction | 19 | 7.9 % |
| BCS | 57 | 23.8 % |
| BCS + LD flap reconstruction | 16 | 6.7 % |
| Bilateral BCS | 2 | 0.8 % |
| Bilateral MRM | 6 | 2.5 % |
| Pre chemo clinical nodal status (%) | ||
| Involved | 202 | 84.2 % |
| Uninvolved | 32 | 13.3 % |
| Missing data | 6 | 12.5 % |
| Skin Involvement by tumor (%) | ||
| Involved | 119 | 49.6 % |
| Uninvolved | 121 | 50.4 % |
| Lymph nodes identified in final HPE (median, range) | 240 | 21 (2–45) |
| Positive lymph nodes in final HPE (median, range) | 240 | 1 (0–30) |
| Estrogen Receptor (%) | ||
| Positive | 170 | 70.8 % |
| Negative | 69 | 28.8 % |
| Missing Data | 1 | 0.4 % |
| Progesterone receptor (%) | ||
| Positive | 128 | 53.3 % |
| Negative | 111 | 46.3 % |
| Missing Data | 1 | 0.4 % |
| HER2 Receptor (%) | ||
| Positive | 93 | 38.8 % |
| Negative | 120 | 50 % |
| Equivocal | 25 | 10.4 % |
| Missing data | 2 | 0.8 % |
| Luminal Classification (%) | ||
| Triple negative | 33 | 13.8 % |
| HER2 enriched | 25 | 10.4 % |
| Luminal type | 175 | 72.8 % |
| Missing | 7 | 3 % |
| Trastuzumab (%) | ||
| Taken | 23 | 24.7 % |
| Not taken | 70 | 75.3 % |
| Type of Chemotherapy (%) | ||
| Anthracycline followed by Taxane | 213 | 88.8 % |
| Anthracyclin or Taxane | 24 | 10 % |
| Missing data | 3 | 1.2 % |
| Pathological complete response (pCR) | ||
| Yes | 56 | 23.3 % |
| No | 184 | 76.7 % |
| Follow up in months (median, range) | 240 | 21 (3–42) |
| Follow up status | ||
| Recurrence | 40 | 16.7 % |
| No Recurrence | 195 | 81.3 % |
| Lost to follow up | 5 | 2 % |
| Relapse free period in months (median, range) | 240 | 21 (3–42) |
| Survival status | ||
| Dead | 16 | 6.7 % |
| Alive | 212 | 88.3 % |
| Unknown | 12 | 5.0 % |
The vast majority of patients (96.3 %) had invasive carcinoma, not otherwise specified, and previously called infiltrating ductal carcinoma [24], with the remainder being lobular (3.3 %) or other (0.4 %) carcinomas. 45 % of tumors were low grade (G1 + G2) and 53.8 %% were high grade (G3). Nodal and skin involvement was present clinically in 84.2 % and 49.6 % of the patients respectively. With regard to hormonal receptor status, 70.8 % of patients were ER positive, and 53.3 % were PR positive. FISH was done for 90 % of the patients with Her2 IHC 2+. Her2 was positive in 38.8 % of the entire group (either IHC 3+, or amplified by FISH). For the purposes of this analysis, patients were classified into three groups. Of the total cohort 13.8 % patients were ER negative, PR negative, Her2 negative or non-amplified (triple negative breast cancer, TNBC), 10.4 % patients were Her2 positive, ER and PR negative (Her2 enriched), and the remaining 72.8 % patients were ER positive and/or PR positive breast cancers (luminal subtype).
As NACT, the majority of patients received sequential anthracycline followed by taxane (88.8 %), and the remaining patients received either anthracycline or taxane only. Due to financial constraints only 23 of 93 suitable patients received trastuzumab. Overall, following NACT, 69 % of patients underwent mastectomy, with the remaining 31 % having breast-conserving surgery. Upon pathologic review of tumor and nodal specimens, 56 of 240 patients (23.3 %) were found to have pCR.
On univariate analysis (Table 2 and 3) factors associated significantly with pCR were high tumor grade (X2 = 4.22, p = 0.04), PR negative hormone receptor status (X2 = 9.36, p = 0.002) and, for suitable patients, administration of trastuzumab (X2 = 6.65, p = 0.01). On multivariate analysis (Table 4) PR negativity (OR 3.2, 95 % CI = 1.6 to 6.04, p = 0.001) and use of trastuzumab (OR 0.24, 95 % CI = 0.1 to 0.6, p = 0.004) were independent predictors of pCR.
