Abstract
Thrombospondin-1 is a secreted matricellular protein that regulates the differentiation and function of many cell types. Thrombospondin-1 is not required for embryonic development, but studies using lineage-committed adult stem cells have identified positive and negative effects of thrombospondin-1 on stem cell differentiation and self-renewal and identified several thrombospondin-1 receptors that mediate these responses. Genetic studies in mice reveal a broad inhibitory role of thrombospondin-1 mediated by its receptor CD47. Cells and tissues lacking thrombospondin-1 or CD47 exhibit an increased capacity for self-renewal associated with increased expression of the stem cell transcription factors c-Myc, Sox2, Klf4, and Oct4. Thrombospondin-1 inhibits expression of these transcription factors in a CD47-dependent manner. However, this regulation differs in some neoplastic cells. Tumor initiating/cancer stem cells express high levels of CD47, but in contrast to nontransformed stem cells CD47 signaling supports cancer stem cells. Suppression of CD47 expression in cancer stem cells or ligation of CD47 by function blocking antibodies or thrombospondin-1 results in loss of self-renewal. Therefore, the therapeutic CD47 antagonists that are in clinical development for stimulating innate anti-tumor immunity may also inhibit tumor growth by suppressing cancer stem cells. These and other therapeutic modulators of thrombospondin-1 and CD47 signaling may also have applications in regenerative medicine to enhance the function of normal stem cells.
Keywords: Self-renewal, c-Myc, tumor initiating cells, cancer, extracellular matrix, matricellular proteins
1. Introduction
The concept that stem cells can give rise to both normal and malignant tissues has attracted growing interest in developing therapeutics to target these cells. Pluripotent cells were first identified in mouse embryos in 1981 (Evans and Kaufman, 1981), and a mouse teratocarcinoma stem cell line was established the same year (Martin, 1981). Pluripotent stem cell lines were first generated from pigs and sheep (Notarianni et al., 1991). Blastocysts produced by in vitro fertilization for clinical purposes were used to develop the first human human embryonic stem cell (ESC) line (Thomson et al., 1998). One approach to circumvent the ethical issues surrounding the use of human embryos for regenerative medicine was to create induced pluripotent stem (iPS) cells using somatic cells isolated from adult tissues. Routine creation of iPS cells was enabled by identification of the four critical stem cell transcription factors cMyc, Sox2, Oct3/4 and Klf4 (Takahashi and Yamanaka, 2006). Forced expression of these four proteins is sufficient to convert various somatic cells into iPS cells. However, the potential of transplanted iPS cells to form teratomas or teratocarcinomas in patients spurred efforts to develop lineage-committed adult stem cells that lack this potential.
Stem cells are also of growing interest in cancer research. Cancers may arise by transformation of tissue stem cells, or transformed somatic cells may activate the self-renewal program of stem cells (Pardal et al., 2003). Regardless of their origin, it is clear that many tumors are sustained by a minor population of tumor initiating cells that share many properties of stem cells. In this review, we examine the role of the matricellular protein thrombospondin-1 (TSP1) and its receptors in stem cell biology, focusing on how TSP1 interactions with its receptors, including CD47, differentially modulate stem cell physiology in normal and neoplastic cells.
2. Stem cells and extracellular matrix
The concept of a stem cell niche encompasses supporting cells, which provide specific cues needed to regulate quiescence of stem cells and maintain their asymmetric division, and a specialized niche extracellular matrix (ECM) that releases growth factors and engages specific ECM receptors on stem cells to control their fate. Signals provided by these ECM receptors mediate dynamic communication between ESCs and their niche (Gattazzo et al., 2014). This ECM is composed of soluble and bound macromolecules that facilitate three-dimensional assembly of stem cells and supporting cells that provides organizing and signaling cues. Changes in stem cell-ECM interactions provide environmental cues that guide wound healing, homeostasis, aging, and stem cell maintenance (Discher et al., 2009; Watt and Fujiwara, 2011).
Thrombospondins are a family of five secreted proteins in vertebrates (Adams and Lawler, 2011). Some thrombospondins are constitutive elements of ECM, but TSP1 and thrombospondin-2 (TSP2) are matricellular proteins that are not constitutive in ECM but are expressed transiently under specific conditions, where they alter cell behavior and ECM remodeling via their interactions with growth factors in the ECM and by engaging specific transmembrane receptors including proteoglycans, integrins, and the nonintegrin receptors CD36, CD47, and CD148 (Calabro et al., 2014; Roberts et al., 2012; Takahashi et al., 2012).
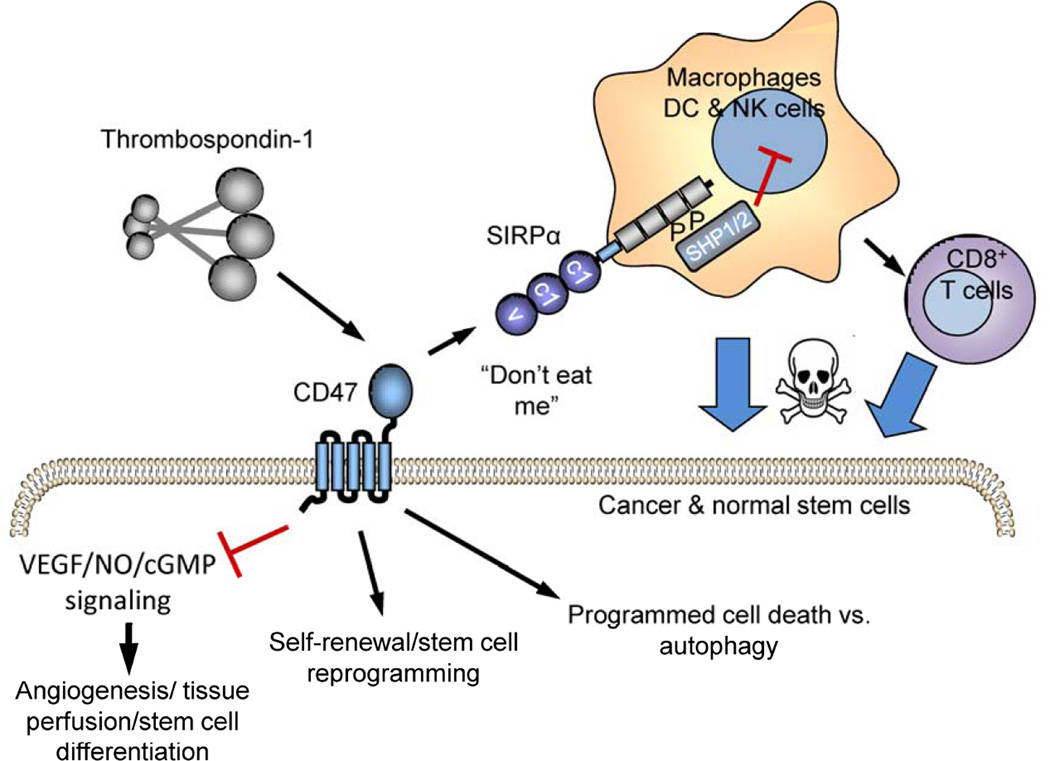
CD47 is a signaling receptor for TSP1 that is ubiquitously expressed, but at a higher level on some stem cells. CD47 consists of an extracellular IgV domain followed by five membrane-spanning segments and a short variably spliced C-terminal cytoplasmic tail (Soto Pantoja, 2013.). TSP1 binding to CD47 results in cell type-specific signaling that can alter cell adhesion, motility, growth, differentiation, and survival (Oldenborg, 2013; Soto-Pantoja et al., 2015) (Fig. 1). Some of these signals are mediated by lateral interactions of CD47 with specific integrins and other signaling receptors in the plasma membrane. CD47 also serves as the counter-receptor for signal regulatory protein-α (SIRPα) and SIRPγ (Barclay and Van den Berg, 2014; Matozaki et al., 2009). SIRPα is highly expressed on phagocytes, and binding of the IgV domain of CD47 on a target cell to the IgV domain of SIRPα on macrophages elicits signaling mediated by the phosphatase SHP1 that blocks phagocytosis of the target cell (Fig. 1) (Oldenborg et al., 2000). Accumulating evidence indicates that CD47 expressed by stem cells serves both as a cell-autonomous signaling receptor and as a SIRPα counter-receptor to limit stem cell clearance. Although less explored in stem cells, SIRPα binding could potentially elicit signaling though CD47 that may be distinct from that produced by TSP1 binding.
Figure 1. CD47 functions as a signaling receptor and a SIRPα counter-receptor.
Cell surface CD47 serves as a signaling receptor for thrombospondin-1. Thrombospondin-1 binding to CD47 limits signaling through the NO/cGMP cascade in vascular and some hematopoietic cell lineages. In nontransformed cells TSP1/CD47 signaling limits stem cell self-renewal. Ligation of CD47 by TSP1 also regulates autophagy signaling and in some cases can induce programmed cell death. CD47 also serves as the counter-receptor for SIRPα and SIRPγ. CD47 binding to SIRPα on macrophages induces phosphorylation of the cytoplasmic domain of SIRPα and recruitment of Src homology region 2 domain-containing phosphatase (SHP)-1 and -2, which inhibits phagocytosis of CD47-expressing stem cells. On dendritic cells, SIRPα signaling inhibits antigen presentation to CD8+ T cells, which can inhibit cytotoxic T cell killing of CSCs. Although not shown here, CD47 is also present and acts as a signaling receptor for TSP1 on macrophages and T cells.
