Abstract
Glycine is a major neurotransmitter that activates inhibitory glycine receptors and is a co-agonist for excitatory glutamatergic N-methyl-D-aspartate (NMDA) receptors. Two transporters, GLYT1 and GLYT2, regulate extracellular glycine concentrations within the CNS. Dysregulation of the extracellular glycine has been associated with hyperekplexia and nonketotic hyperglycinemia. Here, we report four individuals from two families who presented at birth with facial dysmorphism, encephalopathy, arthrogryposis, hypotonia progressing to hypertonicity with startle-like clonus, and respiratory failure. Only one individual survived the respiratory failure and was weaned off ventilation but has significant global developmental delay. Mildly elevated cerebrospinal fluid (CSF) glycine and normal serum glycine were observed in two individuals. In both families, we identified truncating mutations in SLC6A9, encoding GLYT1. We demonstrate that pharmacologic or genetic abolishment of GlyT1 activity in mice leads to mildly elevated glycine in the CSF but not in blood. Additionally, previously reported slc6a9-null mice and zebrafish mutants also display phenotypes consistent with the affected individuals we examined. Our data suggest that truncating SLC6A9 mutations lead to a distinct human neurological syndrome hallmarked by mildly elevated CSF glycine and normal serum glycine.
Keywords: Glycine transporter, GLYT1, SLC6A9, encephalopathy, hypotonia, hypertonicity, arthrogryposis, respiratory failure
Main Text
The amino acid glycine has essential functions in the CNS, both at inhibitory and at excitatory synapses.1, 2 As an inhibitory neurotransmitter, it activates ionotropic glycine receptors (GlyRs), leading to post-synaptic hyperpolarization and inhibition of neuronal activity, predominantly in caudal regions of the CNS.3, 4 In addition, glycine acts as an obligatory co-agonist at excitatory glutamate receptors of the N-methyl-D-aspartate (NMDA) subtype, which are prevalent throughout the CNS.1, 2 Extracellular glycine concentrations are regulated by two glycine transporters, GLYT1 (encoded by SLC6A9 [MIM: 601019]) and GLYT2 (encoded by SLC6A5 [MIM: 604159]). GLYT2 is exclusively found at presynaptic glycinergic terminals and is required for the replenishment of presynaptic glycine reservoirs, whereas GLYT1 is located predominantly on astrocytes and is essential for the clearance of glycine from the extracellular space and termination of glycinergic neurotransmission.5, 6
Several disorders have been associated with dysregulation of glycine-dependent neurotransmission, including nonketotic hyperglycinemia (NKH), hyperekplexia, chronic pain, epilepsy, and psychiatric disorders.5, 6, 7, 8, 9 NKH, also known as glycine encephalopathy (MIM: 605899), is an inborn error of glycine metabolism caused by malfunction of the glycine cleavage system (GCS). This disease is characterized by significant elevation of plasma and cerebrospinal fluid (CSF) glycine, with a CSF-to-plasma glycine ratio above 0.08, manifesting with hypotonia, lethargy, seizures, hiccups, and apnea, which either lead to neonatal death or severe intellectual disability, spasticity and intractable seizures.7, 10 Here, we describe individuals with NKH-like symptoms but lacking the typical elevation of serum glycine and who carry homozygous truncating mutations in the SLC6A9 gene.
The study was approved by the institutional review board of the Rambam Health Care Campus. All participants or their legal guardians provided written, informed consent. Approval for animal studies was obtained from the local animal use and care committees of the University of Erlangen-Nuremberg. All available participants underwent genetic counseling and were thoroughly examined by medical geneticists and a pediatrician specializing in metabolic disorders. Full clinical descriptions of the affected individuals are available in the Supplemental Note.
The proband (family 1, individual III-4, Figure 1, Table 1) was initially referred for genetic consult at the age of 45 days due to encephalopathy, absent neonatal reflexes, hypotonia, multiple limb contractures, dysmorphism, and suspected seizures. She is the first child of healthy double first cousins of Muslim-Arab descent. Prenatal history was significant for increased nuchal translucency of 4.2 mm and cervical cysts, as well as bilateral clubfeet, clenched hands, polyhydramnios, and dilation of the fourth ventricle. The parents declined amniocentesis. She was born at 33 weeks by emergency caesarean section due to bradycardia. She had an abnormal Apgar score and required mechanical ventilation. Skeletal imaging revealed arthrogryposis including hyperextension of knees, dislocation of both hip joints, and bilateral absent patellae. She developed hypertonicity and startle-like clonus at any touch, which resembled seizures and for which she was treated with phenobarbital. An electroencephalogram (EEG) displayed mild generalized slowing of background activity but no evidence of epileptic discharge. Additionally, hypertension was diagnosed at 1.5 months. A full laboratory work-up for hypertension was significant only for increased urinary catecholamines excretion on repeat samples. Brain magnetic resonance imaging (MRI) and magnetic resonance spectrometry displayed lateral and third ventriculomegaly, mildly excessive extra-axial fluid, and a normal corpus callosum. Extensive diagnostic metabolic work-up was normal except for mildly elevated glycine in the CSF (22 μmol/L, normal range up to 8 μmol/L) and normal serum; CSF-to-serum glycine ratio was 0.08. Urinary glycine excretion was significantly elevated (urinary glycine/creatinine 1,453 μmol/mmol, normal range up to 445 μmol/mmol) but resolved thereafter. The proband is currently two years old and receives oxygen supplementation, but has not been ventilated for several months. She is able to track objects and appears to recognize her parents. She smiles socially but does not speak or babble. The startle-like clonus movements of her upper extremities resolved. There are no spontaneous movements of the lower limbs, but she has some motor function of her upper limbs and is able to grasp objects and bring them to the midline.
