Abstract
Numerous pieces of evidence have revealed that oxaliplatin (OXA) evokes mechanical and cold hypersensitivity. However, the mechanism underlying these bothersome side effects needs to be further investigated. It is well known that cyclooxygenase-2 (COX-2) and extracellular signal-regulated kinases (ERK1/2) signaling play crucial roles in several pain states. Our previous data showed that Akt2 in the dorsal root ganglion (DRG) participated in the regulation of OXA-induced neuropathic pain. But it is still unclear whether spinal ERK1/2 signaling is involved in the regulation of OXA-induced hyperalgesia, and the linkage between COX-2 and ERK1/2 signaling in mediating OXA-induced hyperalgesia also remains unclear. In this research, we investigated the possible mechanism of celecoxib, a COX-2 inhibitor, in OXA-induced neuropathic pain. Our results show that single dose of OXA (12 mg/kg) significantly attenuated both the tail withdrawal latency (TWL) and mechanical withdrawal threshold (MWT) at days 4 after the OXA treatment. Administration of celecoxib (30 mg/kg/day) for 4 and 6 days inhibited the decrease in TWL and MWT, and each was significantly higher than that of the OXA+vehicle group and was equivalent to that of the vehicles group. OXA increased the expression of cyclooxygenase-2 (COX-2) mRNA and phosphorylated extracellular signal-regulated kinase1/2 (pERK1/2) protein in the lumbar 4-5 (L4-5) spinal cord dorsal horn neurons. Administration of celecoxib for 7 days suppressed the increase in expression of COX-2 and pERK1/2 induced by OXA. Our findings suggested that COX-2 and ERK1/2 signaling in spinal cord contributed to the OXA-induced neuropathic pain.
Keywords: oxaliplatin, cyclooxygenase-2, pERK1/2, neuropathic pain, celecoxib
Introduction
Oxaliplatin (OXA), a third-generation platinum-based chemotherapy agent, is considered a central component in the treatment of advanced colorectal cancer1. OXA treatment has prolonged the lives of many people diagnosed in advanced stages of the colorectal cancer. Despite its efficacy, there are numerous adverse effects associated with OXA. Neurotoxicity is a common adverse effect of oxaliplatin that usually presents as peripheral neuropathy. The development of a neuropathic syndrome impairs quality of life and potentially results in chemotherapy dose reductions and/or early discontinuation2, 3. There are 2 forms of OXA-induced neurotoxicity: acute neuropathy and chronic neuropathy. The acute form occurs in >90% of patients and may begin during the infusion or within hours of completion. Chronic neuropathy is cumulative and is most commonly seen in patients who have received doses of 540 mg/m2 or more4.
Accumulating studies have reported the important role of OXA in inducing cold and mechanical allodynia. Many studies have focused on the side effects of OXA in the dorsal root ganglion (DRG), which contains the cell bodies of the primary sensory neurons responsible for transduction and modulation of sensory information and transmission of it to the spinal cord5, 6. Our previous research also indicate that celecoxib alleviates OXA-induced neuropathic pain through inhibiting of the PI3K/Akt2 pathway in the mouse DRG7. Recently, an increasing number of data suggested that spinal pathological responses are evoked by OXA, contributing to hyperalgesia. OXA contributes to neuropathic pain through the activation of glias8, 9. OXA-induced mechanical allodynia is associated with spinal NMDA receptor subunit NR2B upregulation, while selective NR2B antagonists Ro25-6981 and ifenprodil attenuate the OXA-induced pain behaviors10. Milnacipran, a serotonin–noradrenaline reuptake inhibitor, is effective against OXA-induced mechanical allodynia, and the anti-allodynic effect is mainly mediated by actions on the spinal cord11. Moreover, bee venom acupuncture treatment alleviates OXA-induced acute cold allodynia in rats via activation of the serotonergic system, especially spinal 5-HT3 receptors12.
