Abstract
Cobblestone lissencephaly (COB) is a severe brain malformation in which overmigration of neurons and glial cells into the arachnoid space results in the formation of cortical dysplasia. COB occurs in a wide range of genetic disorders known as dystroglycanopathies, which are congenital muscular dystrophies associated with brain and eye anomalies and range from Walker-Warburg syndrome to Fukuyama congenital muscular dystrophy. Each of these conditions has been associated with alpha-dystroglycan defects or with mutations in genes encoding basement membrane components, which are known to interact with alpha-dystroglycan. Our screening of a cohort of 25 families with recessive forms of COB identified six families affected by biallelic mutations in TMTC3 (encoding transmembrane and tetratricopeptide repeat containing 3), a gene without obvious functional connections to alpha-dystroglycan. Most affected individuals showed brainstem and cerebellum hypoplasia, as well as ventriculomegaly. However, the minority of the affected individuals had eye defects or elevated muscle creatine phosphokinase, separating the TMTC3 COB phenotype from typical congenital muscular dystrophies. Our data suggest that loss of TMTC3 causes COB with minimal eye or muscle involvement.
Keywords: TMTC3, cobblestone lissencephaly, alpha-dystroglycan, endoplasmic reticulum
Main Text
Cobblestone lissencephaly (COB), also known as type II lissencephaly, is a severe brain malformation characterized by cortical dysplasia, irregular borders between white and gray matter, dysmyelination, cystic cerebellar dysplasia, and brainstem hypoplasia.1 These cortical malformations are due to overmigration of neurons and glial cells beyond the external basement membrane (BM, also known as the glial limitans in the brain) into the subarachnoid space, resulting in the COB appearance.2, 3, 4 The cause of this overmigration defect is an impaired interaction between the glial limitans and the extracellular matrix (ECM) of the BM.5, 6 The dystroglycan-glycoprotein complex (DGC) is a multi-protein complex localized to the glial limitans. In this complex, glycosylated alpha-dystroglycan serves as a binding receptor for ECM proteins of the BM, including laminin, perlecan, or agrin, providing a physical link between the cytoskeleton of the glial cells and the BM.7 Alpha-dystroglycan is a central component of the complex, and loss of its glycosylation reduces DGC binding to the ECM, thus contributing to functional defects during development.8
Type II lissencephaly is associated with disorders ranging from COB without other birth defects to a broader spectrum of conditions referred to as muscular congenital alpha-dystroglycanopathy with brain and eye anomalies (MIM: 253800), including Walker-Warburg syndrome (WWS [MIM: 236670]), muscle-eye-brain disease (MIM: 253280), and Fukuyama congenital muscular dystrophy (MIM: 253800). Symptoms are present at birth and can vary within the spectrum. Affected individuals usually have severe structural brain findings (including lissencephaly and ventriculomegaly), various developmental abnormalities of the eyes (e.g., unilateral or bilateral microphthalmia and retinal dysplasia), hypotonia, progressive muscle weakness, and degeneration. Affected children also display varying degrees of delayed milestones, including delayed speech and motor functions as well as seizures.
All told, COB has been associated with mutations in 16 genes. 12 of them—protein O-mannosyltransferase 1 (POMT1 [MIM: 607423]), protein O-mannosyltransferase 2 (POMT2 [MIM: 607439]), protein O-linked-mannose beta-1,2-N-acetylglucosaminyltransferase 1 (POMGNT1 [MIM: 606822]), fukutin (FKTN [MIM: 607440]), fukutin related protein (FKRP [MIM: 606596]), acetyl glucosaminyl transferase like protein (LARGE [MIM: 603590]), isoprenoid synthase domain-containing protein (ISPD [MIM: 614631]), protein O-linked mannose N-acetylglucosaminyltransferase 2 (GTDC2 [MIM: 614828]), transmembrane protein 5 (TMEM5 [MIM: 605862]), protein O-mannose kinase (POMK [MIM: 615247]), beta-1,3-N-acetylglucosaminyltransferase 3 (B4GAT1 [MIM: 605517]), and beta-1,3-N-acetylgalactosaminyltransferase 2 (B3GALNT2 [MIM: 610194])—are required for the maturation of alpha-dystroglycan into a functional receptor9, 10, 11, 12, 13, 14, 15, 16 and are therefore identified as causing secondary dystroglycanopathies,17 the most common form of congenital muscular dystrophies. Mutations in DAG1 (MIM: 128239), the gene encoding alpha-dystroglycan, are rare and have been described in only a single subject with WWS.18 Additionally, genes encoding BM constituents, including laminin subunit beta 1 (LAMB1 [MIM: 150240]), laminin subunit gamma 3 (LAMC3 [MIM: 614115]), and collagen type IV alpha 1 chain (COL4A1 [MIM 120130]),19, 20, 21 are mutated in COB. Despite intensive research, one-third of COB cases remain unexplained genetically.4
In this report, we show that biallelic mutations in TMTC3 (transmembrane and tetratricopeptide repeat containing 3 [GenBank: NM_181783.3]) result in COB. Individuals harboring mutations in this gene exhibited intellectual disability, hypotonia, and delayed milestones. Most also presented with seizures and severe brain malformations including ventriculomegaly and brainstem and cerebellar hypoplasia, but ocular abnormalities or elevated creatine phosphokinase (CPK) were not a uniform feature.
