Abstract
Purpose
To compare the utility of different staging systems and analyzed independent predictors of survival in patients with hepatocellular carcinoma (HCC) treated with 90Y radioembolization.
Materials and Methods
428 HCC patients were treated with 90Y from 2004-2011. All patients were staged prospectively by Child-Turcotte-Pugh[CTP], United Network for Organ Sharing, Barcelona Clinic Liver Cancer [BCLC], Okuda classification, Cancer of the Liver Italian Program [CLIP], Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire, Chinese University Prognostic Index and the Japan Integrated System; their ability to predict survival was assessed. Staging systems were compared using cox-regression model, linear trend test, Akaike information criterion (AIC) and Concordance Index (C-index). Uni/Multivariate analyses were employed to assess independent predictors of survival.
Results
When tested independently, all staging systems provided significant ability to discriminate early (long survival) from advanced disease (worse survival). CLIP provided the most accurate information in predicting survival outcomes (AIC=2993, C-index=0.8503); CTP was least informative (AIC=3074, C-index=0.6445). Independent predictors of survival included ECOG 0 (HR:0.56, CI:0.34-0.93); non-infiltrative tumors (HR:0.62, CI:0.44-0.89); absence of portal venous thrombosis (HR:0.60, CI:0.40-0.89); absence of ascites (HR:0.56, CI:0.40-0.76); albumin ≥2.8 g/dL (HR:0.72, CI:0.55-0.94); alkaline phosphatase ≤200 U/L (HR:0.68, CI:0.50-0.92); and AFP ≤200 ng/mL (HR:0.67, CI:0.51-0.86).
Conclusion
CLIP was most accurate in predicting HCC survival. Given that not all patients receive the recommended BCLC treatment strategy, this information is relevant for clinical trial design and predicting long-term outcomes following 90Y.
Keywords: staging, hepatocellular carcinoma, radioembolization, chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is the 6th most common malignancy diagnosed worldwide. It is now the 3rd most common cause of cancer-related mortality. Long-term outcomes remain dismal.(1) Depending on tumor stage, patients may be offered surgical, locoregional or systemic therapeutic options. The occurrence of HCC in a cirrhotic background (influencing liver function, performance status, treatment efficacy) has led to the development of multiple staging systems. Currently, there continues to be debate on the single most appropriate and universally applicable HCC staging system.(2)
Yttrium-90 radioembolization (90Y) has assumed an important palliative role in the management of unresectable HCC by producing tumor necrosis and delaying progression.(3-7) While the utility of various staging systems in predicting prognosis of unresectable HCC patients after chemoembolization has been investigated, this has never been performed with radioembolization.(8, 9) Staging systems may exhibit different predictive power depending on the treatment applied. In a study including >2000 Taiwanese patients, the authors concluded that the applicability of HCC staging systems was dependent on treatment methods used.(10, 11) Incorporation of 90Y into Barcelona Clinic Liver Cancer (BCLC) has been suggested by single-center data.(5, 12, 13) 90Y has been widely used in the setting of portal vein thrombosis (PVT) and has generated encouraging outcomes. Physicians utilizing 90Y have trended towards utilizing 90Y in more diffuse/advanced disease, while reserving chemoembolization for earlier disease treatable by selective catheterization. Intuitively, the reality of this selection bias may translate into different predictive abilities of staging systems based on the therapeutic efficacy of 90Y. A recent comparative effectiveness study concluded that 90Y radioembolization leads to lower toxicity and longer time-to-progression (TTP) when compared with chemoembolization.(5) Evidence-based personalized medicine (specific treatment tailored to each patient) has mandated the need for therapy-specific studies assessing the predictive ability of staging systems. This approach enables treating physicians to identify the staging system best fit for the intervention, simplify survival prediction, and permit comparison to other therapies. Finally, since 90Y has yet to be incorporated into BCLC staging, analyzing the prognostic ability of staging systems for 90Y treated patients is of clinical relevance.
A staging system should demonstrate similar outcomes for the same stage (homogeneity), significant survival differences when comparing the stages of a system (discriminatory ability), and longer survival in earlier stages (monotonicity of gradients). Given this, a comprehensive analysis of the eight most widely used HCC staging systems was performed to investigate their prognostic utility in the setting of 90Y radioembolization (Cox-regression, linear trend test, Akaike information criterion [AIC] and Concordance Index [C-index]).(10, 14, 15) Baseline variables independently affecting survival were also analyzed.
Methods
Patient Cohort
428 patients with hepatocellular carcinoma underwent 728 treatments with 90Y radioembolization from January 2004-March 2011 in our center. These patients (and baseline variables) were captured from a prospectively collected database, and all were included in this statistical analysis; no patient was excluded. The study was Health Insurance Portability and Accountability Act compliant and approved by the Institutional Review Board.
Baseline Characteristics
Table 1 summarizes the baseline characteristics. Most patients were treatment naïve (89%), ≥65 years old (52%), male (73%), Caucasian (70%) and ECOG 0 (55%). The mean and median number of 90Y treatment(s)/patient was 1.7 and 1, respectively.