It is well known that cyclooxygenase-2 (COX-2) and extracellular signal-regulated kinases (ERK1/2) signaling play crucial roles in several pain states13, 14. In the present study, we explored the expression levels of COX-2 and ERK1/2 in L4-5 segments of the spinal cord, from which the hind limb receives innervations, of OXA-treated mice. The roles of the COX-2 inhibitor celecoxib in OXA-induced pain behaviors and its underlying mechanisms were also investigated. It is hoped than our novel understanding of OXA-induced neurotoxicity in cancer therapy will provide a new therapeutic strategy to prevent hyperalgesia.
Materials and Methods
Animals
Adult Male C57BL/6J mice (10 weeks old; 49–51 passages from the original colony) were provided by the Center of Laboratory Animal Science of Nanchang University. The mice were fed a standard laboratory diet under controlled temperature and a 12-h light/dark cycle at 20–22°C. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Medical College of Nanchang University and were performed in accordance with the principles outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental protocols
Mice were randomly divided into 3 groups, with 10 mice in each group: a vehicles group, an OXA+vehicle group, and an OXA+celecoxib (30 mg/kg/day) group. In the OXA+vehicle group and the OXA+celecoxib group, the mice were injected intraperitoneally with single doses of 12 mg/kg body weight of OXA (Qilu Pharmaceutical Co., Ltd, Jinan, China) dissolved in 5% glucose solution on day 0 (d0). Celecoxib was dissolved in 0.5% methylcellulose vehicle (Sigma-Aldrich, St Louis, MO, USA) and was delivered twice daily by oral gavage for 7 days, beginning on d1. The same volume of 5% glucose solution was intraperitoneally injected in the vehicles group. The mice in the vehicles and the OXA+vehicle groups were delivered the same volume of 0.5% methylcellulose according to the procedure for the OXA+celecoxib group. The dosage of celecoxib (30 mg/kg/day) was chosen according to a previous report7. This dose of celecoxib was an effective level against the neuropathic pain-induced by OXA. The pain behaviors were tested once every 2 days from d0 before OXA administration to d8. They were tested every day beginning 24h after first dose of celecoxib.
OXA treatment is a well characterized model used for studying neuropathic pain. Based on the OXA concentration used, there are generally two ways to induce hyperalgesia: long-term treatment with a low dose of OXA for 15, 16 and short-term treatment with high dose of OXA7, 17. In this study, the dosage of OXA was chosen according to a previous report6. A single dose of a high concentration of OXA was used to induce hyperalgesia. In brief, single doses of 12 mg/kg body weight of OXA were injected intraperitoneally. At d8, all the mice were deeply anesthetized with pentobarbital sodium (100 mg/kg sodium pentobarbital, i.p.) and sacrificed by decapitation.
Cold-sensitivity detection
The behavioral signs of cold allodynia were measured by a tail immersion test in cold water. Each mouse was lightly immobilized in a plastic holder, and its tail was dipped in cold water. The tail was immersed in 4°C water, and then the tail withdrawal latency (TWL) was counted. The tail immersion test was repeated 3 times at 5 min intervals. The average latency was taken as a measure for the severity of cold allodynia. All behavioral tests were performed blind.
Mechanical-sensitivity detection
Mechanical allodynia measurement was carried out as previously described18. In brief, the mechanical withdrawal threshold (MWT) was determined to evaluate mechanical hyperalgesia using calibrated von Frey filaments (BME-403, Institute of Biomedical Engineering, Tianjin, China). The measurement was repeated 3 times at 30 s intervals. The average was taken as the mechanical withdrawal threshold. All behavioral tests were performed blind.
Real-time PCR quantification
The whole spinal cord was collected by pressure expulsion with ice cold saline. The dorsal and ventral parts of the L4-5 spinal cord were dissected on an ice cooled glass dish. Total RNA from L4-5 spinal cord dorsal segments was isolated using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Reverse transcription was performed using 1,000 ng total RNA as a template and a Applied Biosystems Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Real-time quantitative PCR for COX-2 was performed using an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The expression level of COX-2 was normalized to GAPDH. The probe was purchased from Applied Biosystems. All assays were performed in triplicate. The average fold change relative to the vehicles group was calculated in each group.