Table 2.
Univariate analysis (continuous variables)
| Variables | pCR (mean ± SD) | No pCR (mean ± SD) | t | p (2 tailed) |
|---|---|---|---|---|
| Age | 48.1 ± 9.1 | 48.7 ± 9.8 | −0.377 | 0.71 |
| BMI | 26.1 ± 4.7 | 26.5 ± 4.9 | −0.582 | 0.58 |
| Tumor size | 5.8 ± 2.8 | 6.2 ± 2.5 | −1.211 | 0.23 |
Table 3.
Univariate analysis (categorical variables)
| Variables | pCR | No pCR | X2 | Significance (p value - 2 tailed) |
|---|---|---|---|---|
| Morphology (n = 240)) | ||||
| IDC | 55 | 176 | 0.85 | 0.65 |
| ILC | 1 | 7 | ||
| Others | 0 | 1 | ||
| Grade (n = 237) | ||||
| Low grade(G1 + G2) | 18 | 90 | 4.22 | 0.04 |
| High grade(G3) | 36 | 93 | ||
| Pre chemo clinical nodal status (n = 234) | ||||
| Involved | 44 | 158 | 1.39 | 0.23 |
| Uninvolved | 10 | 22 | ||
| Skin Involvement by tumor (n = 240) | ||||
| Involved | 28 | 93 | 0.005 | 0.94 |
| Uninvolved | 28 | 91 | ||
| Estrogen Receptor (n = 239) | ||||
| Positive | 37 | 133 | 0.911 | 0.3 |
| Negative | 19 | 50 | ||
| Progesterone Receptor (n = 239) | ||||
| Positive | 20 | 108 | 9.36 | 0.002 |
| Negative | 36 | 75 | ||
| Her2 Receptor (n = 238) | ||||
| Positive | 22 | 71 | 0.252 | 0.98 |
| Negative | 27 | 93 | ||
| Equivocal | 6 | 19 | ||
| Luminal Classification (n = 233) | ||||
| Triple negative | 10 | 23 | 1.16 | 0.57 |
| HER2 enriched | 6 | 19 | ||
| Luminal type | 38 | 137 | ||
| Trastuzumab (n = 93) | ||||
| Taken | 10 | 13 | 6.648 | 0.01 |
| Not taken | 12 | 58 | ||
| Type of Chemotherapy (n = 237) | ||||
| Anthracycline followed by Taxane | 50 | 163 | .084 | 0.77 |
| Anthracyclin or Taxane | 5 | 19 | ||
Table 4.
Multivariate analysis
| Variables | B | SE | Sig. | Odds Ratio(OR) | 95 % CI for OR | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Progesterone Receptor | 1.137 | 0.337 | 0.001 | 3.118 | 1.61 | 6.04 | |
| Trastuzumab | −1.404 | 0.493 | 0.004 | 0.24 | 0.10 | 0.64 | |
| Grade | 0.386 | 0.354 | 0.276 | 1.47 | 0.74 | 2.94 | |
| Type of chemotherapy | −0.196 | 0.544 | 0.71 | 0.82 | 0.28 | 2.39 | |
| Constant | 0.853 | 0.215 | 0.000 | 2.35 | |||
B = Coefficient for the constant, SE = Standard error around the coefficient for the constant, OR = odds ratio, CI = Confidence Interval
Survival Analysis
The median follow-up duration for the 240 patients was 21 months. At the cutoff date for follow-up (15 March 2015), 40 patients (16.7 %) had recurrent disease, and 16 patients had died (6.7 %). Of the 40 patients with recurrent disease, 36 had distant metastases, 3 had local relapse, and 1 had developed contralateral breast cancer.
Kaplan-Meier survival curves by pCR, PR status and use of trastuzumab in the subgroup of Her2 positive patients are shown in Fig. 2(a-f). Patients who achieved pCR showed significant positive associations with DFS and OS compared to those without pCR (log-rank test, p < 0.02 for OS and log-rank test, p = 0.02 for DFS). Early OS (21 months) was the same for the two groups.
Fig. 2.
