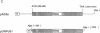Abstract
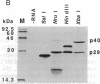
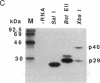
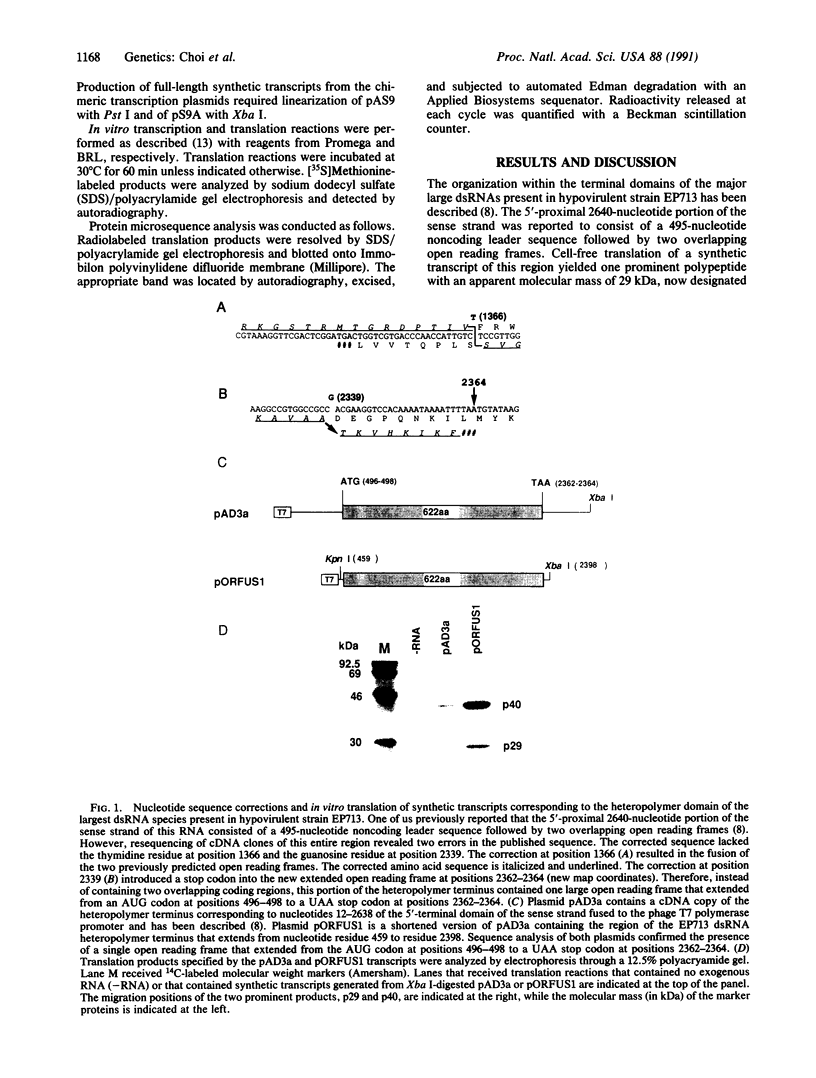
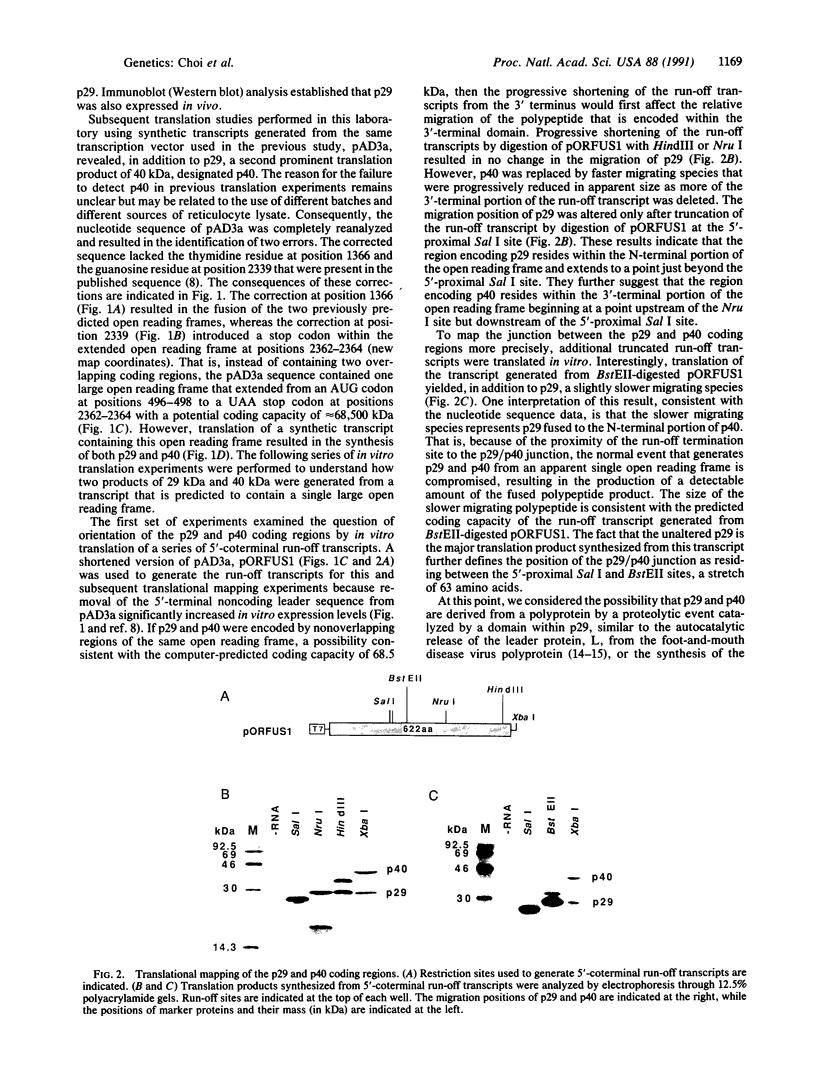
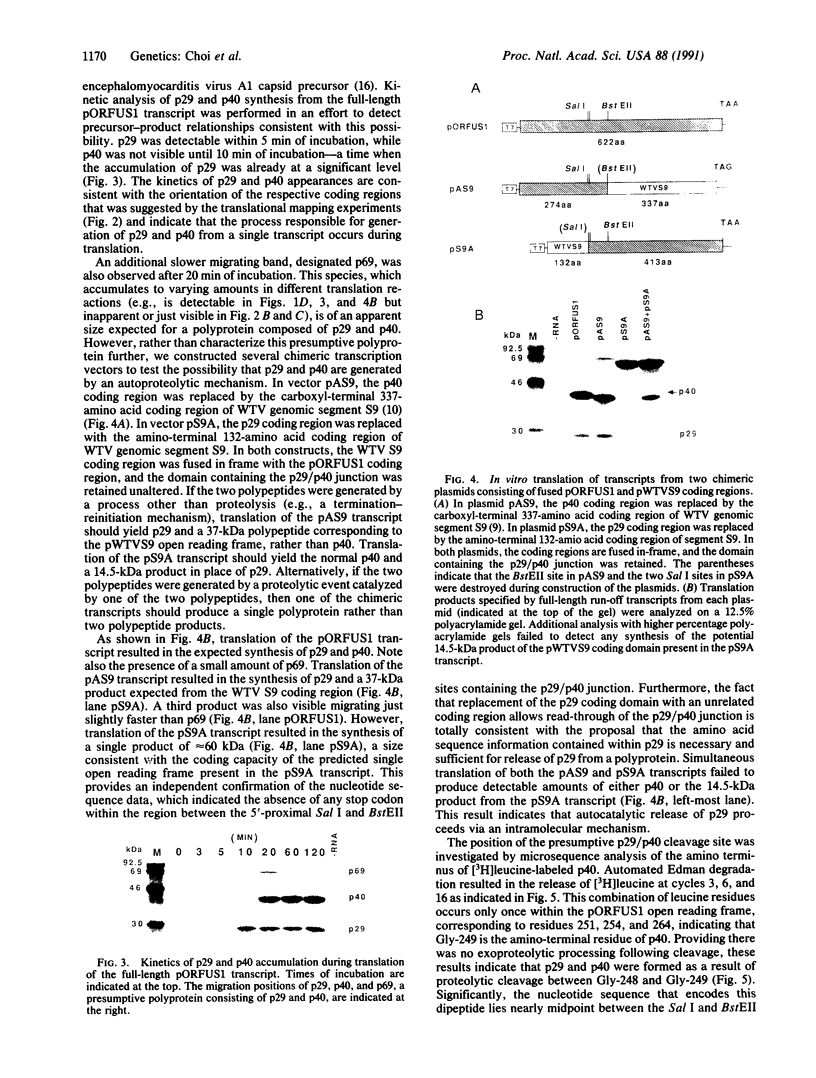
The genetic information responsible for reduced virulence (hypovirulence) of the chestnut blight fungus Cryphonectria parasitica is thought to reside on cytoplasmically replicating double-stranded RNA (dsRNA) molecules. Cell-free translation of synthetic transcripts corresponding to the 5'-terminal 2640 nucleotides of the sense strand of the large dsRNA present in C. parasitica hypovirulent strain EP713 yielded two polypeptides with apparent molecular masses of 29 and 40 kDa. Nucleotide sequence analysis indicated that p29 and p40 were encoded by a single large open reading frame. The coding regions for p29 and p40 were mapped to nonoverlapping portions of the 5'- and 3'-terminal domains of the open reading frame, respectively. Kinetic analysis and in vitro translation studies with chimeric transcripts indicated that p29 is autocatalytically released from a nascent polyprotein during translation. Microsequence analysis of the amino terminus of radiolabeled p40 indicated that cleavage occurred between Gly-248 and Gly-249, consistent with translational mapping analysis. Examination of the p29 amino acid sequence revealed similarity to the Potyvirus-encoded cysteine-type proteinase HC-Pro. These results indicate the types of mechanism that operate during gene expression by hypovirulence-associated dsRNA genetic elements.