Immunohistochemistry
Spinal cord segments of L4-5 were analyzed from 6 mice in each group. The formalin-fixed, paraffin-embedded tissues were cut, and sections (5 µm thick) were used for immunohistochemistry (IHC) detection. Six nonadjacent sections from each specimen of the L4-5 spinal cord were selected. IHC was carried out as previously described19, using primary antibodies against pERK1/2 (1:100; Santa Cruz Biotechnology, Dallas, TX, USA). A rabbit kit (PV-6001, ZSGB-BIO, Beijing, China) was used as a secondary antibody according to the maufacture’s instructions. Protein localization was detected following incubation with diaminobenzidine and H2O2 for 2 min. Finally, sections were dehydrated in graded alcohols and mounted with neutral balsam. The numbers of pERK1/2-positive neurons from one side of spinal cord dorsal horn of each mouse were counted. Data from 6 sections of the same mouse were averaged.
Western blot
L4-5 spinal cord dorsal segments were homogenized in radioimmunoprecipitation assay (RIPA) buffer. Samples of 30 µg of total protein were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. After incubation with primary antibody pERK1/2 (1:1000; Santa Cruz Biotechnology, Dallas, TX, USA) or total extracellular signal-regulated kinase1/2 (tERK,1:1,000; Cell Signaling Technology, Danvers, MA, USA), the membrane was incubated with peroxidase conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA, USA). Immunodetection was completed using Pierce-enhanced chemiluminescence substrate (Thermo Scientific, Waltham, MA, USA), and the membrane was then exposed to X-ray film. The average fold change relative to the vehicles group was calculated in each group.
Statistical analysis
Data are shown as the mean ± SD. Comparisons of means between two groups were carried out using a t-test. Statistical comparisons were performed by analysis of variance (ANOVA) with Dunnett’s test for multiple comparisons. A value of P<0.05 was considered to be significant.
Results
Celecoxib attenuated the cold and mechanical hypersensitivity induced by OXA
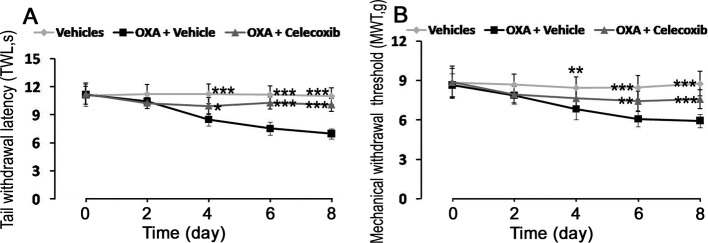
The TWL and MWT were measured to evaluate the effects of celecoxib on pain behaviors of OXA-treated mice. The results showed that both the TWL and MWT thresholds significantly decreased in the OXA+vehicle group (8.50 s and 6.83 g, respectively) when compared with the vehicles group (11.22 s and 8.46 g, respectively) at d4 and remained at low levels. At d8, the TWL and MWT thresholds (6.97 s and 5.93 g, respectively) were still lower when compared with those of the vehicles group (Fig. 1). Celecoxib significantly increased the TWL threshold (from 8.50 s to 9.95 s) beginning at d4. At d6, The TWL threshold continued to increase. Celecoxib significantly upregulated the MWT threshold (from 6.07 g to 7.45 g) beginning at d6. At d8, both the TWL and MWT thresholds still were upregulated by celecoxib (Fig. 1). The results suggested that celecoxib alleviated the OXA-induced pain behaviors.
Fig. 1.
Effects of celecoxib on OXA-induced cold and mechanical hypersensitivity. (A) Celecoxib attenuated the cold hypersensitivity induced by OXA. (B) Celecoxib attenuated the mechanical hypersensitivity induced by OXA. Data are showed as the mean ± SD (n = 10). *P<0.05; **P<0.01; and ***P<0.001 (all vs the OXA+vehicle group).