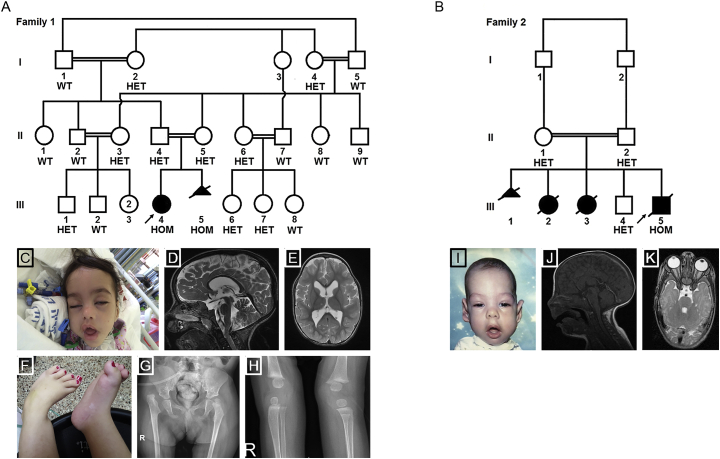
Figure 1.
Family Pedigrees and Clinical Phenotypes of Affected Individuals
(A and B) Pedigrees of families 1 and 2, portraying the segregation of the SLC6A9 variants in the families. HOM, homozygous; HET, heterozygous; WT, wild-type.
(C–H) Proband III-4 from family 1.
(C) Facial image at seven months reveals dysmorphism, including trigonocephaly, long myopathic facies, a tent-shaped mouth, retrognathia, a small upturned nose, depressed nasal bridge, long eyelashes, pronounced eyebrows, ptosis, and low-set ears.
(D and E) Sagittal and axial T2W brain MRI images show dilatation of the lateral and third ventricles and a normal corpus callosum.
(F) Clubfeet and overriding toes.
(G) Pelvis X-ray shows bilateral hip dislocation with redundant epiphysis on the right.
(H) X-ray of knees shows thin, lucent bones, muscle atrophy, and no sign of patellae.
(I–K) Individual III-5 from family 2.
(I) Facial image depicts dysmorphism similar to that of individual III-4 in family 1.
(J) Coronal T1W in the midline shows thin corpus callosum with no signs of myelination at the age of 3 months.
(K) Axial T2W image at the level of the orbits shows thinning of the optic nerves within and posterior to the canal.
Table 1.
Clinical Characteristics of Affected Individuals with SLC6A9 Loss-of-Function
|
Family 1 |
Family 2 |
||||
|---|---|---|---|---|---|
| III-4 (F) | III-5 (M) | III-2 (F) | III-3 (F) | III-5 (M) | |
| Age | 2 years | – | 18 daysa | 2 daysa | 7 monthsa |
| Prenatal skeletal features | bil. clubfeet, clenched fists, overriding toes | none | none (17 weeks) | arthrogryposis (22 weeks) | bil. clubfeet, hyperextension of knees, no movement of elbows |
| Other prenatal features | NT 4.2 cm, cervical cysts, polyhydramnion, 4th ventriculomegaly | NT 6.0 cm, hydrops (abdominal wall and scalp edema) | bone (17 weeks) | bone | bone |
| Delivery | C/S at 33 weeks and 3 days d/t bradycardia | termination at 13 weeks | C/S at 35 weeks and 4 days d/t to fetal distress | C/S at 41 weeks d/t fetal distress | C/S at 30 weeks d/t s/p (3rd C/S) |
| Apgar score at delivery | 3/7 | – | 4/6 | 7/9 | 8/8 |
| Postnatal facial dysmorphism | trigonocephaly, long myopathic facies, tent-shaped mouth, retrognathia, small upturned nose, depressed nasal bridge, long eyelashes, pronounced eyebrows, ptosis, low-set ears | NA | NA | NA | dolichocephaly, long myopathic facies, tent-shaped mouth, small upturned nose, depressed nasal bridge, pronounced eyebrows, ptosis |
| Encephalopathy | yes (resolved at 4 months) | NA | NA | yes | yes |
| Neurological examination | axial hypotonia and absent neonatal reflexes, progressing into hypertonicity and startle-like clonus upon gentle touch or auditory stimulus | NA | NA | prominent head lag | axial hypotonia and absent neonatal reflexes at birth, progressing into hypertonicity and startle-like clonus upon tactile and auditory stimulus |
| MRI findings | lateral and 3rd ventriculomegaly, suspected lt. cerebellar infract, normal corpus callosum | NA | NA | NA | optic nerves atrophy, thin corpus callosum |
| Respiratory failure | yes | NA | yes | yes | yes |
| Skeletal features and/or arthrogryposis | club feet, hyperextension of knees, bil. hip dislocation, contractures of elbows, wrists and hips, overriding toes, absent patellae | NA | lt. club foot, bil. hip dislocation, flexion of elbows and knees, overriding fingers | hyperextension of knees, deformation of hip joints | club feet, hyperextension of knees, bil. hip dislocation, contractures of hips, and wrists, clenched fists |
| Other characteristics | lt. hydronephrosis, HTN with increased urinary catecholamines | NA | NA | NA | bil. hydronephrosis, ASD, rt. cryptorchidism, rt. inguinal hernia, motor and sensory polyneuropathy, abnormal VEP, rt. mild conductive hearing loss |
| CSF glycine (normal < 8 μmol\L) | mildly elevated (22 μmol\L) | NA | elevated (reportedly) | N/A | mildly elevated (25–31 μmol\L) |
| Serum glycine | normal | NA | NA | NA | normal |
Abbreviations are as follows: ASD, atrial septal defect; bil., bilateral; C/S, caesarean section; CSF, cerebrospinal fluid; d/t, due to; F, female; HTN, hypertension; lt., left; NA, not available; M, male; NT, nuchal translucency; rt., right; s/p, status post.