3. TSP1 in hematopoietic stem cell differentiation and self-renewal
A role for TSP1 in hematopoietic stem cells (HSCs) was first reported in 1990 (Long and Dixit, 1990). Nonadherent low density human bone marrow cells were shown to adhere specifically on immobilized TSP1. This was true for mixed-lineage progenitors as well as those from colonies containing erythroid burst-forming cells (BFU-E), and erythroid, granulocyte/macrophage, and megakaryocyte colony-forming cells (CFU-GEMM). This activity was mapped to a large C-terminal region of TSP1 and shown to be inhibited by specific monoclonal antibodies that bind to this domain of TSP1. A subsequent report in 1992 reproduced these results using sorted CD34+DR−CD15− human bone marrow cells that exhibited characteristics of HSCs including self-renewal and the ability to differentiate into multiple hematopoietic lineages (Long et al., 1992). The CD34+DR−CD15− hematopoietic progenitor cells attached on TSP1 but not on immobilized fibronectin. In combination with c-Kit, TSP1 was shown to function as a colony-stimulating factor for CD34+DR−CD15− progenitors. In contrast, TSP1 inhibited colony formation driven by the cytokine IL-3. This suggested that TSP1 is a context-dependent positive and negative regulator of HSC differentiation. Another early study using murine progenitor cells showed that TSP1 significantly inhibited murine megakaryocytopoiesis at a concentration of 1 µg/ml (2.2 nM) (Chen et al., 1997). In contrast to the stimulatory activity described above, this inhibitory activity was reproduced by a recombinant N-terminal domain of TSP1. TSP1 was further shown to inhibit the growth of multipotent hematopoietic colony-forming units (CFU-GEMM) but not those committed to granulocyte/macrophage (CFU-GM) or erythroid (BFU-E) lineages. Studies using a CD36 antibody that inhibits TSP1 binding suggested that the TSP1-induced inhibition of megakaryocytopoiesis is mediated in part by the binding of TSP1 to its receptor CD36 expressed on the megakaryocytic progenitors (Yang et al., 2003). Notably, the N-terminal domain of TSP1 does not contain its CD36-binding site, so it is unclear how the results of these two studies can be rationalized.
4. Thrombospondins in other adult stem cells
Recombinant human TSP1 had a negative effect on the angiogenic potential of human endothelial colony-forming stem cells (Smadja et al., 2011). Suppression of either TSP1 expression or CD47 expression in the endothelial colony-forming cells using siRNA enhanced their angiogenic potential, implicating CD47 as the inhibitory TSP1 receptor in these stem cells. Consistent with the known role of TSP1/CD47 signaling in limiting vascular cell nitric oxide (NO)/cGMP signaling (Soto-Pantoja, 2015), cGMP signaling protected endothelial progenitor cells by suppressing oxidative stress and the expression of TSP1 in a murine salt-sensitive hypertension model (Xie et al., 2010). Salt-induced hypertension was associated with elevated TSP1 expression in this model.
TSP1 promotes the neuronal differentiation of neural progenitor cells (Lu and Kipnis, 2010). Conditioned medium from cultured wild type (WT) astrocytes but not thbs1−/− astrocytes promoted neurogenesis in WT neural progenitor cells. TSP1 also promotes the differentiation of bronchioalveolar stem cells derived from mouse lung into multiple lineages (Lee et al., 2014a). BMP4 signaling induced endothelial cell TSP1 expression via calcineurin/NFATc1 in a 3-dimensional coculture system with bronchioalveolar stem cells. Thbs1−/− endothelial cells were defective in inducing bronchioalveolar stem cell differentiation despite supporting increased colony numbers when cocultured with bronchiolar or alveolar cells. Thbs1−/− stem cells also had no intrinsic differentiation defect. Combined with results from transplantation studies, these data indicate that TSP1 produced by endothelial cells in the lung stem cell niche selectively promotes the alveolar differentiation of bronchioalveolar stem cells in vivo.
Regulation of stem cell differentiation extends to at least one other member of the thrombospondin family. Increased colony-forming activity was found for bone marrow stromal cells isolated from TSP2 null mice (Hankenson et al., 2000). In another study TSP2 secreted by mesenchymal stem cells was reported to promote chondrogenic differentiation in a paracrine manner via PKCα, ERK, p38/MAPK, and Notch signaling pathways (Jeong et al., 2013). TSP1 and TSP2 share several receptors including some integrins, proteoglycans, and CD36 (Calzada and Roberts, 2005), suggesting that both may regulate stem cells via common mechanisms. On the other hand, TSP2 lacks the activities of TSP1 to activate latent TGF-β or bind to α3β1 integrin (Calzada and Roberts, 2005; Schultz-Cherry et al., 1995), so stem cell responses mediated by these pathways may not be shared by TSP2.
5. CD47 is a hematopoietic stem cell marker
Mouse thoracic duct lymph contains HSCs with multilineage potential (Massberg et al., 2007). These cells originate from bone marrow cells that enter into the bloodstream and then enter into peripheral lymphatics and return to bone marrow via the bloodstream. This suggests that a highly adaptive pool of HSCs continuous recirculates and, by differentiating locally, can rapidly generate immune effector cells. CD47 is highly expressed on HSCs and has several proposed functions on these cells. One hypothesis is that CD47 expression on HSCs protects these circulating cells from clearance by macrophages that express SIRPα (van den Berg and van der Schoot, 2008). This is supported by observations that engraftment of human hematopoietic cells into immune-compromised mice is supported by the nonobese diabetic (NOD) SIRPα mutation, which enables high affinity recognition of human CD47 (Kwong et al., 2014; Takenaka et al., 2007; Yamauchi et al., 2013). CD47 binding to SIRPα is generally species-specific (Kwong, 2014; Subramanian et al., 2006; Takenaka, 2007), and CD47 in the parental C57Bl/6 mice used by Yamauchi is known to bind only weakly to human CD47 (Kwong, 2014). Conversely, down-regulation of CD47 expression on HSCs from hemophagocytic lymphohistiocytosis patients correlated with their increased engulfment by macrophages (Kuriyama et al., 2012). Thus, increasing CD47/SIRPα interactions can increase hematopoiesis, and decreasing the interaction can limit hematopoiesis. However, other studies have clearly shown that some tissues lacking CD47 can engraft in a WT host (Wang et al., 2010), and the successful hematopoiesis in cd47−/− and sirpacytΔ mice clearly indicates that HSCs can form and mediate hematopoiesis in the complete absence of CD47/SIRPα signaling. Presumably other don’t eat me signals compensate for the missing CD47 in these models (Oldenborg, 2000).
In addition to highly expressing CD47, HSCs have been reported to express SIRPα (Seiffert et al., 2001). This suggests that CD47/SIRP interactions may modulate intercellular signaling between HSCs, but this idea remains to be explored.
In the context of hematopoietic cell recirculation, evidence that CD47 regulates transmigration of monocytes and T cells through endothelial or epithelial monolayers suggested an additional role for CD47 in the trafficking of bone marrow-derived HSCs (Cooper et al., 1995; de Vries et al., 2002; Liu et al., 2002; Liu et al., 2001). However, another study concluded that cd47−/− polymorphonuclear neutrophils have no defect in transmigration. Rather, the lower number of neutrophils at a site of inflammation resulted from a CD47-dependent defect in granulopoiesis (Bian et al., 2013). This was associated with a deficiency in IL-17 levels, suggesting that CD47 indirectly regulates the differentiation of specific hematopoietic lineages. Activity of the CD47 antibody MAb100.1, which inhibited stroma-supported erythropoiesis in vitro of erythroid progenitor cells co-cultured on stromal cells (Furusawa et al., 1998), further implicates CD47 in the lineage-specific differentiation of HSCs.
6. Mechanisms of CD47 function in nontransformed stem cells
6.1 Function of stem cell CD47 as a SIRPα counter-receptor
As shown in Figure 1, one proposed function of CD47 expressed on stem cells is to protect the stem cells from clearance by phagocytic cells. This is a passive function of CD47, where the active signaling is mediated by SIRPα on the phagocytes. Changes in CD47 expression during HSC mobilization are consistent with this function. Experimental mobilization of HSCs using cyclophosphamide/G-CSF showed increased cell surface CD47 levels on cKit+ cells at day 2 (Jaiswal et al., 2009). The highest expression of CD47 on progenitors occurred just prior to entry into the bloodstream, and was maintained during migration to splenic, liver and bone marrow sinusoids. By day 5 when egress stopped, levels returned to near baseline. HSCs in cord blood and peripheral blood similarly showed elevated cell surface CD47 levels. Parallel experiments in WT versus cd47−/− mice indicated that CD47 is not necessary for the migration of HSCs during mobilization. Consistent with other studies, HSCs from cd47−/− mice did not engraft in irradiated WT recipients, and cd47−/− cells failed to colonize WT bone marrow in a cd47−/−/WT parabiosis model. All of these results are consistent with a protective role of CD47 for inhibiting phagocytic clearance of stem cells.
