Abstract
The symptoms in ovarian cancer are often missed leading to dubious diagnosis and staging. Inguinal lymphadenopathy (ILAP) is reported to be rare and occurring via lymphatic or hematogenous route. The paucity of studies on ILAP in ovarian cancer indicates a scope of refining its staging and management. The present study aims to document the presentation and management of ILAP in ovarian cancer, which may also reflect its incidence and mechanism of spread. All patients of ovarian cancer with inguinal lymphadenopathy presenting to our institute from 1 January 2015 to 31 December 2015 were included. All clinical, treatment, and pathological details were analyzed. Seven patients of ovarian cancer presented with ILAP. The mean age and BMI were 53.29 +/− 8.38 years and 26.23 +/− 3.03 kg/m2. Presentation varied from advanced disease (adnexal, omental, peritoneal, and nodal) to isolated ILAP even without adnexal mass (n = 4). Mean CA 125 was 229.64 +/− 322 (20–924) and ovarian primary was confirmed on microscopy or immunohistochemistry. Six patients underwent surgery with (n = 4) or without neoadjuvant chemotherapy (n = 2). Complete cytoreduction could be achieved in all patients with acceptable operative and perioperative outcomes. Peritoneal surface spread, along hernia track to the groin, was seen in two patients. Histopathology showed advanced disease, isolated ILAP and no residual disease in 3, 2, and 1 patient, respectively. ILAP has diverse clinical presentation in ovarian cancers and is not that uncommon. ILAP may also occur by peritoneal surface spread and shows good results with cytoreductive surgery and chemotherapy.
Keywords: Ovarian cancer, Inguinal, Lymphadenopathy
Introduction
Ovarian cancer is a common but morbid gynecological malignancy with poor survival even with improvement in medical and surgical techniques. Its main symptoms, early satiety, abdominal discomfort, and distension, are vague and are often misinterpreted. This may be the reason for 70 to 80 % of them presenting in stage III or IV, adding to the dismal scenario [1]. Having said this, it becomes imperative for the clinician to carefully identify all symptoms and signs before making the diagnosis and final stage. Inguinal lymphadenopathy (ILAP) in ovarian cancer is one such overlooked clinical situation and deserves some attention.
It is interesting to note that in patients with ovarian cancer, ILAP is reported in up to 3 to 4 % of autopsy and recurrent nodal disease [2, 3]. But very few cases document its occurrence at primary presentation and only some of these describe full cilinico pathological details [4–6]. The question that arises is, if we are missing these cases in primary setting.
Ovarian cancers most commonly spread along the peritoneal surface followed by others. ILAP is considered to occur via lymphatic spread along round ligament or through the blood and is staged with other distant metastasis [7]. However, in most case reports of ILAP in ovarian cancer, management included upfront cytoreductive surgery (CRS), in which complete cytoreduction could be achieved and good survival was seen [3–5, 8]. This controversy demands further studies to confirm its route of spread and refine the staging and management.
We hereby report the presentation and management of inguinal LN in ovarian cancer at our institute, which may also reflect its incidence and mechanism of spread.
Materials and Methods
All the patients of ovarian cancer with ILAP who presented to our institute from 1 January 2015 to 31 December 2015 were included in the study. All the parameters including clinical presentation, investigations, treatment details, and outcomes were recorded. The data was analyzed using descriptive statistics in SPSS version 22.
Results
During the mentioned duration, 324 patients of ovarian cancer were registered at our institute, out of which 7 had ILAP and were included in the study. The mean age and BMI were 53.29 years and 26.23 kg/m2 (Table 1). One of the patients presented with abdominal lump and one with urinary retention, while 4 patients presented with groin swelling as their main symptom (unilateral = 2, bilateral = 2). One patient was a known case of ovarian cancer and presented with rising CA 125, after a disease free interval of 15 months. Other abdominal or systemic symptoms like fever, malaise, anorexia, or weight loss were not seen in any of the patients.
Table 1.
