Abstract
Modifications to an N-methyl-(quinolin-4-yl)oxypropanamide scaffold were explored to discover leads for developing new radioligands for PET imaging of brain TSPO (translocator protein), a biomarker of neuroinflammation. Whereas contraction of the quinolinyl portion of the scaffold or cyclization of the tertiary amido group abolished high TSPO affinity, insertion of an extra nitrogen atom into the 2-arylquinolinyl portion was effective in retaining sub-nanomolar affinity for rat TSPO, while also decreasing lipophilicity to within the moderate range deemed preferable for a PET radioligand. Replacement of a phenyl group on the amido nitrogen with an isopropyl group was similarly effective. Among others, compound 20 (N-methyl-N-phenyl-2-[2-(pyridin-2-yl)-1,8-naphthyridin-4-yloxy]propanamide) appears especially appealing for PET radioligand development, based on high selectivity and high affinity (Ki = 0.5 nM) for rat TSPO, moderate lipophilicity (logD = 2.48), and demonstrated amenability to labeling with carbon-11.
Keywords: Translocator protein, PET, carbon-11, ligand, binding affinity, lipophilic efficiency
Graphical Abstract
INTRODUCTION
Translocator protein 18 kDa (TSPO), formerly known as the peripheral benzodiazepine receptor [1], is located predominantly at the outer mitochondrial membrane in association with a voltage-dependent anion channel and an adenine nucleotide transporter [2]. TSPO is present in several major organs, and is particularly dense in adrenal gland, heart, kidney, and testis [2]. Low amounts are present in normal human brain, primarily in microglia [3]. Activated microglia upregulate TSPO in instances of neuronal damage [4] as seen in many neurological disorders [5–7] including Alzheimer’s disease, movement disorders, stroke, multiple sclerosis, and major depression [8]. Therefore, TSPO can serve as an important biomarker for neuroinflammation. Moreover, ligands for TSPO have also been explored as possible drugs, particularly for anxiety [9].
For more than three decades, PET imaging of human TSPO has been carried out with [11C]PK11195 ([11C]1) [10] or its (R)-enantiomer ([11C](R)-1) [11] (Chart 1) for biomedical investigations of neuroinflammation. [11C](R)-1 has been by far the most employed radioligand for this purpose despite limited brain uptake [12], low specific binding [12], and an undesirable metabolic profile [13]. Efforts to tackle these shortcomings of [11C](R)-1 have resulted in several new structural classes of TSPO radioligand with superior imaging characteristics (Chart 1). Examples include [11C]PBR28 ([11C]2) [14,15], [11C]DAA1106 ([11C]3) [16], [11C]DPA-713 ([11C]4) [17], [18F]DPA-714 ([18F]5) [18], [18F]FBR ([18F]6) [19], [18F]PBR111 ([18F]7) [20], [18F]FEPPA ([18F]8) [21], [18F]FEMPA ([18F]9) [22], and [11C]ER176 ([11C]10) [23]. Nonetheless, many of these new radioligands also suffer particular deficiencies, most prevalent of which is sensitivity to the rs6971 polymorphism in human subjects [20,21,24,25].
Chart 1.
Structures of some notable TSPO PET radioligands.
Successful PET radioligands for imaging specific proteins in brain are required to display a wide array of properties [26]. Among these properties are: i) high affinity and selectivity for the target protein; ii) low molecular weight; iii) intermediate polar surface area for blood-brain barrier penetration; iv) moderate lipophilicity for adequate brain entry in the absence of excessive non-specific binding; and v) amenability to labeling with a positron-emitter, either carbon-11 (t1/2 = 20.4 min) or fluorine-18 (t1/2 = 110 min). This study aimed to develop TSPO ligands as leads with a desirable combination of properties for PET radioligand development. We have previously explored a series of N-methyl-(quinolin-4-yl)oxypropanamides as prospective TSPO ligands [27], and encouragingly many of these ligands have shown low TSPO genotype sensitivity in vitro. Here, we further explore structure-affinity relationships in this structural class. High-affinity TSPO ligands emerged from this effort and a few of these are promising new leads to PET radioligands.
RESULTS and DISCUSSION
In this study, we identified new leads to PET radioligands for imaging TSPO based on modifications to the previously reported [27] N-methyl-(quinolin-4-yl)oxypropanamide TSPO ligand scaffold (Chart 2). These modifications were aimed at exploring, i) variation of substituents on the amide nitrogen, ii) introduction of nitrogen into the quinolin-4-yl group or pendant aryl ring, iii) replacement of the pendant aryl ring with methoxy, 2-pyrimidinyl or N-pyrrolidinyl, iv) the effect of cyclization to eliminate amide bond rotation, and v) contraction of the bicyclic quinolinyl nucleus. Generally, PET radioligands are required to have high affinity with KD in the low nM range, and moderate lipophilicity with measured (or computed) logD in the 2–4 range [26]. Most of the changes that we made to the lead ligand scaffold were intended to retain the very high TSPO affinity (Ki = 0.07 nM for rat TSPO) seen in the previously reported example 11 (Chart 2; scaffold with Y = 2-pyridinyl, and R = Ph), as well as to decrease ligand computed lipophilicity (clogD) from 4.73 towards the desirable range. Usually, the overall shape of the scaffold was modified little to retain high affinity, although the effects of scaffold pruning were also investigated. The main strategy for lowering lipophilicity was to introduce nitrogen into one or more of the aryl rings. The lipophilicity cost for high ligand affinity may be indexed as a lipophilicity efficiency parameter (LipE), defined as ligand pIC50 (or pKi) minus clogD [28]. Therefore, our overall aim encompasses the discovery of ligands with high LipE scores (>6). The amide N-methyl substituent was retained in all new ligands as a site that should be amenable to labeling with carbon-11 through 11C-methylation of N-desmethyl precursors.
Chart 2.
The N-methyl-(quinolin-4-yl)oxypropanamide scaffold used as a basis for new TSPO ligand development.
Chemistry
As prospective TSPO ligands, the 2-heteroaryl-4-alkoxyquinolines 18–23 (Scheme 1) were synthesized in three steps, proceeding with acylations of the requisite 2-aminoarylethanones [29]. The resultant amides 12 and 13 were subjected to Camps cyclization [30] to yield the 2-heteroarylquinolin-4-ones 14 and 15, respectively, which were then chemoselectively O-alkylated to the desired ligands 18–23 (Scheme 1). The required α-bromoamides 16 and 17 were made under Schotten-Baumann conditions.
Scheme 1.
Syntheses of 2-heteroaryl-4-alkoxyquinolines 18–23.a
a Reagents and conditions: (i) for 12 (X = Cl): TEA, CHCl3, 4 °C; for 13 (X = ONa): DMF, DIPEA, PyBroP, CH2Cl2, rt; (ii) NaOH, dioxane, 110 °C; (iii) KOH, EtOAc, H2O, 4 °C; (iv) for 18: NaH, DMSO, 2-bromo-N-phenylpropanamide, 80 °C; for 19: K2CO3, MeCN, 2-bromo-N-phenylpropanamide, 55 °C; for 20–21; K2CO3, MeCN, 2-bromo-N-methyl-N-phenylpropanamide, 55–60 °C; for 22–23: K2CO3, MeCN, 16–17, 55–60 °C.
The 2,4-dialkoxy-1,8-naphthyridine ligand 27 was made by first accomplishing a regioselective methoxylation [31,32] at C2 of 2,4-dichloro-1,8-naphthyridine to yield 2-methoxy-4-chloro-1,8-naphthyridine 26 (Method A, Scheme 2). The position of methoxylation was confirmed by an alternative but much lower yielding regiospecific synthesis wherein methyl 2-aminopyridine-3-carboxylate was first converted into the methyl acetimidate 24. Dieckmann condensation [33] of 24 then gave 2-methoxy-1,8-naphthyridin-4-one 25. Compound 25 was then converted into 26 by treatment with phosphoryl chloride (Method B, Scheme 2), but in only 3% yield. Finally, alkoxylation at C4 of 26 gave 27 (Scheme 2).
Scheme 2.
Synthesis of the 2,4-dialkoxy-1,8-naphthyridine analog 27.a
a Reagents and conditions: (i) MeC(OMe)3, Ac2O, 110 °C; (ii) LDA, THF, hexane, −50 °C; (iii) POCl3, 110 °C; (iv) NaOMe, toluene, rt; (v) NaH, DMF, DMSO, rt.
The 2-pyrrolequinoline ligand 29 was made by chemoselective O-alkylation [34] of 2-amino-4-hydroxyquinoline to yield 28 followed by treatment of its free base with dimethoxytetrahydrofuran in acetic acid (Scheme 3) [35].
Scheme 3.
Synthesis of 2-pyrrolequinoline analog 29.a
a Reagents and conditions: (i) NaOEt, EtOH, 70 °C, then α-bromoamide, DMF, 90 °C; (ii) AcOH, 120 °C.
Two analogs of ligand 22, namely 33 and 34, in which rotation of the amide bond was eliminated through cyclization, were synthesized in two steps. First, an appropriate lactam was subjected to α-chlorination with hexachloroethane to give compounds 31 and 32 in moderate yields [36,37]. Alkylation of 2-(pyridin-2-yl)-quinolin-4(1H)-one with each of these α-chlorolactams required prolonged heating but eventually gave the desired ligands 33 and 34, respectively, in moderate yields (Scheme 4).
Scheme 4.
Synthesis of α-quinolinyloxylactam analogs 33 and 34 with eliminated amide bond rotation.a
a Reagents and conditions: (i) t-BuLi, pentane-THF, −60 to −70 °C, then −20 °C; (ii) THF, −70 °C, then 0 °C for 30 min; iii) C2Cl6 THF, −78 to −70 °C for 1 h, and the rt for1 h; (iv) K2CO3, MeCN, 80 °C, 70 h.
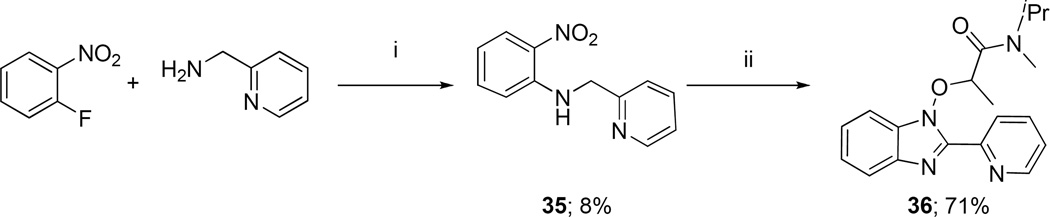
Finally, a ring-contracted analog of 11, namely 36, was made through nucleophilic amination [38] of 1-fluoro-2-nitrobenzene to yield 2-nitro-N-(pyridin-2-ylmethyl)aniline 35, followed by treatment of 35 with two equivalents of sodium hydride to give the cyclized intermediate benzoimidazole-N-oxide, and finally alkylation [39] of this oxide in situ with α-bromoamide 16 (Scheme 5).
Scheme 5.
Synthesis of ring-contracted analog 36.a
a Reagents and conditions: (i) K2CO3, DMF, 90 °C; (ii) 16, NaH, DMF, 55 °C.
Ligand Pharmacology: TSPO Binding Affinity, cLogD, and LipE
The binding affinities (1/KD) of all TSPO ligands, including the previously reported ligand 11, were determined on rat brain homogenates (Table 1). We tested all ligands as racemates only, with the assumption that the high-affinity enantiomer has twofold higher affinity than that recorded for the racemate [27].