Age at death.
Whole-exome sequencing (WES) of the proband’s DNA was performed on a HiSeq2000 platform (Illumina) with the Nextera Rapid Capture Enrichment kit (Illumina) to capture the protein-coding regions. Mapping of the obtained reads to the reference genome (UCSC Genome Browser hg19 and Ensembl Genome browser GRCh37), variant calling, annotation, and data analysis were performed with the Genoox data analysis platform. We first examined genes in which pathogenic variations are known to cause NKH (AMT [MIM: 238310], GCSH [MIM: 238330], and GLDC [MIM: 238300]),10 variant NKH (BOLA3 [MIM: 613183], GLRX5 [MIM: 609588], IBA57 [MIM: 615316], LIAS [MIM: 607031], and NFU1 [MIM: 608100]),11 and hyperekplexia (GLRA1 [MIM: 138491], GLRB [MIM: 138492], GPHN [MIM: 603930], ARHGEF9 [MIM: 300429], and SLC6A5),12 as well as those related to syndromes of congenital patellar aplasia,13 for variations and coverage over exons. Poorly covered exons were Sanger sequenced to exclude pathogenic variants. In view of parental consanguinity, WES results were filtered for rare (MAF < 0.01), homozygous, non-synonymous, and truncating variants (missense, nonsense, frameshift, and splice-site), yielding 54 variants in 53 genes (Table S1). Of these, 50 were missense variants, two were nonsense variants, one was a splice-site variant, and one was a frameshift variant. Further analysis focused on clinically relevant variants and predicted pathogenicity scores. Only one variant was found in a gene that could be functionally linked to the proband’s phenotype—a five nucleotide deletion in SLC6A9. This variant causes a frameshift and leads to a premature stop codon within exon 6 (out of 14) of the gene (Figure 2B). The variant, c.928_932delAAGTC (p.Lys310Phefs∗31) (GenBank: NM_201649.3), was not observed in any of the public databases of common variants (dbSNP, 1000 Genomes, Exome Aggregation Consortium [ExAC] browser, and the NHLBI Exome Sequencing Project [ESP] Exome Variant Server), in the Greater Middle East (GME) Variome Project, or in our database of ∼300 in-house control chromosomes from individuals of different Israeli ethnicities (∼25% of whom are of Muslim-Arab origin). Moreover, no loss-of-function homozygous variants in this gene were seen in ExAC, GME, or our in-house database. The variant was validated by Sanger sequencing and co-segregated as expected in the extended family (Figure 1A), with only the proband displaying homozygosity.
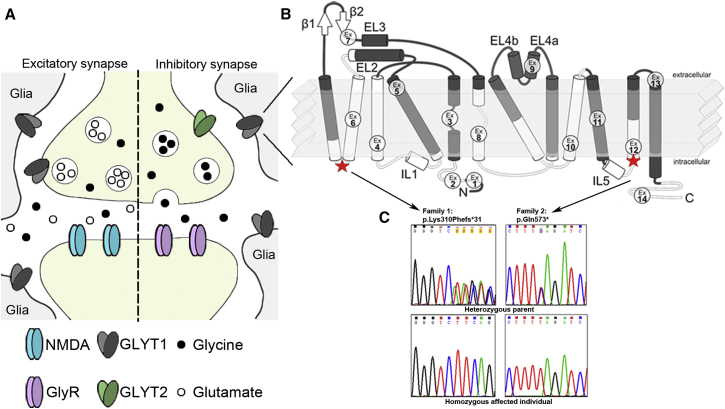
Figure 2.
GLYT1 and SLC6A9 Variants
(A) Localization of GLYT1 on inhibitory glycinergic synapses and excitatory glutamatergic synapses, exhibiting expression mainly on glia cells.
(B) Schematic topological organization of GLYT1, on basis of the crystal structure of the bacterial ortholog LeuTa,14 depicting its 12 transmembrane domains (TMDs) and the intracellular N and C termini. The positions of the respective mutations are indicated. The c.928_932delAAGTC (p.Lys310Phefs∗31) variant is predicted to truncate the protein after the fourth TMD (exon 6), and the c.1717C>T (p.Gln573∗) variant imposes a premature stop in the 11th TMD (exon 12). Ex, exon.
(C) Sequence chromatograms of affected individuals and healthy parents, depicting the identified variants in families 1 and 2 and their location within the protein domains.
The proband has no siblings. However, subsequent pregnancy prenatal ultrasounds at 12 and 13 weeks were significant for increased nuchal translucency of 6.0 mm and hydrops (edema of the abdominal wall and scalp), respectively. The pregnancy was terminated at 13 weeks. DNA analysis revealed a normal male karyotype and homozygosity for the c.928_932delAAGTC (p.Lys310Phefs∗31) variant (family 1, individual III-5, Figure 1A, Table 1).
