Abstract
Limited information exists on the predonation costs incurred by eventual living kidney donors (LKDs). Expenses related to completion of the donation evaluation were collected from 194 LKDs participating in the multi-center, prospective Kidney Donor Outcomes Cohort (KDOC) Study. Most LKDs (n = 187, 96%) reported one or more direct costs, including ground transportation (80%), healthcare (24%), lodging (17%) and air transportation (14%), totaling $101 484 (USD; mean = $523 ± 942). Excluding paid vacation or sick leave, donor and companion lost wages totaled $35 918 (mean = $187 ± 556) and $14 378 (mean = $76 ± 311), respectively. One-third of LKDs used paid vacation or sick leave to avoid incurring lost wages. Few LKDs reported receiving financial support from the transplant candidate (6%), transplant candidate’s family (3%), a nonprofit organization (3%), the National Living Donor Assistance Center (7%), or transplant center (3%). Higher total costs were significantly associated with longer distance traveled to the transplant center (p < 0.001); however, total costs were not associated with age, sex, race/ethnicity, household income, marital status, insurance status, or transplant center. Moderate predonation direct and indirect costs are common for adults who complete the donation evaluation. Potential LKDs should be advised of these possible costs, and the transplant community should examine additional strategies to reimburse donors for them.
Introduction
Live donor kidney transplantation (LDKT) is the optimal treatment for adults with advanced-stage kidney disease, yielding longer patient and graft survival, better quality of life, and lower costs compared to dialysis and deceased donor transplantation (1–3). Some transplant candidates and potential living kidney donors (LKDs) have expressed concern about the financial impact of living donation (4–6). Financial concerns may dissuade transplant candidates from discussing possible living donation with others and may be a disincentive for otherwise willing potential donors.
Despite programs and regulations in the United States that are intended to minimize donation-related expenses (7,8), LKDs may incur costs for transportation, lodging, medical and medication expenses, lost wages and other incidentals (4,9). For some LKDs, these costs are substantial, which have heightened calls for a more comprehensive LKD reimbursement program in the United States, similar to what exists in other countries (10–13). While data are emerging about postdonation expenses, little is known about the costs potential LKDs incur before donation. These front-end expenses are important to study because they are not well characterized and they may represent an immediate economic deterrent for some potential LKDs.
In the United States, the Centers for Medicare and Medicaid Services (CMS) reimburses transplant centers for certain costs that are necessary for evaluating a potential LKD. However, reimbursement does not include expenses for transportation, lodging, parking, meals, lost wages, and medical procedures that may be necessary to complete the evaluation but that are considered part of routine health maintenance (e.g. colonoscopy, mammogram). Potential LKDs may apply for economic assistance from the National Living Donor Assistance Center (NLDAC), which provides travel and lodging reimbursement when both the LKD and intended recipient meet financial eligibility criteria (8). More data about LKD expenses, both before and after donation, are needed to inform the dialogue about reimbursement programs in the United States. Additionally, clearer understanding of these expenses will help transplant programs better inform transplant candidates and potential LKDs about these economic issues.
In this study, we pursued three aims: (1) to identify the direct and indirect costs incurred by LKDs during the predonation evaluation period; (2) to characterize the types of financial assistance received by LKDs for predonation costs; and (3) to determine whether predonation costs incurred by LKDs are associated with sociodemographic characteristics.
Methods
Economic data were collected from LKDs participating in the Kidney Donor Outcomes Cohort (KDOC) study, which is a prospective multi-center study funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to evaluate surgical, medical, psychosocial and cost outcomes in LKDs. Participating transplant centers included: Beth Israel Deaconess Medical Center, Boston, MA; Maine Medical Center, Portland, ME; Montefiore Medical Center, Bronx, NY; Rhode Island Hospital, Providence, RI; University of Arizona, Tucson, AZ; and University of Iowa, Iowa City, IA. All centers performed between 20 and 36 LDKTs annually in the 3 years prior to study enrollment. The 37% LDKT rate across KDOC centers is similar to the 36% LDKTs rate in the United States during this same time period. Across the centers, approximately 25% of LDKTs are preemptive and all centers participate in paired exchange programs.