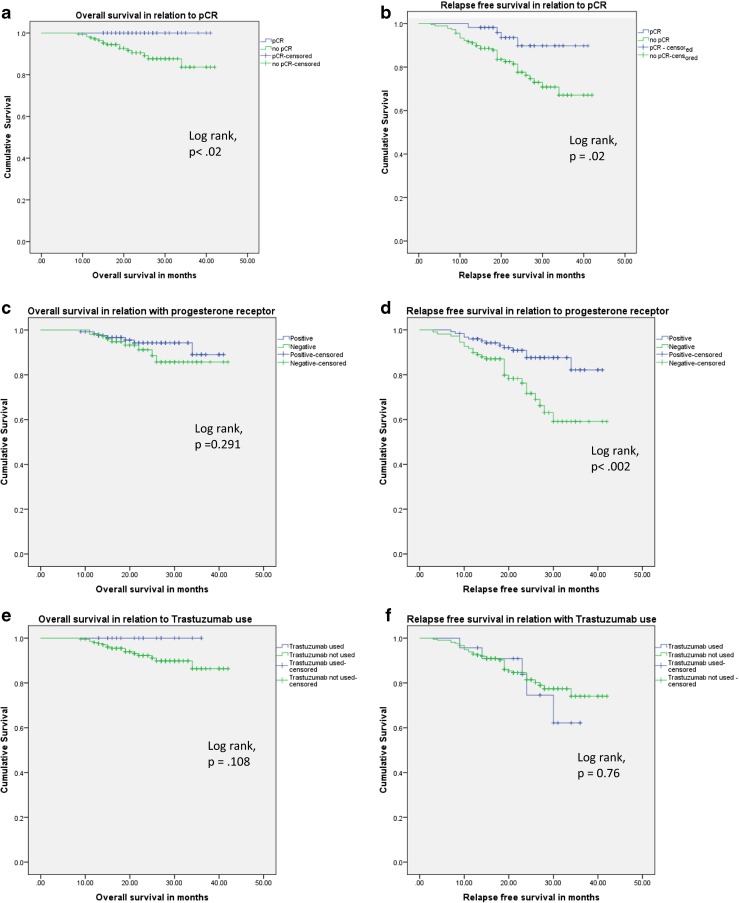
a-f (Kaplan-Meier survival curves showing relation of survival with PCR, PR status and trastuzumab use)
For the entire group, if pCR was not taken into account, Kaplan-Meier analysis showed that PR positivity had a positive association with DFS (log-rank test, p = 0.002). However, subgroup analysis revealed important differences. PR negative patients who achieved pCR had significantly better outcomes, for both DFS and OS, than those who did not (log-rank test, p = 0.048 for DFS, p < 0 .02 for OS) (Fig. 3a-b). For PR positive patients, on the other hand, there was no difference in either DFS or OS, whether pCR was achieved or not (log-rank test, p = 0.08 for DFS, p = 0.22 for OS) (Fig. 3 c-d). Analysing all four of these groups together, (Fig. 3e) the best survival figures were for PR positive patients with pCR and the worst for PR negative patients without pCR, with the other two groups lying between these two curves (log-rank test, p < 0.002 for DFS).
Fig. 3.
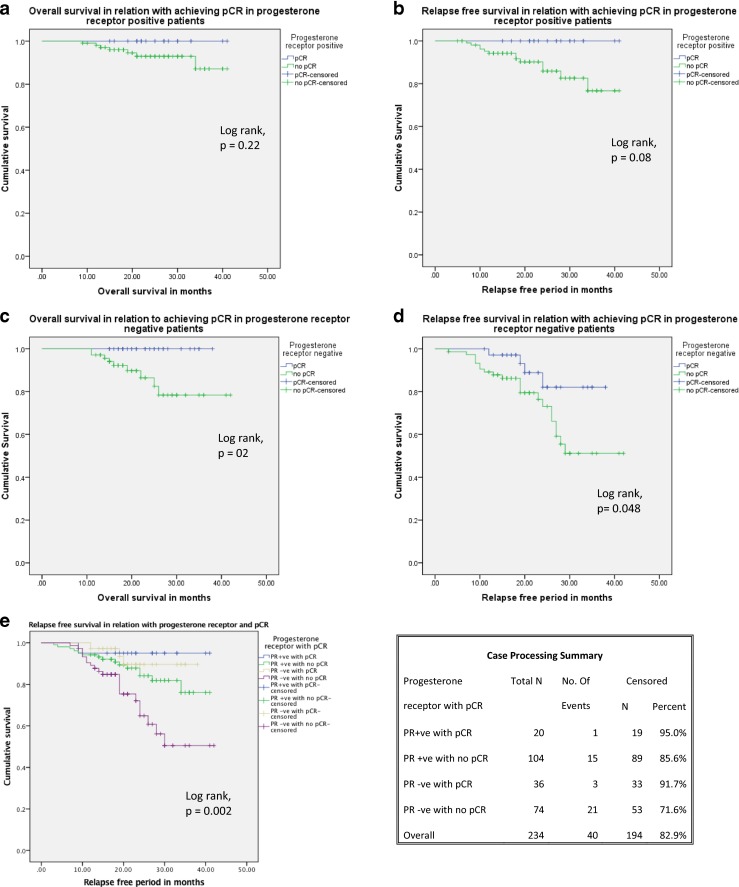
a-d (Subgroup analysis of progesterone receptor with pCR and survival). e Combined analysis of PR status and relapse free survival
Cox regression analysis was also performed. Both pCR (HR = 0.235, p < 0.01) and PR positivity (HR = 0.306, p < 0.01) showed significant positive associations with DFS, but neither of these factors was associated with OS (pCR: HR = 0.01, p = 0.95; PR: HR = 0.45, p = 0.11). In subgroup analysis, pCR was associated with survival in PR negative patients only, (DFS: HR 0·35, p = 0.06; OS: HR 0·024, p = 0·2) and not in PR positive patients (DFS: HR 27.63, p = 0.28, OS: HR 27.43, p = 0.442). Again, overall analysis of all four subgroups (PR positive and negative, with or without pCR) showed that PR negative patients without pCR had the worst prognosis (DFS: HR 0.24, p = 0.02).
Discussion
Neoadjuvant chemotherapy is an effective tool for in vivo testing of chemosensitivity, and if the response to chemotherapy could be predicted at the start of treatment, based on clinical and histopathological variables, it could guide clinicians to either go ahead with NACT followed by BCS, or to consider upfront surgery, including reconstructive procedures if needed, to be followed by adjuvant treatment [1, 2, 5]. Following NACT, pCR has been shown in several clinical trials to correlate with good treatment outcomes, and can be used as a surrogate marker for better survival [9–11]. There is extensive evidence for pCR as a predictor for improved event-free survival [9], and as a powerful, and possibly independent, prognostic variable in aggressive tumor subtypes (luminal B/Her2 negative, Her2 positive/ER negative, and triple negative) [12].