Table 1.
Ki, clogD and LipE values for new oxypropanamide TSPO ligands; comparison with those of the classical ligand 1 and the progenitor ligand 11.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ligand | A | Y | R1 | R2 | R3 | Rat Ki (nM)a | cLogD | LipE |
| 1 (PK11195)b | 0.5 ± 0.3 | 3.97 | 4.3 ± 0.2 | |||||
| 11b | CH | 2-Pyridinyl | Me | Me | Ph | 0.10 ± 0.05 | 4.18 (3.11 ± 0.02)c |
5.9 ± 0.2 |
| 20 | N | 2-Pyridinyl | Me | Me | Ph | 0.5 ± 0.1 | 2.80 | 6.5 ± 0.1 |
| 21 | CH | 2-Pyrimidinyl | Me | Me | Ph | 0.53 ± 0.08 | 2.71 (2.48 ± 0.06)c |
6.6 ± 0.1 |
| 22 | CH | 2-Pyridinyl | Me | Me | iPr | 0.76 ± 0.09 | 2.54 | 6.58 ± 0.05 |
| 23 | CH | 2-Pyridinyl | Me | Me | p-F-C6H4 | 0.09 ± 0.01 | 4.25 | 5.80 ± 0.06 |
| 27 | N | OMe | Me | Me | Ph | 7.8 ± 0.6 | 2.02 | 6.09 ± 0.04 |
| 29 | CH | 1-Pyrrolyl | Me | Me | Ph | 0.22 ± 0.03 | 3.72 | 6.13 ± 0.07 |
| 33 | CH | 2-Pyridinyl | CH2-R2 | CMe2-R1 | Me | >1000 | 2.07 | <3.93 |
| 34 | CH | 2-Pyridinyl | CH2-R2 | CH2-R1 | iPr | >1000 | 2.64 | <3.36 |
| 36 | 2-Pyridinyl | Me | Me | iPr | >1000 | 3.53 | <2.47 | |
The N-(p-fluorophenyl) analog 23 of the previously described high-affinity TSPO ligand 11 maintained very high binding affinity and a comparably high LipE value (Table 1). Ligand 23 opens up the possibility to prepare an 18F-labeled ligand, according to modern 18F-labeling techniques [40,41].
Previously, we achieved an increase in LipE by introducing a nitrogen atom at either the 2’ position of the pendant phenyl group or the 8 position of the quinolinyl scaffold (Chart 2) [26]. We presumed that by having nitrogen atoms at both positions in the same ligand that we might significantly improve LipE, and, indeed, this turned out to be the case in ligand 20 (Table 1). We obtained a similar result when the pendant phenyl group alone was modified to have nitrogen atoms at positions 2’ and 6’, as in ligand 21 (Table 1). Previously, we had established the tolerance of the TSPO binding pocket for ligands containing nitrogen atoms at positions 2’, 3’, and 4’ of the pendant aryl group of the lead scaffold [26]. Nitrogen at the 1’ position in a pyrrolyl ring was no exception, yielding ligand 29 with high affinity (Ki = 0.22 nM) and high LipE (6.3) (Table 1). In addition, we had found that removal of the pendant phenyl group in the scaffold does not seriously affect binding affinity and that LipE was maintained as a result of a decrease in lipophilicity (clogD) by about 2 units [27]. Seeking to exploit the apparent benefit of reducing the size of this substituent and concomitantly molecular weight, we swapped the large and lipophilic pendant aryl group in 20 for the small and polar methoxy group (Table 1, 27). This replacement conserved high LipE despite some decrease in TSPO affinity and established that TSPO is quite tolerant for such substitutions.
Tertiary amides are present in nearly all high-affinity TSPO ligands (e.g., see Chart 1). We sought further improvements to LipE by manipulation of substituents on the tertiary amido group. Previously, we also reported some TSPO ligands based on the scaffold in Chart 2 that incorporated a methylene tether to the aryl ring in place of the oxygen tether [27]. As a group, these methylene-tethered ligands had lower LipE scores than the analogous oxygen-tethered ligands. Because LipE improved by about 1 unit when a phenylamide was exchanged for an isopropylamide in the methylene-tethered series [27], we expected a similar improvement in the oxo-linked series, and indeed this was found when comparing the new ligand 22 with 11 (Table 1).
Previously, we had found that the elimination of amide bond rotation in a methylene-tethered TSPO ligand, 1-methyl-3- [(2-phenylquinolin-4-yl)methyl]pyrrolidin-2-one (30), increased LipE by about 1 unit [26]. Therefore, we were interested to exploit this effect again by making a locked amide rotamer of ligand 22. There are two possible ligands that may result from tethering the α-methyl group in 22 to one of its amide substituents, namely the ligands 34 and 35 (Table 1). Unlike the locked rotamer from our earlier study, both 34 and 35 lacked any appreciable binding affinity for TSPO (Table 1). These results add to previous observations of locked amide rotamers showing much reduced affinity for TSPO [42]. Moreover, evidence indicates that TSPO has a preference to bind the E-rotamer of PK11195 (1) [43,44]. Thus, it seems that TSPO is highly sensitive to the spatial arrangement of the substituents on the requisite tertiary amide in TSPO ligands.
Finally, we tested a truncated scaffold analog of 11. This ligand (Table 1, 36) has a fused bicyclic scaffold whose structure is rather similar to that found in many literature TSPO ligands, as exemplified by DPA-713 (4), PBR111 (7) and IGA-1 (37) [45] (Chart 3). Ligand 36 appears to occupy essentially the same chemical space as these compounds, so we were surprised that 36 showed no binding to TSPO (Ki > 1000 nM). However, simple comparison of the formal 2-dimensional structure of 36 with those of other known TSPO ligands with similar core scaffold shows that the amido carbonyl group, a key pharmacophoric element, is not aligned with the carbonyl groups of the other ligands. Hence, the inability of 36 to form a required directional hydrogen bond with TSPO may explain its lack of affinity.
Chart 3.
Comparison of formal structures of three high-affinity TSPO ligands with 36. Bold bonds indicate scaffold matching that in ligand 4. For ligands 4, 7, and 37, a carbonyl group, shown in red, is part of the matching scaffold, but not for compound 36.
Pharmacological screen
Ligand 11, at 10 µM concentration, showed <50% inhibition of specific binding to all tested receptors and transporters, except the 5-HT1A receptor (Ki = 366 nM).
Radioligand Syntheses
The radioligands [11C]11 and [11C]20 were produced in practically useful radiochemical yields in only five minutes at room temperature by treating their respective N-desmethyl precursors, 19 and 18, with [11C]methyl triflate [46] (Scheme 6). Each radioligand was separated with reversed phase HPLC and readily formulated in sterile ethanol with 10% saline, for possible use in PET scanning. Each radioligand was obtained with high radiochemical purity (>99%) and with high specific activities (244 and 126 GBq/µmol, respectively).
Scheme 6.
Radiosyntheses of [11C]11 and [11C]20.a
a Reagents and conditions: (i) for [11C]11; NaOH, MeCN, rt; for [11C]20; NaH, MeCN, rt.
Lipophilicity
The accessibility of [11C]11 and [11C]20 afforded the opportunity to measure their lipophilicities accurately for comparison to their computed values. Prolonged incubation of these radioligands in phosphate buffer (pH 7.4) at room temperature left them unchanged chemically. Although the measured logD for [11C]11 (3.11) was appreciably lower than the computed value (4.18), the measured logD of [11C]20 (2.48) was quite similar to the computed value (2.8) (Table 1). The computed values are therefore likely to be reasonable estimates (within one log unit of true values), and are also expected to be in approximately correct rank order for the closely related compounds. The measured values for both radioligands placed them near or within the desirable logD range of 2–4.
CONCLUSIONS
Modifications to N-methyl-(quinolin-4-yl)oxypropanamides, especially introduction of nitrogen into the 2-arylquinolin-4-yl scaffold, and use of an isopropyl substituent instead of a phenyl substituent on the tertiary amido nitrogen, were effective in retaining LipE to within a desirable range for PET radioligands. Cyclization of the amido group or contraction of the quinolinyl ring abolished high affinity. Three compounds (20–22) showed favorable properties for PET radioligand development, including high TSPO affinity and moderate computed lipophilicity. Ligands 11 and 20 were shown further to be amenable to labeling with carbon-11, to have acceptable moderate measured lipophilicity, and to be selective for binding to TSPO.
EXPERIMENTAL SECTION
Materials and Methods
Literature methods were used to prepare ligand 11 [27], 2-(pyridin-2-yl)-quinolin-4(1H)-one [30], 2-bromo-N-methyl-N-phenylpropanamide [47], 2-hydroxy-N-methyl-N-phenylpropanamide [48], 1,5,5-trimethylpyrrolidin-2-one [49], and 1-isopropylpyrrolidin-2-one [50]. All other reagents and solvents were purchased. Air-sensitive reagents were stored under N2 in a PureLab HE glovebox (Innovative Technology; Amesbury, MA). Melting points were determined on an SMP30 apparatus (Stuart; Staffordshire, UK). Boiling point vacuum pressures were determined on a DDR-1200 apparatus (J-Kem Scientific Inc.; St. Louis, MO). Reactions in dry solvent were performed with dried reagents under an inert atmosphere. Solutions were taken to dryness by treatment with MgSO4 (unless stated otherwise), followed by filtration and evaporation. 1H (400 MHz), 13C NMR (100 MHz), and 19F NMR (376 MHz) spectra were recorded on an Avance 400 instrument (Bruker; Billerica, MA). Chemical shifts for 19F are reported relative to neat TFA in a coaxial insert (δ = −76.6). HRMS data were obtained at the University of Illinois Urbana-Champaign (Mass Spectrometry Laboratory, School of Chemical Sciences) with a Micromass Q-Tof Ultima instrument for ESI (Waters Corp.; Milford, MA). Preparative HPLC was performed with elution at 30 mL/min on either a Luna PFP(2) column (5 µm; 100 Å; 30 × 250 mm; Phenomenex; Torrance, CA), or a Gemini C18 column (10 µm; 110 Å; 30 × 250 mm; Phenomenex). Mixtures were separated on either an XBridge C18 column (5 µm; 130 Å; 4.6 × 250 mm; Waters: Milford, MA), a Luna C18(2) column (10 µm; 100 Å; 4.6 × 250 mm; Phenomenex), or an XTerra RP18 column (10 µm; 125 Å; 7.8 × 300 mm; Waters). Compound purities were established on either a Luna PFP(2) column (5 µm; 100 Å; 4.6 × 250 mm; Phenomenex) or a Gemini C18 column (5 µm; 110 Å; 4.6 × 250 mm; Phenomenex). All compounds were >95% pure and typically >99% pure, as monitored by absorbance at 220 nm. Radioligands were isolated with HPLC on an XBridge C18 column (5 µm; 130 Å; 10 × 250 mm; Waters) or a Luna C18(2) column (10 µm; 100 Å; 10 × 250 mm; Phenomenex). Radiochemical purities were determined with HPLC on an on a Gold HPLC apparatus (Beckman Coulter, Inc.; Fullerton, CA) equipped with an in-line Flow-Count NaI scintillation detector (Bioscan, Inc.; Washington, DC) and a UV absorbance detector (Beckman Coulter, Inc.) operating at 254 nm. The response of the HPLC system was calibrated for mass of ligand to enable radioligand specific activity to be determined from the detected mass of carrier in a known activity of radioligand. Radioactive decay events were counted in an automatic gamma counter (1480 Wizard 3; PerkinElmer Life Sciences: Wallac Oy; Turku, Finland) with an electronic window set between 360 and 1,800 keV. cLogD values were computed with Pallas for Windows software version 3.8 in default option (CompuDrug; Bal Harbor, FL).