Table 1. Baseline Patient Characteristics.
| Total Patients N=428 | |||||
|---|---|---|---|---|---|
| Demographics | N (%) | Imaging characteristics | N (%) | ||
| Age (years) | <65 | 205 (48) | Ascites | Absent | 350 (82) |
| ≥65 | 223 (52) | Present | 78 (18) | ||
| Gender | Male | 312 (73) | Portal Hypertension | Present | 320 (75) |
| Female | 116 (27) | Absent | 108 (25) | ||
| Ethnic Group | Caucasian | 300 (70) | Infiltrative | Yes | 128 (30) |
| Asian | 41 (10) | No | 300 (70) | ||
| Hispanic | 29 (7) | Tumor Burden | 0-25% | 342 (80) | |
| African-American | 45 (10) | 26-50% | 66 (15) | ||
| Native-American | 2 (0) | >50% | 20 (5) | ||
| Unknown | 11 (3) | Lobar Distribution | Unilobar | 219 (51) | |
| Etiology | Alcohol | 76 (18) | Bilobar | 209 (49) | |
| Cryptogenic | 63 (15) | Tumor Focality | Solitary | 135 (31) | |
| HBV | 40 (9) | Multifocal | 293 (69) | ||
| HCV | 151 (35) | Largest Index Tumor Size (cm) | ≤5 cm | 194 (45) | |
| HCV + Alcohol | 26 (6) | 5.1-10 cm | 165 (39) | ||
| NASH | 18 (4) | >10 cm | 69 (16) | ||
| Primary Biliary Cirrhosis | 8 (2) | Portal Vein Thrombosis | Absent | 272 (64) | |
| Unknown | 26 (6) | Main | 84 (20) | ||
| Miscellaneous | 20 (5) | Lobar | 72 (16) | ||
| Method of Diagnosis | Biopsy | 201 (47) | Extrahepatic Disease | Absent | 377 (88) |
| Imaging | 227 (53) | Present | 51 (12) | ||
| Laboratory values | N (%) | Miscellaneous | N (%) | ||
| Alpha-fetoprotein (ng/mL) | ≤200 | 242 (57) | ECOG | 0 | 235 (55) |
| >200 | 186 (43) | 1 | 163 (38) | ||
| Bilirubin (mg/dL) | < 2 | 360 (84) | |||
| > 2 | 68 (16) | 2 | 30 (7) | ||
| Albumin (g/dL) | ≥ 2.8 | 230 (54) | |||
| < 2.8 | 198 (46) | Previous Liver Directed Therapy | No | 383 (89) | |
| Alkaline Phosphatase (U/L) | ≤ 200 | 349 (82) | |||
| >200 | 79 (18) | Yes | 45 (11) | ||
Patient Evaluation and Staging
All patients provided informed written consent. A history, physical examination, laboratory and imaging studies were obtained. Patients were imaged by magnetic resonance imaging (institutional standard) or computerized tomography (pacemaker, claustrophobia). Diagnostic criteria for HCC followed those defined by the American Association for the Study of Liver Diseases (AASLD) and the National Comprehensive Cancer Networks guidelines.(2, 16, 17) The criteria for treating patients with radioembolization included unresectable HCC as determined by surgery, Eastern Cooperative Oncology Group (ECOG) ≤2 and bilirubin <3.0 mg/dL (unless selective infusion possible).(13)
Radioembolization Treatment
One week before treatment, mesenteric angiography and macroaggregated albumin scans were performed to assess vascular anatomy and lung shunt fraction.(18) The device used was glass-based (Nordion, Canada); this device has regulatory approval for HCC with/without PVT (United States) and liver neoplasia (worldwide). All procedures were performed on an outpatient basis.(18, 19) In brief, target dose was 120 Gy on a lobar (or segmental basis) in HCC patients with bilirubin <3.0 mg/dl with or without portal vein thrombosis. Retreatment was considered when there was persistent enhancement or recurrence.
Patient Follow-up
Toxicity and response assessment were performed at 1 month and subsequently at 2-3 month intervals, with future treatment decisions also made at multidisciplinary conference.All patients were followed; patients alive at the time of data closure (August 15, 2011) were censored on the last date of follow-up. 295 patients received radioembolization as their only treatment (69%). Post radioembolization treatment(s) included (alone or in combination): transplantation (N=64, 15%), radiofrequency ablation (N=13, 3%), bland embolization (N=18, 4.2%), chemoembolization (N=38, 8.8%), Sorafenib (N=21, 5%) and clinical trial (N=16, 3.7%). For those patients transplanted, survival was censored on the date of transplantation. The median follow-up (censored to transplantation) was 23.2 months.
Statistical Analysis
Survival Analyses based on Tumor Stage
We sought to test the ability of various staging systems to prognosticate survival in 90Y treated patients. At presentation, patients were prospectively staged by Child-Turcotte-Pugh (CTP),(20) United Network for Organ Sharing (UNOS), Barcelona Clinic Liver Cancer (BCLC),(21) Okuda classification,(22) Cancer of the Liver Italian Program (CLIP),(23) Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire (GRETCH),(24) Chinese University Prognostic Index (CUPI),(25) and the Japan Integrated System score (JIS)(26). As per previously published data, patients were categorized as BCLC-C when ECOG>0 was due to cancer-related symptoms.
Survival across staging systems: Survival rates within each system were assessed by Kaplan-Meier method to analyze discriminatory ability between early and advanced stages.
Comparison of staging systems: All staging systems were tested for: 1) homogeneity (i.e. small differences in survival among patients within a similar stage across all staging systems) by Cox regression model(14, 27); 2) discriminatory ability (greater survival differences among patients in different stages within each system) by linear trend X2(14, 28); and 3) monotonicity of gradients (patients with earlier stages have longer survival than those in later stages within the same system), by both LR X2 and linear trend X2 with higher value indicating better prognostic ability in predicting survival outcomes. The Akaike information criterion (AIC) within the Cox regression model was used to adjust for the potential bias in comparing prognostic systems with different number of stages. (14, 29) A lower AIC indicates a more informative model for predicting survival.(14) Model comparisons were conducted by comparing the AIC values across staging systems to determine the relative probability of information loss between any two given staging systems. Finally, model validation of different staging systems was compared using C-index as a measure of discrimination.(30) C-index for the survival analysis model is defined as the probability of concordance given that the pairs considered are usable in which at least one had an event. It can be interpreted as the probability that a subject from the event group has a higher predicted probability of having an event than a subject from the non-event group. The C-index tests the ability of a predictive model to separate those who develop event from those who do not, with higher C-index indicating higher predictive discrimination.