In a collaborative effort to identify additional mechanisms explaining the genetics of COB, we recruited 25 families with suspected recessive disease. This study was performed in accordance with the ethical standards set by our institutional review boards, and informed consent was obtained from each individual involved in the study. In-solution exome capture was performed with the SureSelect Human All Exome 50 Mb Kit (Agilent Technologies) with 125-bp paired-end read sequences generated on a HiSeq 2000 or 2500 (Illumina) and then analyzed by standard methods.
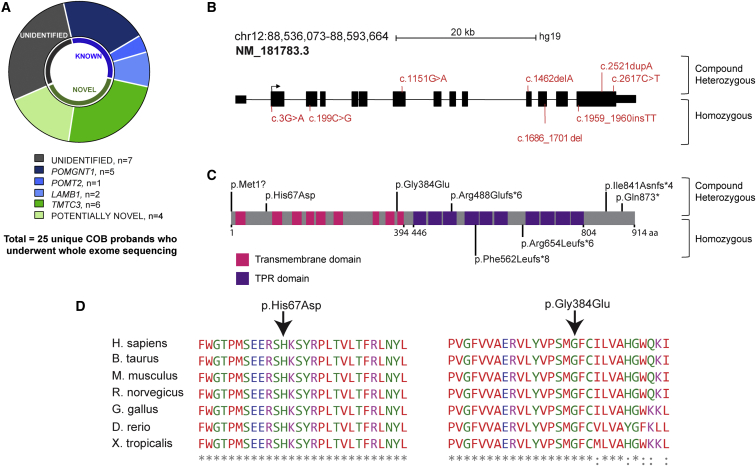
Approximately 32% of the evaluated families were found to harbor biallelic mutations in genes already associated with COB, including POMGNT1 (n = 5), POMT2 (n = 1), and LAMB1 (n = 2) (Figure 1A). Of the remaining screened individuals, there were predicted rare deleterious mutations in genes previously not associated with COB in ten families (40%). Seven (28%) unrelated individuals remained without identified mutations. Six of the ten families harbored biallelic mutations in TMTC3 (24%), which had a total of eight distinct mutations in nine affected individuals from six families (four homozygous and two compound heterozygous) (Figures 1B and 1C and Table S1).
Figure 1.
TMTC3 Is Mutated in COB Brain Malformation
(A) Whole-exome sequencing results for 25 unique families. POMT2 and POMGNT1 mutations were found in one and five and families, respectively, and LAMB1 mutations were found in two families. Six unique probands displayed mutations in TMTC3 (dark green). In one-third of the affected individuals, no causative variant could be identified.
(B) TMTC3, located on chromosome 12, contains 14 exons (blocks), 13 of which are coding. Variants found in individuals are indicated in red (compound heterozygous above and homozygous below the gene). Scale bar represents 20 kb.
(C) TMTC3 (UniProt: Q6ZXV5) has 914 amino acids, 9 transmembrane domains (pink), and 10 TPRs (purple). Variants are indicated in black (compound heterozygous above and homozygous below the protein).
(D) Clustal alignment with multiple species shows conservation of the altered amino acids for the two missense variants. Asterisks indicate amino acids conserved in all noted species, and dots represent amino acid variants in at least one species.