Chemistry
N-(3-Acetylpyridin-2-yl)picolinamide (12)
Pyridine-2-carbonyl chloride hydrochloride (4.89 g, 27.5 mmol) was added portion-wise to a solution of 1-(2-amino-3-pyridinyl)-1-ethanone (5.15 g, 37.8 mmol) and TEA (9.2 mL, 65.0 mmol) in dry CHCl3 (100 mL) at 4 °C. The mixture turned from tan to deep blue-green and the temperature rose to 15 °C. After 29 h, the mixture was diluted with CHCl3 (100 mL) and washed with hydrochloric acid (1 M; 100 mL × 2), water (100 mL), and brine (100 mL × 2), and then dried. The product was recrystallized (MTBE–CHCl3) to give a tan powder (714 mg, 11%). A second recrystallization (MTBE–MeOH) gave 12 as a cream-white powder. mp 151–154 °C. HRMS-ESI (m/z): [M + H]+ calcd for C13H12N3O2, 242.0930; found, 242.0929. 1H NMR (CDCl3): δ 13.22 (bs, 1H), 8.79 (dq, J = 4.8, 0.8 Hz, 1H), 8.74 (dd, J = 4.8, 2.0 Hz, 1H), 8.35 (td, J = 8.0, 1.2 Hz, 1H), 8.24 (dd, J = 8.0, 2.0 Hz, 1H), 7.91 (dt, J = 8.0, 2.0 Hz, 1H), 7.51 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.18 (dd, J = 7.6, 4.8 Hz, 1H), 2.71 (s, 3H). 13C NMR (CDCl3): δ 200.2, 162.6, 153.2, 151.1, 150.1, 148.5, 139.8, 137.5, 126.7, 123.2, 119.3, 118.5, 28.0.
N-(2-Acetylphenyl)pyrimidine-2-carboxamide (13)
DMF (30 mL) was added dropwise to a slurry of sodium pyrimidine-2-carboxylate (5.03 g, 34.4 mmol), 2′-aminoacetophenone (4.6 mL, 37 mmol), DIPEA (6.5 mL, 37 mmol) and PyBroP (17.4 g, 37.4 mmol) in CH2Cl2 (40 mL) held in a water bath, wherein the temperature rose from 21 to 29 °C. As the colorless slurry dissolved, the solution turned from orange to a deep forest green over the course of 18 h. The solvent was removed. The residue was taken up in EtOAc (370 mL), then washed successively with aq KHSO4 (5% w/v, 370 mL × 3), brine (370 mL), aq NaHCO3 (5% w/v, 370 mL × 3), brine (370 mL), and dried (Na2SO4). The residue was washed with ether and dioxane and recrystallized (MTBE–CHCl3; charcoal to decolorize) to give 13 as golden-yellow plates (435 mg, 5%). mp 201–203 °C. HRMS-ESI (m/z): [M + H]+ calcd for C13H12N3O2, 242.0930; found, 242.0928. 1H NMR (CDCl3): δ 13.65 (bs, 1H), 9.09 (dd, J = 8.4, 0.8 Hz, 1H), 9.03 (d, J = 5.2 Hz, 2H), 7.98 (dd, J = 8.0, 1.6 Hz, 1H), 7.66 (ddd, J = 8.4, 7.6, 1.2 Hz, 1H), 7.50 (t, J = 4.8 Hz, 1H), 7.22 (ddd, J = 8.4, 7.6, 1.2 Hz, 1H), 2.74 (s, 3H). 13C NMR (CDCl3): δ 202.4, 161.3, 158.2, 157.8, 139.9, 135.1, 131.7, 123.2, 122.9, 122.6, 121.2, 28.6.
2-(Pyridin-2-yl)-1,8-naphthyridin-4(1H)-one (14)
Compound 12 (716 mg, 2.97 mmol) and NaOH (355 mg, 8.88 mmol) in dry dioxane (40 mL) were heated to 95 °C for 3 h, whereupon the tan solution precipitated a brown solid. The mixture was cooled to rt, and the solid filtered off, washed with toluene (10 mL × 3), and taken up in H2O (20 mL). The blood-red solution was neutralized with hydrochloric acid (1 M, ~7 mL) to precipitate a tan solid, which was filtered off, washed with water (10 mL × 2) followed by ether (10 mL × 2), and then recrystallized (toluene) to give 14 as a pink-tan solid with a cotton candy texture (165 mg). More 14 (60 mg) was obtained from the mother liquor (225 mg total, 34%). mp 205–206 °C. HRMS-ESI (m/z): [M + H]+ calcd for C13H10N3O, 224.0824; found, 224.0829. 1H NMR (CDCl3): δ 10.78 (bs, 1H), 8.76 (td, J = 2.8, 1.2 Hz, 1H), 8.74 (dd, J = 4.4, 1.6 Hz, 1H), 8.68 (dd, J = 7.6, 1.6 Hz, 1H), 8.01 (td, J = 7.2, 0.8 Hz, 1H), 7.92 (dt, J = 7.6, 1.6 Hz, 1H), 7.47 (ddd, J = 7.2, 4.8, 0.8 Hz, 1H), 7.35 (dd, J = 8.0, 3.6 Hz, 1H), 7.00 (br s, 1H). 13C NMR (CDCl3): δ 179.8, 153.6, 150.1, 149.3, 148.2, 145.6, 137.7, 135.5, 125.5, 120.9, 120.5, 120.0, 107.2.
2-(Pyrimidin-2-yl)quinolin-4(1H)-one (15)
Compound 13 (407 mg, 1.69 mmol) and NaOH (202 mg, 5.05 mmol) in dry dioxane (15 mL) were heated in a pressure vessel to 110 °C for 3 h, during which time the pale yellow solution turned yellow-brown and a red-orange solid precipitated. After cooling the mixture to rt, the solid was filtered off, washed with dioxane, taken up in water (20 mL), and brought to pH 5 with acetic acid, whereupon the orange solution gave a salmon-pink precipitate. The solid was filtered off, washed with water, and recrystallized (toluene) to give 15 as a light pink solid (187 mg, 50%). mp 258 °C dec. HRMS-ESI (m/z): [M + H]+ calcd for C13H10N3O, 224.0824; found, 224.0827. 1H NMR (DMSO-d6, keto–enol; 1.4:1.0): δ 12.14 (bs, 1H, keto), 9.10 (d, J = 4.8 Hz, 2H, keto + enol), 8.12 (d, J = 8.8 Hz, 2H, keto + enol), 7.74–7.68 (m, 2H, keto + enol), 7.37 (dt, J = 6.8, 0.8 Hz, 1H, keto + enol), 7.15 (s, 1H, keto + enol), 3.34 (s, 1H, enol). 13C NMR (DMSO-d6): δ 177.7 (keto), 158.3 (enol), 158.0 (keto), 144.7 (enol), 144.6, 140.3 (enol), 140.2 (keto + enol), 132.2 (keto + enol), 125.8 (keto + enol), 124.7 (keto + enol), 123.6 (keto + enol), 122.3 (keto + enol), 119.6 (keto + enol), 108.0 (keto + enol).
2-Bromo-N-isopropyl-N-methylpropanamide (16)
N-Isopropylmethylamine (1.0 mL, 9.8 mmol) was added to a biphasic solution of KOH (1.67 g, 29.7 mmol) in water (10 mL) and EtOAc (10 mL). This mixture was stirred rapidly and cooled to 4 °C whereupon 2-bromopropanoyl chloride (1.5 mL, 15 mmol) was added dropwise with the temperature kept below 11 °C. The ice bath was removed and stirring continued for 1.5 h. The organic phase was separated off and the aqueous phase extracted with EtOAc (5 mL × 2). The combined extracts were washed with brine (10 mL) and dried. The residue was distilled (bp 48–50 °C at 0.2 mmHg), but was still contaminated with 2-bromopropanoic acid. The oil was taken up in CHCl3 (10 mL), washed with NH4OH (5 mL × 3), water (5 mL), and brine (5 mL × 2), and then dried to give 16 as a colorless oil (588 mg, 29%). d 1.38 g/mL. HRMS-ESI (m/z): [M + H]+ calcd for C7H15BrNO, 208.0337; found, 208.0341. 1H NMR (CDCl3, cis–trans; 1.4:1.0): δ 4.84 (sept, J = 6.8 Hz, 1H, cis), 4.60 (q, J = 6.4 Hz, 1H, trans), 4.53 (q, J = 6.4 Hz, 1H, cis), 4.20 (sept, J = 6.8 Hz, 1H, trans), 2.91 (s, 3H, cis), 2.81 (s, 3H, trans), 1.84 (d, J = 5.6 Hz, 3H, trans), 1.83 (d, J = 6.4 Hz, 3H, trans), 1.27 (d, J = 6.8 Hz, 3H, trans), 1.21 (d, J = 6.8 Hz, 3H, cis), 1.12 (d, J = 7.2 Hz, 3H, cis), 1.10 (d, J = 6.8 Hz, 3H, cis). 13C NMR (CDCl3): δ 168.6 (cis), 168.4 (trans), 48.3 (trans), 44.8 (trans), 39.5 (cis), 38.5 (trans), 28.3 (cis), 26.6 (trans), 22.1 (trans), 21.7 (cis), 20.8 (trans), 19.9 (trans), 19.4 (cis), 18.8 (cis).
2-Bromo-N-(4-fluorophenyl)-N-methylpropanamide (17)
The method for 16 was applied to 4-fluoro-N-methylaniline (3.5 mL, 29 mmol) and 2-bromopropanoyl chloride (4.4 mL, 44 mmol). The crude product oil was fractionally distilled. The forerun (bp 37–39 °C at 7.2 mmHg; 0.2 mL) was discarded and 17 (6.7 g, 88%) was collected as a yellow oil with bp 84–88 °C at 5.7 mmHg. d 1.5 g/mL. HRMS-ESI (m/z): [M + H]+ calcd for C10H12BrFNO, 260.0086; found, 260.0090. 1H NMR (CDCl3): δ 7.29 (m, 2H), 7.14 (m, 2H), 4.22 (q, J = 6.8 Hz, 1H), 3.28 (s, 3H), 1.74 (d, J = 6.4 Hz, 3H). 13C NMR (CDCl3): δ 169.6, 162.1 (d, J = 247 Hz), 138.8 (d, J = 3 Hz), 129.1 (d, J = 9 Hz), 116.9 (d, J = 22 Hz), 38.8, 38.2, 21.7. 19F NMR (CDCl3): δ −113.0.