The increased clearance of HSCs with reduced CD47 expression in patients with hemophagocytic lymphohistiocytosis provides additional correlative evidence for a protective function of CD47 in human stem cells (Kuriyama, 2012). This parallels the reduced colonization of hemizygous cd47+/− bone marrow cells relative to WT bone marrow cells when transplanted into an irradiated WT recipient (Jaiswal, 2009). This clearly establishes that reduced CD47 expression on HSC creates a fitness deficit, and this deficit correlates with their increased sensitivity to macrophage phagocytosis. Direct evidence was obtained for preferential macrophage clearance of transplanted cd47+/− cells when macrophages were activated in the recipient mice using lipopolysaccharide. Therefore, under these experimental conditions increased cell surface expression of CD47 on HSCs provides a significant protection against macrophage-mediated clearance.
6.2 CD47 as a signaling receptor for TSP1 in stem cells
Mammalian stem cells resemble the blastomeres of planktonic and benthic organisms with small eggs, and their persistence in adult organisms may contribute to the growth and maintenance of tissues via proliferation and the regulation of organ size via cell loss (Weissman, 2015). Adult stem cells are maintained in a quiescent state but are able to exit quiescence and rapidly expand and differentiate in response to stress or injury to support tissue repair. TSP1 expression is induced in response to certain acute and chronic injuries, and mice lacking TSP1 or CD47 show an increased capacity to recover from such injuries (Isenberg et al., 2007a; Isenberg et al., 2007b; Isenberg et al., 2008a; Isenberg et al., 2008b). CD47 and/or TSP1 inhibition protects tissues in WT mice in the same injury models (Isenberg et al., 2007c; Maxhimer et al., 2009a). Some of these protective responses involve increased NO/cGMP signaling, and other studies demonstrated that TSP1 signaling through CD47 inhibits NO/cGMP signaling (Roberts, 2012). The NO/cGMP pathway also regulates differentiation of stem cells (Mujoo et al., 2011), suggesting that enhanced NO signaling in tissue stem cells may account for some effects of CD47 blockade on tissue regeneration. However, other studies revealed that TSP1 signaling through CD47 regulates stem cell self-renewal by controlling expression of the transcription factors c-Myc, Oct4, Sox2 and Klf4, and blockade of CD47 signaling increases stem cell self-renewal by increasing expression of these four transcription factors (Kaur et al., 2013). Consistent with an inhibitory role of TSP1/CD47 signaling in stem cell maintenance, NO donors were previously shown to dose-dependently induce expression of Oct4 expression in multipotent progenitors isolated from mouse bone marrow (Chu et al., 2008). However, this effect of NO appeared to be independent of cGMP signaling.
thbs1−/− mice have more circulating CD13+/VEGFR-2+/CD45−/CD117+ endothelial progenitor stem cells (EPCs) relative to WT mice (Shaked et al., 2005). The increase in EPCs was suppressed by using a drug targeting CD36, suggesting that this activity of TSP1 is mediated via CD36. On the other hand, EPCs express high levels of CD47, and knockdown of CD47 expression enhanced their cell proliferation and angiogenic potential (Smadja, 2011). Recombinant human TSP1 inhibited the angiogenic potential of EPCs in vitro, which was mediated by CD47 binding. Ischemia and granulocyte macrophage-colony stimulating factor induced mobilization of EPCs for neovascularization (Takahashi et al., 1999). Enhancement of EPCs is a potential strategy for treating ischemic vascular diseases as well as for tissue regeneration, and these studies suggest that therapeutics inhibiting TSP1/CD47 signaling could achieve this goal.
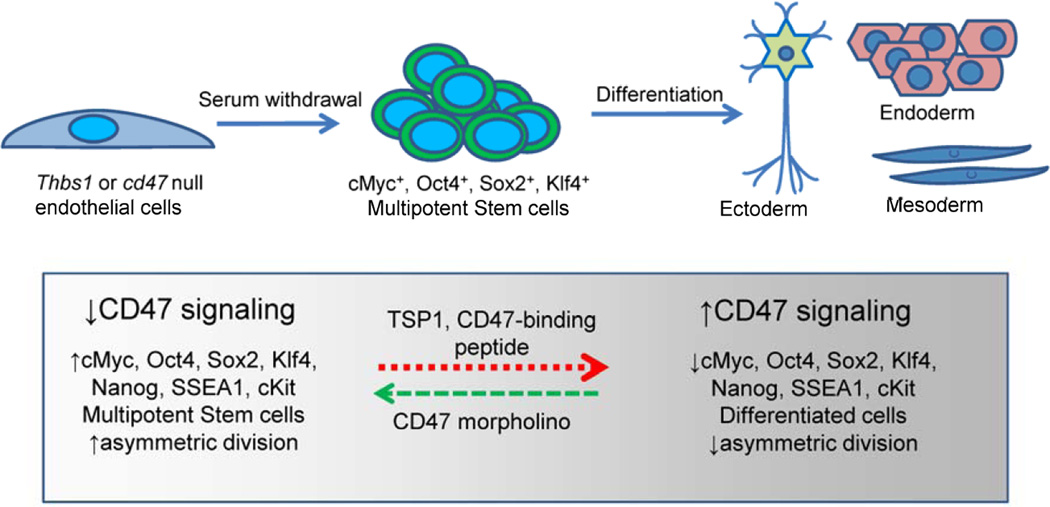
Primary human umbilical vein endothelial cell cultures can be converted to iPS cells by forced expression of the transcription factors Klf4, Oct4, Sox2, and c-Myc (Lagarkova et al., 2010; Panopoulos et al., 2011). We found that primary murine lung endothelial cells isolated from thbs1−/− and cd47−/− mice spontaneously undergo a similar reprogramming to a multipotent stem cell state without requiring transfection or exogenous stem cell growth factors (Kaur, 2013) (Fig. 2). Expression of the four stem cell transcription factors is higher in cd47−/− and thbs1−/− primary lung endothelial cells. WT primary endothelial cells become senescent and stop proliferating when deprived of serum, but the null cells continue growing and spontaneously form multicellular structures consistent with embryoid bodies (EBs). Several pluripotency markers are expressed in these EBs including Nanog, stage-specific embryonic antigen-1 (SSEA1), and cKit. As expected for multipotent stem cells, asymmetric cell division is up-regulated in the induced cd47−/− EBs. The multipotency of these EBs was established by demonstrating their ability to differentiate into mesodermal, ectodermal, and endodermal lineages when exposed to appropriate differentiating growth factors. The four stem cell transcription factors are also expressed at higher levels in some tissues of cd47−/− and thbs1−/− mice, and immunohistochemical analysis indicated that Sox2+ stem cells are more abundant in lung and spleen tissues of these mice.
Figure 2. Model for CD47 and thrombospondin-1 regulation of stem cell reprogramming in nontransformed cells.
Decreased expression or genetic deletion of CD47 or TSP1 leads to spontaneous reprogramming of primary endothelial cells to a multipotent state accompanied by increased expression of the stem cell transcription factors cMyc, Sox2, Oct4 and Klf4. These multipotent stem cells can be differentiated along the three embryonic germ lines. TSP1 and other CD47 ligands can also induce differentiation accompanied by suppression of the stem cell transcription factors and loss of asymmetric cell division.
These studies suggest that suppression CD47 or TSP1 expression could be a therapeutic approach to enhance self-renewal for tissue regeneration. Our previous studies established that therapeutics designed to suppress CD47 expression or TSP1 binding to CD47 enhance the recovery of tissues subjected to ischemic injuries or ionizing radiation in rodents and miniature pigs (Isenberg, 2007c; Isenberg et al., 2008c; Maxhimer, 2009a; Maxhimer et al., 2009b; Soto-Pantoja et al., 2013). Antisense knockdown of CD47 acutely elevated cMyc, Sox2, and Oct4 expression in WT cells (Kaur, 2013). Conversely, re-expression of CD47 in CD47-deficient cells dose-dependently decreased cMyc expression. Dose–dependent suppression of cMyc mRNA by treatment with TSP1 in WT but not in CD47-deficient cells established that TSP1 is the relevant ligand that induces CD47 signaling to suppress stem cell character.
7. TSP1-CD47-SIRPα regulation in bone development
TSP1 is a major regulator of latent transforming growth factor-β1 (TGF-β) activation, and TSP1 control of latent TGF-β activation is critical for regulation of TGF-β activity in some diseases (Nor et al., 2005; Sweetwyne and Murphy-Ullrich, 2012). Following activation from its latent form, TGF-β engages its receptors and induces signaling to regulate bone development and remodeling. Bone marrow-derived mesenchymal stem cells (MSCs) have osteogenic potential and contribute to bone remodeling following injury and during tumor metastasis. Treatment of growing human bone marrow-derived MSCs with TSP1 increases the level of active TGF-β (Bailey Dubose et al., 2012). The MSCs express TSP1, and both TSP1 expression and TGF-β activity decrease during osteoblast differentiation. Exposure to TSP1 and active TGF-β blocks the osteoblastic differentiation of MSCs grown in osteogenic media as measured by decreased expression of Runx2, a key transcription factor associated with osteoblast differentiation, and alkaline phosphatase. The inhibitory effect of TSP1 on osteoblast differentiation results from its ability to activate latent TGF-β. A peptide that blocks TGF-β activation by TSP1 restored osteoblast differentiation assessed by increased Runx2 and alkaline phosphatase expression. A TGF-β neutralizing antibody also increased alkaline phosphatase expression in the presence of TSP1. These studies demonstrate that TSP1 can regulate osteoblast differentiation by activating latent TGF-β. (Bailey Dubose, 2012).