KDOC participants include 194 LKDs and their kidney recipients who were recruited and consented after approval for donation surgery between September 2011 and November 2013. Enrolled LKDs complete comprehensive assessments predonation and at 1, 6, 12, and 24 months postdonation. Inclusion criteria include age ≥18 years, English or Spanish language and eligibility for donation. This sub-study examines economic data from the entire donor cohort at time of predonation assessment. The KDOC study received approval from institutional review boards at all six transplant centers.
Based on a literature review and discussions with transplant professionals (i.e. nephrologists, surgeons, social workers, and coordinators), we identified categories of potential costs and, through an iterative process, classified these as either direct or indirect costs. Direct costs were those expenses that included out-of-pocket cash or credit card payments for travel, lodging, meals, healthcare appointments required by the donor evaluation team, medications, and other incidental expenses. Indirect costs included lost wages for the potential LKD and one companion (e.g. spouse/partner) participating in donation decision-making and/or recovery planning, and costs for dependent (e.g. child, elderly) care assistance while at the transplant center or undergoing medical testing.
LKDs completed a survey with these itemized expenses electronically or via mail prior to surgery to assess predonation costs and again at all assessment time points after surgery to measure postdonation costs. Our analysis here focuses only on the predonation assessment. From the list of possible expenses, LKDs indicated whether they had the expense and, if so, the total cost of the expense. The form allowed the LKD to identify other expenses that were not already itemized and to report costs associated with them as well. Except for personal car use, travel expenses include actual costs as reported by the LKD. For personal car use, we multiplied the number of visits to the transplant center by total roundtrip miles traveled by the federal mileage rate during the study enrollment period ($0.555). Additionally, LKDs reported time away from work, if employed, whether it was paid/unpaid, use of vacation/sick leave, and hourly wage rate. Similar information was gathered for a companion accompanying the LKD for the evaluation. Finally, we asked LKDs whether they received financial assistance for expenses and, if so, from what sources. Follow-up telephone calls and/or emails were made by research assistants for any missing data or to clarify survey responses.
Descriptive data are summarized as means and standard deviations as well as medians and ranges for itemized and total costs. Spearman’s rho was used to assess the relationship between LKD total costs and age, household income, and distance to transplant center. T tests were calculated to examine the relationship between LKD total costs and sex, race (minority, nonminority), work status (employed, not employed), and health insurance status (insured, uninsured). Analysis of variance was used to assess the relationship between LKD total costs and transplant center. All costs are expressed in US dollars (USD). Finally, we assessed financial burden, which we calculated as LKD total costs divided by monthly household income, and examined its association with demographic variables using the same statistical tests as noted above.
Results
Study enrollment rate was 84% (194 study donors/230 eligible donors during enrollment period). Participation rates did not differ significantly by study site. The primary reason for not enrolling LKDs was failure to approach them in clinic. Very few LKDs (n = 11, 5%) refused to take part in the study when informed about it. Participants completed the predonation survey an average of 7 days (SD = 10.8; median = 4) prior to surgery. At time of study enrollment, KDOC LKDs (N = 194) had an average age of 42.7 (±11.8) years, 63% were women, and 76% were white, nonHispanic (Table 1). Most were employed, had health insurance, had household income ≤$75 000, and were biologically related to the intended recipient. Median distance from home to the transplant center was 41.5 miles (25th percentile = 12 miles, 75th percentile = 154 miles). A higher percentage of KDOC LKDs graduated from college compared to the U.S. LKD population during the study enrollment period (p = 0.01); however, there was no significant difference (all p values >0.05) in age, sex, race (white, nonwhite), marital status (married/partnered, not married/partnered), employment, health insurance, or relationship type (biological, nonbiological) (Table 1). The US median household income during study enrollment was $51 300 (14).
Table 1.