N-Phenyl-2-[2-(pyridin-2-yl)-1,8-naphthyridin-4-yloxy]propanamide (18)
NaH (20 mg, 0.50 mmol; 60% in mineral oil) was added to a solution of 14 (60 mg, 0.27 mmol) in dry DMSO (2 mL). The orange effervescent solution was stirred for 1 h, whereupon 2-bromo-N-phenylpropanamide (192 mg, 0.84 mmol) was added. The mixture was heated to 80 °C for 8 h, cooled to rt and quenched with water (15 mL). The precipitate was filtered off, washed with water and purified with HPLC [PFP column; MeOH–NH4CH3CO2 buffer (25 mM, pH 5); 70:30] to give a peach solid (14 mg, 14%), which was recrystallized (toluene–MTBE) to give 18. mp 222–223 °C. HRMS-ESI (m/z): [M + H]+ calcd for C22H19N4O2, 371.1508; found, 371.1503. 1H NMR (CDCl3): δ 9.13 (dd, J = 4.0, 2.0 Hz, 1H), 8.75 (d, J = 8.0 Hz, 1H), 8.74 (ddd, J = 4.8, 2.8, 0.8 Hz, 1H), 8.57 (dd, J = 8.4, 2.0 Hz, 1H), 8.44 (bs, 1H), 8.20 (s, 1H), 7.82 (dt, J = 7.6, 1.6 Hz, 1H), 7.61 (dd, J = 8.8, 1.2 Hz, 2H), 7.46 (dd, J = 8.4, 4.4 Hz, 1H), 7.38–7.31 (3H), 7.14 (tt, J = 7.6, 0.8 Hz, 1H), 5.37 (q, J = 6.8 Hz, 1H), 1.85 (d, J = 6.8 Hz, 3H). 13C NMR (CDCl3): δ 168.4, 160.5, 160.5, 157.0, 154.8, 154.0, 149.0, 137.0, 136.9, 131.2, 129.1, 125.0, 124.9, 122.4, 121.5, 120.4, 116.1, 100.2, 75.7, 18.2.
N-Phenyl-2-[2-(pyridin-2-yl)quinolin-4-yloxy]propanamide (19)
2-(Pyridin-2-yl)quinolin-4(1H)-one (222 mg, 1.00 mmol), 2-bromo-N-phenylpropanamide (255 mg, 1.12 mmol), and K2CO3 (834 mg, 6.03 mmol) in MeCN (35 mL) were heated to 55 °C for 8 h. The mixture was cooled to rt and poured into water (175 mL). The precipitate was filtered off and recrystallized (aq dioxane) to give 19 as a colorless powder (283 mg, 77%). mp 205–206 °C. 1H NMR (CDCl3): δ 8.70 (qd, J = 4.8, 0.8 Hz, 1H), 8.63 (md, J = 9.2, 0.8 Hz, 1H), 8.29 (dd, J = 8.4, 0.8 Hz, 1H), 8.22 (bs, 1H), 8.17 (d, J = 8.4 Hz, 1H), 8.13 (s, 1H), 7.85 (dt, J = 8.0, 2.0 Hz, 1H), 7.80 (ddd, J = 7.2, 5.6, 0.6 Hz, 1H), 7.61 (ddd, J = 8.4, 6.8, 0.6 Hz, 1H), 7.55–7.52 (2H), 7.35 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.34–7.29 (2H), 7.12 (m, 1H), 5.40 (q, J = 6.8 Hz, 1H), 1.87 (d, J = 6.8 Hz, 3H). 13C NMR (CDCl3): δ 168.9, 159.9, 157.6, 155.7, 149.3, 149.1, 136.9, 136.9, 130.3, 129.8, 129.1, 126.5, 124.9, 124.3, 121.7, 121.2, 121.2, 120.2, 99.6, 75.2, 18.4.
N-Methyl-N-phenyl-2-[2-(pyridin-2-yl)-1,8-naphthyridin-4-yloxy]propanamide (20)
2-Bromo-N-methyl-N-phenylpropanamide (122 mg, 0.50 mmol), 14 (100 mg, 0.45 mmol), and K2CO3 (371 mg, 2.69 mmol) in dry MeCN (10 mL) were heated to 55 °C for 18 h. The mixture was cooled to rt and filtered through diatomaceous earth. The solvent was removed, the residue taken up in CH2Cl2 (10 mL), extracted into hydrochloric acid (2 M; 30 mL × 2), and neutralized with satd. NaHCO3. The solution was extracted with CH2Cl2 (50 mL × 3) and the combined organic layers washed with water (50 mL) and brine (50 mL × 2), and then dried. The product was isolated by HPLC [PFP column; MeOH–phosphate buffer (25 mM, pH 6); 80:20] followed by recrystallization (cyclohexane–dioxane) to give 20 as a cream solid (17 mg, 10%). mp 193– 194 °C. HRMS-ESI (m/z): [M + H]+ calcd for C23H21N4O2, 385.1665; found, 385.1664. 1H NMR (CDCl3): δ 9.08 (dd, J = 4.4, 2.4 Hz, 1H), 8.90 (d, J = 8.0 Hz, 1H), 8.78 (d, J = 4.0 Hz, 1H), 8.66 (dd, J = 8.0, 2.0 Hz, 1H), 8.03 (s, 1H), 7.89 (dt, J = 8.0, 2.0 Hz, 1H), 7.68 (bs, 2H), 7.50 (t, J = 7.6 Hz, 2H), 7.45–7.38 (3H), 5.08 (q, J = 6.4 Hz, 1H), 3.33 (s, 3H), 1.69 (d, J = 6.4 Hz, 3H). 13C NMR (CDCl3): δ 169.9, 161.4, 159.9, 157.0, 155.5, 153.7, 148.6, 142.5, 137.0, 132.3, 130.3, 128.8, 127.8, 124.7, 122.5 121.0, 116.3, 99.7, 71.7, 50.7, 38.1, 18.4.
N-Methyl-N-phenyl-2-[2-(pyrimidin-2-yl)quinolin-4-yl]oxypropanamide (21)
2-Bromo-N-methyl-N-phenylpropanamide (216 mg, 0.89 mmol), 15 (179 mg, 0.80 mmol), and K2CO3 (1.04 g, 7.53 mmol) in dry MeCN (10 mL) were heated to 55 °C for 4.5 h, whereupon the colorless slurry turned yellow. The mixture was cooled to rt and filtered through diatomaceous earth. The solvent was removed and the residue taken up in CH2Cl2 (100 mL), washed with water (50 mL × 2) followed by brine (50 mL), dried, and recrystallized (cyclohexane–dioxane) to give 21 as a colorless solid (263 mg, 86%). mp 211–212 °C. HRMS-ESI (m/z): [M + H]+ calcd for C23H21N4O2, 385.1665; found, 385.1665. 1H NMR (CDCl3): δ 9.02 (d, J = 4.8 Hz, 2H), 8.32 (d, J = 8.4 Hz, 1H), 8.26 (d, J = 8.4 Hz, 1H), 7.87 (s, 1H), 7.72 (dt, J = 6.8, 1.2 Hz, 1H), 7.55– 7.51 (3H), 7.41–7.37 (3H), 7.32 (t, J = 7.2 Hz, 1H), 5.09 (q, J = 6.8 Hz, 1H), 3.34 (s, 3H), 1.69 (d, J = 6.4 Hz, 3H). 13C NMR (CDCl3): δ 170.0, 163.7, 161.0, 157.6, 155.3, 149.4, 142.6, 130.2, 130.1, 130.0, 128.6, 127.7, 126.6, 122.1, 121.7, 120.6, 100.6, 71.5, 38.2, 18.3.
N-Isopropyl-N-methyl-2-[2-(pyridin-2-yl)quinolin-4-yloxy]propanamide (22)
The method for 21 was applied to 2-(pyridin-2-yl)-quinolin-4(1H)-one (222 mg, 1.00 mmol) and 16 (170 µL, 1.12 mmol). The crude product was recrystallized (aq EtOH) to give 22 as white sea urchin-shaped clusters (219 mg, 63%). mp 175–176 °C. HRMS-ESI (m/z): [M + H]+ calcd for C21H24N3O2, 350.1869; found, 350.1868. 1H NMR (CDCl3, cis–trans; 1.1:1.0): δ 8.67–8.64 (4H, cis + trans), 8.31 (d, J = 8.4 Hz, 2H, cis + trans), 8.10 (d, J = 8.4 Hz, 2H, cis + trans), 7.95 (s, 1H, trans), 7.90 (s, 1H, cis), 7.85 (tt, J = 7.6, 2.0 Hz, 2H, cis + trans), 7.72 (dd, J = 6.8, 1.6 Hz, 1H, cis), 7.71 (dd, J = 6.8, 1.6 Hz, 1H, trans), 7.53 (dd, J = 6.8, 1.2 Hz, 1H, cis), 7.51 (dd, J = 6.8, 1.2 Hz, 1H, trans), 7.35–7.31 (2H, cis + trans), 5.55 (q, J = 6.8 Hz, 1H, trans), 5.41 (q, J = 6.4 Hz, 1H, cis), 4.91 (sept, J = 6.8 Hz, 1H, cis), 4.35 (sept, J = 6.4 Hz, 1H, trans), 3.02 (s, 3H, cis), 2.84 (s, 3H, trans), 1.78 (d, J = 6.4 Hz, 3H, trans), 1.77 (d, J = 6.8 Hz, 3H, cis), 1.38 (d, J = 6.4 Hz, 3H, trans), 1.17 (d, J = 6.4 Hz, 3H, trans), 1.16 (d, J = 6.8 Hz, 3H, cis), 1.10 (d, J = 6.8 Hz, 3H, cis). 13C NMR (CDCl3): δ 169.4 (cis), 169.2 (trans), 160.8 (cis), 160.7 (trans), 157.3 (trans), 157.2 (cis), 156.2 (trans), 156.1 (cis), 149.2 (cis + trans), 148.9 (cis + trans), 136.8 (cis + trans), 129.9 (cis), 129.3 (trans), 125.9 (cis + trans), 124.1 (cis + trans), 122.1 (cis + trans), 121.7 (trans), 121.6 (cis), 121.3 (cis + trans), 98.7 (cis), 98.6 (trans), 72.3 (cis), 72.1 (trans), 47.7 (trans), 44.8 (cis), 39.5 (cis), 38.5 (trans), 28.3 (cis), 26.6 (trans), 22.1 (trans), 21.7 (cis), 20.8 (trans), 19.9 (trans), 19.4 (cis), 18.8 (cis).
N-(4-Fluorophenyl)-N-methyl-2-[2-(pyridin-2-yl)quinolin-4-yloxy]propanamide (23)
The method for 21 was applied to 2-(pyridin-2-yl)-quinolin-4(1H)-one (222 mg, 1.00 mmol) and 17 (195 µL, 1.12 mmol). The crude product was recrystallized (aq dioxane) to give white crystals. These were dried in an Abderhalden pistol under high vacuum (T = 110 °C) in the presence of P2O5 for 1 d to give 23 (293 mg, 73%). mp 179–180 °C. HRMS-ESI (m/z): [M + H]+ calcd for C24H21FN3O2, 402.1618; found, 402.1613. 1H NMR (CDCl3): δ 8.75 (d, J = 4.0 Hz, 1H), 8.70 (d, J = 8.0 Hz, 1H), 8.27 (dd, J = 8.4, 0.8 Hz, 1H), 8.08 (d, J = 8.0 Hz, 1H), 7.88 (dt, J = 7.6, 1.6 Hz, 1H), 7.82 (s, 1H), 7.70 (dt, J = 8.0, 1.2 Hz, 1H), 7.68 (br s, 2H), 7.50 (dt, J = 8.0, 0.8 Hz, 1H), 7.38 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.15 (dt, J = 8.0, 1.2 Hz, 2H), 5.04 (q, J = 6.4 Hz, 1H), 3.31 (s, 3H), 1.87 (d, J = 6.4 Hz, 3H). 13C NMR (CDCl3): δ 170.3, 162.3 (d, J = 248 Hz), 160.7, 156.9, 156.2, 149.2, 148.6, 138.6 (d, J = 3 Hz), 137.0, 130.0, 129.8 (d, J = 8 Hz), 129.1, 125.9, 124.2, 122.3, 121.8, 121.3, 117.2 (d, J = 22 Hz), 98.6, 71.1, 38.3, 18.5. 19F NMR (CDCl3): δ −112.9.