Celecoxib decreased pERK1/2-positive neurons in the L4-5 spinal cord dorsal horn
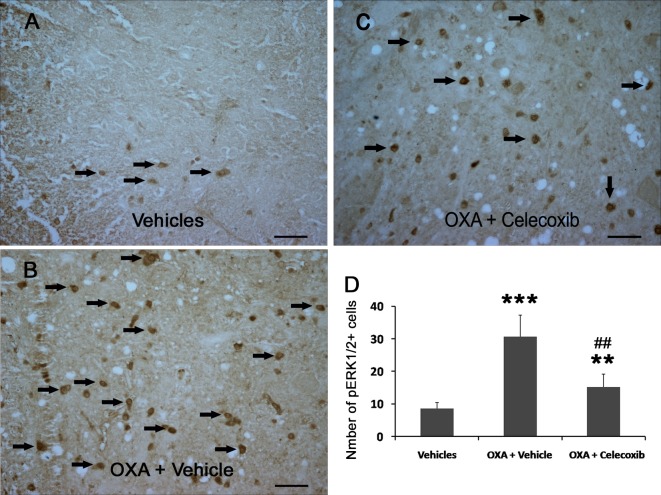
The immunoreactivity of pERK1/2 was detected using immunohistochemistry. The results showed that OXA increased the number of pERK1/2-positive neurons from 8.67 to 30.67 neurons and that administration of celecoxib efficiently decreased the number of pERK1/2-positive neurons from 30.67 to 15.17 neurons (Fig. 2). The results imply that ERK1/2 signaling may be involved in the regulation of OXA-induced hypersensitivity.
Fig. 2.
Celecoxib decreased pERK1/2-positive neurons in the L4-5 spinal cord dorsal horn of OXA-treated mice. (A–C) pERK1/2 immunoreactivities (brown) were detected. Arrowheads indicate representative pERK1/2-positive neurons. Scale bar is 40 µm. (D) The numbers of pERK1/2-positive neurons on one side of the L4-5 spinal cord dorsal horn. Data are shown as the mean ± SD (n = 6). **P<0.01; ***P<0.001 (both vs the vehicles group). ##P<0.01 (vs the OXA+vehicle group).
Celecoxib suppressed the OXA-induced COX-2 mRNA expression
COX-2 mRNA was detected by real-time PCR. COX-2 mRNA expression was significantly upregulated (2.10 folds) in the OXA+vehicle group as compared with the vehicles group. Administration of celecoxib significantly suppressed the expression of COX-2 mRNA compared with the OXA+vehicle group (Fig. 3).
Fig. 3.
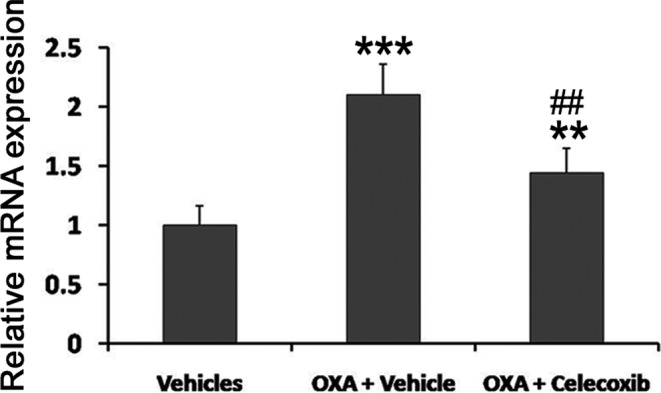
OXA increased the COX-2 mRNA in L4-5 spinal cord dorsal horn. COX-2 mRNA was detected by real-time PCR. Data are shown as the mean ± SD (n = 6). **P<0.01; ***P<0.001 (both vs the vehicles group). ##P<0.01 (vs the OXA+vehicle group).