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aliperti G., Schlesinger M. J. Evidence for an autoprotease activity of sindbis virus capsid protein. Virology. 1978 Oct 15;90(2):366–369. doi: 10.1016/0042-6822(78)90321-5. [DOI] [PubMed] [Google Scholar]
- Anagnostakis S. L. Biological control of chestnut blight. Science. 1982 Jan 29;215(4532):466–471. doi: 10.1126/science.215.4532.466. [DOI] [PubMed] [Google Scholar]
- Anzola J. V., Dall D. J., Xu Z. K., Nuss D. L. Complete nucleotide sequence of wound tumor virus genomic segments encoding nonstructural polypeptides. Virology. 1989 Jul;171(1):222–228. doi: 10.1016/0042-6822(89)90529-1. [DOI] [PubMed] [Google Scholar]
- Burroughs J. N., Sangar D. V., Clarke B. E., Rowlands D. J., Billiau A., Collen D. Multiple proteases in foot-and-mouth disease virus replication. J Virol. 1984 Jun;50(3):878–883. doi: 10.1128/jvi.50.3.878-883.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Cary S. M., Parks T. D., Dougherty W. G. A second proteinase encoded by a plant potyvirus genome. EMBO J. 1989 Feb;8(2):365–370. doi: 10.1002/j.1460-2075.1989.tb03386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G. H., Marek E. T., Schardl C. L., Richey M. G., Chang S. Y., Smith D. A. sti35, a stress-responsive gene in Fusarium spp. J Bacteriol. 1990 Aug;172(8):4522–4528. doi: 10.1128/jb.172.8.4522-4528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaney M. A., Vakharia V. N., Lloyd R. E., Ehrenfeld E., Grubman M. J. Leader protein of foot-and-mouth disease virus is required for cleavage of the p220 component of the cap-binding protein complex. J Virol. 