Characteristics of KDOC cohort and living donors in the United States
| Variable | KDOC cohort (N = 194) | US donors (N = 12 799)1 |
|---|---|---|
| Age, years, mean (SD) | 42.7 (11.8) | |
| Age, years | ||
| 18–34 | 55 (28%) | 3774 (29%) |
| 35–49 | 76 (39%) | 5323 (42%) |
| 50–64 | 63 (33%) | 3435 (27%) |
| ≥65 | 0 (0%) | 267 (2%) |
| Sex, female | 123 (63%) | 7926 (62%) |
| Race | ||
| White, nonHispanic | 148 (76%) | 8964 (70%) |
| Hispanic | 17 (9%) | 1775 (14%) |
| Black | 12 (6%) | 1409 (11%) |
| Other | 6 (3%) | 552 (4%) |
| More than one race | 11 (6%) | 99 (1%) |
| Education, college degree | 98 (51%) | 5210 (41%) |
| Marital status, married/partnered | 97 (50%) | 6783 (53%) |
| Employment status, working | 152 (80%) | 10 386 (81%) |
| Health insurance, insured | 172 (89%) | 10 977 (86%) |
| Household income | ||
| <$25 000 | 29 (15%) | |
| $25 000 to $49 999 | 41 (21%) | |
| $50 000 to $74 999 | 34 (18%) | |
| $75 000 to $99 999 | 26 (13%) | |
| ≥ $100 000 | 52 (27%) | |
| Unknown | 12 (6%) | |
| Relationship to recipient | ||
| Parent | 12 (6%) | 1280 (10%) |
| Child | 35 (18%) | 2176 (17%) |
| Sibling | 34 (18%) | 2944 (23%) |
| Other relative | 29 (15%) | 1024 (8%) |
| Spouse | 32 (17%) | 1792 (14%) |
| Friend/acquaintance | 40 (21%) | 3199 (25%) |
| Nondirected stranger | 12 (6%) | 384 (3%) |
| Transplant center | ||
| Beth Israel Deaconess Medical Center (MA) | 56 (29%) | |
| Maine Medical Center (ME) | 32 (17%) | |
| Montefiore Medical Center (NY) | 26 (13%) | |
| Rhode Island Hospital (RI) | 24 (12%) | |
| University of Arizona (AZ) | 28 (14%) | |
| University of Iowa (IA) | 28 (14%) | |
| Resides within same state as transplant center | 127 (66%) | |
Living kidney donors in the United States, September 2011 and November 2013. Data obtained from http://optn.transplant.hrsa.gov. Empty cells reflect data that are not available for the population of US donors. KDOC, Kidney Donor Outcomes Cohort.
Correction made after online publication May 5, 2015: “Health insurance, insured” row has been updated in Table 1.
Tables 2 and 3 summarize direct and indirect costs incurred by LKDs and are presented in a format similar to Klarenbach et al (15) to facilitate comparison. Most (n = 187, 96%) reported at least one direct expense type: 38% reported only 1 expense type, 31% reported 2, 20% reported 3 and 7% reported 4–5. No LKD reported out-of-pocket expenses in all six categories. While ground transportation was the most common expense type (80%), the highest average cost, both for those LKDs who incurred the cost and for all LKDs combined, was for air transportation ($975 and $183, respectively). For those who incurred the expense, average lodging ($649), and nonreimbursed healthcare ($190) were higher than those for ground transportation ($177), meals ($89), and medications ($34). Across the entire cohort (N = 194), LKDs reported a sum total of $101 484 in direct expenses (mean $523; median $174).
Table 2.
Direct costs incurred during living donation evaluation period (N = 194)
| Costs $USD (in donors who incurred the expense type)
|
Costs $USD (all donors)
|
|||||
|---|---|---|---|---|---|---|
| Expense type | n (%) | Mean (SD) | Median (25th, 75th percentile) | Sum | Mean (SD) | Median (25th, 75th percentile) |
| Ground transportation | 155 (80) | 177 (216) | 103 (37, 226) | 27 434 | 141 (206) | 67 (9, 203) |
| Air transportation | 28 (14) | 1265 (999) | 975 (538, 1875) | 35 422 | 183 (582) | 0 (0, 0) |
| Lodging | 33 (17) | 649 (862) | 300 (108, 500) | 21 425 | 110 (428) | 0 (0, 0) |
| Meals | 87 (45) | 89 (122) | 50 (20, 100) | 7771 | 40 (93) | 0 (0, 40) |
| Healthcare | 47 (24) | 190 (221) | 95 (40, 250) | 8927 | 46 (135) | 0 (0, 0) |
| Medications | 15 (8) | 34 (23) | 25 (22, 50) | 505 | 3 (11) | 0 (0, 0) |
| Any direct costs | 187 (96) | 543 (954) | 205 (68, 547) | 101 484 | 523 (942) | 179 (59, 506) |
Table 3.