A review of the hospital’s medical records for undiagnosed individuals with similar clinical features led to the identification of family 2 (Figure 1, Table 1), a consanguineous Muslim-Arab family that is unrelated to family 1. Three of their children were born with encephalopathy, severe arthrogryposis, respiratory failure, and hypotonia that rapidly transformed into hypertonicity with pronounced startle-like clonus. After the birth of the first child with severe arthrogryposis, similar findings were noted on second trimester ultrasounds in subsequent pregnancies. One child died at two days of age and the other two were mechanically ventilated but eventually died at 18 days and at seven months of age. Data available from medical records revealed that individual III-2 in family 2 had elevated CSF glycine (levels are unavailable) and individual III-3 had normal muscle histology (light and electron microscopy). Individual III-5 had mildly elevated CSF glycine (25–31 μmol/L) with normal serum glycine. These findings raised the diagnosis of atypical NKH, prompting a therapeutic trial with ketamine and sodium benzoate with no improvement. Further evaluation of individual III-5 identified motor and sensory polyneuropathy, abnormal visual evoked responses, and mild conductive hearing impairment on brain evoked auditory response. Brain MRI showed a very thin corpus callosum and bilateral optic nerve atrophy. He had dysmorphic features, as well as neurological signs and skeletal findings, that were remarkably similar to those of the proband in family 1; both individuals required tracheostomy and gastrostomy. Sanger sequencing of SLC6A9 in individual III-5 in family 2 (see Table S2 for primer sequences) revealed a homozygous nonsense variant in exon 12, c.1717C>T (p.Gln573∗). This variant was not observed in public or internal databases and co-segregated among available family members (Figure 1B).
SLC6A9 comprises 14 exons that encode GLYT1, a transporter belonging to the Na+/Cl−-dependent superfamily, which consists of 12 transmembrane domains (TMDs) and intracellular N- and C-termini (Figure 2). Binding of 2Na+/Cl− and glycine induces the transporter’s activity and imports glycine predominantly into glial cells.1, 15 Both SLC6A9 variants identified in this study are predicted to cause truncation of the protein; one variant causes truncation after the fourth TMD and the second in the eleventh TMD (according to sequence alignment to the previously crystallized bacterial ortholog LeuTa, Figure 2B, Table S3).14 Similarly to disease-causing mutations in SLC6A5, these mutations are predicted to cause a complete loss of the transporter’s function.16, 17
Slc6a9-null mice have been previously described and appear to share similar phenotypic characteristics with the individuals affected by GLYT1 encephalopathy, including lethargy, pronounced hypotonia, and hyporesponsivity. Slc6a9-null mice die several hours after birth as a result of generalized hypotonia and respiratory failure.18, 19 Interestingly, the phenotype in mice appears to be caused by glycinergic over-inhibition due to excess activation of the ionotropic inhibitory GlyRs, rather than over-excitation via NMDA receptors.18, 20, 21 Knockout mice presented with an impaired respiratory rhythm, as demonstrated by plethysmographic and electrophysiological recordings, which eventually led to their early demise.18 Likewise, slc6a9 mutant zebrafish are deemed shocked (sho) because they exhibit a neuromuscular phenotype that includes embryonic reduced spontaneous coiling of the trunk, diminished escape responses when touched, and an absence of swimming.22
Glycinergic neurons are involved in coordinating motor function, spinal reflex responses, and sensory signal processing by activating GlyRs localized mainly in the brain stem and spinal cord.4, 23, 24 Immunoreactivity analysis using previously described GlyT1-specific antibodies on the wild-type (WT) mouse brain confirmed the highest protein concentration in caudal regions of the CNS known to be rich in glycinergic neurons (Figure 3A).20, 25 This is in line with Slc6a9 expression by RNA in in situ hybridization in the mouse brain (Allen Mouse Brain Atlas). In peripheral tissues, including liver, muscle, and skin, no GlyT1 immunoreactivity was observed (data not shown). Both the characterization of GlyT1-deficient animals as and the expression pattern of GlyT1 suggest that the transporter functions predominantly in the CNS, which corresponds with the phenotype observed in all four affected individuals described here. We suggest that the elevation of brain glycine leads to the encephalopathy and hypotonia observed in the affected individuals through the over-activation of GlyRs.
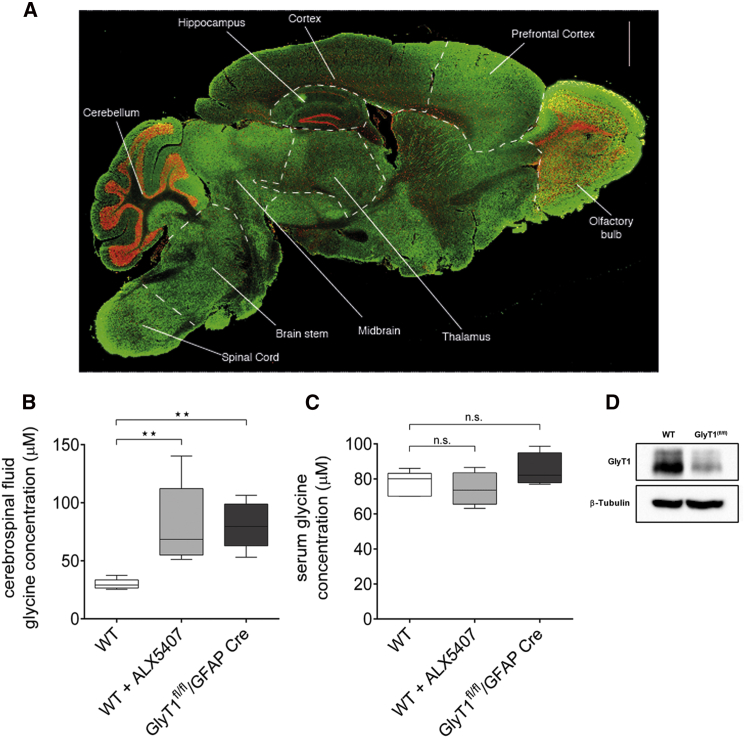
Figure 3.