All studies do not use the same definition of pCR, but a recent meta-analysis by Gunter Minckwitz et al. [12] showed that only ypT0N0 was associated with a favorable outcome, and all other definitions (ypTis, ypNis, ypN-residual etc.) were associated with increased relapse risk. In our study, using ypT0N0 as the definition, predictors for pCR were high grade disease (univariate), PR negative status and for Her2 positive breast cancers, trastuzumab therapy [9, 13–15]. Our results were similar to those of Perez et al. [16], where PR status and trastuzumab treatment were the only independent predictors of pCR. We could not demonstrate an association between pCR and triple negative disease, although this has previously been reported by other groups [9, 10]. For the whole group, pCR was significantly associated with both DFS and OS in Kaplan-Meier survival curves but on Cox regression analysis there was a significant positive association with DFS only [11].
Our results showed that adding trastuzumab to the neoadjuvant regimen significantly improved pCR in both univariate and multivariate analysis, but did not alter either DFS or OS. Our pCR rate of 43 % for patients receiving trastuzumab was higher than that in published data, (30 % in the trastuzumab alone arm of Neo ALLTO [17], 31.7 % in Gepar Quattro [18], both studies giving trastuzumab with anthracycline/taxane based NACT). However the number of patients receiving trastuzumab in our study was very small and no conclusions can be drawn from this data. International opinion, including the St. Gallen’s consensus [19], supports the inclusion of one anti-Her2 agent in neoadjuvant treatment regimes, and the use of two anti Her2 agents in combination has also been studied [20]. However the cost of treatment with trastuzumab was prohibitive for the majority of our patients.
In our study, PR negativity was significantly associated with pCR, and pCR was also accurate in predicting outcome in this subgroup. Paradoxically however, PR positive patients had better DFS. This result initially appears contradictory, but is in line with other published data [16, 21–23]. Low PR expression has been associated with low DFS in ER positive/Her2 negative patients [21], and poor outcome in the Luminal B subgroup [22]. PR status may even be a powerful, independent prognostic variable in early breast cancer management [23].