The affected individual in family I carried a frameshift variant in exon 11 (c.1462delA [p.Arg488Glufs∗6]) and a stop variant in exon 14 (c.2617C>T [p.Gln873∗]), whereas the affected individual in family VI carried a missense variant in exon 8 (c.1151G>A [p.Gly384Glu]) and a frameshift variant in exon 14 (c.2521dupA [p.Ile841Asnfs∗4]). Frameshift variants were found in exon 14 (c.1959_1960insTT [p.Arg654Leufs∗6]) and exon 12 (c.1686_1701del [p.Phe562Leufs∗8]) of families II and III, respectively. Affected individuals in family IV carried a missense variant in exon 3 (c.199C>G [p.His67Asp]), and affected individuals in family V had a variant affecting the initiation methionine (c.3G>A [p.Met1?]). The missense variants disrupt amino acids that are highly conserved across evolution (Figure 1D). The variants were unique in our dataset of >5,000 exomes from individuals with neurodevelopmental phenotypes; were not represented in dbSNP138, the Greater Middle Eastern Variome, 1000 Genomes, or the Exome Aggregation Consortium (ExAC) Browser; were confirmed by Sanger sequencing; and fully segregated with disease according to a recessive mode of inheritance for the four families with available samples (Figure S1). All known COB-associated genes were well covered, and no deleterious variants or deletions were found in any of these genes.
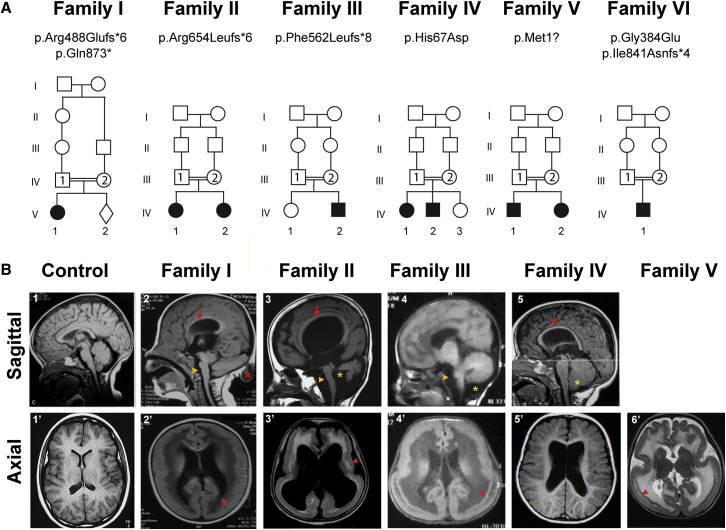
All nine affected children from the six families were born to consanguineous Arabic families except for family V (from Turkey) and family VI (from North America) (Figures 2 and S1 and Table 1). The study included five females and four males with ages ranging from 9 months to 15 years. All children were born full term without complications during pregnancy and delivery, except the child from family VI had occipital encephalocele, not infrequently seen in COB. Measurements at birth, including birth weight and height, were in the normal range for those reported. Head circumference at birth was available for three of nine subjects and ranged from −1 to +1 SD from the mean.
Figure 2.
Family Pedigrees and Brain MRI of Individuals with TMTC3 Variants
(A) Pedigrees harboring biallelic damaged variants in TMTC3. Double bars indicate consanguinity, and filled symbols represent affected individuals.
(B) Sagittal MRI (upper panel) showing hypoplasia of the corpus callosum (red arrow in B2, B3, and B5), hypoplastic brainstem (yellow arrowhead in B2–B4), cerebellum (yellow star in B3), or dysplastic cerebellum (yellow star in B4 and B5). Note the occipital meningocele (red asterisk in B2). Axial MRI (lower panel) reveals COB with heterotopic neurons (red arrow in B2′, B3′, B5′, and B6’). Ventriculomegaly was also observed in most individuals.
Table 1.