Methyl 2-(1-methoxyethylideneamino)nicotinate (24)
Methyl 2-aminopyridine-3-carboxylate (5.17 g, 34.0 mmol) and acetic anhydride (20 mL) were heated to 110 °C for 1 h in a mixture of trimethyl orthoacetate (50 mL) and acetic anhydride (20 mL), whereupon the colorless solution turned yellow. After 1 h, methyl acetate began to distill off and heating was continued for 5 h. The excess reagents were removed in vacuo leaving a red oil and white syrup. This was taken up in Et2O (100 mL) and washed with aq Na2CO3 (2 M, 50 mL × 2), water (50 mL), and brine (50 mL × 2), and then dried. The residue was purified by Kugelrohr distillation (140–160 °C at 1.3 mmHg) to yield 24 as a yellow oil which smelled like sugar snap peas (2.00 g, 28%). HRMS-ESI (m/z): [M + H]+ calcd for C10H13N2O3, 209.0926; found, 209.0930. 1H NMR (CDCl3): δ 8.51 (dd, J = 4.8, 1.6 Hz, 1H), 8.21 (dd, J = 7.6, 2.0 Hz, 1H), 7.06 (dd, J = 8.0, 4.8 Hz, 1H), 3.87 (s, 3H), 3.86 (s, 3H), 1.87 (s, 3H). 13C NMR (CDCl3): δ 165.9, 163.1, 160.7, 152.3, 140.0, 118.3, 117.6, 53.9, 52.1, 17.2.
2-Methoxy-1,8-naphthyridin-4(1H)-one (25)
A solution of 24 (1.9 g, 9.1 mmol) in dry THF (10 mL) was added dropwise to a slurry of LDA (10 mmol) in THF–hexane (10 mL) at −50 °C under Ar, whereupon the solution turned bright orange. After 1 h, the temperature was raised to 0 °C. The solution was stirred for another 30 min and quenched with cold satd. NH4Cl solution (25 mL) followed by Na2CO3 solution (2 M, 50 mL). The aqueous layer was separated off, washed with Et2O (50 mL × 2), and carefully neutralized with hydrochloric acid (1 M) to give a white precipitate. Product remaining in the mother liquor was extracted into BuOH (50 mL × 4), which was washed with brine (50 mL × 2), and dried. The product was isolated by HPLC [PFP column; MeOH–NH4HCO2 buffer (25 mM, pH 4); 35:65] to give 25 as a cream solid (1.2 g, 76%). mp ~160 °C (dec; if ramping was omitted: ~200 °C dec). HRMS-ESI (m/z): [M + H]+ calcd for C9H9N2O2, 177.0664; found, 177.0665. 1H NMR (HFIP-d2): δ 8.82 (dd, J = 8.0, 1.2 Hz, 1H), 8.64 (dd, J = 4.8, 1.6 Hz, 1H), 7.57 (dd, J = 8.0, 5.2 Hz, 1H), 6.27 (s, 1H), 4.11 (s, 3H). 13C NMR (HFIP-d2): δ 164.2, 149.9, 146.8, 136.9, 119.5, 116.7, 90.6, 55.3.
4-Chloro-2-methoxy-1,8-naphthyridine (26)
Method A: A slurry of 2,4-dichloro-1,8-naphthyridine (4.95 g, 24.9 mmol) in dry toluene (50 mL) was added to a slurry of NaOMe (5.0 g, 93 mmol) in toluene (50 mL) at rt. The temperature rose to 32 °C as the yellow solid dissolved to give a brown solution [Note-NaOMe should be broken up periodically if needed]. After 17 h, the mixture was filtered through diatomaceous earth, and washed with toluene. The solvent was removed and the residue recrystallized (aq EtOH) to give 26 as fine, light yellow needles (3.87 g). Concentration of the mother liquor yielded more product (250 mg; 4.12 g; total, 85%). mp 134–135 °C. HRMS-ESI (m/z): [M + H]+ calcd for C9H8ClN2O, 195.0325; found, 195.0327. 1H NMR (CDCl3): δ 8.99 (dd, J = 4.4, 2.0 Hz, 1H), 8.48 (dd, J = 8.0, 2.0 Hz, 1H), 7.45 (dd, J = 8.0, 4.4 Hz, 1H), 7.12 (s, 1H), 4.16 (s, 3H). 13C NMR (CDCl3): δ 164.5, 155.4, 153.6, 143.7, 133.6, 120.5, 118.2, 114.0, 54.5. Method B: Compound 25 (1.20 g, 6.82 mmol) in POCl3 (10 mL) was heated to 110 °C under Ar for 19 h to give an orange solution. The solution was cooled and carefully quenched by slow addition to water (100 mL) and neutralized with NH4OH. This product was extracted into CHCl3 (100 mL), washed with water (100 mL) followed by brine (100 mL × 2), and then dried. The product was isolated by HPLC [Gemini column; MeOH– NH4HCO2 buffer (25 mM, pH 7); 65:35] of the residue gave 26 as a pale yellow solid (41 mg, 3%). mp 132–133 °C.
2-(2-Methoxy-1,8-naphthyridin-4-yloxy)-N-methyl-N-phenylpropanamide (27)
2-Hydroxy-N-methyl-N-phenylpropanamide (197 mg, 1.10 mmol) and NaH (44 mg, 1.10 mmol; 60%) were stirred in dry DMF (1.0 mL) at rt for 4 h. The red solution was transferred via cannula into a mixture of 26 (195 mg, 1.00 mmol) in DMSO–DMF (1:1; 2.0 mL). The reaction stalled after 30 min so the product was isolated with HPLC [PFP column; MeOH–NH4HCO2 buffer (25 mM, pH 7); 75:25] followed by [Gemini column; MeOH–NH4HCO2 buffer (25 mM, pH 7); 70:30] to yield 27 as small, colorless needles (112 mg, 33%). mp 151–152 °C. HRMS-ESI (m/z): [M + H]+ calcd for C19H20N3O3, 338.1505; found, 338.1499. 1H NMR (CDCl3): δ 8.88 (dd, J = 4.4, 2.0 Hz, 1H), 8.39 (dd, J = 8.4, 2.0 Hz, 1H), 7.43–7.34 (3H), 7.28–7.25 (3H), 5.94 (s, 1H), 4.88 (q, J = 6.8 Hz, 1H), 4.11 (s, 3H), 3.33 (s, 3H), 1.61 (d, J = 6.8 Hz, 3H). 13C NMR (CDCl3): δ 169.3, 165.9, 161.5, 156.0, 152.9, 142.3, 132.0, 130.2, 128.7, 127.2, 119.0, 113.8, 92.5, 71.9, 54.1, 38.2, 17.9.
2-(2-Aminoquinolin-4-yloxy)-N-methyl-N-phenylpropanamide (28)
2-Amino-4-hydroxyquinoline hydrate was dried in an Abderhalden pistol under high vacuum (T = 110 °C) in the presence of P2O5. A slurry of this quinoline (3.70 g, 23.1 mmol) with sodium ethoxide (24 mmol) in ethanol (9.1 mL) was heated to 70 °C, whereupon a red solution formed. The solvent was distilled off, leaving a white solid, which was dried under vacuum until the internal temperature returned to 70 °C. Dry DMF (20 mL) was added to the solid and the slurry heated to 90 °C. 2-Bromo-N-methyl-N-phenylpropanamide (5.87 g, 24.3 mmol) was added in one portion. Heating was continued for 30 min, whereupon NaBr gradually precipitated. The mixture was cooled to rt poured into water (200 mL), and extracted into CH2Cl2 (150 mL × 3). The organic extracts were washed with water (40 mL × 5), brine (250 mL × 2), and finally dried (K2CO3). Recrystallization (aq EtOH) of the white solid gave light yellow crystals, which were dried in an Abderhalden pistol under high vacuum (T = 110 °C) in the presence of P2O5 to give 28 (4.50 g, 61%). mp 182–184 °C. HRMS-ESI (m/z): [M + H]+ calcd for C19H20N3O2, 322.1556; found, 322.1552. 1H NMR (CDCl3): δ 7.83 (d, J = 8.0 Hz, 1H), 7.54 (q, J = 8.0 Hz, 1H), 7.54 (s, 1H), 7.30–7.28 (3H), 7.19–7.14 (3H), 5.67 (s, 1H), 4.90 (q, J = 6.4 Hz, 1H), 4.67 (s, 2H), 3.31 (s, 3H), 1.60 (d, J = 6.4 Hz, 3H). 13C NMR (CDCl3): δ 169.7, 161.0, 158.0, 148.6, 142.4, 130.2, 129.9, 128.4, 127.2, 125.3, 122.3, 121.9, 117.7, 90.5, 71.9, 38.4, 17.8.
2-[2-(1H-Pyrrol-1-yl)quinolin-4-yloxy]-N-methyl-N-phenylpropanamide (29)
2,5-Dimethoxytetrahydrofuran (195 µL, 1.50 mmol) was added to a pale yellow solution of 28 (321 mg, 1.00 mmol) in acetic acid (4 mL) at 120 °C (bath temp.), causing the solution to turn red. This solution was heated for 25 min, cooled to rt, poured into CH2Cl2 (100 mL), and washed with NH4OH (50 mL × 2), water (50 mL), and brine (50 mL × 2), and finally dried. The product was isolated with HPLC [PFP column; MeCN–NH4CH3CO2 buffer (25 mM, pH 7); 70:30] to give 29 as a pale pink solid (90 mg, 24%). mp 130 °C dec. HRMS-ESI (m/z): [M + H]+ calcd for C23H22N3O2, 372.1712; found, 372.1713. 1H NMR (CDCl3): δ 8.07 (dd, J = 8.0, 0.4 Hz, 1H), 7.88 (d, J = 8.4 Hz, 1H), 7.88 (ddd, J = 8.4, 7.2, 1.6 Hz, 1H), 7.52 (d, J = 4.4 Hz, 1H), 7.52 (s, 1H), 7.40 (ddd, J = 8.0, 6.8, 1.2 Hz, 1H), 7.37–7.31 (3H), 7.23–7.21 (2H), 6.41 (s, 1H), 6.40 (t, J = 2.0 Hz, 2H), 5.04 (q, J = 6.4 Hz, 1H), 3.32 (s, 3H), 1.67 (d, J = 6.4 Hz, 3H). 13C NMR (CDCl3): δ 169.4, 161.9, 150.7, 148.0, 142.3, 130.7, 130.2, 128.7, 127.9, 127.3, 124.7, 122.4, 120.0, 118.6, 111.4, 91.8, 71.8, 38.3, 18.1.