Celecoxib downregulated the level of OXA-induced pERK1/2 protein
To investigate the potential mechanism of celecoxib in reversing of OXA-induced hyperalgesia in term of protein level, the pERK1/2 protein was measured by Western blot. The level pERK1/2 protein significantly increased to 261% in the OXA+vehicle group as compared with the vehicles group. Administration of celecoxib significantly decreased pERK1/2 protein expression (Fig. 4). These data further illustrated that the spinal COX-2 and pERK1/2 pathway mediated OXA-induced hypersensitivity.
Fig. 4.
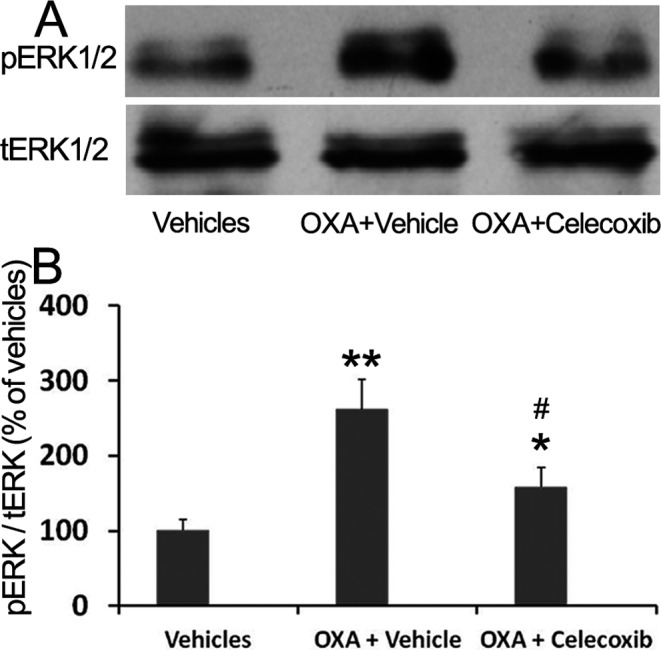
Celecoxib decreased the expression of pERK1/2 protein in the L4-5 spinal cord dorsal horn of OXA-treated mice. pERK1/2 protein was measured by Western blot. Data are shown as the mean ± SD (n = 4). *P<0.05; **P<0.01 (both vs the vehicles group). #P<0.05 (vs the OXA+vehicle group).
Discussion
The present study demonstrated the following novel findings: (1) Administration of oxaliplatin (OXA) increased spinal cyclooxygenase-2 (COX-2) mRNA and ERK1/2 protein. (2) Celecoxib suppressed the COX-2 and ERK1/2 pathway in the spinal cord of OXA-treated mice. (3) Celecoxib alleviated OXA-induced hyperalgesia through inhibition of spinal pERK1/2 protein. These results reveal a critical role of spinal COX-2 and ERK1/2 signaling in OXA-induced neuropathic pain.
Neuropathic pain, arising from lesions to peripheral nerves, is present in many neurological diseases and occurs in patients with diabetes, cancer, and AIDS. Moreover, it is frequently induced by chemotherapy20. It is caused by an injury to the peripheral or central nervous system. Characteristic features of neuropathic pain are hyperalgesia, allodynia, and spontaneous pain. Numerous pieces of evidence have shown that upregulation of spinal pain mediators and related receptors contributes to neuropathic pain21, 22.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are strong cyclooxygenase (COX) inhibitors that are widely used in the management of acute and inflammatory pain. Two isoforms of COX, COX-1 and COX-2, are targets of NSAIDs. A variety of studies have shown that COX-2 inhibitors play a pivotal role in neuropathic pain. Ibuprofen attenuates hyperalgesia following chronic constriction injury by suppressing the expression of P2X3 receptors in the DRG23. Celecoxib produces an antihyperalgesic and antiallodynic effect in diabetic rats24. Furthermore, spinal COX-2 contributes to neuropathic pain. Spinal nerve ligation causes mechanical allodynia, which is accompanied by increased expression of spinal COX-2 immunoreactivity25. Intrathecal administrations of the COX-2 inhibitors attenuate streptozotocin-induced mechanical hyperalgesia through inhibition of spinal COX-2 protein13. It has been revealed that the COX-2 inhibitor celecoxib can inhibit tumor growth and enhance the anti-tumor effect of OXA through their synergistic role in inhibiting different targets26.