Clinical and treatment details of patients with ILAP in ovarian cancers
| Patient characteristics | N = 7 |
|---|---|
| Age (years) | 53.29 +/− 8.38 (46–68) |
| BMI (kg/m2) | 26.23+/−3.03 (20.93–30) |
| Clinical presentation Abdominal symptoms Groin swelling (U/L, B/L) Biochemical relapse |
2 4 (2, 2) 1 |
| Examination Abdominopelvic mass Inguinal LN (U/L, B/L) NED |
2 6 (4, 2) 1 |
| Imaging Adnexal mass RPLN Inguinal (U/L, B/L) |
3 4 7 (2, 5) |
| CA 125 (IU/ml) | 229.64 +/− 322 ( 20–924) |
| Management | N = 6 |
| PDS - > Adj chemo 3#NACT - > ICS - > Adj chemo 3# NACT - > SDS |
2 3 1 |
| Operative findings Adnexal mass Peritoneal and omental deposits Pelvic LAP Inguinal LAP Incisional hernia with deposit |
N = 6 3 2 3 6 (U/L = 2, B/L = 4) 1 |
| PCI | 6.0 +/− 4.14 (2–13) |
| CC score = 0 | 6 (100 %) |
| Duration of surgery (hours) | 4.33 +/− 1.50 (3–6) |
| Blood loss (ml) | 458.33 +/− 188.193 (250–750) |
| ICU stay (days) | 3.5 +/− 0.54 (3–4) |
| Hospital stay (days) | 7.33 +/− 2.33 (6–12) |
| Complications Seroma (grade2) Wound dehiscence (grade 3) |
N = 6 2 1 |
ILAP inguinal lymphadenopathy, BMI body mass index, U/L unilateral, B/L bilateral, NED no evidence of disease, NACT neoadjuvant chemotherapy, ICS interval cytoreductive surgery, SDS secondary debulking surgery, PCI peritoneal carcinomatosis index, CC score completeness of cytoreduction score
On examination, all patients had good performance status. Abdominopelvic mass was palpable in 2 patients. Abdominal and pelvic examination was essentially normal in rest of the patients. All patients had palpable inguinal lymph nodes (U/L = 4, B/L = 2) except the one with biochemical relapse. There was no lesion in perineum or lower limbs and no cervical or axillary LAP.
Trucut biopsy was done in all 7 patients, which confirmed the metastasis from ovarian origin on microscopic or immunohistochemistry examination. Imaging revealed adnexal mass with retroperitoneal lymph nodes in only 3 patients, and one of these had extensive upper abdominal disease. In the remaining 4 patients, there was no evidence of adnexal mass or other pathology, which was confirmed with whole body PET scan. One had pelvic and inguinal while the other 3 had ILAP only. Two patients also had anterior abdominal wall nodule near incisional hernia site. There was no suspicion of disease in other nodal regions or distant sites.
CA 125 was raised in all but 2 patients, while CEA and CA 19–9 were normal. Two patients underwent primary CRS, while 3 patients underwent interval CRS after 3 cycles of neoadjuvant chemotherapy. The patient with recurrence was also given neoadjuvant chemotherapy before secondary CRS. One patient did not follow advice. Follow-up imaging after neoadjuvant chemotherapy showed good response.
Exploratory laparotomy after pre anesthesia workup revealed adnexal mass in 3 of 6 patients. Two of these also had extensive omental and peritoneal deposits, enlarged para aortic and pelvic lymph nodes. In remaining 3 patients, gross disease was limited to nodes only (pelvic + inguinal in 1, inguinal only in 3). One patient had incisional hernia with omentum reaching up to the groin, which collaborated with abdominal wall deposit seen on imaging. The mean peritoneal carcinomatosis index (PCI) was 6.0 +/− 4.14 ranging from 2 to 13. Primary CRS comprising of hysterectomy with bilateral salpingo-oophorectomy, omentectomy and pelvic, para aortic and inguinal lymph node dissection was done in 5 patients (Fig. 1a, b, c ). Peritonectomy was done in 2 patients with peritoneal deposits. For patient undergoing secondary CRS, inguinal LN dissection was done (Fig. 2). Complete cytoreduction (CC score = 0) was achieved in all patients. The average duration of surgery was 4.33 h and blood loss was 458.33 ml. The patients recovered well and were discharged after an average stay of 7.33 days. Two patients had seroma (Fig. 3) which could be managed with aspiration, and one had wound dehiscence, which required debridement and resuturing.
Fig. 1.
a Cancer ovary with B/L inguinal lymph nodes. b After complete cytoreduction. c Placement of bilateral suction drains
Fig. 2.
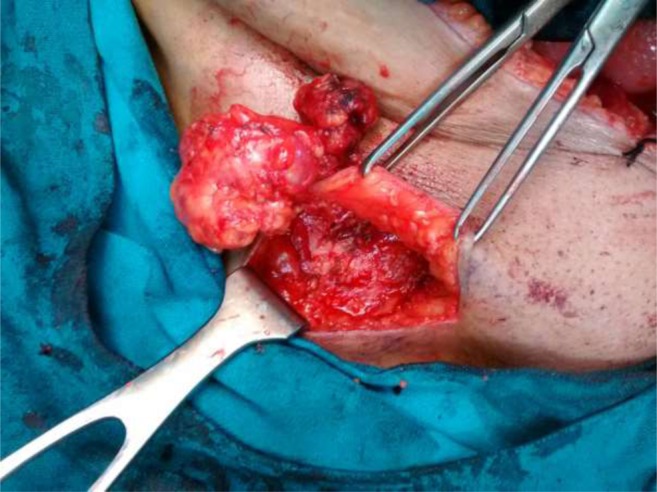
Superficial inguinal lymph node dissection
Fig. 3.