GlyT1 Levels in Mouse Brain and Glycine Concentrations in Mouse Cerebrospinal Fluid (CSF) and Serum
(A) Immunohistochemical analysis of GlyT1 localization in the adult C57BL/6 mouse brain. Cryosections (12 μm) derived from paraformaldehyde-fixed adult mouse brain were subjected to immunohistochemical analysis with a GlyT1-specific antibody (green).25 For orientation, nuclei were visualized with TO-PRO-3 iodide (red).
(B and C) CSF and blood samples were collected from naive and ALX5407-treated (12 hr after bolus 10 mg/kg, intraperitoneally) C57BL/6 mice, as well as from GlyT1fl/fl/GFAP Cre mice20 (n = 4–6 for each group, duplicate or triplicate for each sample). Protein-free samples were prepared by TCA treatment and the amino acid conjugated to fluorescent products by ortho-phthaldialdehyde treatment. Amino acid concentration was determined by high-pressure liquid chromatography coupled to a fluorescent detector.26 Data are expressed as box plots with whiskers from minimum to maximum. Statistical analysis of wild-type (WT) versus ALX5407 and WT versus GlyT1fl/fl/GFAP Cre was performed with the Mann-Whitney test, ∗∗p < 0.01.
(D) Western blot analysis of GlyT1 in samples derived from WT and GlyT1fl/fl/GFAP Cre mouse spinal cords, using a GlyT1-specific antibody.20 Antibody against β-tubulin was used as a control.
Consistent with the mouse knockout model, all four affected individuals described in this study displayed early respiratory failure, and three died between the ages of two days and seven months. The shared respiratory depression phenotype in both mice and humans in early postnatal stages is in line with the pattern of expression of slc6a9 in the mouse brain, with high levels found in the medulla and pons, which contain the respiratory control centers (Figure 3A).27 One child survived to the age of two years and is currently ventilation free. This was also observed in several glial GlyT1-deficient mice (GlyT1fl/fl/GFAP-Cre mice) that survived the postnatal period, which might reflect changes in the function of GLYT1.20 It has been suggested that GlyT1 is only critical for glycine turnover during the early postnatal period in mice but can be compensated for by other mechanisms in adult animals.20 Further studies are required to clarify the function of GLYT1 in human CNS development and the possible compensatory mechanisms that allow survival of the respiratory crisis.
In the probands of both pedigrees, the CSF-to-serum glycine ratio was consistent with that observed in NKH;7 yet, in contrast to NKH, which is characterized by significant elevation of glycine in both plasma and CSF, the affected individuals in this study had only mild elevation of CSF glycine and normal serum glycine. This might result from the confined expression pattern of GLYT1 to the CNS, with only low expression in selected peripheral tissues.5, 18, 28, 29 To test the effect of GlyT1 dysfunction on the metabolic profile, we analyzed CSF and blood samples that were collected from naive, ALX5407-treated, and GlyT1fl/fl/GFAP Cre mice (Figures 3B and 3C). Glycine amounts were determined by a method originally developed by Graser et al. (1985)30 and modified by Hannappel et al. (1988).26 For better reproducibility, the derivatization reaction was carried out at 26°C. In samples from WT mice, median CSF glycine concentrations of 29 μM were observed, whereas bolus application of the irreversible GlyT1 inhibitor ALX5407 (10 mg/kg), essentially abolishing GlyT1 activity, caused an elevation of the median CSF glycine concentration to 68.5 μM within 12 hr (p < 0.01) (Figure 3B). At this time point, the mice developed pronounced hypoactivity and labored breathing with intermittent hypertonic seizures, more so of the hind limbs (data not shown). To test for the effects of a chronic reduction in GlyT1 activity on the CSF and blood glycine concentrations, we made use of the previously described symptom-free adult GlyT1fl/fl/GFAP-Cre mice, which show a more than 90% reduction in GlyT1 activity in the brain stem and spinal cord (Figure 3D).20 Here too, only a mild but significant elevation of the extracellular glycine concentration (median 79.5 μM) was observed (p < 0.01) (Figure 3B).
These data support the idea that the mild elevation in CSF glycine, as observed in both probands with homozygous GLYT1 deficiency, is indeed caused by a loss of GLYT1 activity and not by additional possibly unrecognized mechanisms. Furthermore, total blood glycine concentrations were similar in samples from WT, ALX5407-treated WT, and GlyT1fl/fl/GFAP-Cre mice (Figure 3C), confirming that the effect of GlyT1 deficiency on glycine concentration is restricted to the CNS at large. The relatively mild elevation of the CSF glycine concentrations both in mice with reduced GlyT1 activity and in humans carrying GLYT1 mutations might result from the compensatory activity of other glycine-specific transporters, e.g., GLYT2, or non-specific amino acid transporters, e.g., the alanine-serine-cysteine-1 (Asc-1) transporter.31 Moreover, considering that GCS activity is unaffected in both humans and mice with GLYT1 dysfunction, intracellular glycine can be normally metabolized, thus accounting for the relative mild increase in CSF glycine and normal serum.