Indirect costs incurred during living donation evaluation period (N = 194)
| Donors who incurred the expense type
|
All donors
|
|||||
|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | Median (25th, 75th percentile) | Sum | Mean (SD) | Median (25th, 75th percentile) | |
| Donors1 | ||||||
| Work hours missed | 120 (62) | 36 (49) | 24 (15, 36) | 4278 | 22 (42) | 11 (0, 26) |
| Hours missed, unpaid | 52 (27) | 38 (51) | 28 (12, 40) | 1982 | 10 (31) | 0 (0, 5) |
| Paid vacation hours used | 46 (24) | 23 (30) | 16 (8, 24) | 1333 | 7 (27) | 0 (0, 0) |
| Unpaid vacation hours used | 32 (17) | 28 (33) | 20 (10, 32) | 906 | 5 (17) | 0 (0, 0) |
| Paid sick time hours used | 31 (16) | 25 (39) | 18 (10, 24) | 790 | 4 (18) | 0 (0, 0) |
| Unpaid sick time hours used | 25 (13) | 18 (12) | 16 (10, 22) | 448 | 2 (7) | 0 (0, 0) |
| Lost wages, excluding paid vacation/sick time, USD2 | 52 (27) | 691 (900) | 440 (273, 812) | 35 918 | 187 (556) | 0 (0, 140) |
| Lost wages, including paid vacation/sick time, USD2 | 120 (62) | 876 (1217) | 511 (276, 836) | 105 095 | 545 (1048) | 240 (0, 608) |
| Companion | ||||||
| Work hours missed | 61 (32) | 26 (26) | 16 (9, 31) | 1568 | 8 (19) | 0 (0, 8) |
| Hours missed, unpaid | 24 (12) | 24 (24) | 16 (9, 32) | 578 | 3 (12) | 0 (0, 0) |
| Paid vacation hours used | 23 (12) | 32 (28) | 24 (15, 48) | 735 | 4 (14) | 0 (0, 0) |
| Unpaid vacation hours used | 17 (9) | 23 (29) | 10 (7, 28) | 388 | 2 (11) | 0 (0, 0) |
| Paid sick time hours used | 9 (5) | 14 (11) | 8 (8, 20) | 126 | 1 (4) | 0 (0, 0) |
| Unpaid sick time hours used | 6 (3) | 12 (11) | 9 (4, 20) | 73 | 0 (3) | 0 (0, 0) |
| Lost wages, excluding paid vacation/sick time, USD2 | 24 (12) | 599 (692) | 283 (183, 842) | 14 378 | 76 (311) | 0 (0, 0) |
| Lost wages, including paid vacation/sick time, USD2 | 61(32) | 705 (853) | 326 (177, 915) | 42 978 | 222 (578) | 0 (0, 130) |
| Dependent care expense, USD | 15 (8) | 251 (263) | 150 (60, 400) | 3760 | 19 (97) | 0 (0, 0) |
One donor travelled from another country, remained in the United States for several weeks between evaluation and surgery, and thus missed an unusually high amount of work. This donor was excluded from subsequent calculations pertaining to lost work time and wages.
The hourly wage reported by the donor/companion was used in calculations. If hourly wage was missing, the US federal minimum wage ($7.25) was imputed.
Two-thirds (n = 122, 63%) reported missing work to complete the donation evaluation (Table 3). Among those LKDs who reported lost wages (27%), the mean number of work hours lost was 36 and mean lost wages, excluding paid vacation and sick leave, was $691. Mean lost wages if paid vacation and sick leave are included was $876. Across the entire cohort (N = 194), LKDs reported missing a sum total of 4278 work hours and $35 918 in lost wages ($105 095 if paid vacation and sick leave are included).