Similarly, CD47 has been implicated in the differentiation of osteoclasts. Parathyroid hormone-stimulated bone marrow cultures derived from cd47−/− mice showed a significant reduction in multinuclear osteoclasts expressing tartrate-resistant acid phosphatase (TRAP+) and production of M-CSF and RANKL as compared with WT bone marrow cultures (Lundberg et al., 2007). Tyrosine phosphorylation of SIRPα was reduced in cd47−/− bone marrow stromal cells. Stromal cells lacking the cytoplasmic signaling domain of SIRPα (sirpacytΔ) also showed defective osteogenic differentiation, and both the cd47−/− and non-signaling sirpacytΔ stromal cells showed reduced ability to support osteoclastogenesis by WT bone marrow macrophages. Thus, CD47-induced SIRPα signaling is critical for stromal cell support of osteoclast formation. These findings were supported by in vivo evidence that femoral bones of 18- or 28-week-old cd47−/− mice contained significantly reduced osteoclast and osteoblast numbers and exhibited an osteopenic bone phenotype. Thus, a lack of CD47 strongly impairs SIRPα-dependent osteoblast differentiation, resulting in impaired bone formation and reduced formation of osteoclasts (Lundberg, 2007). Blocking antibodies against CD47 and SIRPα also reduced the numbers of TRAP+ osteoclasts formed in cultures of murine hematopoietic cells stimulated by M-CSF and RANKL. Further study identified reduced expression of the osteoclastogenic genes nfatc1, Oscar, Trap/Acp, ctr, catK, and dc-stamp in bone marrow cultures from cd47−/− mice stimulated with parathyroid hormone or 1α,25(OH)2-vitamin D3 (Koskinen et al., 2013). Stromal cells lacking either CD47 or the cytoplasmic tail of SIRPα were defective in supporting osteoclastogenesis in WT bone marrow-derived macrophages. Therefore, CD47/SIRPα signaling in stromal cells is necessary for supporting the osteoclast differentiation of bone marrow stem cells.
Proliferation and differentiation of osteoclasts depends upon cell-cell fusion. CD47/ SIRPα interactions have been implicated for macrophage fusion (Han et al., 2000), which plays an important role for bone development and differentiation of osteoclasts (Hobolt-Pedersen et al., 2014; Yagi et al., 2006). Further studies are needed to define how TSP1/CD47 interactions and CD47/SIRPα interaction alter osteoclast survival, differentiation, and regeneration.
8. CD47 in cancer stem cells
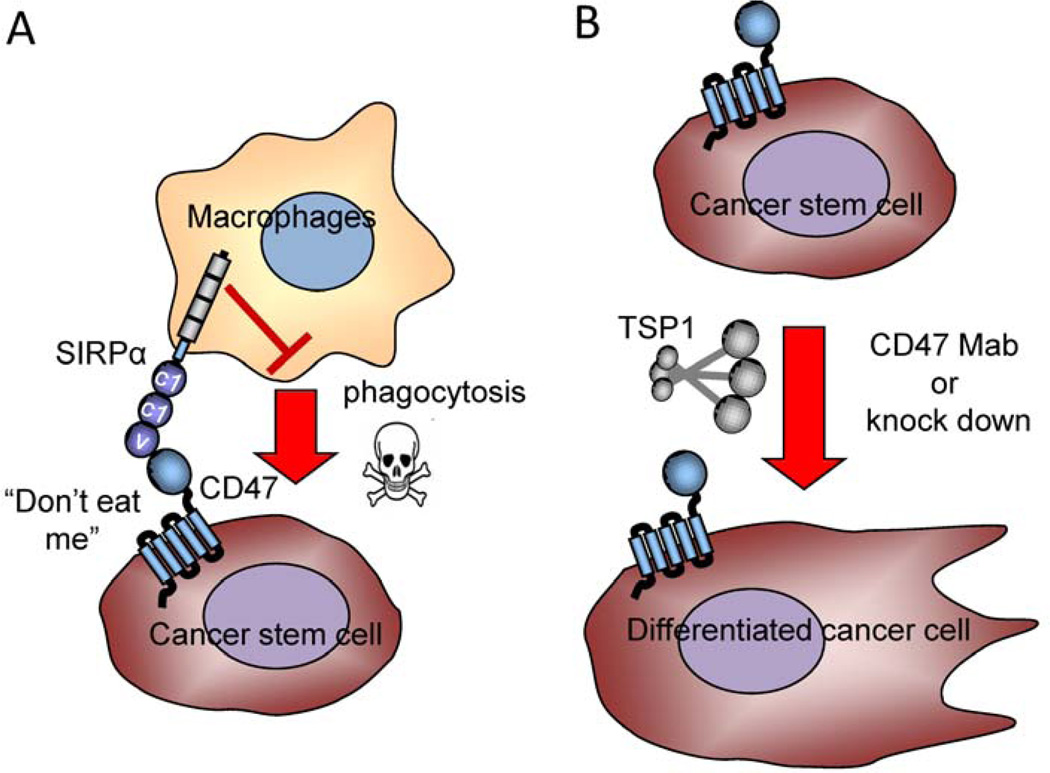
Although decreased expression of CD47 can be advantageous for nontransformed stem cells, data from a number of laboratories has established that cancer stem cells (CSCs) frequently express higher levels of CD47. Furthermore, clinical data for a number of malignancies indicates that high CD47 expression is a negative prognostic factor (Majeti et al., 2009; Willingham et al., 2012; Yoshida et al., 2015; Zhao et al., 2011). Finally, murine xenograft and syngeneic murine tumor models show favorable responses to CD47 knockdown or treatment with function-blocking CD47 antibodies (Lee et al., 2014b; Majeti, 2009; Maxhimer, 2009b; Soto-Pantoja et al., 2014; Zhao, 2011). One fundamental question raised by these data is why CSCs appear to respond differently than normal stem cells to modulation of CD47 expression or signaling. Currently, there are two major hypotheses to explain a selective advantage of high CD47 expression in CSCs (Fig. 3). The “don’t eat me” hypothesis proposes that resistance to macrophage-mediated phagocytic clearance provides a selective pressure for CSCs to maintain high CD47 expression. According to this hypothesis, CD47 serves a passive role as a counter-receptor for SIRPα, resulting in a myeloid-specific immune checkpoint (Fig. 3A). The second hypothesis posits that CD47 has a cell-autonomous function in CSCs that promotes their maintenance (Fig. 3B). The “don’t eat me” hypothesis is analogous to that discussed above for adult stem cells and in the context of cancer has been reviewed elsewhere (Chao et al., 2012a; Chao et al., 2012b; Weiskopf and Weissman, 2015). Here we will focus on the second hypothesis, which has gained experimental support from several recent publications.
Figure 3. Models for CD47 function in cancer stem cells.
A) Elevated expression of CD47 on cancer stem/tumor-initiating cells can protect the cells from phagocytic clearance by macrophages. B) Signaling by CD47 expressed on CSCs maintains their stem cell character. Knock down of CD47 or ligation by TSP1 or the blocking antibody B6H12 induces their differentiation.
8.1 Increased CD47 expression in cancer stem cells
Cell surface CD47 expression is elevated on human and mouse myeloid leukemia cells (Jaiswal, 2009) and on a subset of self-renewing leukemia stem cells (Majeti, 2009). This increased CD47 expression predicted decreased overall survival in three independent cohorts of adult acute myeloid leukemia patients. CD47 was also identified in an unbiased proteomics screen of plasma membrane proteins on CD34+ acute myeloid leukemias from two patients (Bonardi et al., 2013). This finding was extended to tumor-initiating cells from primary human bladder carcinomas defined as Lineage-CD44+CK5+CK20− (Chan et al., 2009). These cells showed higher CD47 expression than the bulk tumor cells. Similar up-regulation of CD47 was found in the granulocyte-macrophage progenitor population of patients with myelodysplastic syndrome at the high-risk refractory anemia with excess blasts (RAEB) stage (Pang et al., 2013). Another study found that elevated CD47 on erythroblasts of myelodysplastic syndrome patients positively correlated with their peripheral red blood cell count, consistent with HSC function (Jiang et al., 2013). Association of high CD47 expression with stem cell/tumor-initiating populations has also been reported in gastric cancer (Yoshida, 2015), hepatocellular carcinoma (Lee, 2014b), and pancreatic ductal adenocarcinoma (Cioffi et al., 2015).
Elevated CD47 expression has been associated with a metastasis-initiating population of human luminal breast cancers (Baccelli et al., 2013). The metastasis-initiating cells expressed EPCAM, CD44, CD47 and MET. In patients with metastases, the number of EPCAM+CD44+CD47+MET+ circulating tumor cells, but not of bulk EPCAM+ circulating tumor cells, correlated with decreased overall survival and an increased number of metastasic sites. Basal breast cancers are known to have a higher percentage of tumor initiating cells and to express a higher level of CD47 than luminal breast cancers (Zhao, 2011). Recently we reported that CD47 is expressed higher on breast CSCs than differentiated cells derived from the triple negative MDA-MB-231 cell line (Kaur et al., 2016). Analysis of TCGA data for patients with basal triple-negative breast carcinoma showed higher CD47 expression relative to tumors from patients with HER2+ and ER+ luminal breast cancers, but CD47 expression within the triple negative group did not correlate significantly with overall survival.