The staging systems were compared using intention-to-treat (survival calculated from first treatment to death including all post 90Y treatments) and censored methodologies (survival calculated from first treatment to death but censored on date of curative transplantation), the latter only including the 364 patients who did not receive transplantation (minimizing the confounding effect of censoring to curative treatment).(10, 14)
Uni/Multivariate analyses
Uni/multivariate analyses were performed using the Cox proportional hazards model to capture the effects of different variables on survival. Uni/multivariate analyses were performed for all 428 patients using censored survival (transplantation). Composite variables were not included in uni/multivariate analyses. Hazard ratio estimates were based on simultaneous analysis of all variables. Type I error of multiple comparisons on univariate analyses were corrected using Bonferroni methodology.(31) Variables with a p-value ≤0.15 by univariate analysis (after Bonferroni correction) were included in the multivariate model. All analyses were performed using SAS 9.2; P<.05 was considered significant.
Results
Survival analyses
At the time of analysis, 302 patients had died. Median survival for the whole cohort was 10.6 months (95% confidence interval [CI]:9.0–15.2 months).
Survival within staging systems: Table 2 summarizes the Kaplan-Meier analysis where all systems were analyzed using censored survival. All could predict survival differences by disease stage and had a significant discriminatory ability (p<0.05). The Kaplan-Meier curves for overall survival are presented in Figures 1 and 2.
Comparison of staging systems: Table 3 summarizes the comparison of all staging systems.
Table 2. Survival across HCC staging systems.
| Staging System | Categories | Number of Patients (%) | Median Survival in Months | P-value (log rank) |
|---|---|---|---|---|
| Child-Pugh | A | 201 (47) | 15.7 | <0.0001 |
| B | 215 (50) | 7.7 | ||
| C | 12 (3) | 2.5 | ||
| BCLC | A | 98 (23) | 26.9 | <0.0001 |
| B | 122 (28) | 15.1 | ||
| C | 196 (46) | 7.4 | ||
| D | 12 (3) | 2.5 | ||
| UNOS | T1 | 5 (2) | - | <0.0001 |
| T2 | 99 (23) | 20.5 | ||
| T3 | 65 (15) | 19.9 | ||
| T4a | 82 (19) | 13.5 | ||
| T4b | 126 (29) | 6.5 | ||
| M | 51 (12) | 6 | ||
| CLIP Score | 0 | 44 (10) | 30.3 | <0.0001 |
| 1 | 113 (26) | 17.1 | ||
| 2 | 115 (27) | 14.1 | ||
| 3 | 79 (18) | 7.7 | ||
| 4 | 58 (14) | 3.6 | ||
| 5 | 17 (4) | 4.8 | ||
| 6 | 2 (1) | 2.4 | ||
| GRETCH | A | 95 (22) | 22.7 | <0.0001 |
| B | 239 (56) | 11.8 | ||
| C | 94 (22) | 4.5 | ||
| CUPI | Low Risk | 347 (81) | 14.1 | <0.0001 |
| Intermediate Risk | 72 (17) | 4.9 | ||
| High Risk | 9 (2) | 2.3 | ||
| JIS Score | 0 | 2 (1) | - | <0.0001 |
| 1 | 53 (12) | 26.9 | ||
| 2 | 85 (20) | 27.5 | ||
| 3 | 139 (32) | 11.2 | ||
| 4 | 140 (33) | 6.3 | ||
| 5 | 9 (2) | 2.5 | ||
| Okuda | 1 | 151 (35) | 17.7 | <0.0001 |
| 2 | 266 (62) | 8.7 | ||
| 3 | 11 (3) | 2.3 |
Abbreviations: BCLC: Barcelona Clinic Liver Cancer; CLIP: Cancer of the Liver Italian Program; CTP: Child-Turcotte- Pugh; CUPI: Chinese University Prognostic Index; GRETCH: Group d'Etude et de Traitement du Carcinome Hepatocellulaire; JIS: Japanese Integrated System; UNOS: United Network for Organ Sharing
Figure 1.
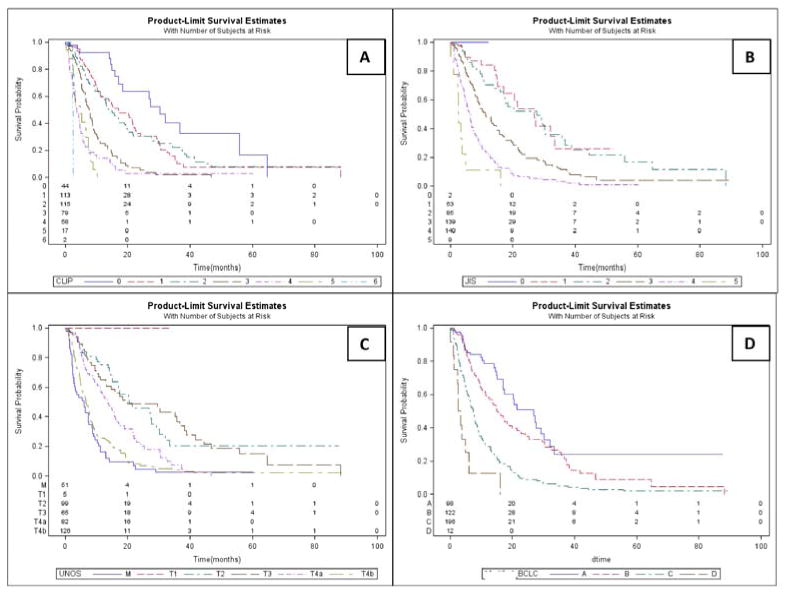
Kaplan Meier Survival Curves. a) Cancer of the Italian Liver Program, b) Japanese integrated System, c) United Network for Organ Sharing, d) Barcelona Clinic Liver Cancer.