Clinical Features of Individuals with TMTC3 Variants
| I-V-1 | II-V-1 | II-V-2 | III-IV-2 | IV-IV-1 | IV-IV-2 | V-IV-1 | V-IV-2 | VI-IV-1 | |
|---|---|---|---|---|---|---|---|---|---|
| cDNA mutations | c.1462delA, c.2617C>T | c.1959_1960insTT | c.1959_1960insTT | c.1686_1701del | c.199C>G | c.199C>G | c.3G>A | c.3G>A | c.1151G>A, c.2521dupA |
| Proteins variants | p.Arg488Glufs∗6, p.Gln873∗ | p.Arg654Leufs∗6 | p.Arg654Leufs∗6 | p.Phe562Leufs∗8 | p.His67Asp | p.His67Asp | p.Met1? | p.Met1? | p.Gly384Glu, p.Ile841Asnfs∗4 |
| Gender | female | female | female | male | female | male | male | female | male |
| Country of origin | Egypt | Yemen | Yemen | Egypt | Lebanon | Lebanon | Turkey | Turkey | US |
| Consanguinity | + | + | + | + | + | + | + | + | − |
| Weight at birth (kg) | NA | NA | NA | 2 | 3.55 | 3.63 | 3.45 | 2.6 | 2.7 |
| Length at birth (cm) | NA | NA | NA | 47 | 55 | 53 | NA | NA | NA |
| HC at birth (SD) | NA | NA | NA | −1 | −1 | +1 | NA | NA | NA |
| Psychomotor Development | |||||||||
| Gross motor skillsa | delayed | delayed | delayed | delayed | delayed | delayed | delayed | delayed | delayed |
| Fine motor skillsa | delayed | delayed | delayed | absent | delayed | delayed | delayed | delayed | delayed |
| Languagea | delayed | delayed | delayed | absent | absent | absent | delayed | delayed | absent |
| Sociala | delayed | delayed | delayed | delayed | delayed | delayed | delayed | delayed | delayed |
| Neurological Examination | |||||||||
| HC at last examination (cm) | 41 (−7.5 SDs) | NA | NA | 39.5 (−4 SDs) | 46 (−1.34 SDs) | 46.5 (−0.22 SD) | 49.5 (−1 SD) | 45 (−2 SDs) | 53 (+1.5 SDs) |
| Age at diagnosis | NA | NA | NA | 6 months | 18 months | 6 months | 1 month | 5 months | prenatal: encephalocele on ultrasound |
| Hypotonia | + | + | + | + | + | + | + | + | + |
| Intellectual disability | + | + | + | + | + | + | + | + | + |
| Autistic-like behavior | NA | + | + | − | NA | NA | − | − | NA |
| Seizures | |||||||||
| Onset | 4 months | 6 months | 8 months | 7 months | − | − | − | 5 months | 6 months |
| Type | GTC | GTC | GTC | myoclonic | − | − | − | GTC | infantile spasms |
| Frequency | weekly | weekly | under control | infrequent | − | − | − | weekly | daily |
| Ophthalmologic Findings | |||||||||
| Cataract | − | + (L) | NA | − | − | − | + (R) | − | − |
| Micropthalmia | − | − | NA | − | − | − | + (L) | − | − |
| Buphthalmos | − | − | NA | − | − | − | − | − | − |
| Megalocornea | − | − | NA | − | − | − | − | − | − |
| Optic atrophy | − | + | NA | − | − | − | − | − | − |
| Retinal dysplasia | − | − | NA | − | − | − | − | − | − |
| Other Systemic Findings | |||||||||
| Cardiovascular | NA | − | NA | − | − | − | NA | − | − |
| Respiratory | NA | − | NA | RD at birth | − | − | NA | − | − |
| Musculoskeletal | NA | − | NA | club feet | club feet | − | NA | contractures of elbow | G-tube feeding due to hypotonia |
| Genitourinary | NA | unilateral pubic adhesion | − | − | − | − | NA | − | − |
| Other | − | − | occipital lipoma | squint | − | − | facial asymmetry | hypertelorismus, strabismus, sacral hemangioma | squint |
| MRI | |||||||||
| Agyria | + | + | + | + | − | − | − | − | − (PMG) |
| Cobblestone lissencephaly | + | + | + | + | − | − | + | + | + |
| Ventriculomegaly | + | + | + | − | + | + | + | − | + |
| Encephalocele | + (occipital) | − | − | − | − | − | − | − | + (occipital) |
| Corpus callosum | hypoplasia | − | − | hypoplasia | hypoplasia | hypoplasia | − | − | partial hypoplasia |
| Brainstem | dysplasia, hypoplasia | hypoplasia | hypoplasia | hypoplasia | hypoplasia | hypoplasia | − | − | − |
| Cerebellum | dysplasia | hypoplasia | hypoplasia | hypoplasia | dysplasia | dysplasia | − | − | − |
| Retrocerebellar cysts | − | − | − | + | − | − | − | − | − |
| Investigations | |||||||||
| CPK level (units/liter) | normal | NA | NA | normal | normal | normal | normal | elevated (650) | elevated (1,025) |
| Other metabolic findings | NA | normal | increased GGT and ALP | normal | normal | normal | normal | normal | normal |
| VEP/ERG | NA | NA | NA | normal | NA | NA | NA | NA | normal |
| EMG | normal | NA | NA | normal | NA | NA | NA | NA | NA |
| Muscle biopsy | NA | NA | NA | NA | NA | NA | NA | NA | mild myopathic change, increased interstitial collagen, fiber-size variation |
Abbreviations: ALP, alkaline phosphatase; CPK, creatine phosphokinase; EMG, electromyography; GGT, gamma glutamate transferase; GTC, generalized tonic clonic; HC, head circumference; L, left; NA, not available; PMG, polymicrogyria; R, right; RD, respiratory distress; and VEP/ERG, visual evoked potential and electroretinogram.