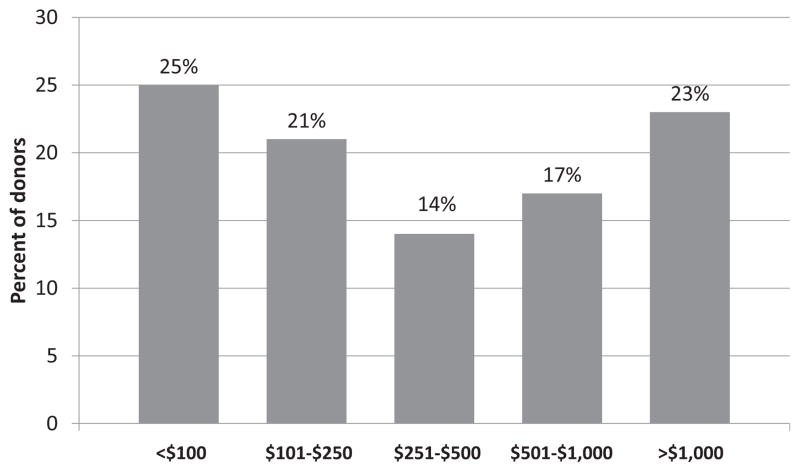
One-third (n = 61, 32%) of companions missed a mean of 26 work hours. Companions who missed work lost a mean of $599, excluding paid vacation and sick leave. Across the entire cohort (N = 194), companions missed a sum total of 1568 work hours and $14 378 in lost wages ($42 978 if paid vacation and sick leave are included). Additionally, 8% of LKDs reported dependent care expenses, totaling $3760. When considering direct costs, LKD and companion lost wages (excluding paid vacation and sick leave), and dependent care expenses, 189 (97%) LKDs experienced some financial loss for completion of the donation evaluation. The mean and sum total financial loss for the entire cohort was $801 (median $306) and $154 567, respectively. While most LKDs (60%) had total costs <$500, 17% reported costs between $501 and $1000, and 23% experienced costs >$1000 to complete the evaluation (Figure 1). Higher total costs were significantly associated with longer distance traveled to the transplant center (Spearman’s rho = 0.73, p < 0.001); however, total costs were not associated with age (p = 0.13), household income (p = 0.88), sex (p = 0.81), race/ethnicity (p = 0.96), marital status (p = 0.60), insurance status p = 0.07), or transplant center (p = 0.53). LKDs with a household income <$50 000 were more likely to have unpaid missed work hours than those with higher income (37% vs. 21%, p = 0.02).
Figure 1.
Total costs incurred by living kidney donors during the donation evaluation.
Median financial burden for LKDs (total costs/monthly household income) was 6% (25th percentile = 2%, 75th percentile = 23%). Financial burden was higher for those with less household income (Spearman’s rho = −0.42, p < 0.001) and greater travel distance to the transplant center (Spearman’s rho = 0.63, p < 0.001). Financial burden was not significantly associated with other demographic characteristics (all p values >0.05).
Some LKDs (n = 35, 18%) reported receiving financial assistance for expenses related to donation evaluation, with almost all (n = 30) reporting support from only one source. The most common source of financial support was the NLDAC (7%, mean = $2412), followed by the transplant candidate (6%, mean = $641), transplant candidate’s family (3%, mean = $1283), a nonprofit organization (3%, mean = $1617) and the transplant center (3%, mean = $22). The cohort (N = 194) reported receiving a sum total of $58 341 (mean = $300) (Table 4).
Table 4.
Financial support received during living donation evaluation period (N = 194)
| Donors who incurred the support type
|
All donors
|
|||||
|---|---|---|---|---|---|---|
| Source of support | n (%) | Mean (SD) | Median (25th, 75th percentile) | Sum | Mean (SD) | Median (25th, 75th percentile) |
| Transplant candidate | 11 (6) | 641 (630) | 300 (150, 1000) | 7050 | 36 (207) | 0 (0, 0) |
| Transplant candidate family | 6 (3) | 1283 (1892) | 650 (50, 2225) | 7700 | 40 (377) | 0 (0, 0) |
| Nonprofit organization | 6 (3) | 1617 (1093) | 1750 (600, 2550) | 9700 | 50 (331) | 0 (0, 0) |
| National Living Donor Assistance Center | 14 (7) | 2412 (2326) | 1850 (438, 5000) | 33 761 | 174 (869) | 0 (0, 0) |
| Transplant center | 6 (3) | 22 (13) | 0 (0, 28) | 130 | 0 (3) | 0 (0, 0) |
Discussion
This is the first study in the United States to describe the predonation direct and indirect costs of eventual LKDs. Direct costs, such as transportation to the transplant center, and work hours missed are common for LKD candidates, particularly those who must travel greater distances and who have less household income. The entire cohort of 194 LKDs incurred $154 567 in total costs (average of $804 per donor) during the evaluation period, with considerable variability in both expense type and amount. Surprisingly few LKDs received financial reimbursement of their expenses. If these data are extrapolated to the 5620 LKDs in the United States in 2013 (1), total expenses related to the donation evaluation would exceed $4 million.