In line with current evidence, Allred score > 2 was considered positive, and all patients received endocrine treatment if either ER or PR was positive [24]. The PR receptor is often seen as secondary in importance to ER receptor status. Newer evidence suggests that this approach may change. Retrospective studies suggest that PR positivity can be categorized into 3 groups (< 10, 10 to 60 and ≥60 fmol/mg), which can predict the response to adjuvant tamoxifen, and might also be the second most critical factor, equal or even of greater value that ER expression, in predicting DFS [25, 26]. The combination of PR expression with Ki-67 seems to improve the accuracy of IHC-based classification of luminal A and luminal B breast cancer, especially for postmenopausal women [21].
The mechanism for the difference in outcome related to PR status has been studied. There is evidence that PR positivity may be associated with taxane resistance [16]. This might have contributed to the lower pCR rate in PR positive patients in our study, as 89 % of our patients received taxane-based chemotherapy. Other studies have demonstrated an up-regulation of the anti-apoptotic gene BCL-XL in breast cancer cells as a consequence of PR expression [27]. The main cytotoxic mechanism of taxanes is apoptosis, and this might contribute to the relative resistance of PR positive carcinomas to taxanes. Besides BCL-XL, several other genes have also been shown to be PR dependent in breast cancer cells [28].
This study supports growing evidence that the PR receptor, often considered just a surrogate marker of ER, may have an independent role, and might be a potentially useful target for improved breast cancer therapy. PR non-expression could be used to identify patients, otherwise in good prognostic groups, who might benefit from additional therapy, such as more chemotherapy, extended endocrine therapy or treatments targeting growth factor receptor pathways. The question of more aggressive therapy for the subset of PR negative patients who do not achieve pCR may warrant clinical trials.
These results were derived from sub-group, post-hoc analysis of data from a pre-existing cohort without ‘a-priori’ hypothesis for survival analysis of data on DFS and OS in relation to the PR. Another limitation of this study was that Ki67 status was not available for analysis, so, on the basis of the ER, PR and Her2 results, patients were placed in a combined group including all luminal patients. In addition, Her2 status remained equivocal for some patients, and few Her2 positive patients received trastuzumab. Survival data is for a limited follow-up period, with a median of 21 months, which is relatively brief for luminal subtypes, which may recur 15 years or later after primary treatment [29]. Strong points are a large, single institution dataset, receiving a standardized neoadjuvant regimen, completed before surgery, for the majority of patients.
Conclusion
Our study supported the impact of PR status on the outcome of patients having neoadjuvant chemotherapy for primary breast cancer. Absent PR expression was strongly and independently associated with pCR, but also with worse prognosis. Patients who attained pCR, defined as ypT0 ypN0, had improved event-free survival compared to patient with residual disease. Achieving pCR was particularly important for patients with PR negative tumors, with residual disease indicating significantly worse prognosis in this group. However, in view of the study’s limitations, at best these results are “hypothesis generating” and need confirmation by a well-designed prospective cohort, or a randomized trial where PR status could serve as a “stratifying variable” and patients may be randomized to groups treated with or without taxane.
Compliance with Ethical Standards
Conflict of Interests
The authors have no conflict of interests.
References
- 1.Tewari M, Krishnamurthy A, Shukla HS. Predictive markers of response to neoadjuvant chemotherapy in breast cancer. Surg Oncol. 2008;17:301–311. doi: 10.1016/j.suronc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Colleoni M, Zahrieh D, Gelber RD, Viale G, Luini A, Veronesi P, et al. Preoperative systemic treatment: prediction of responsiveness. Breast. 2003;12:538–542. doi: 10.1016/S0960-9776(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 3.Guarneri V, Broglio K, Kau SW, et al. Prognostic value of pathological complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037–1044. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 4.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 5.Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22:2303–2312. doi: 10.1200/JCO.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 6.Hammond MEH, Hayes DF, Wolff AC, Mangu PB, Temin S. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Journal of Oncology Practice. 2010;6(4):195–197. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 8.Tavassoli FA, Devilee P. World Health Organization classification of Tumours. Lyon: Pathology and Genetics of Tumours of the Breast and Female Genital Organs. IARC Press; 2003. [Google Scholar]