Normal, delayed, or absent.
All affected individuals presented with moderate to severe psychomotor delay. They were able to achieve sitting but much later than unaffected peers. Most were able to ambulate between the ages of 4 and 5 years. All individuals had truncal hypotonia with variable appendicular spasticity and were able to achieve head control and visual tracking but lacked language skills typical for their age. Individuals from families III, IV, and VI presented with no verbal speech. None of the individuals could be placed at the severe end of the dystroglycanopathy spectrum, and unlike WWS individuals, they continued to achieve developmental milestones.
Follow-up measurements of head circumference showed microcephaly in children from families I and III. There were no individuals with macrocephaly or craniofacial dysmorphisms, and none showed progressive loss of milestones, which can be seen in severe forms of dystroglycanopathies.
The majority of individuals (six of nine) had seizures: four had generalized tonic-clonic seizures, whereas III-IV-2 had myoclonic seizures and VI-IV-1 had infantile spasms with secondarily generalized epilepsy. The onset of seizures was between 4 and 8 months, and they were intractable with medication. The frequency of epilepsy in dystroglycanopathies is 30%–62%, which is in accordance with our findings.22, 23
Truncal hypotonia with hyperreflexia and mild to severe appendicular spasticity but without muscular atrophy was present in all children. No retinal dysplasia, optic nerve hypoplasia, megalocornea, or buphthalmos was observed. One individual (V-IV-1) had microphthalmia and a cataract, and another (II-V-1) had a unilateral cataract. This is strikingly low in comparison to the reported high incidence of ocular abnormalities in the COB spectrum: 94% microphthalmia, 43% retinal dysplasia, 95% optic nerve hypoplasia, 50% glaucoma, and 58% pupil abnormalities.24
MRI of eight individuals showed features typical of COB (Figure 2), whereas individual IV-2 from family IV had subcortical and periventricular hypomyelination. Some individuals reported within the COB spectrum have shown infantile hypomyelination that later evolved to patchy dysmyelination, as well as overmigration of neuronal cells in the leptomeningeal space.25 Keeping in mind that this affected individual was 10 months old at the time, this finding is suggestive of COB spectrum. Ventriculomegaly and hypoplastic corpus callosum were encountered in seven and five individuals (partially in VI-IV-1), respectively. Pontocerebellar and cerebellar hypoplasia were both seen in six of nine individuals. Two children, I-V-1 and VI-IV-1, had occipital encephalocele, and one child, III-IV-2, had a retrocerebellar cyst. Overall imaging findings were compatible with those of previous COB cases in the literature.26 CPK, a muscle-damage marker that is usually elevated in muscular dystrophies, was elevated only in children V-IV-2 and VI-IV-1 (Table 1).
TMTC3 encodes a 914 aa protein composed of nine transmembrane domains and ten tetratricopeptide repeats (TPRs), a pattern conserved across evolution, at least to the fly. This protein was first identified in the context of renal transplant surgeries, where it was found to be upregulated in the blood of operationally tolerant subjects.27 TMTC3 localizes to the endoplasmic reticulum (ER) in cultured human odontoblasts and acts as a binding partner of the protein disulfide isomerase family A member 3 (PDIA3 or ERp57) in a yeast two-hybrid screen.28 PDIA3, in turn, is an ER protein involved in the folding of glycoproteins by disulfide-bond formation in association with the chaperone calnexin, and it is overexpressed in ER stress conditions.29 An in vitro study showed that, consistent with its interaction with PDIA3, silencing TMTC3 in HeLa cells sensitizes the cells to ER stress by modulating the proteasome activity and XBP-1 transcript expression, a key player in the unfolded protein response.28 In addition, knockout of mSmile, the murine homolog of TMTC3, results in early postnatal death and altered Tgf-β signaling in embryonic fibroblasts.30 However, the brain phenotype of knockout mice was not evaluated.
TMTC3 is one of four human paralogs (TMTC1–TMTC4) known or predicted to be transmembrane proteins with TPRs.28, 31 TPRs, which are found in many proteins, mediate protein-protein interactions in various cellular processes, such as synaptic vesicle fusion, protein folding, and protein translocation.32 TMTC1 and TMTC2 are ER membrane proteins that interact with the ER calcium uptake pump SERCA2B (both) and calnexin (TMTC2 only).31 Although no clear function has been associated with any of these TMTC family members, their ER localization and association with known ER essential proteins suggest that they actively participate in ER function and homeostasis. Except for the transmembrane and TPR domains, TMTC3 does not have any recognized domains. Because the glycosylation of alpha-dystroglycan begins in the ER, it is tempting to speculate that TMTC3 regulates the glycosylation of alpha-dystroglycan, potentially explaining the functional defects observed during the development in the absence of a functional TMTC3 protein.