Our findings complement those reported by Klarenbach et al (15), who reported that some Canadian LKDs experience substantial financial consequences associated with the donation experience overall. However, it is difficult to compare our two studies, since they were conducted in different countries, with different currencies and healthcare practices and systems. Also, while expense data during the predonation period were collected, Klarenbach et al did not report these data separately. Nevertheless, both studies highlight the need to develop policies and practices that move toward financial neutrality for LKDs by offsetting their direct and indirect costs, which would help to reduce financial disincentives in living kidney donation. The US lags behind several other countries, including Canada, Australia, the Netherlands and Israel, which have implemented programs to reimburse LKDs for such costs (11,16–18).
The NLDAC program provided travel and lodging reimbursement totaling $33 761 to 14 (7%) LKDs in our cohort, which is consistent with the 5% of LKDs it has reached nationally since its inception (8). Legislation that authorized NLDAC stipulates that eligibility criteria include evaluation of both the LKD and recipient household income, with the explicit expectation that recipients should reimburse LKD expenses if they have sufficient financial resources. Our study shows that few LKDs (n = 11, 6%) report receiving financial assistance from the recipient to cover donation evaluation costs. It is possible that some LKDs are reluctant to disclose reimbursement from the recipient, fearing an implication of coercion or possible violation of federal law. Although we did not collect household income data on all recipients of LKDs in our cohort, we can conservatively estimate that 34 (18%) LKDs and recipients reported household income that would meet NLDAC’s financial eligibility (i.e. 300% of federal poverty level). This suggests that as many as two-thirds of LKDs may not be applying to NLDAC for expense reimbursement. In the context of these data, we support ongoing policy discussions about the economics of living kidney donation, eliminating financial means testing for reimbursement of LKD expenses, and removing the expectation and burden of reimbursement from transplant recipients (12).
We also found that transportation costs are common for LKDs during the evaluation process. Modifying NLDAC to enable reimbursement for travel expenses for all donors, regardless of income, would ease the financial burden substantially. Moreover, greater distance from the transplant center is associated with more financial burden. There may be a need for a more coordinated system to facilitate LKD evaluation at transplant centers in closer proximity to the more distant donor’s home, thus reducing travel expenses and financial burden. All of our centers allowed for most evaluation components to be completed at centers that were more geographically convenient for the donor, but still required at least one predonation visit for imaging studies and to meet the team. As part of the transplant community, centers should cooperate on potential donor evaluations. To the degree that finances represent a barrier to performing donor evaluations for other centers, the development of a standard acquisition charge should be considered (19). Moreover, giving LKDs the option of surgery closer to home and shipment of the kidney to the transplanting center (much like in kidney paired exchange) as well as using mobile health technology to complete some aspects of the evaluation remotely warrants consideration by the transplant community if minimizing costs to the donor is an important endpoint.
Lost wages following living donation have been identified as a substantial donation-related expense (9). The Organ Procurement and Transplantation Network (OPTN) now requires that such financial exigencies be included in the informed consent process for all potential LKDs (20). However, the focus has been exclusively on lost wages during post-donation recovery. We found that, for the entire KDOC cohort, predonation lost wages for LKDs and companions totaled $35 918 and $14 378, respectively. If one considers the use of paid vacation or sick leave to be an indirect expense to the LKD, then we found a threefold increase in lost wages. We did not ask study participants to identify whether financial support received was used to offset lost wages, although we know that NLDAC is prohibited from reimbursing LKDs for lost wages. Considering that the decline in living kidney donation in the United States mirrors the economic downturn in the country and may be more pronounced among those with lower household incomes (21,22), consideration should be given to expanding NLDAC coverage to include some reimbursement of lost wages, as is currently done in several other countries.