Figure 2.
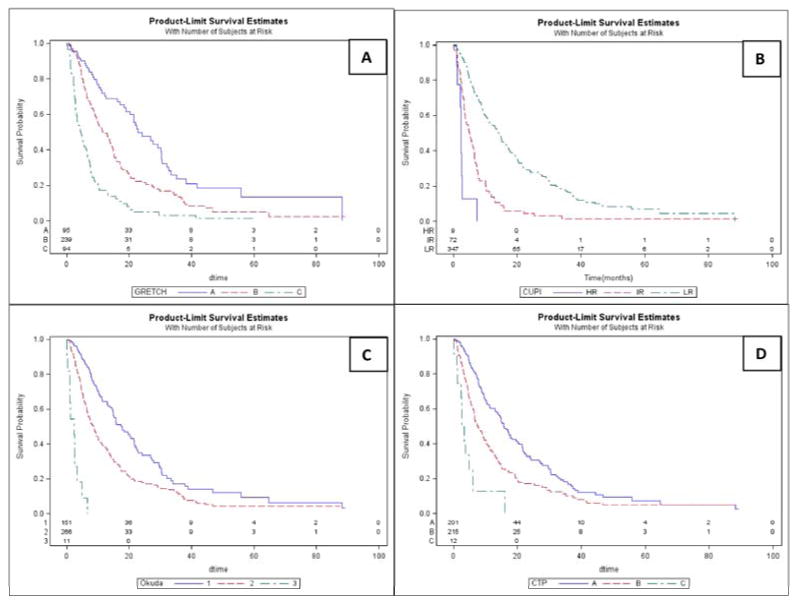
Kaplan Meier Survival Curves. a) Groupe D'Etude et du Traitement Carcinome Hepatocellulaire, b) Chinese University Prognostic Index, c) OKUDA, d) Child-Turcotte-Pugh.
Table 3. Comparison of HCC Staging Systems.
| Staging System | Discriminatory Ability Linear Trend X2 | Homogeneity Likelihood Ratio X2 Test | Akaike Information Criterion; P value of comparison of CLIP to Model | Discrimination for Model Validation Cindex |
|---|---|---|---|---|
| All patients censored to transplantation (N=428) | ||||
| CLIP | 118.18 | 127.22 | 2992.80; N/A | 0.8503 |
| JIS Score | 101.37 | 103.98 | 3014.04; <0.0001 | 0.8474 |
| UNOS | 93.14 | 94.61 | 3023.41; <0.0001 | 0.8393 |
| BCLC | 79.49 | 81.97 | 3032.05; <0.0001 | 0.7749 |
| GRETCH | 72.98 | 73.40 | 3038.61; <0.0001 | 0.7726 |
| CUPI | 56.20 | 64.45 | 3047.57; <0.0001 | 0.7037 |
| Okuda | 48.99 | 53.13 | 3058.89; <0.0001 | 0.6889 |
| CTP | 35.69 | 38.00 | 3074.02; <0.0001 | 0.6445 |
| All patients with intention to treat survival (N=428) | ||||
| CLIP | 154.91 | 164.43 | 3057.86; N/A | 0.8157 |
| UNOS | 136.97 | 156.90 | 3073.79; <0.0001 | 0.7885 |
| JIS Score | 130.20 | 143.95 | 3080.56; <0.0001 | 0.7822 |
| BCLC | 118.78 | 133.89 | 3087.99; <0.0001 | 0.7761 |
| GRETCH | 95.41 | 111.55 | 3109.36; <0.0001 | 0.6927 |
| CUPI | 71.06 | 71.92 | 3133.70; <0.0001 | 0.6885 |
| Okuda | 49.45 | 50.21 | 3155.31; <0.0001 | 0.6314 |
| CTP | 34.32 | 35.33 | 3170.45; <0.0001 | 0.5287 |
| Patients who did not receive transplantation (N=364) | ||||
| CLIP | 108.36 | 114.83 | 2793.34; N/A | 0.8519 |
| JIS Score | 92.88 | 94.34 | 2811.83; <0.0001 | 0.8328 |
| UNOS | 80.40 | 81.58 | 2824.58; <0.0001 | 0.8256 |
| BCLC | 70.12 | 70.57 | 2831.59; <0.0001 | 0.7586 |
| GRETCH | 67.14 | 68.03 | 2832.14; <0.0001 | 0.7545 |
| CUPI | 52.01 | 58.40 | 2841.76; <0.0001 | 0.7057 |
| Okuda | 47.90 | 50.44 | 2849.73; <0.0001 | 0.6887 |
| CTP | 35.04 | 36.18 | 2863.99; <0.0001 | 0.6610 |
Abbreviations: BCLC: Barcelona Clinic Liver Cancer; CLIP: Cancer of the Liver Italian Program; CTP: Child-Turcotte- Pugh; CUPI: Chinese University Prognostic Index; GRETCH: Group d'Etude et de Traitement du Carcinome Hepatocellulaire; JIS: Japanese Integrated System; UNOS: United Network for Organ Sharing
All patients with censored survival (N=428): CLIP exhibited the highest discriminatory ability (Linear Trend X2=118.2) and homogeneity (LR X2=127.2) indicating highest ability to discriminate early from advanced disease, as well as small survival differences when compared to corresponding stages of other systems. AIC value was also the lowest for CLIP (2993), indicating that the model was the most informative in explaining the patient survival. C-index was highest for CLIP (0.8503) indicating higher predictive discrimination for this system. CLIP was followed by JIS score (AIC=3014, C-index=0.8474)) and UNOS (AIC=3023, C-index=0.8393). Of note, CTP classification yielded the lowest discriminatory ability and homogeneity, with the lowest predictive ability for this specific population.