However, the effects of spinal COX-2 on OXA-induced neuropathic pain are poorly understood. In the present investigation, we found that administration of celecoxib could alleviate both mechanical and cold hypersensitivity induced by OXA through inhibition of spinal COX-2 mRNA. Our findings may provide a clinically useful evidence for the dual roles of celecoxib in cancer therapy: enhancing the anti-tumor effect of OXA and alleviating OXA-induced hyperalgesia.
Chemotherapy-induced peripheral neuropathy could trigger the pathophysiological changes in the spinal cord, as evidenced by neuroinflammatory processes including the release of pro-inflammatory cytokines27. OXA increases production of pro-inflammatory and neuroexcitatory cytokines (TNF, IL-1b) in the dorsal horn of the spinal cord28. COX-2, an inflammatory mediator, increases in the spinal cord dorsal horn neurons following L5 spinal nerve ligation25. Our previous data show that OXA can increase the expression of COX-2 in the DRG7. In the present study, we also found that OXA increased the COX-2 mRNA expression in the spinal dorsal horn. However, administration of 2.4 mg/kg OXA for 5 consecutive days every week for 3 weeks slightly increased the expression of COX-2 protein, with no significant difference in the rat spinal cord15. Therefore, OXA increased the spinal COX-2 level in a dose-dependent manner. The mechanisms of the OXA-induced increase in COX-2 mRNA are still not clear and need to be further investigated.
The mitogen-activated protein kinase (MAPK) cascade is a highly conserved module that is involved in various pathological functions, including neuropathic pain29, 30. At least four members of the MAPK family, ERK1/2, JNK, p38, and ERK5, have been identified31. The role of spinal ERKs in nociception had been explored in the recent years. It is reported that the immunoreactivity of pERK1/2 can be used as a quantitative marker for sensitization or inhibition in the pain pathway at the spinal level14.
It has been revealed that COX-2 is involved in the regulation of MAP kinase signaling. The p-ERK Level is downregulated after celecoxib treatment in the cirrhotic liver model of rats32. The COX-2 inhibitor parecoxib exerts its analgesic effect on surgical pain through inhibition of neuronal ERK activation in the spinal cord33. In the present study, we investigated the role of spinal ERK1/2 signaling in OXA-induced neuropathic pain. OXA increased the expression of pERK1/2 in the spinal dorsal horn neurons while celecoxib alleviated the OXA-induced hyperalgesia accompanied by the downregulation of pERK1/2. Thus, spinal ERK1/2 plays a vital role in OXA-induced neuropathic pain.
In conclusion, our study was the first to show that COX-2 and pERK1/2 were increased within the spinal cord after administration of OXA. A COX-2 inhibitor alleviated the neuropathic pain caused by OXA through inhibition of COX-2 and pERK1/2. These data illustrated that OXA can induce neuropathic pain, and this role of OXA was mediated by the COX-2 and pERK1/2 pathway.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81260318), Educational Department of Jiangxi Province (No. GJJ14162), and the Health and Family Planning Commission of Jiangxi Province (No. 20143198).
Footnotes
Disclosure of Potential Conflicts of Interest: We have no conflicts of interest.