- 9.Von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804.24. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 10.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 11.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 12.Fumagalli D, Bedard PL, Nahleh Z, et al. A common language in neoadjuvant breast cancer clinical trials: proposals for standard definitions and endpoints. Lancet Oncol. 2012;13:e240–e248. doi: 10.1016/S1470-2045(11)70378-3. [DOI] [PubMed] [Google Scholar]
- 13.Kim MM, Allen P, Gonzalez-Angulo AM, et al. Pathologic complete response to neoadjuvant chemotherapy with trastuzumab predicts for improved survival in women with HER2-overexpressing breast cancer. Ann Oncol. 2013;24(8):1999–2004. doi: 10.1093/annonc/mdt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peintinger F, Buzdar AU, Kuerer HM, et al. Hormone receptor status and pathologic response of HER2-positive breast cancer treated with neoadjuvant chemotherapy and trastuzumab. Ann Oncology. 2008;19(12):2020–2025. doi: 10.1093/annonc/mdn427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lips EH, Mulder L, de Ronde JJ, Mandjes IAM, Koolen BB, Wessels LFA, Rodenhuis S, Wesseling J. Breast cancer subtyping by immunohistochemistry and histological grade outperforms breast cancer intrinsic subtypes in predicting neoadjuvant chemotherapy response. Breast Cancer Res Treat. 2013;140:63–71. doi: 10.1007/s10549-013-2620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.J. M. Perez Garcia, C. Saura, E. Muñoz, G. Sanchez-Olle, P. Gomez, V. Peg, D. Sabadell, J. Cortes, J. Baselga, M. Bellet. (2009) Role of progesterone receptor status (PR) as predictive factor of pathological complete response (pCR) to neoadjuvant chemotherapy (NACT) in breast cancer (BC) patients (pts). J Clin Oncol 27:15 s, (suppl; abstr 637)
- 17.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;18:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, Bauerfeind I, Hilfrich J¨r, Eidtmann H, Gerber B, Hanusch C, Ku¨hn T, du Bois A, Blohmer J-U, Thomssen C, Costa SD, Jackisch C, Kaufmann M, Mehta K, von Minckwitz G. Neoadjuvant TreatmentWith trastuzumab in HER2-positive breast cancer: results from the Gepar Quattro study. J Clin Oncol. 2010;28(12):2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 19.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 21.Nishimukai A, Yagi T, Yanai A, Miyagawa Y, Enomoto Y, Murase K, Imamura M, Takatsuka Y, Sakita I, Hatada T, Miyoshi Y. (2014) High Ki-67 Expression and Low Progesterone Receptor Expression Could Independently Lead to a Worse Prognosis for Postmenopausal Patients With Estrogen Receptor-Positive and HER2-Negative Breast Cancer, Clin breast cancer Dec 24. pii: S1526–8209(14)00288–2. doi: 10.1016/j.clbc.2014.12.007. [DOI] [PubMed]
- 22.G. Cancello, P. Maisonneuve,, N. Rotmensz, G. Viale, M. Colleon, Progesterone receptor loss identifies Luminal B breast cancer subgroups at higher risk of relapse: Ann Oncol, 2013:661–668 [DOI] [PubMed]
- 23.Purdie CA, Quinlan P, Jordan LB, Ashfield A, Ogston S, Dewar JA, Thompson AM. Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study. British Journal of Cancer. 2014;110:565–572. doi: 10.1038/bjc.2013.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burstein HJ (2010) Ann Alexis Prestrud, Jerome Seidenfeld, Holly Anderson, Thomas a. Buchholz, Nancy E. Davidson, Karen E. Gelmon et al. “American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor–positive breast cancer.”Journal of. Clin Oncol JCO-2009 [DOI] [PMC free article] [PubMed]
- 25.Lamy P-J, Pujol P, Thezenas S, Kramar A, Guilleux PRF, Grenier J. Progesterone receptor quantification as a strong prognostic determinant in postmenopausal breast cancer women under tamoxifen therapy. Breast Cancer Res Treat. 2002;76:65–71. doi: 10.1023/A:1020228620173. [DOI] [PubMed] [Google Scholar]
- 26.Clark GM, McGuire WL, Hubay CA, et al. Progesterone receptor as a prognostic factor instage II breast cancer. N Engi J Med. 1983;309:1343–1347. doi: 10.1056/NEJM198312013092240. [DOI] [PubMed] [Google Scholar]
- 27.Daniel AR, Hagan CR, Lange CA. Progesterone receptor action: defining a role in breast cancer. Expert Rev Endocrinol Metab. 2011;6(3):359–369. doi: 10.1586/eem.11.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 29.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomize trials. Lancet. 2005; 365:1687–1717. doi:10.1016/S0140–6736(05)66544–0. [DOI] [PubMed]