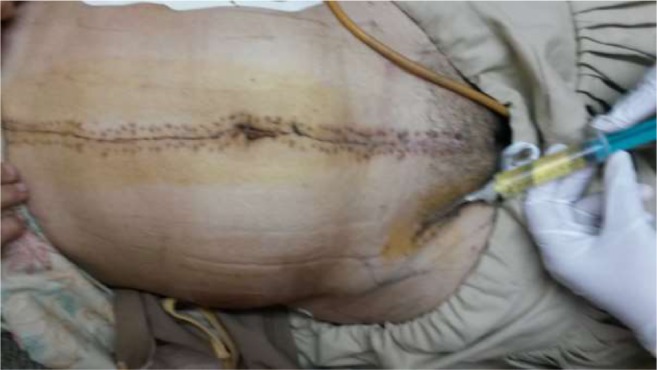
Seroma aspiration
In the two patients undergoing upfront CRS, histopathology revealed high-grade serous carcinoma with metastatic deposits in pelvic peritoneum, pouch of Douglas and pelvic, para aortic and inguinal lymph nodes. Out of three patients undergoing interval CRS, one showed extensive disease, i.e., bilateral ovarian serous carcinoma with metastasis in lymph nodes and abdominal wall deposits. In the second patient, only inguinal lymph nodes were positive for high-grade serous carcinoma. In the third patient, complete response to neoadjuvant treatment was seen with no evidence of disease in adnexa, uterus, omentum, or lymph nodes. In the patient undergoing secondary CRS after chemotherapy, residual disease was seen in the inguinal lymph nodes only. Adjuvant chemotherapy was given to all the patients.
On follow-up, one of the patients had rising CA 125 and PET scan revealed recurrent disease in peripancreatic, para aortic and axillary lymph nodes. The rest 5 patients are alive without disease and are under close follow up with 3 monthly clinical evaluation, CA 125, USG abdomen and chest X ray.
Discussion
Presentation and Incidence
In the present study, seven cases of ILAP in ovarian cancer were seen over a single year with bizarre presentations varying from patients with extensive upper abdominal and nodal disease to isolated inguinal nodal metastasis. Four of our patients were seen even without adnexal mass. Only few case reports of isolated inguinal LN in early ovarian cancer are found in literature [4, 5, 9]. However, report of ILAP preceding the adnexal mass by 33 months and the present study point towards the under reporting of inguinal lymph nodes and suggest a higher incidence in early ovarian cancer [10]. An upsurge in the incidence may be seen with patient awareness, meticulous clinical evaluation, increasing use of PET scan and immunohistochemistry.
Route of Spread
In ovarian cancer, spread to para aortic, pelvic and inguinal lymph nodes may occur via lymphatics along infundibulo-pelvic, broad and round ligament, respectively. But isolated ILAP is seldom seen without others [7]. Spread via hematogenous route to solitary sites like inguinal or axillary lymph nodes, breast, and brain is reported [11].
The commonest route of spread in ovarian cancer, i.e., along the peritoneum, seems to play role in ILAP also. This is suggested by the finding of incisional hernia and lower abdominal wall deposits seen in two of our patients. The track reaching up to the groin was confirmed at surgery in one patient while the other defaulted. This deserves a special mention as consideration of peritoneal metastasis as loco regional by Sugarbaker brought a revolution in the treatment approach [12]. Thus, a close watch is required to recommend or negate peritoneal spread leading to ILAP in ovarian cancer.
Management and Staging
In our patients, a good response to neoadjuvant chemotherapy was seen with complete response in one of them. Complete cytoreduction could be achieved in all patients with acceptable intraoperative and perioperative outcomes. With similar results in literature, chemotherapy and surgery seem to have a definite role in ovarian cancer with ILAP, but their sequence remains to be confirmed.
The prognosis of nodal metastasis is controversial, especially if found as an isolated metastatic site. Be it the isolated presence of para aortic nodes, or the ILAP, the studies differ in survival outcomes [13, 14]. Though complete cytoreduction without increase in perioperative morbidity, a good response to chemotherapy and a 5-year survival of up to 64 % is reported, ILAP is presently staged asIVb [4]. With the limited follow-up, survival outcomes cannot be documented in the present study, but the other aspects mentioned make it significant.
Conclusion
Inguinal lymphadenopathy is not that uncommon in ovarian cancer and can occur via spread along hernia track. A vigilant evaluation and documentation of all cases may refine staging and management of ovarian cancers with ILAP.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
The authors have strictly adhered to the ethical norms.
Contributor Information
Shveta Giri, Phone: 00 9560420752, Email: drshvetagiri@yahoo.co.in.