Two possible mechanisms might cause the subsequent transition to hypertonicity and startle-like clonus: either the potentiation of the NMDA receptors or downregulation of the GlyR activity due to negative feedback and loss of glycinergic transmission.32 Interestingly, a similar hypotonic phenotype with intermittent hypertonic episodes was observed in WT animals treated with ALX5407, thus further corroborating the hypothesis that both the hypotonicity and the hypertonic episodes are a direct cause of GLYT1 dysfunction and are not caused by additional maladaptive processes induced by enhanced glycine neurotransmission during development. However, further studies are needed to decipher the precise cellular mechanisms by which GLYT1 dysfunction causes the different facets of the phenotype.
Interestingly, individual III-4 in family 1 demonstrated hypertension accompanied by elevated urine catecholamines, which cannot be attributed to the elevated glycine-dependent neurotransmission in the caudal CNS. Given that pheochromocytoma and neuroblastoma were ruled out, these finding suggest a dysfunction of the adrenal medulla. Glycine has been previously shown to induce release of catecholamines from chromaffin cells in the adrenal medulla.33, 34 Studies of glycine uptake and transport in the adrenal medulla have produced similar transport profiles to those previously identified for glycine in the brain.33 Considering that chromaffin cells are embryonically derived from the neural crest, and structurally resemble post-synaptic sympathetic neurons,35 it is plausible that they might have a glycinergic system similar to that of the CNS, including GLYT1 serving as a transporter in charge of glycine clearance. Consistently, SLC6A9 polymorphisms have previously been associated with hypertension.36
Individual III-5 from family 2 exhibited motor and sensory polyneuropathy and optic atrophy. Whether these symptoms are a result from additional maladaptive processes induced by excess glycine-dependent neurotransmission or are caused by an alternative mechanism is unclear at present.
The skeletal phenotype, marked by arthrogryposis, is not specific for the loss of GLYT1, but rather, can be associated with the childrens' neuro-metabolic condition causing severe hypotonia.37 In addition, Eyal et al. (2015) have recently shown, in mouse embryos, that the developmental separation of the patella from the femur is regulated by mechanical load caused by muscle contraction.38 Therefore, the hyperextension of the knees and lack of muscle contraction in the affected individuals could have prevented normal patella and knee joint formation.
Though NKH has no curative treatment, it appears that in individuals with residual GCS enzymes activity, early aggressive treatment with sodium benzoate, to reduce plasma glycine, and NMDA receptor blockers can improve neurodevelopmental outcomes.39, 40 The subtype of glycine encephalopathy described here did not respond to NKH-targeted therapy in individual III-5 in family 2. The unique metabolic phenotype underscores the significance in studying the profile of CSF amino acids, even when plasma amino acids are within normal range. This highlights the importance of accurate clinical and genetic diagnoses of the different glycine encephalopathy subtypes, enabling suitable treatment, genetic counseling, and family planning, including the possibility of prenatal or preimplantation genetic diagnosis.
In conclusion, we describe a unique human neurological syndrome caused by loss of GLYT1. Although GLYT1 has been previously proposed in the etiology of NKH,5, 6 this study demonstrates that lack of GLYT1 leads to a similar yet distinct phenotype. The syndrome is marked by facial dysmorphism, arthrogryposis, encephalopathy, hypotonia progressing to hypertonicity, and respiratory insufficiency. The mild elevation in CSF glycine and normal serum glycine might constitute a diagnostic marker for GLYT1 defects, even in the presence of an elevated CSF-to-plasma glycine ratio, which is customarily used in the diagnosis of NKH. We propose that glycine encephalopathy should be regarded as an entity with several subtypes, of which NKH is one and GLYT1 encephalopathy, described here, is another.
Acknowledgments
We would like to thank the families for their participation and full cooperation with this study. We would also like to thank the expert consultation and technical help of the Cytogenetics and Molecular Genetics Laboratories and the clinical staff at the Genetics Institute, as well as the Pathology Department at Rambam Health Care Campus, Haifa, Israel, and Noa Weisbrot for her graphical assistance. This research was supported by the Rambam-Ofakim Research Program (to T.H.), and by the DFG (EU110/3-1 to V.E.) and the Interdisciplinary Center for Clinical Research (IZKF) at the University Hospital of the University of Erlangen-Nuremberg (TP E15 to V.E.).
Published: October 20, 2016
Footnotes
Supplemental Data include a Supplemental Note and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.09.004.
Contributor Information
Volker Eulenburg, Email: volker.eulenburg@fau.de.
Hagit N. Baris, Email: hb_feldman@rambam.health.gov.il.