3-Chloro-1,5,5-trimethylpyrrolidin-2-one (31)
t-Butyl lithium (1.64 M; 17 mmol) in pentane (2.3 mL) was added dropwise to a solution of 2-bromomesitylene (fractionally distilled from CaH2) (2.8 mL, 18 mmol) in dry THF (30 mL) held between −70 °C and −60 °C, whereupon a white precipitate formed. The suspension was warmed to −20 °C and then cooled back to −70 °C. A solution of 1,5,5-trimethylpyrrolidin-2-one (fractionally distilled from BaO) (2.3 mL, 17 mmol) in dry THF (25 mL) was added dropwise. The reaction mixture was warmed to 0 °C for 30 min, and then transferred dropwise via a cannula into a solution of hexachloroethane (4.17 g, 17.6 mmol) in dry THF (40 mL) held between −78 °C and −70 °C. After 1 h, the solution was warmed to rt and stirred for another 1 h. The solvent was removed and the residue taken up in CH2Cl2 (200 mL), and then filtered through diatomaceous earth several times until the solution was no longer cloudy. The solvent was removed and the residual yellow oil shaken with water (100 mL), causing separation of a white oil. The liquid layer was decanted from this oil, filtered through diatomaceous earth, and extracted with CHCl3 (100 mL × 2). The extracts were washed with brine (100 mL ×2) and dried to give a white waxy solid that was contaminated with 3-bromo-1,5,5-trimethylpyrrolidin-2-one (~13%). This byproduct could not be removed either by recrystallization (hexanes) or by sublimation (65 °C at 0.2 mmHg). Therefore, the solid was taken up in DMF (2 mL) and stirred with LiCl (510 mg, 12 mmol) for several hours. The mixture was diluted with water (10 mL), extracted into CHCl3 (10 mL × 2), washed with brine (10 mL × 2), and dried to give white crystals which were triturated with cold hexanes to give 31 (1.12 g, 41%). mp 74–75 °C. HRMS-ESI (m/z): [M + H]+ calcd for C7H13ClNO, 162.0686; found, 162.0691. 1H NMR (CDCl3): δ 4.45 (ddd, J = 8.4, 5.6, 0.4 Hz, 1H), 2.81 (s, 3H), 2.48 (dd, J = 14.0, 8.4 Hz, 1H), 2.17 (dd, J = 14.0, 5.6 Hz, 1H), 1.37 (s, 3H), 1.24 (s, 3H). 13C NMR (CDCl3): δ 169.2, 58.8, 53.9, 44.3, 26.5, 26.3, 24.9.
3-Chloro-1-isopropylpyrrolidin-2-one (32)
This compound was synthesized in the same manner as 31 using 1-isopropylpyrrolidin-2-one (9.3 g, 73 mmol). The workup was modified slightly in that the reaction mixture was quenched with satd. NaHCO3 (100 mL) and filtered through diatomaceous earth. The organic layer was separated off, and the aqueous layer extracted with CHCl3 (100 mL × 3). The combined extracts were washed with brine (300 mL × 2) and dried. The residual oil was worked up as for 31. No brominated byproduct was present so the product was fractionally distilled (bp 68–70 °C at 0.9 mmHg) to give 32 as a colorless oil (8.2 g, 70%). HRMS-ESI (m/z): [M + H]+ calcd for C7H13ClNO, 162.0686; found, 162.0690. 1H NMR (CDCl3): δ 4.38 (dd, J = 7.6, 4.4 Hz, 1H), 4.36 (sept, J = 6.8 Hz, 1H), 3.48 (ddd, J = −13.6, 6.8, 6.4 Hz, 1H), 3.32 (ddd, J = −13.6, 7.6, 4.0 Hz, 1H), 2.53 (dddd, J = −14.0, 7.6, 7.6, 6.4 Hz, 1H), 2.23 (dddd, J = −14.0, 7.6, 4.0, 4.0 Hz, 1H), 1.18 (d, J = 6.8 Hz, 3H), 1.16 (d, J = 6.8 Hz, 3H). 13C NMR (CDCl3): δ 169.4, 55.7, 43.4, 39.4, 29.8, 19.8, 19.4.
1,5,5-Trimethyl-3-[2-(pyridin-2-yl)quinolin-4-yloxy]pyrrolidin-2-one (33)
2-(Pyridin-2-yl)-quinolin-4(1H)-one (222 mg, 1.00 mmol), 31 (210 mg, 1.40 mmol), and K2CO3 (866 mg, 6.28 mmol) in dry MeCN (35 mL) were heated to 80 °C for 70 h. The mixture was cooled to rt, poured into water (300 mL) and kept at 4 °C overnight. The precipitate that formed was recrystallized (hexanes–Et2O) to give 33 as large white prisms (201 mg, 58%). mp 131–133 °C. HRMS-ESI (m/z): [M + H]+ calcd for C21H22N3O2, 348.1712; found, 348.1712. 1H NMR (CDCl3): δ 8.72 (ddd, J = 4.8, 1,6, 0.8 Hz, 1H), 8.66 (d, J = 8.0 Hz, 1H), 8.26 (dd, J = 8.4, 0.8 Hz, 1H), 8.12 (s, 1H), 8.12 (d, J = 8.4 Hz, 1H), 7.90 (dt, J = 8.0, 2.0 Hz, 1H), 7.71 (ddd, J = 8.4, 6.8, 1.6 Hz, 1H), 7.50 (ddd, J = 8.4, 6.8, 1.2 Hz, 1H), 7.36 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 5.40 (dd, J = 7.6, 5.6 Hz, 1H), 2.90 (s, 3H), 2.67 (dd, J = 13.6, 8.0 Hz, 1H), 2.19 (dd, J = 13.6, 8.0 Hz, 1H), 1.40 (s, 3H), 1.37 (s, 3H). 13C NMR (CDCl3): δ 169.3, 161.1, 157.2, 156.3, 149.1, 149.0, 136.9, 130.0, 129.2, 125.9, 124.1, 122.2, 121.9, 121.5, 99.5, 75.0, 58.3, 41.3, 27.3, 26.6, 24.6.
1-Isopropyl-3-[2-(pyridin-2-yl)quinolin-4-yloxy]pyrrolidin-2-one (34)
2-(Pyridin-2-yl)-quinolin-4(1H)-one (222 mg, 1.00 mmol), 32 (160 µL, 1.12 mmol), and K2CO3 (834 mg, 6.03 mmol) in dry MeCN (35 mL) were heated to 80 °C for 7 d. The red mixture was cooled to rt, filtered through diatomaceous earth, taken up into CH2Cl2 (100 mL), washed with water (50 mL), and brine (50 mL × 2), and then dried. The product was isolated with HPLC [PFP column; MeOH–NH4CH3CO2 buffer (25 mM, pH 7); 75:25] followed by trituration with aq MeOH (50% v/v) to give 34 as a white solid (167 mg, 48%). mp 108–110 °C. HRMS-ESI (m/z): [M + H]+ calcd for C21H22N3O2, 348.1712; found, 348.1712. 1H NMR (CDCl3): δ 8.70 (ddd, J = 4.8, 1,6, 0.8 Hz, 1H), 8.66 (ddd, J = 8.0, 1.2, 1.2 Hz, 1H), 8.28 (dd, J = 8.4, 1.2 Hz, 1H), 8.14 (s, 1H), 8.10 (d, J = 8.4 Hz, 1H), 7.86 (dt, J = 8.0, 2.0 Hz, 1H), 7.71 (ddd, J = 8.4, 6.8, 1.2 Hz, 1H), 7.50 (ddd, J = 8.0, 6.8, 0.8 Hz, 1H), 7.34 (ddd, J = 7.2, 4.8, 1.2 Hz, 1H), 5.38 (dd, J = 7.6, 7.2 Hz, 1H), 4.50 (sept, J = 6.8 Hz, 1H), 3.48 (ddd, J = −13.2, 10.0, 3.6 Hz, 1H), 3.42 (ddd, J = −13.6, 7.2, 2.4 Hz, 1H), 2.83 (dddd, J = −13.6, 10.0, 7.6, 3.6 Hz, 1H), 2.26 (dddd, J = −13.6, 8.8, 7.2, 1.6 Hz, 1H), 1.26 (d, J = 6.8 Hz, 3H), 1.22 (d, J = 6.8 Hz, 3H). 13C NMR (CDCl3): δ 169.1, 161.2, 157.3, 156.3, 149.2, 148.9, 136.9, 130.0, 129.2, 125.9, 124.1, 122.3, 121.8, 121.5, 99.5, 75.9, 43.2, 38.5, 26.2, 19.8, 19.5.
2-Nitro-N-(pyridin-2-ylmethyl)aniline (35)
2-(Aminomethyl)pyridine (4.9 mL, 48 mmol), 1-fluoro-2-nitrobenzene (5.5 mL, 52 mmol), and K2CO3 (13 g, 95 mmol) in DMF (35 mL) were heated for 19 h (90 °C, bath temp.). The blood red mixture was then allowed to cool, poured into citrate buffer (pH 7; 450 mL), and extracted with CH2Cl2 (250 mL × 2). The organic layer was then washed with water (350 mL × 5), followed by brine (350 mL), and then dried. The residue was sonicated in Et2O (100 mL × 2), filtered through diatomaceous earth, and taken up into rapidly stirring hydrochloric acid (0.1 M; 500 mL). The biphasic mixture was filtered through diatomaceous earth, and the aqueous layer was separated off, washed with Et2O (200 mL × 2), and neutralized with NH4OH. The resultant precipitate was recrystallized twice (aq EtOH) to yield 35 as tiny golden needles (862 mg, 8%). mp 92–93 °C. HRMS-ESI (m/z): [M + H]+ calcd for C12H12N3O2, 230.0930; found, 230.0937. 1H NMR (CDCl3): δ 8.87 (s, 1H), 8.64 (ddd, J = 4.8, 1.6, 0.8 Hz, 1H), 8.21 (dd, J = 8.4, 1.6 Hz, 1H), 7.68 (ddd, J = 7.6, 7.6, 1.6 Hz, 1H), 7.40 (ddd, J = 8.4, 6.8 Hz, 1H), 7.32 (d, J = 8.0 Hz, 1H), 7.23 (ddd, J = 7.6, 4.8, 0.8 Hz, 1H), 6.83 (dd, J = 8.4, 0.8 Hz, 1H), 6.68 (ddd, J = 8.4, 6.8, 1.2 Hz, 1H), 4.68 (d, J = 5.2 Hz, 1H). 13C NMR (CDCl3): δ 156.6, 149.6, 145.0, 136.9, 136.2, 132.4, 126.9, 122.6, 121.2, 115.8, 114.3, 48.4.