References
- 1.Petrioli R, Licchetta A, Roviello G, Pascucci A, Francini E, Bargagli G, Conca R, Miano ST, Marzocca G, and Francini G. Multidisciplinary Oncology Group On Gastrointestinal Tumors CEA and CA19.9 as early predictors of progression in advanced/metastatic colorectal cancer patients receiving oxaliplatin-based chemotherapy and bevacizumab. Cancer Invest. 30: 65–71. 2012. [DOI] [PubMed] [Google Scholar]
- 2.Xiao WH, and Bennett GJ. Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain. 153: 704–709. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouwers EE, Huitema AD, Boogerd W, Beijnen JH, and Schellens JH. Persistent neuropathy after treatment with cisplatin and oxaliplatin. Acta Oncol. 48: 832–841. 2009. [DOI] [PubMed] [Google Scholar]
- 4.Cersosimo RJ. Oxaliplatin-associated neuropathy: a review. Ann Pharmacother. 39: 128–135. 2005. [DOI] [PubMed] [Google Scholar]
- 5.Mi Y, Zhang X, Zhang F, Qi J, Gao H, Huang D, Li L, Zhang H, and Du X. The role of potassium channel activation in celecoxib-induced analgesic action. PLoS One. 8: e54797 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Wang Q, Lei Q, Zhang L, and Kang L. Ontogenic expression profiles and oxaliplatin regulation of leptin expression in mice dorsal root ganglion. Neuroreport. 26: 870–876. 2015. [DOI] [PubMed] [Google Scholar]
- 7.Jiang SP, Zhang ZD, Kang LM, Wang QH, Zhang L, and Chen HP. Celecoxib reverts oxaliplatin-induced neuropathic pain through inhibiting PI3K/Akt2 pathway in the mouse dorsal root ganglion. Exp Neurol. 275: 11–16. 2016. [DOI] [PubMed] [Google Scholar]
- 8.Di Cesare Mannelli L, Pacini A, Bonaccini L, Zanardelli M, Mello T, and Ghelardini C. Morphologic features and glial activation in rat oxaliplatin-dependent neuropathic pain. J Pain. 14: 1585–1600. 2013. [DOI] [PubMed] [Google Scholar]
- 9.Di Cesare Mannelli L, Pacini A, Micheli L, Tani A, Zanardelli M, and Ghelardini C. Glial role in oxaliplatin-induced neuropathic pain. Exp Neurol. 261: 22–33. 2014. [DOI] [PubMed] [Google Scholar]
- 10.Mihara Y, Egashira N, Sada H, Kawashiri T, Ushio S, Yano T, Ikesue H, and Oishi R. Involvement of spinal NR2B-containing NMDA receptors in oxaliplatin-induced mechanical allodynia in rats. Mol Pain. 7: 8 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andoh T, Kitamura R, and Kuraishi Y. Milnacipran inhibits oxaliplatin-induced mechanical allodynia through spinal action in mice. Biol Pharm Bull. 38: 151–154. 2015. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Li DX, Yoon H, Go D, Quan FS, Min BI, and Kim SK. Serotonergic mechanism of the relieving effect of bee venom acupuncture on oxaliplatin-induced neuropathic cold allodynia in rats. BMC Complement Altern Med. 14: 471 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsunaga A, Kawamoto M, Shiraishi S, Yasuda T, Kajiyama S, Kurita S, and Yuge O. Intrathecally administered COX-2 but not COX-1 or COX-3 inhibitors attenuate streptozotocin-induced mechanical hyperalgesia in rats. Eur J Pharmacol. 554: 12–17. 2007. [DOI] [PubMed] [Google Scholar]
- 14.Donnerer J, and Liebmann I. pERK1/2 immunofluorescence in rat dorsal horn and paraventricular nucleus neurons as a marker for sensitization and inhibition in the pain pathway. Tissue Cell. 47: 55–60. 2015. [DOI] [PubMed] [Google Scholar]
- 15.Di Cesare Mannelli L, Pacini A, Corti F, Boccella S, Luongo L, Esposito E, Cuzzocrea S, Maione S, Calignano A, and Ghelardini C. Antineuropathic profile of N-palmitoylethanolamine in a rat model of oxaliplatin-induced neurotoxicity. PLoS One. 10: e0128080 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renn CL, Carozzi VA, Rhee P, Gallop D, Dorsey SG, and Cavaletti G. Multimodal assessment of painful peripheral neuropathy induced by chronic oxaliplatin-based chemotherapy in mice. Mol Pain. 7: 29 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohsawa M, Otake S, Murakami T, Yamamoto S, Makino T, and Ono H. Gabapentin prevents oxaliplatin-induced mechanical hyperalgesia in mice. J Pharmacol Sci. 125: 292–299. 