All patients with intention-to-treat survival (N=428): CLIP continued to demonstrate the best predictive capability, with highest discriminatory ability, homogeneity, lowest AIC and highest C-index.
Non-transplanted patients (N=364): CLIP outperformed all other systems by predictive and discriminatory ability, homogeneity, AIC and C-index.
In all three analyses, CLIP exhibited significantly lower AIC (p<.0001) than other staging systems, suggesting that CLIP was a superior model in predicting survival. The rank order of predictive ability of a specific staging system was consistent across all four statistical methods used in the analyses (i.e. Cox-regression, linear trend test, AIC and C-index)
Uni/Multivariate analyses
Univariate analyses demonstrated age ≥65, ECOG 0/1, lack of symptomatic disease, tumor burden ≤25%, tumor size <10 cm, unilobar/solitary tumors, absence of extrahepatic metastases, non-infiltrative disease, absent/branch PVT, no ascites, albumin ≥2.8 g/dL, bilirubin <2 mg/dL, alkaline phosphatase ≤200 U/L and alpha-fetoprotein (AFP) <200 ng/mL as predictors of better survival outcomes. On multivariate analyses, ECOG 0, non-infiltrative tumors, absence of PVT or ascites, albumin ≥2.8 g/dL, alkaline phosphatase ≤200 U/L, and AFP ≤200 ng/mL predicted better outcomes (Table 4).
Table 4. Uni/Multivariate analyses.
| UNIVARIATE (Kaplan Meier and Logrank Test) | MULTIVARIATE (Cox Proportional Hazards Model)** | |||||
|---|---|---|---|---|---|---|
| Predictor | Category | Hazard Ratio (CI) | P-Value | Adjusted P Value* | Hazard Ratio (CI) | P-value |
| Age | ≥65 | 0.73 (0.58 – 0.92) | 0.0089 | 0.15 | 0.85 (0.67 – 1.09) | 0.208 |
| <65 | 1.00 | 1.00 | ||||
| Gender | F | 0.89 (0.68 – 1.16) | 0.39 | |||
| M | 1.00 | |||||
| ECOG | 0 | 0.23 (0.15 – 0.34) | <0.0001 | 0.0017 | 0.56 (0.34 – 0.93) | 0.027 |
| 1 | 0.45 (0.30 – 0.68) | 0.0002 | 0.0034 | 0.72 (0.46 – 1.51) | 0.174 | |
| 2 | 1.00 | 1.00 | ||||
| Symptomatic Disease | No | 0.61 (0.47 – 0.80) | 0.0004 | 0.0068 | 1.10 (0.80 – 1.51) | 0.552 |
| Yes | 1.00 | 1.00 | ||||
| Tumor Burden | >0-25% | 0.35 (0.22 – 0.56) | <0.0001 | 0.0017 | 0.68 (0.38 – 1.22) | 0.198 |
| 26-50% | 0.75 (0.45 – 1.25) | 0.277 | 0.79 (0.46 – 1.36) | 0.409 | ||
| >50% | 1.00 | 1.00 | ||||
| Tumor Distribution | Unilobar | 0.56 (0.44 – 0.71) | <0.0001 | 0.0017 | 0.82 (0.60 – 1.11) | 0.202 |
| Bilobar | 1.00 | 1.00 | ||||
| Tumor Size | ≤5 cm | 0.43 (0.32 – 0.60) | <0.0001 | 0.0017 | 0.94 (0.60 – 1.47) | 0.797 |
| >5-10 cm | 0.72 (0.53 – 0.97) | 0.035 | 0.59 | 1.06 (0.72 – 1.55) | 0.754 | |
| >10 cm | 1.00 | 1.00 | ||||
| Number of Lesions | Solitary | 0.48 (0.36 – 0.62) | <0.0001 | 0.0017 | 0.77 (0.54 – 1.10) | 0.162 |
| Multifocal | 1.00 | 1.00 | ||||
| Extrahepatic Metastases | Absent | 0.41 (0.30 – 0.56) | <0.001 | 0.0017 | 0.74 (0.52 – 1.06) | 0.101 |
| Present | 1.00 | 1.00 | ||||
| Infiltrative Tumor | No | 0.29 (0.22 – 0.37) | <0.0001 | 0.0017 | 0.62 (0.44 – 0.89) | 0.008 |
| Yes | 1.00 | 1.00 | ||||
| Portal Venous Thrombosis | Absent | 0.25 (0.19 – 0.33) | <0.0001 | 0.0017 | 0.60 (0.40 – 0.89) | 0.012 |
| Branch | 0.46 (0.33 – 0.65) | <0.0001 | 0.0017 | 0.76 (0.51 – 1.13) | 0.178 | |
| Main | 1.00 | 1.00 | ||||
| Ascites | Absent | 0.35 (0.26 – 46) | <0.0001 | 0.0017 | 0.56 (0.40 – 0.76) | 0.0003 |
| Present | 1.00 | 1.00 | ||||
| Portal Hypertension | Absent | 0.81 (0.63 – 1.05) | 0.120 | 0.78 (0.57 – 1.05) | 0.101 | |
| Present | 1.00 | 1.00 | ||||
| Albumin (g/dL) | ≥ 2.8 | 0.54 (0.43 – 0.69) | <0.0001 | 0.0017 | 0.72 (0.55 – 0.94) | 0.016 |
| < 2.8 | 1.00 | 1.00 | ||||
| Bilirubin (mg/dL) | < 2 | 0.76 (0.55 – 1.05) | 0.1004 | 1.12 (0.78 – 1.61) | 0.512 | |
| ≥ 2 | 1.00 | 1.00 | ||||
| Alkaline Phosphatase (U/L) | ≤ 200 | 0.43 (0.33 – 0.57) | <0.0001 | 0.0017 | 0.68 (0.50 – 0.92) | 0.013 |
| >200 | 1.00 | 1.00 | ||||
| AFP (ng/mL) | ≤200 | 0.54 (0.42 – 0.68) | <0.0001 | 0.0017 | 0.67 (0.51 – 0.86) | 0.002 |
| >200 | 1.00 | 1.00 | ||||
Abbreviations: AFP: Alpha-fetoprotein; CI, confidence interval; ECOG: Eastern Cooperative Oncology Group; HR, hazard ratio
Adjusted for multiple comparisons using Bonferroni methodology (correction factor n=17)
Factors were included in multivariate analysis if P < 0.15 in univariate analysis (unadjusted for multiple comparisons).