This study benefited from a few notable strengths: the inclusion of multiple sites in three distinct regions of the United States, a large prospective cohort that is representative of the national LKD population (1), and the inclusion of companion expenses in the analysis. Additionally, this is the first comprehensive examination of direct and indirect costs focused on the predonation evaluation period. These strengths notwithstanding, study findings should be considered in the context of a few methodological limitations. First, our cohort includes only those adults who subsequently proceeded to donation. While their costs may more accurately reflect the total direct and indirect predonation expenses, average costs reported in this study are likely higher than if the sample had also included those who did not complete all elements of the donation evaluation. Nevertheless, many potential donors may incur costs and not actually complete the process. The expenses for nondonors who have incurred evaluation-related costs should be considered in any system of reimbursement. Second, we relied on self-reporting of donation-related expenses and did not verify these reports with documentation (e.g. receipts, expense diary entries, etc.), which raises the possibility of recall bias. Third, two-thirds of our sample exceeded the median household income of the US general population (14). It is possible that the financial impact shown in this study is greater for LKDs with less household income, which may have been a contributing factor in deciding not to pursue donation. Fourth, there are potential donation-related costs that were not assessed as part of this study (e.g. pet boarding, overnight stays with family or friends). Finally, our study cohort derives from small to moderate transplant centers and may not be representative of larger volume programs. Because of these limitations, costs for other donor cohorts may be very different than those found in this study.
In summary, this is the first multi-center study to delineate the direct and indirect costs incurred by eventual LKDs during the donation evaluation process. By providing transplant candidates with high-quality organs and a quicker pathway to transplantation, LKDs not only provide tangible benefits to patients but also cost savings to the healthcare system. However, nearly all LKDs experience some out-of-pocket costs and, for some, these costs are substantial. The potential direct and indirect costs associated with the donation evaluation should be integrated into the informed consent process for all potential LKDs. Also, although we will report on postdonation expenses as our cohort progresses, more research is needed to identify the total costs associated with donation (pre and post) and effective strategies to attenuate these costs. Study findings support national efforts to achieve financial neutrality for LKDs (12).
Acknowledgments
This study was supported by Award No. R01DK085185 from the NIDDK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the National Institutes of Health. Preparation of this manuscript was also supported, in part, by the Julie Henry Research Fund and the Center for Transplant Outcomes and Quality Improvement, The Transplant Institute, Beth Israel Deaconess Medical Center, Boston, MA. The authors gratefully acknowledge the hard work and dedication of the study coordinators and others at the six KDOC transplant centers who assisted in the completion of this project: Aws Aljanabi, Jonathan Berkman, Tracy Brann, Rochelle Byrne, Lauren Finnigan, Krista Garrison, Ariel Hodara, Tun Jie, Scott Johnson, Nicole McGlynn, Maeve Moore, Matthew Paek, Henry Simpson, Carol Stuehm, Denny Tsai and Carol Weintroub.
Abbreviations
- CMS
Centers for Medicare and Medicaid Services
- KDOC
Kidney Donor Outcomes Cohort
- LDKT
live donor kidney transplantation
- LKD
living kidney donor
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- NLDAC
National Living Donor Assistance Center
- USD
United States dollars
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2012 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services; Administration, Healthcare Systems Bureau, Division of Transplantation; 2014. [Google Scholar]
- 2.U S Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease, End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Diseases; 2013. [Google Scholar]