2014. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Kang L, Li G, Zeng H, Zhang L, Ling X, Dong H, Liang S, and Chen H. Intrathecal leptin inhibits expression of the P2X2/3 receptors and alleviates neuropathic pain induced by chronic constriction sciatic nerve injury. Mol Pain. 9: 65 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen HP, Zhou W, Kang LM, Yan H, Zhang L, Xu BH, and Cai WH. Intrathecal miR-96 inhibits Nav1.3 expression and alleviates neuropathic pain in rat following chronic construction injury. Neurochem Res. 39: 76–83. 2014. [DOI] [PubMed] [Google Scholar]
- 20.Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): what we need and what we know. J Peripher Nerv Syst. 19: 66–76. 2014. [DOI] [PubMed] [Google Scholar]
- 21.Barragán-Iglesias P, Pineda-Farias JB, Cervantes-Durán C, Bravo-Hernández M, Rocha-González HI, Murbartián J, and Granados-Soto V. Role of spinal P2Y6 and P2Y11 receptors in neuropathic pain in rats: possible involvement of glial cells. Mol Pain. 10: 29 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim G, Wang S, Zhang Y, Tian Y, and Mao J. Spinal leptin contributes to the pathogenesis of neuropathic pain in rodents. J Clin Invest. 119: 295–304. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Zhang X, Guo QL, Zou WY, Huang CS, and Yan JQ. Cyclooxygenase inhibitors suppress the expression of P2X(3) receptors in the DRG and attenuate hyperalgesia following chronic constriction injury in rats. Neurosci Lett. 478: 77–81. 2010. [DOI] [PubMed] [Google Scholar]
- 24.Juárez-Rojop IE, Morales-Hernández PE, Tovilla-Zárate CA, Bermúdez-Ocaña DY, Torres-Lopez JE, Ble-Castillo JL, Díaz-Zagoya JC, and Granados-Soto V. Celecoxib reduces hyperalgesia and tactile allodynia in diabetic rats. Pharmacol Rep. 67: 545–552. 2015. [DOI] [PubMed] [Google Scholar]
- 25.Lau YM, Wong SC, Tsang SW, Lau WK, Lu AP, and Zhang H. Cellular sources of cyclooxygenase-1 and -2 up-regulation in the spinal dorsal horn after spinal nerve ligation. Neuropathol Appl Neurobiol. 40: 452–463. 2014. [DOI] [PubMed] [Google Scholar]
- 26.Zhao S, Cai J, Bian H, Gui L, and Zhao F. Synergistic inhibition effect of tumor growth by using celecoxib in combination with oxaliplatin. Cancer Invest. 27: 636–640. 2009. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Yoon SY, Zhang H, and Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J Pain. 13: 293–303. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janes K, Wahlman C, Little JW, Doyle T, Tosh DK, Jacobson KA, and Salvemini D. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav Immun. 44: 91–99. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crown ED. The role of mitogen activated protein kinase signaling in microglia and neurons in the initiation and maintenance of chronic pain. Exp Neurol. 234: 330–339. 2012. [DOI] [PubMed] [Google Scholar]
- 30.Stenberg L, Kanje M, Mårtensson L, and Dahlin LB. Injury-induced activation of ERK 1/2 in the sciatic nerve of healthy and diabetic rats. Neuroreport. 22: 73–77. 2011. [DOI] [PubMed] [Google Scholar]
- 31.Nishimoto S, and Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 7: 782–786. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao JH, Wen SL, Yang WJ, Lu YY, Tong H, Huang ZY, Liu ZX, and Tang CW. Celecoxib ameliorates portal hypertension of the cirrhotic rats through the dual inhibitory effects on the intrahepatic fibrosis and angiogenesis. PLoS One. 8: e69309 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo YJ, Shi XD, Fu D, Yang Y, Wang YP, and Dai RP. Analgesic effects of the COX-2 inhibitor parecoxib on surgical pain through suppression of spinal ERK signaling. Exp Ther Med. 6: 275–279. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]