In addition to TMTC3, other genes encoding TPR-containing proteins have been associated with human diseases. In AIPL1 (MIM: 604393), nonsense mutations affecting the TPR of the encoded protein, aryl-hydrocarbon-interacting protein-like 1, cause Leber congenital amaurosis (MIM: 20400),33 and in neutrophil cytosolic factor 2 (NCF2 [MIM: 608515]), a missense mutation affecting the encoded TPR is linked to chronic granulomatous disease (MIM: 233710).34
In summary, we implicate TMTC3 in COB with moderate to severe intellectual disability and intractable, infantile-onset epilepsy without eye or muscle involvement. We previously reported mutations in LAMB1 in families with individuals similarly presenting with a pure form of COB lacking eye and muscle defects.20 Similarly, recessive mutations in LAMC3 showed isolated brain involment.21 These shared phenotypes suggest common functions for TMTC3 and the laminin genes.
Acknowledgments
We thank the children and their families for their contributions to this study. This work was supported by the Francois Wallace Mohanan fellowship (J.J.); NIH grant R01GM077243 (J.A.-A.); Deutsche Forschungsgemeinschaft grants AB393/2-1 and AB393/2-2 (R.A.J.); NIH grants R01NS041537, R01NS048453, R01NS052455, and P01HD070494, the Simons Foundation Autism Research Initiative, and Qatar National Research Fund grant 6-1463 (T.B.-O., M.A., and J.G.G.); and Paul D. Wellstone Muscular Dystrophy Cooperative Research Center grant 1U54NS053672 (K.P.C.). K.P.C. and J.G.G. are Howard Hughes Medical Institute Investigators. We thank the Broad Institute (U54HG003067 to E. Lander and UM1HG008900 to D. MacArthur), the Yale Center for Mendelian Disorders (U54HG006504 to R. Lifton and M.G.), and the Gregory M. Kiez and Mehmet Kutman Foundation (M.G.). We acknowledge M. Gerstein, S. Mane, A.B. Ekici, and S. Uebe; the Yale Biomedical High Performance Computing Center for data analysis and storage; the Yale Program on Neurogenetics; and the Yale Center for Human Genetics and Genomics.
Published: October 20, 2016
Footnotes
Supplemental Data include one figure and one table and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.09.007.
Web Resources
1000 Genomes, http://www.1000genomes.org/
Clustal Omega, http://www.ebi.ac.uk/Tools/msa/clustalo/
ExAC Browser, http://exac.broadinstitute.org/
GenBank, http://www.ncbi.nlm.nih.gov/genbank/
OMIM, http://www.omim.org/
UniProt, http://www.uniprot.org/uniprot/
Supplemental Data
References
- 1.van der Knaap M.S., Smit L.M., Barth P.G., Catsman-Berrevoets C.E., Brouwer O.F., Begeer J.H., de Coo I.F., Valk J. Magnetic resonance imaging in classification of congenital muscular dystrophies with brain abnormalities. Ann. Neurol. 1997;42:50–59. doi: 10.1002/ana.410420110. [DOI] [PubMed] [Google Scholar]
- 2.Olson E.C., Walsh C.A. Smooth, rough and upside-down neocortical development. Curr. Opin. Genet. Dev. 2002;12:320–327. doi: 10.1016/s0959-437x(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 3.Kerjan G., Gleeson J.G. Genetic mechanisms underlying abnormal neuronal migration in classical lissencephaly. Trends Genet. 2007;23:623–630. doi: 10.1016/j.tig.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Devisme L., Bouchet C., Gonzalès M., Alanio E., Bazin A., Bessières B., Bigi N., Blanchet P., Bonneau D., Bonnières M. Cobblestone lissencephaly: neuropathological subtypes and correlations with genes of dystroglycanopathies. Brain. 2012;135:469–482. doi: 10.1093/brain/awr357. [DOI] [PubMed] [Google Scholar]