- 3.Purnell TS, Auguste P, Crews DC, et al. Comparison of life participation activities among adults treated by hemodialysis, peritoneal dialysis, and kidney transplantation: A systematic review. Am J Kidney Dis. 2013;62:953–973. doi: 10.1053/j.ajkd.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dew MA, Jacobs CL. Psychosocial and socioeconomic issues facing the living kidney donor. Adv Chronic Kidney Dis. 2012;19:237–243. doi: 10.1053/j.ackd.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigue JR, Cornell DL, Kaplan B, Howard RJ. Patients’ willingness to talk to others about living kidney donation. Prog Transplant. 2008;18:25–31. doi: 10.1177/152692480801800107. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigue JR, Schold JD, Mandelbrot DA. Concern for lost income following living kidney donation: Lost opportunity? Am J Transplant. 2014;14:480. [Google Scholar]
- 7.Department of Health and Human Services. Reimbursement of travel and subsistence expenses toward living organ donation eligibility guidelines. Fed Regist. 2009;74:29218–29220. [cited 17 Oct 2014] Available from: http://www.gpo.gov/fdsys/pkg/FR-2009-06-19/html/E9-14425.htm. [Google Scholar]
- 8.Warren PH, Gifford KA, Hong BA, Merion RM, Ojo AO. Development of the National Living Donor Assistance Center: Reducing financial disincentives to living organ donation. Prog Transplant. 2014;24:76–81. doi: 10.7182/pit2014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke KS, Klarenbach S, Vlaicu S, et al. The direct and indirect economic costs incurred by living kidney donors—A systematic review. Nephrol Dial Transplant. 2006;21:1952–1960. doi: 10.1093/ndt/gfl069. [DOI] [PubMed] [Google Scholar]
- 10.Schulz-Baldes A, Delmonico FL. Improving institutional fairness to live kidney donors: Donor needs must be addressed by safeguarding donation risks and compensating donation costs. Transpl Int. 2007;20:940–946. doi: 10.1111/j.1432-2277.2007.00542.x. [DOI] [PubMed] [Google Scholar]
- 11.Sickand M, Cuerden MS, Klarenbach SW, et al. Reimbursing live organ donors for incurred non-medical expenses: A global perspective on policies and programs. Am J Transplant. 2009;9:2825–2836. doi: 10.1111/j.1600-6143.2009.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaPointe Rudow D, Hays R, Baliga P, et al. Consensus conference on best practices in live kidney donation: Recommendations to optimize education, access, and care. Am J Transplant. doi: 10.1111/ajt.13173. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klarenbach S, Garg AX, Vlaicu S. Living organ donors face financial barriers: A national reimbursement policy is needed. CMAJ. 2006;174:797–798. doi: 10.1503/cmaj.051168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Census. Household Income. 2012 [cited 30 Oct 2014]. Available from: http://www.census.gov/prod/2013pubs/acsbr12-02.pdf.
- 15.Klarenbach S, Gill JS, Knoll G, et al. Economic consequences incurred by living kidney donors: A Canadian multi-center prospective study. Am J Transplant. 2014;14:916–922. doi: 10.1111/ajt.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Australian Department of Health. Leave for Living Organ Donors. Canberra, Australia: Australian Department of Health; [cited 17 Jul 2014] Available from: http://www.health.gov.au/internet/main/publishing.nsf/Content/Leave-for-living-organ-donors. [Google Scholar]
- 17.The Kidney Foundation of Canada. Living Organ Donor Expense Reimbursement Program (LODERP) British Columbia, Canada: The Kidney Foundation of Canada; [cited 6 Jun 2014]. Available from: http://www.kidn[12]ey.ca/BC/LODERP. [Google Scholar]
- 18.Lavee J, Ashkenazi T, Stoler A, Cohen J, Beyar R. Preliminary marked increase in the national organ donation rate in Israel following implementation of a new organ transplantation law. Am J Transplant. 2013;13:780–785. doi: 10.1111/ajt.12001. [DOI] [PubMed] [Google Scholar]
- 19.Rees MA, Schnitzler MA, Zavala EY, et al. Call to develop a standard acquisition charge model for kidney paired donation. Am J Transplant. 2012;12:1392–1397. doi: 10.1111/j.1600-6143.2012.04034.x. [DOI] [PubMed] [Google Scholar]
- 20.Organ Procurement and Transplantation Network (OPTN) Policies. [cited 17 Oct 2014]. Available from: http://optn.transplant.hrsa.gov/ContentDocuments/OPTN_Policies.pdf.
- 21.Rodrigue JR, Schold JD, Mandelbrot DA. The decline in living kidney donation in the United States: Random variation or cause for concern? Transplantation. 2013;96:767–773. doi: 10.1097/TP.0b013e318298fa61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gill J, Dong J, Rose C, et al. The effect of race and income on living kidney donation in the United States. J Am Soc Nephrol. 2013;24:1872–1879. doi: 10.1681/ASN.2013010049. [DOI] [PMC free article] [PubMed] [Google Scholar]