N-Isopropyl-N-methyl-2-(2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yloxy)propanamide (36)
NaH (480 mg, 2.00 mmol) in mineral oil (60% w/v) was added to a solution of 35 (229 mg, 1.00 mmol) in DMF (20 mL) at rt, which turned from bright yellow to deep red-brown. The solution was heated to 55 °C for 1 h. A solution of 16 (315 µL, 2.00 mmol) in DMF (5 mL) was then added and heating continued for another 1 h. The solution was then cooled and the solvent removed under reduced pressure. The residue was taken up into water (100 mL) and extracted with CHCl3 (50 mL × 3). The combined extracts were then washed with water (50 mL) followed by brine (50 mL × 2), and then dried. The product was isolated with HPLC [PFP column; MeOH–NH4HCO2 buffer (25 mM, pH 7); 70:30] to give 36 as an orange syrup (240 mg, 71%). HRMS-ESI (m/z): [M + H]+ calcd for C19H23N4O2, 339.1821; found, 339.1825. 1H NMR (CDCl3, cis–trans; 1.3:1.0): δ 8.76–8.73 (m, 2H, cis + trans), 8.32 (s, 1H, cis), 8.30 (s, 1H, trans), 7.90–7.85 (m, 2H, cis + trans), 7.76–7.72 (m, 3H, 2 cis + trans), 7.68 (m, 1H, trans) 7.41 (ddd, J = 7.6, 4.0, 1.2 Hz, 1H, trans), 7.40 (ddd, J = 7.6, 4.0, 1.2 Hz, 1H, cis), 7.35–7.28 (4H, 2 cis + 2 trans), 6.06 (q, J = 6.4 Hz, 1H, trans), 5.99 (q, J = 6.4 Hz, 1H, cis), 4.83 (sept, J = 6.8 Hz, 1H, cis), 4.13 (sept, J = 6.8 Hz, 1H, trans), 2.74 (s, 3H, trans), 2.60 (s, 3H, cis), 1.63 (d, J = 6.0 Hz, 3H, cis), 1.62 (d, J = 6.0 Hz, 3H, trans), 1.10 (d, J = 6.4 Hz, 3H, trans), 1.04 (d, J = 6.8 Hz, 3H, cis), 0.78 (d, J = 6.8 Hz, 3H, cis), 0.63 (d, J = 6.4 Hz, 3H, trans). 13C NMR (CDCl3): δ 169.5 (cis), 169.4 (trans), 149.2 (cis), 148.7 (trans), 147.3 (trans), 147.2 (cis), 137.8 (cis + trans), 136.9 (cis + trans), 133.7 (trans), 133.5 (cis), 124.7 (trans), 124.6 (cis), 124.3 (cis + trans), 123.3 (cis + trans), 120.2 (trans), 120.1 (cis), 111.1 (cis), 111.0 (trans), 80.8 (cis), 80.1 (trans), 47.7 (trans), 44.5 (cis), 27.8 (cis), 26.6 (trans), 20.8 (trans), 19.9 (trans), 19.3 (cis), 18.7 (cis), 16.8 (trans), 16.6 (cis).
Radiochemistry
[11C]Methyl triflate
[11C]Methyl triflate was prepared from cyclotron-produced [11C]carbon dioxide, via conversion into [11C]methane by reduction with hydrogen over palladium, direct iodination of [11C]methane, and passage of the generated [11C]methyl iodide over heated silver triflate [43].
[11C]11
Compound 19 (0.55 mg, 1.5 µmol) was treated with [11C]methyl triflate in the presence of aq NaOH (0.5 M, 1 eq.) in dry MeCN (300 µL) at rt for 5 min. The radioligand was isolated with HPLC [XBridge column; MeCN–NH4OH buffer (1 mM, pH 7.7); 65:35] at 6 mL/min (tR = 11 min). The isolated product was taken up in ethanol–saline (10:90, v/v) containing polysorbate 80 (12 mg) and sterile filtered (Millex-MP 0.22 µm, 25 mm). The radiochemical purity of [11C]11 was >99% as established by HPLC [Xbridge column; MeCN– NH4HCO2 buffer (0.1 M, pH 6.3); 50:50] at 2 mL/min (tR = 9.2 min). Product identity was also confirmed by LC-MS of associated carrier. The average decay-corrected radiochemical yield of [11C]11 was 17% from cyclotron-produced [11C]carbon dioxide and the average specific activity was 244 GBq/µmol at the end of synthesis (n = 7), corresponding to about 40 min from the end of radionuclide production.
[11C]20
Compound 18 (0.80 mg, 2.2 µmol) was treated with [11C]MeOTf in the presence of excess NaH in dry MeCN (300 µL) at rt for 5 min The radioligand was isolated by HPLC [Luna column; MeCN–NH4HCO2 (0.1 mM); 45:55] at 6 mL/min (tR = 12 min). The isolated product was formulated as for [11C]11. HPLC analysis of [11C]20, as for [11C]11, showed > 99% radiochemical purity (tR = 6.1 min). LC-MS of associated carrier confirmed product identity. The average decay-corrected radiochemical yield of [11C]20 was 21% from cyclotron-produced [11C]carbon dioxide and the average specific activity was 126 GBq/µmol at the end of radiosynthesis (n = 4).
LogD determinations
These were performed on [11C]11 and [11C]20 of high radiochemical purity (>99.4%), for distribution between n-octanol and sodium phosphate buffer (pH 7.4, 0.15 M) at rt, essentially by the methodology that we have described previously [14]. The chemical stabilities of these radioligands in the buffer for the duration of the measurements were verified by HPLC analysis. Neither radioligand was adsorbed on the wall of the test tube during distribution between phases.
Determination of ligand binding affinities for rat brain TSPO
All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the NIMH Animal Care and Use Committee.
Binding assays were performed as previously described [27], except that crude rat brain homogenates were used instead of mitochondrial fractions. Data were analyzed with Prism 5 nonlinear regression curve-fitting software (GraphPad Prism; San Diego, CA). Briefly, whole rat brains from Sprague-Dawley rats were homogenized in cold HEPES buffer (50 mM; pH 7.4) with a Teflon pestle and Glas-Col Homogenizing System. The homogenates were centrifuged at 20,000g for 15 min at 4 °C. The pellets were then resuspended, aliquotted into various vials, and stored at −80 °C. A self-displacement assay on 1 was used as a control along with each assay of test ligand with [3H]1 as reference radioligand. The individually calculated control KD values for 1 were compared to the reported value of 0.707 nM [27] as an assurance of the correctness of results obtained on test ligands. The KD value of 0.707 nM for 1 was used as the dissociation constant to calculate Ki values for test ligands.
Pharmacological Screening
Compound 11 was screened at the Psychoactive Drug Screening Program [51] for inhibition of binding at 10 µM concentration (n = 4) to a wide range of human receptors: AMY 1A, 1B, 1D, 2A, 2B and 2C; benzodiazepine; β2; serotonin 1A, 1B, 1D, 1E, 2A, 2B, 2C, 3, 5A, 6, and 7; dopamine 2–5; opiate δ, κ, and µ; muscarinic 1–5; σ1R and σ2R; GABAA; histamine 1 and 4; and human transporters (DAT, NET, SERT).
Supplementary Material
N-methyl-(quinolin-4-yl)oxypropanamides were synthesized for development of improved radioligands for PET imaging of brain TSPO
Lead analogs were modified to optimize in vitro binding affinity and computed lipophilicity
Two TSPO ligands with high affinity and moderate lipophilicity were radiolabeled with C-11 in high purity
Acknowledgments
This study was supported by the Intramural Research Program of the National Institutes of Health (NIH), specifically the NIMH (ZIA MH0023793 and ZIAMH002852). We thank the NIH Clinical Center PET Department (Chief: Dr. Peter Herscovitch) for radioisotope production.
ABBREVIATIONS
- DIPEA
diisopropylethylamine
- HFIP
hexafluoroisopropyl alcohol
- LDA
lithium diisopropylamide
- MTBE
methyl tert-butyl ether
- PFP
pentafluorophenyl
- PyBroP
bromotripyrrolidinophosphonium hexafluorophosphate
- TEA
triethylamine
- TSPO
translocator protein (18 kDa)
- LipE
lipophilic efficiency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
This paper was composed with contributions from all authors. All authors approved the final version of the manuscript. CB, synthesized, and characterized the compounds; KJJ tested the ligands in vitro; SSZ measured the lipophilicities; CLM prepared the radioligands; RBI supervised in vitro tests; VWP initiated and supervised the project.
Conflict of Interest
Each author declares there were not any actual or potential conflicts of interest that could have influenced this study.
Appendix A. Supplementary data
Supplementary data related to this article can be found at
REFERENCES
- 1.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang M-R, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 2.McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. U.S.A. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doble A, Malgouris C, Daniel M, Daniel N, Imbault F, Basbaum A, Uzan A, Guérémy C, Le Fur G. Labelling of peripheral-type benzodiazepine binding sites in human brain with [3H]PK 11195: anatomical and subcellular distribution. Brain Res. Bull. 1987;18:49–61. doi: 10.1016/0361-9230(87)90033-5. [DOI] [PubMed] [Google Scholar]
- 4.Benavides J, Fage D, Carter C, Scatton B. Peripheral type benzodiazepine binding sites are a sensitive indirect index of neuronal damage. Brain Res. 1987;421:167–172. doi: 10.1016/0006-8993(87)91287-x. [DOI] [PubMed] [Google Scholar]
- 5.Owen DRJ, Matthews PM. Imaging brain microglial activation using positron emission tomography and translocator protein-specific radioligands. In: Guest PC, Bahn S, editors. Biomarkers of Neurological and Psychiatric Disease. Vol. 101. Waltham, MA: Academic Press; 2011. pp. 19–39. [DOI] [PubMed] [Google Scholar]
- 6.Stefaniak J, O’Brien J. Imaging of neuroinflammation in dementia: a review. J. Neurol., Neurosurg. Psychiatry. 2016;87:21–28. doi: 10.1136/jnnp-2015-311336. [DOI] [PubMed] [Google Scholar]
- 7.Vivash L, O’Brien TJ. Imaging microglial activation with TSPO PET: lighting up neurologic diseases? J. Nucl. Med. 2016;57:165–168. doi: 10.2967/jnumed.114.141713. [DOI] [PubMed] [Google Scholar]
- 8.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72:268–275. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa B, Da Pozzo E, Martini C. Translocator protein as a promising target for novel anxiolytics. Curr. Top. Med. Chem. 2012;12:270–285. doi: 10.2174/156802612799078720. [DOI] [PubMed] [Google Scholar]
- 10.Camsonne R, Moulin MA, Crouzel C, Syrota A, Mazière M, Comar D. Marquage au carbone 11 du PK11195 & visualisation des récepteurs périphériques des benzodiazepines par tomographie à émission positrons. J. Pharmacol. 1986;17:383–383. [Google Scholar]
- 11.Shah F, Hume SP, Pike VW, Ashworth S, McDermott J. Synthesis of the enantiomers of [N-methyl-11C]PK 11195 and comparison of their behaviors as radioligands for PK binding sites in rats. Nucl. Med. Biol. 1994;21:573–581. doi: 10.1016/0969-8051(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 12.Debruyne JC, Van Laere KJ, Versijpt J, De Vos F, Eng JK, Strijckmans K, Santens P, Achten E, Slegers G, Korf J, Dierckx RA, De Reuck JL. Semiquantification of the peripheral-type benzodiazepine ligand [11C]PK11195 in normal human brain and application in multiple sclerosis patients. Acta Neurol. Belg. 2002;102:127–135. [PubMed] [Google Scholar]