Web Resources
1000 Genomes, http://browser.1000genomes.org/index.html
Allen Mouse Brain Atlas, http://mouse.brain-map.org
Ensembl Genome Browser, http://www.ensembl.org/index.html
ExAC Browser, http://exac.broadinstitute.org/
Genoox data analysis platform, http://genoox.com/
GenBank, http://www.ncbi.nlm.nih.gov/genbank/
GME Variome, http://igm.ucsd.edu/gme
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim.org/
UCSC Genome Browser, http://genome.ucsc.edu
UniProt, http://www.uniprot.org/
Supplemental Data
References
- 1.Eulenburg V., Armsen W., Betz H., Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem. Sci. 2005;30:325–333. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Chen N.H., Reith M.E.A., Quick M.W. Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch. 2004;447:519–531. doi: 10.1007/s00424-003-1064-5. [DOI] [PubMed] [Google Scholar]
- 3.Jiang J., Alstermark B. Not GABA but glycine mediates segmental, propriospinal, and bulbospinal postsynaptic inhibition in adult mouse spinal forelimb motor neurons. J. Neurosci. 2015;35:1991–1998. doi: 10.1523/JNEUROSCI.1627-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betz H., Laube B. Glycine receptors: recent insights into their structural organization and functional diversity. J. Neurochem. 2006;97:1600–1610. doi: 10.1111/j.1471-4159.2006.03908.x. [DOI] [PubMed] [Google Scholar]
- 5.Harvey R.J., Carta E., Pearce B.R., Chung S.K., Supplisson S., Rees M.I., Harvey K. A critical role for glycine transporters in hyperexcitability disorders. Front. Mol. Neurosci. 2008;1:1–6. doi: 10.3389/neuro.02.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey R.J., Yee B.K. Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence and pain. Nat. Rev. Drug Discov. 2013;12:866–885. doi: 10.1038/nrd3893. [DOI] [PubMed] [Google Scholar]
- 7.Swanson M.A., Coughlin C.R., Jr., Scharer G.H., Szerlong H.J., Bjoraker K.J., Spector E.B., Creadon-Swindell G., Mahieu V., Matthijs G., Hennermann J.B. Biochemical and molecular predictors for prognosis in nonketotic hyperglycinemia. Ann. Neurol. 2015;78:606–618. doi: 10.1002/ana.24485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen H.Y., van Vliet E.A., Bright K.A., Hanthorn M., Lytle N.K., Gorter J., Aronica E., Boison D. Glycine transporter 1 is a target for the treatment of epilepsy. Neuropharmacology. 2015;99:554–565. doi: 10.1016/j.neuropharm.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Javitt D.C., Hashim A., Sershen H. Modulation of striatal dopamine release by glycine transport inhibitors. Neuropsychopharmacology. 2005;30:649–656. doi: 10.1038/sj.npp.1300589. [DOI] [PubMed] [Google Scholar]
- 10.Chang Y.T., Lin W.-D., Chin Z.N., Wang C.-S., Chou I.C., Kuo H.T., Tsai F.J. Nonketotic hyperglycinemia: A case report and brief review. BioMedicine. 2012;2:80–82. [Google Scholar]
- 11.Baker P.R., 2nd, Friederich M.W., Swanson M.A., Shaikh T., Bhattacharya K., Scharer G.H., Aicher J., Creadon-Swindell G., Geiger E., MacLean K.N. Variant non ketotic hyperglycinemia is caused by mutations in LIAS, BOLA3 and the novel gene GLRX5. Brain. 2014;137:366–379. doi: 10.1093/brain/awt328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey R.J., Topf M., Harvey K., Rees M.I. The genetics of hyperekplexia: more than startle! Trends Genet. 2008;24:439–447. doi: 10.1016/j.tig.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Bongers E.M.H.F., van Kampen A., van Bokhoven H., Knoers N.V.A.M. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin. Genet. 2005;68:302–319. doi: 10.1111/j.1399-0004.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita A., Singh S.K., Kawate T., Jin Y., Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 15.Olivares L., Aragón C., Giménez C., Zafra F. Analysis of the transmembrane topology of the glycine transporter GLYT1. J. Biol. Chem. 1997;272:1211–1217. doi: 10.1074/jbc.272.2.1211. [DOI] [PubMed] [Google Scholar]
- 16.Rees M.I., Harvey K., Pearce B.R., Chung S.K., Duguid I.C., Thomas P., Beatty S., Graham G.E., Armstrong L., Shiang R. Mutations in the gene encoding GlyT2 (SLC6A5) define a presynaptic component of human startle disease. Nat. Genet. 2006;38:801–806. doi: 10.1038/ng1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eulenburg V., Becker K., Gomeza J., Schmitt B., Becker C.M., Betz H. Mutations within the human GLYT2 (SLC6A5) gene associated with hyperekplexia. Biochem. Biophys. Res. Commun. 2006;348:400–405. doi: 10.1016/j.bbrc.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 18.Gomeza J., Hülsmann S., Ohno K., Eulenburg V., Szöke K., Richter D., Betz H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron. 2003;40:785–796. doi: 10.1016/s0896-6273(03)00672-x. [DOI] [PubMed] [Google Scholar]
- 19.Tsai G., Ralph-Williams R.J., Martina M., Bergeron R., Berger-Sweeney J., Dunham K.S., Jiang Z., Caine S.B., Coyle J.T. Gene knockout of glycine transporter 1: characterization of the behavioral phenotype. Proc. Natl. Acad. Sci. USA. 2004;101:8485–8490. doi: 10.1073/pnas.0402662101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eulenburg V., Retiounskaia M., Papadopoulos T., Gomeza J., Betz H. Glial glycine transporter 1 function is essential for early postnatal survival but dispensable in adult mice. Glia. 2010;58:1066–1073. doi: 10.1002/glia.20987. [DOI] [PubMed] [Google Scholar]