8.2 Modulation of cancer stem cells by altering CD47 expression
Microarray analysis of tumor initiating cells from hepatocellular carcinoma identified multiple genes with altered expression when CD47 expression was suppressed using shRNA (Lee, 2014b). A number of these genes showed parallel changes in expression when the tumor initiating cells were induced to differentiate. Among these, cathepsin-S mRNA in the tumor initiating cells was decreased when the cells differentiated and when CD47 expression was suppressed. CD47 expression correlated with that of cathepsin-S in hepatocellular carcinoma patient specimens, and CD47 regulated cathepsin-S expression in tumor initiating cells through activation of NFκB. Cathepsin-S in turn controlled tumor initiating cell migration and invasion through activation of protease-activated receptor-2, which is a substrate for this protease. Protease-activated receptor-2 is a G protein-coupled receptor that regulates cell survival, proliferation, and motility (Gieseler et al., 2013) . Consistent with these in vitro results, antisense morpholino suppression of CD47 suppressed the growth and metastasis of hepatocellular carcinoma xenografts in NOD/SCID mice, and combining morpholino knockdown of CD47 with doxorubicin treatment enhanced tumor growth inhibition. This study established that decreasing CD47 expression can cell-autonomously suppress CSCs.
The mechanisms that elevate CD47 expression in stem cells remain poorly understood. Elements in the CD47 promoter have been defined that respond to aPal/NRF1 and NFκB (Chang and Huang, 2004; Lo et al., 2015), and miR-141 targets the 3’-UTR of CD47 mRNA to inhibit CD47 expression (Tang et al., 2013). High CD47 expression was recently associated with high Hif expression in breast cancers and correlated with stem cell character (Zhang et al., 2015). CD47 mRNA and cell surface protein expression were induced by hypoxia. Chromatin immunoprecipitation demonstrated hypoxia-induced binding of Hif1α and Hif1β to the CD47 promoter. Hif-deficient cells were subject to increased phagocytosis by mouse bone marrow-derived macrophages. Notably, SUM159 breast cancer cells cultured as nonadherent spheroids (mammospheres), which enriches for CSCs, expressed twofold higher CD47 mRNA levels than control adherent cultures. Conversely, shRNA knockdown of CD47 in SUM159 cells reduced their formation of mammospheres and reduced expression of the CSC marker aldehyde dehydrogenase. These data indicate that elevated CD47 expression promotes the specification and survival of breast CSCs in a cell-autonomous manner, independent of CD47 interactions with SIRPα on phagocytes.
8.3 Modulation of cancer stem cells using CD47 ligands
Repeated passage in immune-competent mice to select resistant cells from Lewis lung carcinoma resulted in the isolation of cells with increased CSC characteristics as well as increased CD47 expression (Zheng et al., 2015). Notably, the selected cells also had very low TSP1 expression. Treatment of the selected cells with recombinant TSP1 reduced cell proliferation and was associated with increased expression of the cell cycle inhibitor p21 and decreased expression of cMyc, Klf4, Sox2 and Oct4. TSP1 also increased levels of cleaved caspase-3. Notably, knockdown of CD47 using a shRNA vector blocked these responses to TSP1. TSP1 treatment also inhibited proliferation and suppressed sphere formation in human colon cancer (HCT116), non-small cell lung cancer (A549), and cervical cancer (HeLa) cell lines (Zheng, 2015). These data further support a cell-autonomous function of CD47 signaling in CSCs and implicate TSP1 signaling through CD47 in regulating CSC fate.
CD47 is highly expressed by pancreatic ductal adenocarcinomas and their metastases as compared to normal pancreatic tissues, but CD47 protein expression in the cancers was not significantly correlated with clinical outcome (Cioffi, 2015). However, CD47 expression was significantly elevated when pancreatic adenocarcinoma cells were induced to form nonadherent spheres, relative to the same cell lines grown as adherent cultures. CD47+ and CD133+ stem-like cells exhibited more sphere formation than CD47− and CD133− cells. Consistent with the “don’t eat me” hypothesis, treating the pancreatic CSCs with a CD47 antibody that blocks SIRPα binding (B6H12) specifically induced phagocytosis by macrophages. However, the CD47 antibody induced death of pancreatic CSCs that was independent of macrophages. Pancreatic CSCs treated with the CD47 blocking antibody B6H12 exhibited higher annexin-V binding, suggesting that the antibody cell-autonomously induces apoptosis, although other forms of programmed cell death were not excluded. Finally, treatment of mice bearing pancreatic tumor xenografts with B6H12 either as a single agent or in combination with chemotherapy significantly reduced the percentage of tumor cells expressing the CSC surface markers CD133 and SSEA1. These data suggest that, in addition to enhancing innate immune clearance, this CD47 blocking antibody can directly eliminate pancreatic CSCs in vitro and in vivo (Cioffi, 2015).
We recently found that the CD47 blocking antibody B6H12 directly alters the expression of many genes in human breast CSCs (CD44hi/CD24low) derived from the MDA-MB-231 cell line (Kaur, 2016). B6H12 inhibited asymmetric cell division and cell proliferation of breast CSCs, which is consistent with the pancreatic CSC data (Cioffi, 2015). Treatment with the B6H12 antibody down-regulated the expression of Klf4 mRNA and protein, which contrasts with the elevated Klf4 expression in endothelial cells and tissues of cd47−/− mice (Kaur, 2013). This indicates that CD47 induces different signaling pathways to regulate Klf4 in cancer cells versus normal cells. B6H12 also inhibited proliferation of T47D breast carcinoma cells, but proliferation of the ER+ line MCF7 and the nontransformed breast line MCF10A was enhanced rather than inhibited by B6H12. The latter cell lines contain very low percentages of stem cells, suggesting that this CD47 antibody may be selectively inhibitor for more malignant breast cancers that have a high percentage of CSCs. The positive proliferative responses of MCF10A mammary epithelial cells to the blocking antibody B6H12 is consistent with our previous findings that nontransformed cd47−/− cells proliferate faster than WT cells (Kaur, 2013). The MCF7 data indicates that there may be exceptions to the rule that CD47 signaling triggers opposing responses in nontransformed stem cells versus CSCs.
The changes in gene expression induced in triple negative breast CSCs following B6H12 treatment also provided some insight into the molecular mechanism by which CD47 signaling regulates breast CSC differentiation. B6H12 strongly suppressed mRNA levels of EGF and EGFR and acutely inhibited EGFR phosphorylation in breast CSCs (Kaur, 2016). EGFR and Klf4 are known targets of the micro-RNA miR-7 (Okuda et al., 2013; Webster et al., 2009), and we further observed that B6H12 increased the expression of miR-7-5P in breast CSCs but not in differentiated cells from the same cancer cell line. Other studies have identified miR-7 as a suppressor of CSC and normal stem cells (Cui et al., 2014; Okuda, 2013; Pek et al., 2009; Zhang, 2015). Therefore, regulation of this noncoding RNA may mediate some of the effects of CD47 signaling on CSCs.
We also compared genes exhibiting altered expression in bCSC following B6H12 treatment with a list of genes that correlate with CD47 mRNA expression in the Cancer Genome Atlas RNA-Seq data for triple-negative breast cancers (Kaur, 2016). Sixty genes achieved significant correlation with CD47 in both bCSC and triple-negative breast cancer tumor data. Consistent with our in vitro data, EGFR mRNA expression positively correlated with CD47 mRNA expression in triple-negative breast cancer primary tumors. Several known stem cell markers and regulators correlated with CD47 mRNA expression in the TCGA breast carcinoma dataset including a positive correlation with cKit protein expression, negative correlation with phosphorylation of PDK1 at Ser241, and negative correlation with mRNA expression of the transcription factor PATZ1, which maintains stem cells by its regulation of Pou5f1, Nanog, cMyc, and global changes in histone modification (Ma et al., 2014; Ow et al., 2014). Significant negative correlations between PATZ1 and CD47 mRNA expression were also found in TCGA datasets for melanoma, head and neck squamous cell carcinoma, and bladder carcinoma, suggesting a more general regulation of CSCs by CD47.
9. Future Prospects
Specific signaling pathways that are regulated by CD47 have been identified in normal stem cells and in several types of neoplastic stem cells (Figs. 2 & 4). However, it is not clear which of these signaling pathways account for the differential responses of normal and neoplastic stem cells. cMyc is one obvious candidate in that Myc signaling is dysregulated in many cancers. We have shown that Burkitt’s lymphoma cells, where the 5’ promoter region of cMyc is replaced with Ig heavy chain promoter sequence, are resistant to growth inhibition by CD47 over-expression, and cMyc expression is unperturbed by CD47 in these cells (Kaur, 2013). Others have identified NFκB dependent regulation of cystatin-S/protease activated receptor-2, p21, Gβγ-dependent activation of the PI3-kinase/Akt pathway, and ubiquitin-like with PHD and ring finger domains 1 (UHRF1)-dependent down-regulation of p16INK4A as targets of CD47 signaling in specific cancer cell lines (Boukhari et al., 2015; Lee, 2014b; Sick et al., 2011; Zheng, 2015). Further study is needed to determine which of these CD47 target pathways generalize to other cancer types, and which may differ in neoplastic versus nontransformed stem cells.