1988 Nov;62(11):4407–4409. doi: 10.1128/jvi.62.11.4407-4409.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J. A detailed kinetic analysis of the in vitro synthesis and processing of encephalomyocarditis virus products. Virology. 1986 Feb;149(1):114–127. doi: 10.1016/0042-6822(86)90092-9. [DOI] [PubMed] [Google Scholar]
- Kräusslich H. G., Nicklin M. J., Toyoda H., Etchison D., Wimmer E. Poliovirus proteinase 2A induces cleavage of eucaryotic initiation factor 4F polypeptide p220. J Virol. 1987 Sep;61(9):2711–2718. doi: 10.1128/jvi.61.9.2711-2718.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C. S., Carrington J. C. Identification of essential residues in potyvirus proteinase HC-Pro by site-directed mutagenesis. Virology. 1989 Dec;173(2):692–699. doi: 10.1016/0042-6822(89)90582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae B. P., Hillman B. I., Tartaglia J., Nuss D. L. Characterization of double-stranded RNA genetic elements associated with biological control of chestnut blight: organization of terminal domains and identification of gene products. EMBO J. 1989 Mar;8(3):657–663. doi: 10.1002/j.1460-2075.1989.tb03423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K., Beck E. A second protease of foot-and-mouth disease virus. J Virol. 1986 Jun;58(3):893–899. doi: 10.1128/jvi.58.3.893-899.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]