- 21.Obrenovitch T.P., Hardy A.M., Urenjak J. High extracellular glycine does not potentiate N-methyl-D-aspartate-evoked depolarization in vivo. Brain Res. 1997;746:190–194. doi: 10.1016/s0006-8993(96)01197-3. [DOI] [PubMed] [Google Scholar]
- 22.Cui W.W., Low S.E., Hirata H., Saint-Amant L., Geisler R., Hume R.I., Kuwada J.Y. The zebrafish shocked gene encodes a glycine transporter and is essential for the function of early neural circuits in the CNS. J. Neurosci. 2005;25:6610–6620. doi: 10.1523/JNEUROSCI.5009-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du J., Lü W., Wu S., Cheng Y., Gouaux E. Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature. 2015;526:224–229. doi: 10.1038/nature14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legendre P. The glycinergic inhibitory synapse. Cell. Mol. Life Sci. 2001;58:760–793. doi: 10.1007/PL00000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlösser L., Barthel F., Brandenburger T., Neumann E., Bauer I., Eulenburg V., Werdehausen R., Hermanns H. Glycine transporter GlyT1, but not GlyT2, is expressed in rat dorsal root ganglion--Possible implications for neuropathic pain. Neurosci. Lett. 2015;600:213–219. doi: 10.1016/j.neulet.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Hannappel E., Kalbacher H., Voelter W. Thymosin β 4Xen: a new thymosin β 4-like peptide in oocytes of Xenopus laevis. Arch. Biochem. Biophys. 1988;260:546–551. doi: 10.1016/0003-9861(88)90480-8. [DOI] [PubMed] [Google Scholar]
- 27.Richter D.W., Spyer K.M. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 2001;24:464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- 28.Pramod A.B., Foster J., Carvelli L., Henry L.K. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol. Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howard A., Tahir I., Javed S., Waring S.M., Ford D., Hirst B.H. Glycine transporter GLYT1 is essential for glycine-mediated protection of human intestinal epithelial cells against oxidative damage. J. Physiol. 2010;588:995–1009. doi: 10.1113/jphysiol.2009.186262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graser T.A., Godel H.G., Albers S., Földi P., Fürst P. An ultra rapid and sensitive high-performance liquid chromatographic method for determination of tissue and plasma free amino acids. Anal. Biochem. 1985;151:142–152. doi: 10.1016/0003-2697(85)90064-8. [DOI] [PubMed] [Google Scholar]
- 31.Safory H., Neame S., Shulman Y., Zubedat S., Radzishevsky I., Rosenberg D., Sason H., Engelender S., Avital A., Hülsmann S. The alanine-serine-cysteine-1 (Asc-1) transporter controls glycine levels in the brain and is required for glycinergic inhibitory transmission. EMBO Rep. 2015;16:590–598. doi: 10.15252/embr.201439561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J.Y., Pearl P.L. Metabolic causes of epileptic encephalopathy. Epilepsy Res. Treat. 2013;2013 doi: 10.1155/2013/124934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yadid G., Youdim M.B., Zinder O. Uptake and receptor sites for glycine in isolated bovine adrenal medulla chromaffin cells. Neuroscience. 1993;55:1147–1152. doi: 10.1016/0306-4522(93)90328-d. [DOI] [PubMed] [Google Scholar]
- 34.Yadid G., Youdim M.B.H., Zinder O. Preferential release of epinephrine by glycine from adrenal chromaffin cells. Eur. J. Pharmacol. 1992;221:389–391. doi: 10.1016/0014-2999(92)90729-n. [DOI] [PubMed] [Google Scholar]
- 35.Huber K., Kalcheim C., Unsicker K. The development of the chromaffin cell lineage from the neural crest. Auton. Neurosci. 2009;151:10–16. doi: 10.1016/j.autneu.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Ueno T., Tabara Y., Fukuda N., Tahira K., Matsumoto T., Kosuge K., Haketa A., Matsumoto K., Sato Y., Nakayama T. Association of SLC6A9 gene variants with human essential hypertension. J. Atheroscler. Thromb. 2009;16:201–206. doi: 10.5551/jat.e125. [DOI] [PubMed] [Google Scholar]
- 37.Hall J.G. Arthrogryposis (multiple congenital contractures): diagnostic approach to etiology, classification, genetics, and general principles. Eur. J. Med. Genet. 2014;57:464–472. doi: 10.1016/j.ejmg.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Eyal S., Blitz E., Shwartz Y., Akiyama H., Schweitzer R., Zelzer E. On the development of the patella. Development. 2015;142:1831–1839. doi: 10.1242/dev.121970. [DOI] [PubMed] [Google Scholar]
- 39.Korman S.H., Wexler I.D., Gutman A., Rolland M.O., Kanno J., Kure S. Treatment from birth of nonketotic hyperglycinemia due to a novel GLDC mutation. Ann. Neurol. 2006;59:411–415. doi: 10.1002/ana.20759. [DOI] [PubMed] [Google Scholar]
- 40.Flusser H., Korman S.H., Sato K., Matsubara Y., Galil A., Kure S. Mild glycine encephalopathy (NKH) in a large kindred due to a silent exonic GLDC splice mutation. Neurology. 2005;64:1426–1430. doi: 10.1212/01.WNL.0000158475.12907.D6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.