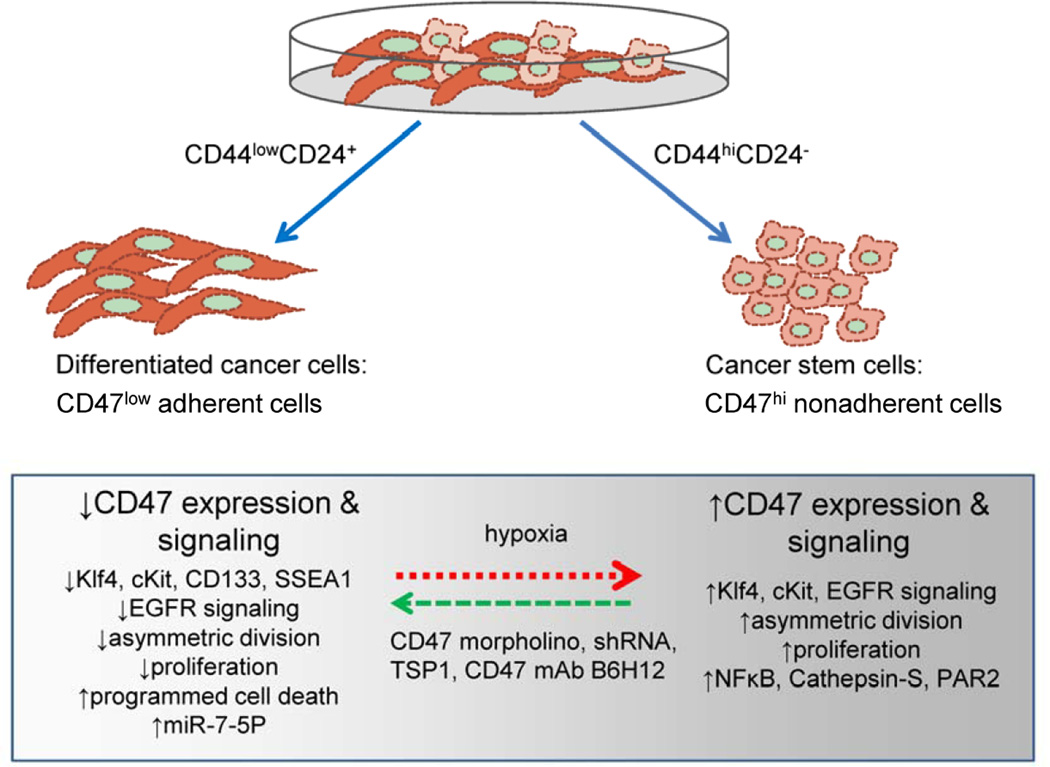
Figure 4. Signal transduction pathways regulated by CD47 in cancer stem cells.
CSCs can be separated from differentiated cancer cells and typically express CD47 at a 2–4-fold higher level. CSC are typically less adherent. Studies in breast, liver, and pancreatic cancers have shown that decreasing CD47 expression in CSC or exposure to CD47 ligands including TSP1 and the antibody B6H12 convert CSC to differentiated cancer cells with a resulting decrease in expression of stem cell markers (e.g. cKit, Klf4, CD133, SSEA1), loss of asymmetric division, increased NFκB activation, and in some cases increased cell death. The Hif pathway has been implicated in the elevated expression of CD47 in CSC.
Quiescence may preserve the self-renewal of normal stem cells and is a critical factor in the resistance of CSCs to chemotherapy that leads to relapse of cancer. Improved knowledge of quiescence mechanisms is needed to target drug-resistant quiescent CSCs. For example, quiescent CD34+ cells from CML patients are resistant to the tyrosine kinase inhibitor Imatinib mesylate (Bhatia et al., 2003; Cheung and Rando, 2013). Thus, an improved understanding of the molecular mechanisms of quiescence in adult stem cells is critical for success of molecular targeted therapies (Li, 2011; Maugeri-Sacca et al., 2011). Although TSP1/CD47 signaling clearly plays roles in both normal and neoplastic stem cells, no studies have examined its potential role in stem cell quiescence. Considering that at least some organs in cd47−/− mice have a higher abundance of stem cells, the remarkable radioresistance of cd47−/− and thbs1−/− tissues could result in part from higher stem cell numbers. Validating this hypothesis will require evidence that the null stem cells are more quiescent or intrinsically more radioresistant.
Studies are beginning to identify the intrinsic and extrinsic regulatory mechanisms that control stem cell quiescence (Hittelman et al., 2010). p53 plays an important role in self-renewal for regulating stem cell quiescence (Lin et al., 2005; Meletis et al., 2006) and is known to regulate TSP1 expression (Dameron et al., 1994; Watnick et al., 2015). Other intrinsic regulatory mechanisms include Hif-1α (Takubo et al., 2010) and negative regulators of the mTOR pathway (Ito et al., 2008; Thompson et al., 2008; Yilmaz et al., 2006). As previously discussed, Hif1α regulates CD47 expression, and other studies have shown that CD47 signaling controls autophagy (Soto-Pantoja et al., 2012), which is downstream of mTOR. Many extrinsic stimuli from the ECM in the stem cell niche also regulate stem cell quiescence including TGF-β and integrin signaling (Li and Bhatia, 2011), activation of which is controlled by TSP1 and CD47, respectively. These studies may guide future efforts to define signaling controlled by TSP1, CD47, and other TSP1 receptors in the stem cell niche.
Although the origin of CSCs from transformation of normal stem cells rather than de-differentiation of tumor cells into stem-like cells remains controversial, solid tumors clearly contain mixed populations of terminally differentiated cells with a minor population of cancer cells that can initiate tumor growth (Pignalosa and Durante, 2012). It is important to understand the signaling pathways that control self-renewal and survival of CSCs to develop novel therapies to prevent regrowth of tumors following radiation therapy. CD47 is a promising candidate that can act differentially in normal versus CSCs. For this therapeutic strategy to succeed, we need to better understand how the TSP1/CD47 axis regulates stem cells in normal tissues verses tumors.
It is also important to further investigate how CD47 signaling in CSC regulates their responses to cytotoxic chemotherapy and targeted therapeutics. Published studies have reported synergism between CD47 blockade and doxorubicin (Lee, 2014b) and synergism with some therapeutic antibodies including those targeting CD20 and HER2/Neu (Chao et al., 2010; Zhao, 2011). The latter synergism has been attributed to enhancement of ADCC responses, but another possibility is that altered CD47 signaling in CSC increases their sensitivity to these targeted therapies in a cell-autonomous manner.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Abbreviations
- CFU
colony-forming units
- CSCs
cancer stem cells
- EBs
embryoid bodies
- ECM
extracellular matrix
- EPCs
endothelial progenitor cells
- ESC
embryonic stem cells
- HSC
hematopoietic stem cells
- iPS
inducible pluripotent stem
- MSCs
mesenchymal stem cells
- NO
nitric oxide
- NOD
nonobese diabetic
- SIRPα
signal regulatory protein-α
- SSEA1
stage-specific embryonic antigen-1
- TGF-β
transforming growth factor-β1
- TSP1
thrombospondin-1
- TSP2
thrombospondin-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- Bailey Dubose K, Zayzafoon M, Murphy-Ullrich JE. Thrombospondin-1 inhibits osteogenic differentiation of human mesenchymal stem cells through latent TGF-beta activation. Biochem Biophys Res Commun. 2012;422:488–493. doi: 10.1016/j.bbrc.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–4707. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- Bian Z, Guo Y, Luo Y, Tremblay A, Zhang X, Dharma S, et al. CD47 deficiency does not impede polymorphonuclear neutrophil transmigration but attenuates granulopoiesis at the postacute stage of colitis. J Immunol. 2013;190:411–417. doi: 10.4049/jimmunol.1201963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonardi F, Fusetti F, Deelen P, van Gosliga D, Vellenga E, Schuringa JJ. A proteomics and transcriptomics approach to identify leukemic stem cell (LSC) markers. Mol Cell Proteomics. 2013;12:626–637. doi: 10.1074/mcp.M112.021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhari A, Alhosin M, Bronner C, Sagini K, Truchot C, Sick E, et al. CD47 activation-induced UHRF1 over-expression is associated with silencing of tumor suppressor gene p16INK4A in glioblastoma cells. Anticancer Res. 2015;35:149–157. [PubMed] [Google Scholar]
- Calabro NE, Kristofik NJ, Kyriakides TR. Thrombospondin-2 and extracellular matrix assembly. Biochim Biophys Acta. 2014;1840:2396–2402. doi: 10.1016/j.bbagen.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzada MJ, Roberts DD. Novel integrin antagonists derived from thrombospondins. Curr Pharm Des. 2005;11:849–866. doi: 10.2174/1381612053381792. [DOI] [PubMed] [Google Scholar]
- Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WT, Huang AM. Alpha-Pal/NRF-1 regulates the promoter of the human integrin-associated protein/CD47 gene. J Biol Chem. 2004;279:14542–14550. doi: 10.1074/jbc.M309825200. [DOI] [PubMed] [Google Scholar]
- Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Majeti R, Weissman IL. Programmed cell removal: a new obstacle in the road to developing cancer. Nat Rev Cancer. 2012a;12:58–67. doi: 10.1038/nrc3171. [DOI] [PubMed] [Google Scholar]
- Chao MP, Weissman IL, Majeti R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012b;24:225–232. doi: 10.1016/j.coi.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YZ, Incardona F, Legrand C, Momeux L, Caen J, Han ZC. Thrombospondin, a negative modulator of megakaryocytopoiesis. J Lab Clin Med. 1997;129:231–238. doi: 10.1016/s0022-2143(97)90144-x. [DOI] [PubMed] [Google Scholar]
- Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L, Jiang Y, Hao H, Xia Y, Xu J, Liu Z, et al. Nitric oxide enhances Oct-4 expression in bone marrow stem cells and promotes endothelial differentiation. Eur J Pharmacol. 2008;591:59–65. doi: 10.1016/j.ejphar.2008.06.066. [DOI] [PubMed] [Google Scholar]
- Cioffi M, Trabulo S, Hidalgo M, Costello E, Greenhalf W, Erkan M, et al. Inhibition of CD47 Effectively Targets Pancreatic Cancer Stem Cells via Dual Mechanisms. Clin Cancer Res. 2015;21:2325–2337. doi: 10.1158/1078-0432.CCR-14-1399. [DOI] [PubMed] [Google Scholar]
- Cooper D, Lindberg FP, Gamble JR, Brown EJ, Vadas MA. Transendothelial migration of neutrophils involves integrin-associated protein (CD47) Proc Natl Acad Sci U S A. 1995;92:3978–3982. doi: 10.1073/pnas.92.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Xiao Z, Chen T, Wei J, Chen L, Liu L, et al. The miR-7 identified from collagen biomaterial-based three-dimensional cultured cells regulates neural stem cell differentiation. Stem Cells Dev. 2014;23:393–405. doi: 10.1089/scd.2013.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Hendriks JJ, Honing H, De Lavalette CR, van der Pol SM, Hooijberg E, et al. Signal-regulatory protein alpha-CD47 interactions are required for the transmigration of monocytes across cerebral endothelium. J Immunol. 2002;168:5832–5839. doi: 10.4049/jimmunol.168.11.5832. [DOI] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Furusawa T, Yanai N, Hara T, Miyajima A, Obinata M. Integrin-associated protein (IAP, also termed CD47) is involved in stroma-supported erythropoiesis. J Biochem. 1998;123:101–106. doi: 10.1093/oxfordjournals.jbchem.a021895. [DOI] [PubMed] [Google Scholar]
- Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieseler F, Ungefroren H, Settmacher U, Hollenberg MD, Kaufmann R. Proteinase-activated receptors (PARs) - focus on receptor-receptor-interactions and their physiological and pathophysiological impact. Cell Commun Signal. 2013;11:86. doi: 10.1186/1478-811X-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Sterling H, Chen Y, Saginario C, Brown EJ, Frazier WA, et al. CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. J Biol Chem. 2000;275:37984–37992. doi: 10.1074/jbc.M002334200. [DOI] [PubMed] [Google Scholar]
- Hankenson KD, Bain SD, Kyriakides TR, Smith EA, Goldstein SA, Bornstein P. Increased marrow-derived osteoprogenitor cells and endosteal bone formation in mice lacking thrombospondin 2. J Bone Miner Res. 2000;15:851–862. doi: 10.1359/jbmr.2000.15.5.851. [DOI] [PubMed] [Google Scholar]
- Hittelman WN, Liao Y, Wang L, Milas L. Are cancer stem cells radioresistant? Future Oncol. 2010;6:1563–1576. doi: 10.2217/fon.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobolt-Pedersen AS, Delaisse JM, Soe K. Osteoclast fusion is based on heterogeneity between fusion partners. Calcif Tissue Int. 2014;95:73–82. doi: 10.1007/s00223-014-9864-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, et al. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007a;109:1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Hyodo F, Pappan LK, Abu-Asab M, Tsokos M, Krishna MC, et al. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler Thromb Vasc Biol. 2007b;27:2582–2588. doi: 10.1161/ATVBAHA.107.155390. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Maxhimer JB, Hyodo F, Pendrak ML, Ridnour LA, DeGraff WG, et al. Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am J Pathol. 2008a;173:1100–1112. doi: 10.2353/ajpath.2008.080237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Pappan LK, Romeo MJ, Abu-Asab M, Tsokos M, Wink DA, et al. Blockade of thrombospondin-1-CD47 interactions prevents necrosis of full thickness skin grafts. Ann Surg. 2008b;247:180–190. doi: 10.1097/SLA.0b013e31815685dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, et al. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007c;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Romeo MJ, Maxhimer JB, Smedley J, Frazier WA, Roberts DD. Gene silencing of CD47 and antibody ligation of thrombospondin-1 enhance ischemic tissue survival in a porcine model: implications for human disease. Ann Surg. 2008c;247:860–868. doi: 10.1097/SLA.0b013e31816c4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453:1072–1078. doi: 10.1038/nature07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SY, Kim DH, Ha J, Jin HJ, Kwon SJ, Chang JW, et al. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;31:2136–2148. doi: 10.1002/stem.1471. [DOI] [PubMed] [Google Scholar]
- Jiang H, Fu R, Wang H, Li L, Liu H, Shao Z. CD47 is expressed abnormally on hematopoietic cells in myelodysplastic syndrome. Leuk Res. 2013;37:907–910. doi: 10.1016/j.leukres.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Kaur S, Elkahloun AG, Singh SP, Chen QR, Meerzaman DM, Song T, et al. A function-blocking CD47 antibody suppresses stem cell and EGF signaling in triple-negative breast cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Soto-Pantoja DR, Stein EV, Liu C, Elkahloun AG, Pendrak ML, et al. Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci Rep. 2013;3:1673. doi: 10.1038/srep01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen C, Persson E, Baldock P, Stenberg A, Bostrom I, Matozaki T, et al. Lack of CD47 impairs bone cell differentiation and results in an osteopenic phenotype in vivo due to impaired signal regulatory protein alpha (SIRPalpha) signaling. J Biol Chem. 2013;288:29333–29344. doi: 10.1074/jbc.M113.494591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama T, Takenaka K, Kohno K, Yamauchi T, Daitoku S, Yoshimoto G, et al. Engulfment of hematopoietic stem cells caused by down-regulation of CD47 is critical in the pathogenesis of hemophagocytic lymphohistiocytosis. Blood. 2012;120:4058–4067. doi: 10.1182/blood-2012-02-408864. [DOI] [PubMed] [Google Scholar]
- Kwong LS, Brown MH, Barclay AN, Hatherley D. Signal-regulatory protein alpha from the NOD mouse binds human CD47 with an exceptionally high affinity-- implications for engraftment of human cells. Immunology. 2014;143:61–67. doi: 10.1111/imm.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarkova MA, Shutova MV, Bogomazova AN, Vassina EM, Glazov EA, Zhang P, et al. Induction of pluripotency in human endothelial cells resets epigenetic profile on genome scale. Cell Cycle. 2010;9:937–946. doi: 10.4161/cc.9.5.10869. [DOI] [PubMed] [Google Scholar]
- Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014a;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Cheung VC, Lu P, Lau EY, Ma S, Tang KH, et al. Blockade of CD47-mediated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology. 2014b;60:179–191. doi: 10.1002/hep.27070. [DOI] [PubMed] [Google Scholar]
- Li J. Quiescence regulators for hematopoietic stem cell. Exp Hematol. 2011;39:511–520. doi: 10.1016/j.exphem.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Li L, Bhatia R. Stem cell quiescence. Clin Cancer Res. 2011;17:4936–4941. doi: 10.1158/1078-0432.CCR-10-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- Liu Y, Buhring HJ, Zen K, Burst SL, Schnell FJ, Williams IR, et al. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J Biol Chem. 2002;277:10028–10036. doi: 10.1074/jbc.M109720200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. J Biol Chem. 2001;276:40156–40166. doi: 10.1074/jbc.M104138200. [DOI] [PubMed] [Google Scholar]
- Lo J, Lau EY, Ching RH, Cheng BY, Ma MK, Ng IO, et al. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology. 2015;62:534–545. doi: 10.1002/hep.27859. [DOI] [PubMed] [Google Scholar]
- Long MW, Briddell R, Walter AW, Bruno E, Hoffman R. Human hematopoietic stem cell adherence to cytokines and matrix molecules. J Clin Invest. 1992;90:251–255. doi: 10.1172/JCI115844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MW, Dixit VM. Thrombospondin functions as a cytoadhesion molecule for human hematopoietic progenitor cells. Blood. 1990;75:2311–2318. [PubMed] [Google Scholar]
- Lu Z, Kipnis J. Thrombospondin 1--a key astrocyte-derived neurogenic factor. FASEB J. 2010;24:1925–1934. doi: 10.1096/fj.09-150573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg P, Koskinen C, Baldock PA, Lothgren H, Stenberg A, Lerner UH, et al. Osteoclast formation is strongly reduced both in vivo and in vitro in the absence of CD47/SIRPalpha-interaction. Biochem Biophys Res Commun. 2007;352:444–448. doi: 10.1016/j.bbrc.2006.11.057. [DOI] [PubMed] [Google Scholar]
- Ma H, Ow JR, Tan BC, Goh Z, Feng B, Loh YH, et al. The dosage of Patz1 modulates reprogramming process. Sci Rep. 2014;4:7519. doi: 10.1038/srep07519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 2009;19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Maugeri-Sacca M, Vigneri P, De Maria R. Cancer stem cells and chemosensitivity. Clin Cancer Res. 2011;17:4942–4947. doi: 10.1158/1078-0432.CCR-10-2538. [DOI] [PubMed] [Google Scholar]
- Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD. Thrombospondin-1/CD47 blockade following ischemia-reperfusion injury is tissue protective. Plast Reconstr Surg. 2009a;124:1880–1889. doi: 10.1097/PRS.0b013e3181bceec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, et al. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med. 2009b;1:3ra7. doi: 10.1126/scitranslmed.3000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- Mujoo K, Krumenacker JS, Murad F. Nitric oxide-cyclic GMP signaling in stem cell differentiation. Free Radic Biol Med. 2011;51:2150–2157. doi: 10.1016/j.freeradbiomed.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Nor JE, Dipietro L, Murphy-Ullrich JE, Hynes RO, Lawler J, Polverini PJ. Activation of Latent TGF-beta1 by Thrombospondin-1 is a Major Component of Wound Repair. Oral Biosci Med. 2005;2:153–161. [PMC free article] [PubMed] [Google Scholar]