Swati H Shah, Email: drswati2002@gmail.com.
Kanika Batra, Email: kanica.batra@gmail.com.
Anu-Bajracharya, Email: dr.anubajracharya42@gmail.com.
Vandana Jain, Email: dr.vandana.j@gmail.com.
Himanshu Shukla, Email: dr.himanshushukla@gmail.com.
Rupinder Sekhon, Email: rupysekhon@hotmaiil.com.
Sudhir Rawal, Email: sudhirrawal85@gmail.com.
References
- 1.Di Giorgio A, Naticchioni E, Biacchi D, et al. Cytoreductive surgery (peritonectomy procedures) combined with HIPEC in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer. 2008;113:315–325. doi: 10.1002/cncr.23553. [DOI] [PubMed] [Google Scholar]
- 2.Dvoretsky PM, Richards KA, Angel C, Rabinowitz L, Stoler MH, Beecham JB, Bonfiglio TA. Distribution of disease at autopsy in 100 women with ovarian cancer. Hum Pathol. 1988;19(1):57–63. doi: 10.1016/S0046-8177(88)80316-2. [DOI] [PubMed] [Google Scholar]
- 3.Ferrero A, Ditto A, Giorda G, et al. Secondary cytoreductive surgery for isolated lymph node recurrence of epitheliovarian cancer: a multicenter study. Eur J Surg Oncol. 2014;40(7):891–898. doi: 10.1016/j.ejso.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Yang XJ, Zheng FY, Xu YS, Ou RY. Ovarian cancer initially presenting with isolated ipsilateral superficial inguinal lymph node metastasis: a case study and review of the literature. J Ovarian Res. 2014;7(1):1. doi: 10.1186/1757-2215-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang D, Ng K, Tan H, Chung AY, Yew B, Lee VK. Ovarian carcinoma presenting with isolated contralateral inguinal lymph node metastasis: a case report. Ann Acad Med Singap. 2007;36(6):427. [PubMed] [Google Scholar]
- 6.Rose PG, Piver MS, Tsukada Y, Lau T. Metastatic patterns in histologic variants of ovarian cancer. An autopsy study. Cancer. 1989;64(7):1508–1513. doi: 10.1002/1097-0142(19891001)64:7<1508::AID-CNCR2820640725>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 7.Eichner E, Bove ER. In vivo studies on the lymphatic drainage of the human ovary. Obstet Gynecol. 1954;3(3):287–297. [PubMed] [Google Scholar]
- 8.Fotiou S, Aliki T, Petros Z, Ioanna S, Konstantinos V, Vasiliki M, George C. Secondary cytoreductive surgery in patients presenting with isolated nodal recurrence of epithelial ovarian cancer. Gynecol Oncol. 2009;114(2):178–182. doi: 10.1016/j.ygyno.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Scholz HS, Lax S, Tamussino KF, Petru E. Inguinal lymph node metastasis as the only manifestation of lymphatic spread in ovarian cancer: a case report. Gynecol Oncol. 1999;75(3):517–518. doi: 10.1006/gyno.1999.5592. [DOI] [PubMed] [Google Scholar]
- 10.Kehoe S, Luesley D, Rollason T. Ovarian carcinoma presenting with inguinal metastatic lymphadenopathy 33 months prior to intraabdominal disease. Gynecol Oncol. 1993;50(1):128–130. doi: 10.1006/gyno.1993.1177. [DOI] [PubMed] [Google Scholar]
- 11.Loredo DS, Powell JL, Reed WP, Rosenbaum JM. Ovarian carcinoma metastatic to breast: a case report and review of the literature. Gynecol Oncol. 1990;37(3):432–436. doi: 10.1016/0090-8258(90)90382-U. [DOI] [PubMed] [Google Scholar]
- 12.Sugarbaker PH, Gianola FJ, Speyer JL, Wesley R, Barofsky I, Meyers CE. Prospective randomized trial of intravenous vs intraperitoneal 5—Fuin patients with advanced primary colon or rectal cancer. SeminOncol. 1985;12:101–111. [PubMed] [Google Scholar]
- 13.Ferrandina G, Legge F, Petrillo M, Salutari V, Scambia G. Ovarian cancer patients with “node-positive-only” stage IIIC disease have a more favorable outcome than stage IIIA/B. Gynecol Oncol. 2007;107(1):154–156. doi: 10.1016/j.ygyno.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Blanchard P, Plantade A, Pagès C, Afchain P, Louvet C, Tournigand C, de Gramont A. Isolated lymph node relapse of epithelial ovarian carcinoma: outcomes and prognostic factors. Gynecol Oncol. 2007;104(1):41–45. doi: 10.1016/j.ygyno.2006.06.039. [DOI] [PubMed] [Google Scholar]