Discussion
Unlike other solid organ malignancies where tumor characteristics, burden and biology play a critical role in predicting survival, HCC is different since survival is also affected by underlying cirrhosis.(32) HCC staging systems therefore account for tumor burden, performance status, liver function and in one (BCLC), efficacy of the treatment. Currently, there is no international consensus on the most appropriate HCC staging system; BCLC appears to be the leading contender.(2) Although CTP classification system is not strictly an HCC staging system, it is widely used when considering therapeutic options in HCC. On the other hand, UNOS does not consider liver function and relies solely on tumor characteristics.
BCLC is a classification system categorizing patients in four stages by tumor characteristics, ECOG and CTP class.(21) It is one of the most commonly used systems and has been endorsed by the AASLD.(14, 16, 33) A major strength of BCLC staging system is the simple association of a stage with a recommended treatment strategy. A recent study however, outlined the frequency with which the suggested treatment by BCLC could not implemented (35-45%).(34) Further clarification/substratification of BCLC stages may be necessary in the future; this is supported in part by the recent reports of 48-month median survival in hyperselected BCLC B patients.(35) Despite the imperfect treatment allocation process in BCLC staging, the clear depiction of burden, liver function, tumor size and performance status permits excellent description (compared with other systems) of overall HCC status.
In this study, all eight staging systems could significantly discriminate 90Y patients with early (long survival) and late stage disease (worse survival). However, the CLIP score appeared as best fit in prognosticating survival outcomes. CLIP was developed in a 435 patient multicenter study, (23) combining CTP and tumor characteristics (size, PVT, AFP). It is based on a simple scoring system providing 7 categories (0-6) of patients at baseline. CLIP has been validated in Japanese, Canadian, Italian and American cohorts.(15, 36-38) CLIP was also endorsed by a consensus conference on staging of HCC.(39) Our observations were maintained after excluding patients who received curative transplantation (highlighting outcomes with 90Y as primary therapy), as well as by intention-to-treat. However, UNOS outperformed JIS by intention-to-treat, potentially as a result of T1-T3 being transplanted and further diverging survival curves from T4a-N/M. Another possible reason for the superiority of these three systems may be the higher number of stage levels within each system (CLIP:7, JIS:6, UNOS:6); hence, they may better dissect patients into several small homogenous groups rather than few, large heterogeneous groups.
Results of studies comparing HCC systems have varied greatly. In a review article, investigators reported that European/American studies found BCLC/CLIP superior, while Eastern studies reported on the superiority of JIS/TNM/CLIP.(10) Comparison of these systems is challenging given differences in etiology/geography and treatment strategies. Recently, CLIP has been advocated for advanced HCC patients receiving chemotherapy or best supportive care.(10, 15) In contradistinction, CTP classification was found to be the most accurate system in patients treated with chemoembolization.(8) In this study, CLIP exhibited the best predictive ability while CTP had the least; this may be due to selection bias of more advanced disease (e.g. PVT, diffuse disease) for 90Y. Uni/multivariate analyses confirmed the rationale for CLIP, with 3 of 7 variables that independently predicted better survival involved tumor characteristics (non-infiltrative lesions, AFP≤200 ng/mL, absence of PVT). In this study, tumor size/number/burden and extrahepatic metastases were not independent predictors of survival; this may be related to the relatively advanced patient population with tumor burden (rather than metastases) being the predominant cause of death.