- 5.Barkovich A.J., Guerrini R., Kuzniecky R.I., Jackson G.D., Dobyns W.B. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135:1348–1369. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegenthaler J.A., Pleasure S.J. We have got you ‘covered’: how the meninges control brain development. Curr. Opin. Genet. Dev. 2011;21:249–255. doi: 10.1016/j.gde.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barresi R., Campbell K.P. Dystroglycan: from biosynthesis to pathogenesis of human disease. J. Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 8.Michele D.E., Barresi R., Kanagawa M., Saito F., Cohn R.D., Satz J.S., Dollar J., Nishino I., Kelley R.I., Somer H. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 9.Buysse K., Riemersma M., Powell G., van Reeuwijk J., Chitayat D., Roscioli T., Kamsteeg E.-J., van den Elzen C., van Beusekom E., Blaser S. Missense mutations in β-1,3-N-acetylglucosaminyltransferase 1 (B3GNT1) cause Walker-Warburg syndrome. Hum. Mol. Genet. 2013;22:1746–1754. doi: 10.1093/hmg/ddt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beltran-Valero de Bernabé D., Voit T., Longman C., Steinbrecher A., Straub V., Yuva Y., Herrmann R., Sperner J., Korenke C., Diesen C. Mutations in the FKRP gene can cause muscle-eye-brain disease and Walker-Warburg syndrome. J. Med. Genet. 2004;41:e61. doi: 10.1136/jmg.2003.013870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godfrey C., Clement E., Mein R., Brockington M., Smith J., Talim B., Straub V., Robb S., Quinlivan R., Feng L. Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain. 2007;130:2725–2735. doi: 10.1093/brain/awm212. [DOI] [PubMed] [Google Scholar]
- 12.Jae L.T., Raaben M., Riemersma M., van Beusekom E., Blomen V.A., Velds A., Kerkhoven R.M., Carette J.E., Topaloglu H., Meinecke P. Deciphering the glycosylome of dystroglycanopathies using haploid screens for lassa virus entry. Science. 2013;340:479–483. doi: 10.1126/science.1233675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manzini M.C., Tambunan D.E., Hill R.S., Yu T.W., Maynard T.M., Heinzen E.L., Shianna K.V., Stevens C.R., Partlow J.N., Barry B.J. Exome sequencing and functional validation in zebrafish identify GTDC2 mutations as a cause of Walker-Warburg syndrome. Am. J. Hum. Genet. 2012;91:541–547. doi: 10.1016/j.ajhg.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens E., Carss K.J., Cirak S., Foley A.R., Torelli S., Willer T., Tambunan D.E., Yau S., Brodd L., Sewry C.A., UK10K Consortium Mutations in B3GALNT2 cause congenital muscular dystrophy and hypoglycosylation of α-dystroglycan. Am. J. Hum. Genet. 2013;92:354–365. doi: 10.1016/j.ajhg.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vuillaumier-Barrot S., Bouchet-Séraphin C., Chelbi M., Devisme L., Quentin S., Gazal S., Laquerrière A., Fallet-Bianco C., Loget P., Odent S. Identification of mutations in TMEM5 and ISPD as a cause of severe cobblestone lissencephaly. Am. J. Hum. Genet. 2012;91:1135–1143. doi: 10.1016/j.ajhg.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willer T., Lee H., Lommel M., Yoshida-Moriguchi T., de Bernabe D.B.V., Venzke D., Cirak S., Schachter H., Vajsar J., Voit T. ISPD loss-of-function mutations disrupt dystroglycan O-mannosylation and cause Walker-Warburg syndrome. Nat. Genet. 2012;44:575–580. doi: 10.1038/ng.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godfrey C., Foley A.R., Clement E., Muntoni F. Dystroglycanopathies: coming into focus. Curr. Opin. Genet. Dev. 2011;21:278–285. doi: 10.1016/j.gde.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Riemersma M., Mandel H., van Beusekom E., Gazzoli I., Roscioli T., Eran A., Gershoni-Baruch R., Gershoni M., Pietrokovski S., Vissers L.E. Absence of α- and β-dystroglycan is associated with Walker-Warburg syndrome. Neurology. 2015;84:2177–2182. doi: 10.1212/WNL.0000000000001615. [DOI] [PubMed] [Google Scholar]