- 13.Roivainen A, Någren K, Hirvonen J, Oikonen V, Virsu P, Tolvanen T, Rinne JO. Whole-body distribution metabolism of [N-methyl-11C](R)-1-(2-chlorophenyl)-N-(1-methylpropyl)-3-isoquinolinecarboxamide in humans; an imaging agent for in vivo assessment of peripheral benzodiazepine receptor activity with positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:671–682. doi: 10.1007/s00259-008-1000-1. [DOI] [PubMed] [Google Scholar]
- 14.Briard E, Zoghbi SS, Imaizumi M, Gourley JP, Shetty HU, Hong J, Cropley V, Fujita M, Innis RB, Pike VW. Synthesis and evaluation in monkey of two sensitive 11C-labeled aryloxyanilide ligands for imaging brain peripheral benzodiazepine receptors in vivo. J. Med. Chem. 2008;51:17–30. doi: 10.1021/jm0707370. [DOI] [PubMed] [Google Scholar]
- 15.Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T, Hong J, Pike VW, Innis RB. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage. 2008;40:43–52. doi: 10.1016/j.neuroimage.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasuno F, Ota M, Kosaka J, Ito H, Higuchi M, Doronbekov TK, Nozaki S, Fujimura Y, Koeda M, Asada T, Suhara T. Increased binding of peripheral benzodiazepine receptor in Alzheimer’s disease measured by positron emission tomography with [11C]DAA1106. Biol. Psychiatry. 2008;64:835–841. doi: 10.1016/j.biopsych.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Endres CJ, Pomper MG, James M, Uzuner O, Hammoud DA, Watkins CC, Reynolds A, Hilton J, Dannals RF, Kassiou M. Initial evaluation of 11C-DPA-713, a novel TSPO PET ligand, in humans. J. Nucl. Med. 2009;50:1276–1282. doi: 10.2967/jnumed.109.062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golla SSV, Boellaard R, Oikonen V, Hoffmann A, van Berckel BNM, Windhorst AD, Virta J, Haaparanta-Solin M, Luoto P, Savisto N, Solin O, Valencia R, Thiele A, Eriksson J, Schuit RC, Lammertsma AA, Rinne JO. Quantification of [18F]DPA-714 binding in the human brain: initial studies in healthy controls and Alzheimer's disease patients. J. Cereb. Blood Flow Metab. 2015;35:766–772. doi: 10.1038/jcbfm.2014.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickstein LP, Zoghbi SS, Fujimura Y, Imaizumi M, Zhang Y, Pike VW, Innis RB, Fujita M. Comparison of 18F- and 11C-labeled aryloxyanilide analogs to measure translocator protein in human brain using positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:352–357. doi: 10.1007/s00259-010-1622-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Q, Colasanti A, Owen DR, Onega M, Kamalakaran A, Bennacef I, Matthews PM, Rabiner EA, Turkheimer FE, Gunn RN. Quantification of the specific translocator protein signal of 18F-PBR111 in healthy humans: a genetic polymorphism effect on in vivo binding. J. Nucl. Med. 2013;54:1915–1923. doi: 10.2967/jnumed.113.121020. [DOI] [PubMed] [Google Scholar]
- 21.Mizrahi R, Rusjan PM, Kennedy J, Pollock B, Mulsant B, Suridjan I, De Luca V, Wilson AA, Houle S. Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [18F]-FEPPA. J. Cereb. Blood Flow Metab. 2012;32:968–972. doi: 10.1038/jcbfm.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varrone A, Oikonen V, Forsberg A, Joutsa J, Takano A, Solin O, Haaparanta-Solin M, Nag S, Nakao R, Al-Tawil N, Wells LA, Rabiner EA, Valencia R, Schultze-Mosgau M, Thiele A, Vollmer S, Dyrks T, Lehmann L, Heinrich T, Hoffmann A, Nordberg A, Halldin C, Rinne JO. Positron emission tomography imaging of the 18-kDa translocator protein (TSPO) with [18F]FEMPA in Alzheimer's disease patients and control subjects. Eur. J. Nucl. Med. Mol. Imaging. 2015;42:438–446. doi: 10.1007/s00259-014-2955-8. [DOI] [PubMed] [Google Scholar]
- 23.Zanotti-Fregonara P, Zhang Y, Jenko KJ, Gladding RL, Zoghbi SS, Fujita M, Sbardella G, Castellano S, Taliani S, Martini C, Innis RB, Da Settimo F, Pike VW. Synthesis and evaluation of translocator 18 kDa protein (TSPO) positron emission tomography (PET) radioligands with low binding sensitivity to human single nucleotide polymorphism rs6971. ACS Chem. Neurosci. 2014;5:963–971. doi: 10.1021/cn500138n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL, Zoghbi SS, Hyde T, Kleinman JE, Pike VW, McMahon FJ, Innis RB. Biomarkers Consortium PET Radioligand Project Team. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J. Cereb. Blood Flow Metab. 2013;33:53–58. doi: 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen DRJ, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC, Innis RB, Pike VW, Reynolds R, Matthews PM, Parker CA. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J. Nucl. Med. 2011;52:24–32. doi: 10.2967/jnumed.110.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pike VW. Consideration in the development of reversibly binding PET radioligands for brain imaging. Curr. Med. Chem. 2016 doi: 10.2174/0929867323666160418114826. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brouwer C, Jenko K, Zhogbi SS, Innis RB, Pike VW. Development of N-methyl-(2-arylquinolin-4-yl)oxypropanamides as leads to PET radioligands for translocator protein (18 kDa) J. Med. Chem. 2014;57:6240–6251. doi: 10.1021/jm5007947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shultz MD. Setting expectations in molecular optimizations: strengths and limitations of commonly used composite parameters. Bioorg. Med. Chem. Lett. 2013;23:5980–5991. doi: 10.1016/j.bmcl.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Frérot E, Coste J, Pantaloni A, Dufour M-N, Jouin P. PyBOP and PyBroP: two reagents for the difficult coupling of the α,α-dialkyl amino acid, Aib. Tetrahedron. 1991;47:259–270. [Google Scholar]
- 30.Jones CP, Anderson KW, Buchwald SL. Sequential Cu-catalyzed amidation-base-mediated Camps cyclization: a two-step synthesis of 2-aryl-4-quinolones from o-halophenones. J. Org. Chem. 2007;72:7968–7973. doi: 10.1021/jo701384n. [DOI] [PubMed] [Google Scholar]
- 31.Osborne AG, Miller LAD. Regioselective alkoxydehalogenation of 2,4-dihalogenoquinolines and a reinvestigation of the bromination of 2-methoxyquinoline. J. Chem. Soc, Perkin Trans. 1993;1:181–184. [Google Scholar]
- 32.Czuba W, Woźniak M. Róznice w zdolności do wymiany nukleofilowej chlorowców podstawionych w położeniu 2 i 4 1,8-naftyrydyny. Zesz. Nauk. Uniw. Jagielloh., Pr. Chem. 1975;20:61–70. [Google Scholar]
- 33.M’rabet H, M’hirsi S, Zantour H, Baccar B. Action de quelques réactifs électrophiles sur les N-aryl acétimidates. J. Soc. Chim. Tunis. 1994;3:513–521. [Google Scholar]
- 34.Actor P, Berkoff CE, Craig PN, Julius M, Redl G, Grout RJ, Hynam BM, Partridge MW. Antimicrobial 2-amino-4-alkoxyquinolines: synthesis and analysis of structure-activity correlations. Arzneim.-Forsch. 1974;24:8–11. doi: 10.1002/chin.197416334. [DOI] [PubMed] [Google Scholar]
- 35.Clauson-Kaas N, Tyle Z. Preparation of cis and trans 2,5-dimethoxy-2-(acetamidomethyl)-2,5-dihydrofuran, of cis and trans 2,5-dimethoxy-2-(acetamidomethyl)-tetrahydrofuran and of 1-phenyl-2-(acetamidomethyl)-pyrrole. Acta Chem. Scand. 1952;6:667–670. [Google Scholar]
- 36.Beck AK, Hoekstra MS, Seebach D. 1,3-Diketones by 1:1-reactions of Li-enolates with acid chlorides generation of kinetic enolates with mesityl lithium. Tetrahedron Lett. 1977;18:1187–1190. [Google Scholar]
- 37.Vedejs E, Galante RJ, Goekjian PG. Athio-Diels-Alder route to the azocine ring system. Total synthesis of (±)-otonecine. J. Am. Chem. Soc. 1998;120:3613–3622. [Google Scholar]
- 38.Stacy GW, Ettling BV, Papa AJ. Reactions of benzaldehyde with o-nitroaniline. J. Org. Chem. 1964;29:1537–1540. [Google Scholar]
- 39.Takahashi S, Kanō H. Benzimidazole N-oxides. I. The structure of benzimidazole N-oxide and synthesis of its derivatives. Chem. Pharm. Bull. 1963;11:1375–1381. doi: 10.1248/cpb.11.1375. [DOI] [PubMed] [Google Scholar]
- 40.Cai L, Lu S, Pike VW. Chemistry with [18F]fluoride ion. Eur. J. Org. Chem. 2008:2853–2873. [Google Scholar]
- 41.Preshlock S, Tredwell M, Gouverneur V. 18F-Labeling of arenes and heteroarenes for applications in positron emission tomography. Chem. Rev. 2016;116:719–766. doi: 10.1021/acs.chemrev.5b00493. [DOI] [PubMed] [Google Scholar]
- 42.Cappelli A, Anzini M, Vomero S, De Benedetti PG, Menziani MC, Giorgi G, Manzoni C. Mapping the peripheral benzodiazepine receptor binding site by conformationally restrained derivatives of 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide (PK11195) J. Med. Chem. 1997;40:2910–2921. doi: 10.1021/jm960516m. [DOI] [PubMed] [Google Scholar]
- 43.Lee Y-S, Siméon FG, Briard E, Pike VW. Solution structures of the prototypical 18 kDa translocator protein ligand, PK 11195, elucidated with 1H/13C NMR spectroscopy and quantum chemistry. ACS Chem. Neurosci. 2012;3:325–335. doi: 10.1021/cn3000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaremko Ł, Jaremko M, Giller K, Becker S, Zweckstetter M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science. 2014;343:1363–1366. doi: 10.1126/science.1248725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Primofiore G, Da Settimo F, Taliani S, Simorini F, Patrizi MP, Novellino E, Greco G, Abignente E, Costa B, Chelli B, Martini C. N,N-Dialkyl-2-phenylindol-3-ylglyoxylamides. A new class of potent and selective ligands at the peripheral benzodiazepine receptor. J. Med. Chem. 2004;47:1852–1855. doi: 10.1021/jm030973k. [DOI] [PubMed] [Google Scholar]
- 46.Jewett DM. A simple synthesis of [11C]methyl triflate. Appl. Radiat. Isot. 1992;43:1383–1385. doi: 10.1016/0883-2889(92)90012-4. [DOI] [PubMed] [Google Scholar]
- 47.Bischoff CA. Studien über Verkettungen XXVI. Das Aethylanilin. Ber. Dtcsh. Chem. Ges. 1897;30:3178–3180. [Google Scholar]
- 48.Pohmakotr M, Winotai C. α-Hydroxylation of carboxylic acids and amides using bis(trimethylsilyl)peroxide. Synth. Commun. 1988;18:2141–2146. [Google Scholar]
- 49.Williams PD, Perlow DS, Payne LS, Holloway MK, Siegl PKS, Schorn TW, Lynch RJ, Doyle JJ, Strouse JF, Vlasuk GP, Hoogsteen K, Springer JP, Bush BL, Halgren TA, Richards AD, Kay J, Veber DF. Renin inhibitors containing conformationally restricted P1-P1 dipeptide mimetics. J. Med. Chem. 1991;34:887–900. doi: 10.1021/jm00107a004. [DOI] [PubMed] [Google Scholar]
- 50.Kramarova EP, Shipov AG, Orlova NA, Artamkina OB, Belavin IY, Baukov YI. Simple method for the N-alkylation of lactams. J. Gen. Chem. USSR. 1988;58:970–979. [Google Scholar]
- 51.Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang X-P, Norval S, Sassano MF, Shin AI, Webster LA, Simeons FRC, Stojanovski L, Prat A, Seidah NG, Constam DB, Bickerton GR, Read KD, Wetsel WC, Gilbert IH, Roth BL, Hopkins AL. Automated design of ligands topolypharmacological profiles. Nature. 2012;492:215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.