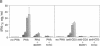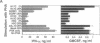Abstract
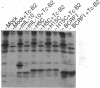
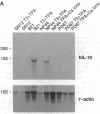
We have demonstrated the existence of human cytokine synthesis inhibitory factor (CSIF) [interleukin 10 (IL-10)]. cDNA clones encoding human IL-10 (hIL-10) were isolated from a tetanus toxin-specific human T-cell clone. Like mouse IL-10, hIL-10 exhibits strong DNA and amino acid sequence homology to an open reading frame in the Epstein-Barr virus, BCRFI. hIL-10 and the BCRFI product inhibit cytokine synthesis by activated human peripheral blood mononuclear cells and by a mouse Th1 clone. Both hIL-10 and mouse IL-10 sustain the viability of a mouse mast cell line in culture, but BCRFI lacks comparable activity in this assay, suggesting that BCRFI may have conserved only a subset of hIL-10 activities.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchetta R., de Waal Malefijt R., Yssel H., Abrams J., de Vries J. E., Spits H., Roncarolo M. G. Host-reactive CD4+ and CD8+ T cell clones isolated from a human chimera produce IL-5, IL-2, IFN-gamma and granulocyte/macrophage-colony-stimulating factor but not IL-4. J Immunol. 1990 Feb 1;144(3):902–908. [PubMed] [Google Scholar]
- Badaro R., Jones T. C., Carvalho E. M., Sampaio D., Reed S. G., Barral A., Teixeira R., Johnson W. D., Jr New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986 Dec;154(6):1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bloom B. R. Learning from leprosy: a perspective on immunology and the Third World. J Immunol. 1986 Jul 1;137(1):i–x. [PubMed] [Google Scholar]
- Bloom B. R., Mehra V. Immunological unresponsiveness in leprosy. Immunol Rev. 1984 Aug;80:5–28. doi: 10.1111/j.1600-065x.1984.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Boom W. H., Liano D., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. II. Effects of interleukin 4- and interleukin 2-producing T cell clones on resting B lymphocytes. J Exp Med. 1988 Apr 1;167(4):1350–1363. doi: 10.1084/jem.167.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calender A., Billaud M., Aubry J. P., Banchereau J., Vuillaume M., Lenoir G. M. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8060–8064. doi: 10.1073/pnas.84.22.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Badaró R., Reed S. G., Jones T. C., Johnson W. D., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985 Dec;76(6):2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Lebman D. A., Hiraki D. D., Christiansen J. A., Shrader B., Cherwinski H. M., Savelkoul H. F., Finkelman F. D., Bond M. W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988 Feb;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R., Sanders V. M., Mosmann T. R., Vitetta E. S. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J Exp Med. 1988 Aug 1;168(2):543–558. doi: 10.1084/jem.168.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Go N. F., Castle B. E., Barrett R., Kastelein R., Dang W., Mosmann T. R., Moore K. W., Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990 Dec 1;172(6):1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin J., Menezes J., Mercier G., Lamoureux G., Oth D. Differential interleukin-2 and interferon-gamma production by human lymphocyte cultures exceptionally resistant to Epstein-Barr virus immortalization. Cell Immunol. 1989 Sep;122(2):440–449. doi: 10.1016/0008-8749(89)90090-7. [DOI] [PubMed] [Google Scholar]
- Hasler F., Bluestein H. G., Zvaifler N. J., Epstein L. B. Analysis of the defects responsible for the impaired regulation of Epstein-Barr virus-induced B cell proliferation by rheumatoid arthritis lymphocytes. I. Diminished gamma interferon production in response to autologous stimulation. J Exp Med. 1983 Jan 1;157(1):173–188. doi: 10.1084/jem.157.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. F., Fox M., Schmid C., Shen C. K. Molecular evolution of the human adult alpha-globin-like gene region: insertion and deletion of Alu family repeats and non-Alu DNA sequences. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5970–5974. doi: 10.1073/pnas.80.19.