There are several approaches to the interpretation and practical application of staging systems. One approach is to devise a system that allocates treatment based on the highest level of available evidence (BCLC). Although useful for clinical trial design, it is not surprising that 4 disparate conditions with very different natural histories (BCLC A, B, C, D) would result in a low AIC, since it is itself designed to highlight these differences. The limitation is that these suggested options may not be available/applicable (e.g. transplantation) or patients may not be candidates for the allocated treatment, limiting the universal applicability in clinical practice.(34) Another approach is to study a staging system and investigate survival associated with each score within a stage for a given treatment.(11) For example, while it is recognized that HCC patients should ideally be treated in specialized centers where all treatments are available, this is not always feasible. At the local center, chemoembolization may be the only option available (no transplantation, ablation, 90Y). Knowledge of expected outcomes in that center treated with that one available modality (chemoembolization) is relevant, permitting a discussion of long-term outcomes with patients. Alternatively, in some cancer centers, bed unavailability has resulted in limited use of chemoembolization (and other inpatient procedures), given the need for 1 or more days of hospitalization. Hence, these centers have adopted outpatient radioembolization. The reality is that not all small lesions are ablatable, many patients within Milan criteria will never receive transplantation, and most solitary HCCs will not undergo resection. Hence, staging systems best suited to predict outcomes for a specific treatment should be investigated. The versatility of 90Y is highlighted in these situations, as the applicability of this new treatment has been described in small segmental lesions, multifocal disease and portal vein invasion.(5). This study suggests the addition of CLIP to reporting standards for 90Y and an integration of this system in clinical practice when treating patients with 90Y.(19)
This introduces the concept of using staging systems in a dynamic fashion. The BCLC framework is extremely useful as it permits the clear categorization of HCC by size/liver function/performance status with optimal treatment strategies and expected survival outcomes (useful for clinical trial design). From there, if the allocated treatment is not available/feasible in that treatment center, or the therapy is not included in the guidelines (e.g. 90Y, external beam radiotherapy), the staging system most correlated with the treatment being offered may be quite beneficial. This study suggests that once we have framed patient status by BCLC, if they are not candidates for the suggested treatment and 90Y has been selected, rather than inform a patient of 16-22 months expected survival (e.g. BCLC B), a more accurate prediction may be available through CLIP. Similarly, Taiwanese investigators may structure initial patient assessment by BCLC, but then use Taiwanese score.(10) Other investigators have also reported on the combined use of CLIP in BCLC C patients.(15) Hence, there is clinical rationale for the use of BCLC plus a system optimally suited for a specific therapy; examples of this include UNOS for transplantation and TNM for resection.
There are strengths to this study. First, this was a prospectively followed 90Y cohort, analyzed with thorough 4 robust statistical tools (likelihood ratio, AIC, C-index, linear trend), all 4 yielding the same conclusion. Second, the population reflected diverse baseline characteristics (infiltrative disease, PVT, ECOG 1-2, metastases, tumor size) resulting in sufficient patient numbers at each level within staging systems. Third, minor technical improvements were made as the science behind this approach evolved (ex: segmentectomy). Finally, this analysis is of clinical relevance and may be used by clinicians and investigators when estimating expected survival of 90Y patients by CLIP (given its absence in BCLC).
are limitations. First, there are inherent limitations to the clinical value of prediction models evaluating staging systems. Second, although CLIP was mathematically superior to BCLC by discriminatory, homogeneity, AIC criteria and C-index, the practical application should be kept in perspective. There was a 13.2-month survival difference between CLIP 0 and 1, followed by 3 and 6.4 months for CLIP 2 and 3, respectively. Hence, the differences in survival by CLIP stages were not evenly distributed. Third, CLIP generates higher stage levels with smaller differences in survival between levels; other systems have fewer levels with wider survival differences, resulting in more intra-stage variability; this may only be applicable to a similar Western patient population. Despite the recognized intragroup heterogeneity of survival outcomes within BCLC stages (recent reports of 48-month median survival in BCLC A/B), individual stages clearly depict imaging/functional status of the patient; CLIP cannot provide this valuable information.(35) This construct has permitted clinical trial design and the definition of specific populations to be studied with various therapies. While the therapeutic recommendations made by BCLC staging remain points of debate and discussion, the descriptive strength of tumor/liver/functional status of each BCLC stage remains unparalleled.
In conclusion, all staging systems exhibited significant ability to discriminate survival across different stages within a system. CLIP demonstrated the highest ability to prognosticate survival in 90Y treated patients. Although the seminal role of BCLC is recognized with treatment allocation, 90Y remains absent. Hence, the importance of assessing prognosis using a staging system in patients not receiving the recommended modality by BCLC is of clinical interest. For 90Y, CLIP appears to accomplish this goal.
Acknowledgments
Role of Funding: RS is supported in part by NIH grant CA126809.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- AFP
Alpha-fetoprotein
- AIC
Akaike information criterion
- BCLC
Barcelona Clinic Liver Cancer
- C-index
Concordance Index
- CLIP
Cancer of the Liver Italian Program
- CTP
Child-Turcotte-Pugh
- CUPI
Chinese University Prognostic Index
- ECOG
Eastern Cooperative Oncology Group
- GRETCH
Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire
- HCC
Hepatocellular Carcinoma
- JIS
Japan Integrated System score
- 90Y
Yttrium-90 radioembolization
- PVT
Portal vein thrombosis
- TNM
Tumor node metastases
- TTP
time-to-progression
- UNOS
United Network for Organ Sharing
Footnotes