- Notarianni E, Galli C, Laurie S, Moor RM, Evans MJ. Derivation of pluripotent, embryonic cell lines from the pig and sheep. J Reprod Fertil Suppl. 1991;43:255–260. [PubMed] [Google Scholar]
- Okuda H, Xing F, Pandey PR, Sharma S, Watabe M, Pai SK, et al. miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res. 2013;73:1434–1444. doi: 10.1158/0008-5472.CAN-12-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg PA. CD47: A Cell Surface Glycoprotein Which Regulates Multiple Functions of Hematopoietic Cells in Health and Disease. ISRN Hematol. 2013;2013:614619. doi: 10.1155/2013/614619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- Ow JR, Ma H, Jean A, Goh Z, Lee YH, Chong YM, et al. Patz1 regulates embryonic stem cell identity. Stem Cells Dev. 2014;23:1062–1073. doi: 10.1089/scd.2013.0430. [DOI] [PubMed] [Google Scholar]
- Pang WW, Pluvinage JV, Price EA, Sridhar K, Arber DA, Greenberg PL, et al. Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes. Proc Natl Acad Sci U S A. 2013;110:3011–3016. doi: 10.1073/pnas.1222861110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panopoulos AD, Ruiz S, Yi F, Herrerias A, Batchelder EM, Izpisua Belmonte JC. Rapid and highly efficient generation of induced pluripotent stem cells from human umbilical vein endothelial cells. PLoS One. 2011;6:e19743. doi: 10.1371/journal.pone.0019743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- Pek JW, Lim AK, Kai T. Drosophila maelstrom ensures proper germline stem cell lineage differentiation by repressing microRNA-7. Dev Cell. 2009;17:417–424. doi: 10.1016/j.devcel.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Pignalosa D, Durante M. Overcoming resistance of cancer stem cells. Lancet Oncol. 2012;13:e187–e188. doi: 10.1016/S1470-2045(12)70196-1. [DOI] [PubMed] [Google Scholar]
- Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, et al. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270:7304–7310. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- Seiffert M, Brossart P, Cant C, Cella M, Colonna M, Brugger W, et al. Signal-regulatory protein alpha (SIRPalpha) but not SIRPbeta is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34(+)CD38(−) hematopoietic cells. Blood. 2001;97:2741–2749. doi: 10.1182/blood.v97.9.2741. [DOI] [PubMed] [Google Scholar]
- Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, et al. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Sick E, Boukhari A, Deramaudt T, Ronde P, Bucher B, Andre P, et al. Activation of CD47 receptors causes proliferation of human astrocytoma but not normal astrocytes via an Akt-dependent pathway. Glia. 2011;59:308–319. doi: 10.1002/glia.21102. [DOI] [PubMed] [Google Scholar]
- Smadja DM, d’Audigier C, Bieche I, Evrard S, Mauge L, Dias JV, et al. Thrombospondin-1 is a plasmatic marker of peripheral arterial disease that modulates endothelial progenitor cell angiogenic properties. Arterioscler Thromb Vasc Biol. 2011;31:551–559. doi: 10.1161/ATVBAHA.110.220624. [DOI] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Kaur S, Roberts DD. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol. 2015;50:212–230. doi: 10.3109/10409238.2015.1014024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Miller TW, Pendrak ML, DeGraff WG, Sullivan C, Ridnour LA, et al. CD47 deficiency confers cell and tissue radioprotection by activation of autophagy. Autophagy. 2012;8:1628–1642. doi: 10.4161/auto.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Ridnour LA, Wink DA, Roberts DD. Blockade of CD47 increases survival of mice exposed to lethal total body irradiation. Sci Rep. 2013;3:1038. doi: 10.1038/srep01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Terabe M, Ghosh A, Ridnour LA, DeGraff WG, Wink DA, et al. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. 2014;74:6771–6783. doi: 10.1158/0008-5472.CAN-14-0037-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto Pantoja DR, Kaur S, Miller TW, Isenberg JS, Roberts DD. Leukocyte surface antigen CD47. USCD Molecule Pages. 2013;2:1. [Google Scholar]
- Subramanian S, Tsai R, Discher DE. The ‘metabolon,’ CD47, and the ‘phagocytic synapse’: molecular co-localization and species divergence. Transfus Clin Biol. 2006;13:31–38. doi: 10.1016/j.tracli.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Mernaugh RL, Friedman DB, Weller R, Tsuboi N, Yamashita H, et al. Thrombospondin-1 acts as a ligand for CD148 tyrosine phosphatase. Proc Natl Acad Sci U S A. 2012;109:1985–1990. doi: 10.1073/pnas.1106171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Tang W, Qin J, Tang J, Zhang H, Zhou Z, Li B, et al. Aberrant reduction of MiR-141 increased CD47/CUL3 in Hirschsprung’s disease. Cell Physiol Biochem. 2013;32:1655–1667. doi: 10.1159/000356601. [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Jankovic V, Gao J, Buonamici S, Vest A, Lee JM, et al. Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7. J Exp Med. 2008;205:1395–1408. doi: 10.1084/jem.20080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- van den Berg TK, van der Schoot CE. Innate immune ‘self’ recognition: a role for CD47-SIRPalpha interactions in hematopoietic stem cell transplantation. Trends Immunol. 2008;29:203–206. doi: 10.1016/j.it.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang H, Wang S, Fu Y, Yang YG. Survival and function of CD47-deficient thymic grafts in mice. Xenotransplantation. 2010;17:160–165. doi: 10.1111/j.1399-3089.2010.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick RS, Rodriguez RK, Wang S, Blois AL, Rangarajan A, Ince T, et al. Thrombospondin-1 repression is mediated via distinct mechanisms in fibroblasts and epithelial cells. Oncogene. 2015;34:2823–2835. doi: 10.1038/onc.2014.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Fujiwara H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RJ, Giles KM, Price KJ, Zhang PM, Mattick JS, Leedman PJ. Regulation of epidermal growth factor receptor signaling in human cancer cells by microRNA-7. J Biol Chem. 2009;284:5731–5741. doi: 10.1074/jbc.M804280200. [DOI] [PubMed] [Google Scholar]
- Weiskopf K, Weissman IL. Macrophages are critical effectors of antibody therapies for cancer. MAbs. 2015;7:303–310. doi: 10.1080/19420862.2015.1011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman I. Evolution of normal and neoplastic tissue stem cells: progress after Robert Hooke. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140364. doi: 10.1098/rstb.2014.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie HH, Zhou S, Chen DD, Channon KM, Su DF, Chen AF. GTP cyclohydrolase I/BH4 pathway protects EPCs via suppressing oxidative stress and thrombospondin-1 in salt-sensitive hypertension. Hypertension. 2010;56:1137–1144. doi: 10.1161/HYPERTENSIONAHA.110.160622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M, Miyamoto T, Toyama Y, Suda T. Role of DC-STAMP in cellular fusion of osteoclasts and macrophage giant cells. J Bone Miner Metab. 2006;24:355–358. doi: 10.1007/s00774-006-0697-9. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Takenaka K, Urata S, Shima T, Kikushige Y, Tokuyama T, et al. Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment. Blood. 2013;121:1316–1325. doi: 10.1182/blood-2012-06-440354. [DOI] [PubMed] [Google Scholar]
- Yang M, Li K, Ng MH, Yuen PM, Fok TF, Li CK, et al. Thrombospondin-1 inhibits in vitro megakaryocytopoiesis via CD36. Thromb Res. 2003;109:47–54. doi: 10.1016/s0049-3848(03)00142-7. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Tsujimoto H, Matsumura K, Kinoshita M, Takahata R, Matsumoto Y, et al. CD47 is an adverse prognostic factor and a therapeutic target in gastric cancer. Cancer Med. 2015;4:1322–1333. doi: 10.1002/cam4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D, et al. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A. 2015;112:E6215–E6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XW, van Beek EM, Schornagel K, Van der Maaden H, Van Houdt M, Otten MA, et al. CD47-signal regulatory protein-alpha (SIRPalpha) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci U S A. 2011;108:18342–18347. doi: 10.1073/pnas.1106550108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Zou F, Wang J, Yin G, Le V, Fei Z, et al. Photodynamic therapy-mediated cancer vaccination enhances stem-like phenotype and immune escape, which can be blocked by thrombospondin-1 signaling through CD47 receptor protein. J Biol Chem. 2015;290:8975–8986. doi: 10.1074/jbc.M114.624965. [DOI] [PMC free article] [PubMed] [Google Scholar]