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. B., Kaye J., Conrad P. J., Katz M. E., Janeway C. A., Jr Autocrine growth inhibition of a cloned line of helper T cells. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1886–1890. doi: 10.1073/pnas.83.6.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Chan-Liew W. L. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 1980 Winter;2(4):303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Genetically determined susceptibility to Leishmania tropica infection is expressed by haematopoietic donor cells in mouse radiation chimaeras. Nature. 1980 Nov 13;288(5787):161–162. doi: 10.1038/288161a0. [DOI] [PubMed] [Google Scholar]
- Hsu D. H., de Waal Malefyt R., Fiorentino D. F., Dang M. N., Vieira P., de Vries J., Spits H., Mosmann T. R., Moore K. W. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990 Nov 9;250(4982):830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- Hudson G. S., Bankier A. T., Satchwell S. C., Barrell B. G. The short unique region of the B95-8 Epstein-Barr virus genome. Virology. 1985 Nov;147(1):81–98. doi: 10.1016/0042-6822(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Katsura Y. Cell-mediated and humoral immune responses in mice. III. Dynamic balance between delayed-type hypersensitivity and antibody response. Immunology. 1977 Mar;32(3):227–235. [PMC free article] [PubMed] [Google Scholar]
- Killar L., MacDonald G., West J., Woods A., Bottomly K. Cloned, Ia-restricted T cells that do not produce interleukin 4(IL 4)/B cell stimulatory factor 1(BSF-1) fail to help antigen-specific B cells. J Immunol. 1987 Mar 15;138(6):1674–1679. [PubMed] [Google Scholar]
- Lotz M., Tsoukas C. D., Fong S., Carson D. A., Vaughan J. H. Regulation of Epstein-Barr virus infection by recombinant interferons. Selected sensitivity to interferon-gamma. Eur J Immunol. 1985 May;15(5):520–525. doi: 10.1002/eji.1830150518. [DOI] [PubMed] [Google Scholar]
- MacNeil I. A., Suda T., Moore K. W., Mosmann T. R., Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990 Dec 15;145(12):4167–4173. [PubMed] [Google Scholar]
- Moore K. W., Vieira P., Fiorentino D. F., Trounstine M. L., Khan T. A., Mosmann T. R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990 Jun 8;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- O'Garra A., Stapleton G., Dhar V., Pearce M., Schumacher J., Rugo H., Barbis D., Stall A., Cupp J., Moore K. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2(9):821–832. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Parish C. R. The relationship between humoral and cell-mediated immunity. Transplant Rev. 1972;13:35–66. doi: 10.1111/j.1600-065x.1972.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Peltz G. A., Grundy H. O., Lebo R. V., Yssel H., Barsh G. S., Moore K. W. Human Fc gamma RIII: cloning, expression, and identification of the chromosomal locus of two Fc receptors for IgG. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1013–1017. doi: 10.1073/pnas.86.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo M. G., Yssel H., Touraine J. L., Bacchetta R., Gebuhrer L., De Vries J. E., Spits H. Antigen recognition by MHC-incompatible cells of a human mismatched chimera. J Exp Med. 1988 Dec 1;168(6):2139–2152. doi: 10.1084/jem.168.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Lal S. L., Shrivastava S. N., Blackwell J., Neva F. A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987 Feb 1;138(3):908–913. [PubMed] [Google Scholar]
- Stout R. D., Bottomly K. Antigen-specific activation of effector macrophages by IFN-gamma producing (TH1) T cell clones. Failure of IL-4-producing (TH2) T cell clones to activate effector function in macrophages. J Immunol. 1989 Feb 1;142(3):760–765. [PubMed] [Google Scholar]
- Suda T., O'Garra A., MacNeil I., Fischer M., Bond M. W., Zlotnik A. Identification of a novel thymocyte growth-promoting factor derived from B cell lymphomas. Cell Immunol. 1990 Aug;129(1):228–240. doi: 10.1016/0008-8749(90)90200-b. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]