Conflict of Interest: None of the other authors have any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.El-Serag HB. Hepatocellular carcinoma. The New England journal of medicine. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. Epub 2011/10/14. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium(90) radioembolization for intermediate-advanced hepatocarcinoma: A phase II study. Hepatology. 2012 doi: 10.1002/hep.26014. Epub 2012/08/23. [DOI] [PubMed] [Google Scholar]
- 4.Salem R, Mazzaferro V, Sangro B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: Biological lessons, current challenges and clinical perspectives. Hepatology. 2013 doi: 10.1002/hep.26382. Epub 2013/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization Results in Longer Time-to-Progression and Reduced Toxicity Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2011;140:497–507 e492. doi: 10.1053/j.gastro.2010.10.049. Epub 2010/11/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: A European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 7.Memon K, Kulik L, Lewandowski RJ, et al. Radioembolization for hepatocellular carcinoma with portal vein thrombosis: Impact of liver function on systemic treatment options at disease progression. J Hepatol. 2013;58:73–80. doi: 10.1016/j.jhep.2012.09.003. Epub 2012/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgiades CS, Liapi E, Frangakis C, et al. Prognostic accuracy of 12 liver staging systems in patients with unresectable hepatocellular carcinoma treated with transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:1619–1624. doi: 10.1097/01.RVI.0000236608.91960.34. Epub 2006/10/24. [DOI] [PubMed] [Google Scholar]
- 9.Brown DB, Fundakowski CE, Lisker-Melman M, et al. Comparison of MELD and Child-Pugh scores to predict survival after chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2004;15:1209–1218. doi: 10.1097/01.RVI.0000128123.04554.C1. Epub 2004/11/05. [DOI] [PubMed] [Google Scholar]
- 10.Chen CH, Hu FC, Huang GT, et al. Applicability of staging systems for patients with hepatocellular carcinoma is dependent on treatment method – Analysis of 2010 Taiwanese patients. European Journal of Cancer. 2009;45:1630–1639. doi: 10.1016/j.ejca.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Trinchet JC, Beaugrand M. Is there an ideal prognostic classification for hepatocellular carcinoma? The quest for the Holy Grail. Journal of Hepatology. 2010;53:23–24. doi: 10.1016/j.jhep.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–1749. doi: 10.1002/hep.23944. Epub 2010/11/03. [DOI] [PubMed] [Google Scholar]
- 13.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. Epub 2009/09/22. [DOI] [PubMed] [Google Scholar]
- 14.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–716. doi: 10.1002/hep.20636. Epub 2005/03/30. [DOI] [PubMed] [Google Scholar]
- 15.Huitzil-Melendez FD, Capanu M, O'Reilly EM, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:2889–2895. doi: 10.1200/JCO.2009.25.9895. Epub 2010/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. Journal of the National Cancer Institute. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 18.Salem R, Thurston KG. Radioembolization with 90Yttrium Microspheres: A State-of-the-Art Brachytherapy Treatment for Primary and Secondary Liver Malignancies: Part 1: Technical and Methodologic Considerations. J Vasc Interv Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 19.Salem R, Lewandowski RJ, Gates VL, et al. Research reporting standards for radioembolization of hepatic malignancies. J Vasc Interv Radiol. 2011;22:265–278. doi: 10.1016/j.jvir.2010.10.029. Epub 2011/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 21.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Seminars in liver disease. 1999;19:329–338. doi: 10.1055/s-2007-1007122. Epub 1999/10/13. [DOI] [PubMed] [Google Scholar]
- 22.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 24.Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire. J Hepatol. 1999;31:133–141. doi: 10.1016/s0168-8278(99)80173-1. Epub 1999/07/29. [DOI] [PubMed] [Google Scholar]
- 25.Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. Epub 2002/03/29. [DOI] [PubMed] [Google Scholar]
- 26.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. Epub 2003/04/04. [DOI] [PubMed] [Google Scholar]
- 27.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;16:965–980. doi: 10.1002/(sici)1097-0258(19970515)16:9<965::aid-sim509>3.0.co;2-o. Epub 1997/05/15. [DOI] [PubMed] [Google Scholar]
- 28.Feinstein AR. Clinical biostatistics. XVI. The process of prognostic stratification. 2. Clin Pharmacol Ther. 1972;13:609–624. doi: 10.1002/cpt1972134609. Epub 1972/07/01. [DOI] [PubMed] [Google Scholar]
- 29.Forster MR. Key Concepts in Model Selection: Performance and Generalizability. J Math Psychol. 2000;44:205–231. doi: 10.1006/jmps.1999.1284. Epub 2000/03/29. [DOI] [PubMed] [Google Scholar]
- 30.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Statistics in Medicine. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 31.Cleophas TJ, Zwinderman AH. Clinical trials are often false positive: a review of simple methods to control this problem. Curr Clin Pharmacol. 2006;1:1–4. doi: 10.2174/157488406775268228. [DOI] [PubMed] [Google Scholar]
- 32.Ghassan KAA. Hepatocellular Carcinoma: Molecular Biology and Therapy. Seminars in Oncology. 2006;33(11):79–83. doi: 10.1053/j.seminoncol.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723–731. doi: 10.1016/j.jhep.2005.12.015. Epub 2006/02/21. [DOI] [PubMed] [Google Scholar]
- 34.D'Avola D, Iñarrairaegui M, Pardo F, et al. Prognosis of Hepatocellular Carcinoma in Relation to Treatment Across BCLC Stages. Annals of Surgical Oncology. 2011;18:1964–1971. doi: 10.1245/s10434-011-1551-4. [DOI] [PubMed] [Google Scholar]
- 35.Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–1335. doi: 10.1016/j.jhep.2012.01.008. Epub 2012/02/09. [DOI] [PubMed] [Google Scholar]
- 36.Ueno S, Tanabe G, Sako K, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001;34:529–534. doi: 10.1053/jhep.2001.27219. Epub 2001/08/30. [DOI] [PubMed] [Google Scholar]
- 37.Levy I, Sherman M. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002;50:881–885. doi: 10.1136/gut.50.6.881. Epub 2002/05/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farinati F, Rinaldi M, Gianni S, Naccarato R. How should patients with hepatocellular carcinoma be staged? Validation of a new prognostic system. Cancer. 2000;89:2266–2273. Epub 2001/01/09. [PubMed] [Google Scholar]
- 39.Henderson JM, Sherman M, Tavill A, Abecassis M, Chejfec G, Gramlich T. AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: consensus statement. HPB (Oxford) 2003;5:243–250. doi: 10.1080/13651820310015833. Epub 2008/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]