- 19.Labelle-Dumais C., Dilworth D.J., Harrington E.P., de Leau M., Lyons D., Kabaeva Z., Manzini M.C., Dobyns W.B., Walsh C.A., Michele D.E., Gould D.B. COL4A1 mutations cause ocular dysgenesis, neuronal localization defects, and myopathy in mice and Walker-Warburg syndrome in humans. PLoS Genet. 2011;7:e1002062. doi: 10.1371/journal.pgen.1002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radmanesh F., Caglayan A.O., Silhavy J.L., Yilmaz C., Cantagrel V., Omar T., Rosti B., Kaymakcalan H., Gabriel S., Li M. Mutations in LAMB1 cause cobblestone brain malformation without muscular or ocular abnormalities. Am. J. Hum. Genet. 2013;92:468–474. doi: 10.1016/j.ajhg.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barak T., Kwan K.Y., Louvi A., Demirbilek V., Saygı S., Tüysüz B., Choi M., Boyacı H., Doerschner K., Zhu Y. Recessive LAMC3 mutations cause malformations of occipital cortical development. Nat. Genet. 2011;43:590–594. doi: 10.1038/ng.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barkovich A.J. Neuroimaging manifestations and classification of congenital muscular dystrophies. AJNR Am. J. Neuroradiol. 1998;19:1389–1396. [PMC free article] [PubMed] [Google Scholar]
- 23.Ishigaki K. [Central Nervous Involvement in Patients with Fukuyama Congenital Muscular Dystrophy] Brain Nerve. 2016;68:119–127. doi: 10.11477/mf.1416200361. [DOI] [PubMed] [Google Scholar]
- 24.Dobyns W.B., Truwit C.L. Lissencephaly and other malformations of cortical development: 1995 update. Neuropediatrics. 1995;26:132–147. doi: 10.1055/s-2007-979744. [DOI] [PubMed] [Google Scholar]
- 25.Bahi-Buisson N., Poirier K., Boddaert N., Fallet-Bianco C., Specchio N., Bertini E., Caglayan O., Lascelles K., Elie C., Rambaud J. GPR56-related bilateral frontoparietal polymicrogyria: further evidence for an overlap with the cobblestone complex. Brain. 2010;133:3194–3209. doi: 10.1093/brain/awq259. [DOI] [PubMed] [Google Scholar]
- 26.Kirschner J., Bönnemann C.G. The congenital and limb-girdle muscular dystrophies: sharpening the focus, blurring the boundaries. Arch. Neurol. 2004;61:189–199. doi: 10.1001/archneur.61.2.189. [DOI] [PubMed] [Google Scholar]
- 27.Brouard S., Mansfield E., Braud C., Li L., Giral M., Hsieh S.C., Baeten D., Zhang M., Ashton-Chess J., Braudeau C. Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc. Natl. Acad. Sci. USA. 2007;104:15448–15453. doi: 10.1073/pnas.0705834104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Racapé M., Duong Van Huyen J.-P., Danger R., Giral M., Bleicher F., Foucher Y., Pallier A., Pilet P., Tafelmeyer P., Ashton-Chess J. The involvement of SMILE/TMTC3 in endoplasmic reticulum stress response. PLoS ONE. 2011;6:e19321. doi: 10.1371/journal.pone.0019321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parakh S., Atkin J.D. Novel roles for protein disulphide isomerase in disease states: a double edged sword? Front. Cell Dev. Biol. 2015;3:30. doi: 10.3389/fcell.2015.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yun E.J., Vu T.H. mSmile is necessary for bronchial smooth muscle and alveolar myofibroblast development. Anat. Rec. (Hoboken) 2012;295:167–176. doi: 10.1002/ar.21475. [DOI] [PubMed] [Google Scholar]
- 31.Sunryd J.C., Cheon B., Graham J.B., Giorda K.M., Fissore R.A., Hebert D.N. TMTC1 and TMTC2 are novel endoplasmic reticulum tetratricopeptide repeat-containing adapter proteins involved in calcium homeostasis. J. Biol. Chem. 2014;289:16085–16099. doi: 10.1074/jbc.M114.554071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeytuni N., Zarivach R. The TPR Motif as a Protein Interaction Module – A Discussion of Structure and Function. In: Cai J., Wang R.E., editors. Protein Interactions. Intech; 2012. pp. 103–118. [DOI] [PubMed] [Google Scholar]
- 33.Sohocki M.M., Bowne S.J., Sullivan L.S., Blackshaw S., Cepko C.L., Payne A.M., Bhattacharya S.S., Khaliq S., Qasim Mehdi S., Birch D.G. Mutations in a new photoreceptor-pineal gene on 17p cause Leber congenital amaurosis. Nat. Genet. 2000;24:79–83. doi: 10.1038/71732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsukahara F., Hattori M., Muraki T., Sakaki Y. Identification and cloning of a novel cDNA belonging to tetratricopeptide repeat gene family from Down syndrome-critical region 21q22.2. J. Biochem. 1996;120:820–827. doi: 10.1093/oxfordjournals.jbchem.a021485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.