Abstract
Mott cells are a variant form of plasma cells in humans and laboratory animals. This report describes the morphological characteristics of Mott cells observed in a 33-week-old female CB6F1-Tg rasH2 mouse. Microscopically, a large number of round cells with abundant eosinophilic globules, which were variable in size, were observed in the spleen and were densely distributed in the red pulp adjacent to the marginal zone. A few similar cells were present in the submandibular lymph node and bone marrow. Neither systemic nor local chronic inflammatory changes were seen in this animal. These cells were positive for mouse immunoglobulins. Ultrastructurally, the dilated rough endoplasmic reticulum had a homogenous substances with an intermediate electron density. On the basis of the above findings, these cells were identified as Mott cells. The present lesion is thought to be a spontaneous lesion, an unusual appearance of Mott cells without any associated pathological conditions.
Keywords: Mott cell, plasma cell, Tg rasH2 mice
Mott cells (grape cells or morular cells) are a variant form of plasma cells that are characterized by a reddish cytoplasm located peripherally and are commonly observed in the spleen of an autoimmune disease mouse models such as New Zealand Black (NZB) mice and the IgM-Fc receptor (FcμR)-deficient autoimmune mice1, 2. This types of cells is known to produce immunoglobulin (Ig), which, rather than being secreted, accumulates in rough endoplasmic reticulum-derived vesicles known as Russell bodies.
The CB6F1-Tg rasH2 (Tg rasH2) mouse is a hemizygous transgenic mouse carrying multiple copies of the human c-Ha-ras gene with its own promoter and enhancer3. A short term carcinogenicity assay using this mouse model was endorsed and validated as an alternative to conventional 2-year carcinogenicity bioassays in mice. However, there have been few published reports about the spontaneous lesions in Tg rasH2 mice4. Recently, we encountered unusual accumulation of Mott cells in hematopoietic tissues, especially in the spleen, in a female Tg rasH2 mouse in the control group of a 26-week carcinogenicity study. Here, we report on the histological features of this splenic change.
The experimental procedures were approved by the Institutional Animal Care and Use Committees of Shonan Research Center, Takeda Pharmaceutical Company Limited. A 6-week-old female CB6F1 Tg rasH2 mouse was purchased from CLEA Japan (Shizuoka, Japan), housed in a metal cage in an animal room at Takeda Pharmaceutical Company Limited (Kanagawa, Japan) with a temperature of 20°C to 26°C, a relative humidity of 40% to 80% and a 12-hour light/dark cycle, and fed a commercial diet (CE-2, CLEA Japan., Tokyo, Japan) and tap water ad libitum. A methylcellulose solution (0.5 w/v%), which is generally used as a vehicle in toxicity studies, was administered once daily via oral gavage at 10 mL/kg to the mouse for 26 weeks begining at 7-weeks of age. At 33 weeks of age, the animal was euthanized by exsanguination from the abdominal aorta under inhalation anesthesia with isoflurane. There were no clinical signs or necropsy findings. In addition, no abnormalities were observed in its blood chemistry, including the serum albumin and albumin/globulin ratio (data not shown), and hematology compared with the other vehicle control animals in the same study (Table 1). All organs were fixed in 10 vol% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE; all organs) and periodic acid-Schiff (PAS; spleen only). For identification of cell type, spleen sections were immunohistochemically stained with anti-mouse immunoglobulin G (IgG), anti-mouse immunoglobulins-complex (Igs; react with IgG, IgA, and IgM; fluorescein isothiocyanate (FITC) labelling), anti-mouse CD45R/B220 monoclonal antibody, and anti-mouse F4/80 polyclonal antibody. Details of the primary antibodies used are summarized in Table 2. Briefly, after the pretreatments and incubation with primary antibodies, the sections were immunohistochemically stained by the polymer immunocomplex method using Histofine Simple Stain Mouse MAX PO (Rat) (Nichirei, Tokyo, Japan) for CD45R/B220 and F4/80 and a VECTASTAIN Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) for IgG, and then the sections were counterstained with hematoxylin.
Table 1. Hematological Parameters of the Present Case and the Control Data from the Same Study.
Table 2. Primary Antibodies and Reaction Conditions for Immunohistochemistry.
For electron microscopy, formalin-fixed spleen tissues were trimmed and fixed with 2.5% glutaraldehyde, postfixed in 1% osmium tetroxide solution (pH 7.4) for 2 hours, and processed into resin. Semithin sections were cut and stained with toluidine blue. Ultrathin sections were cut and stained with uranyl acetate and lead citrate and then examined under an electron microscope (H-7600, Hitachi, Tokyo, Japan).
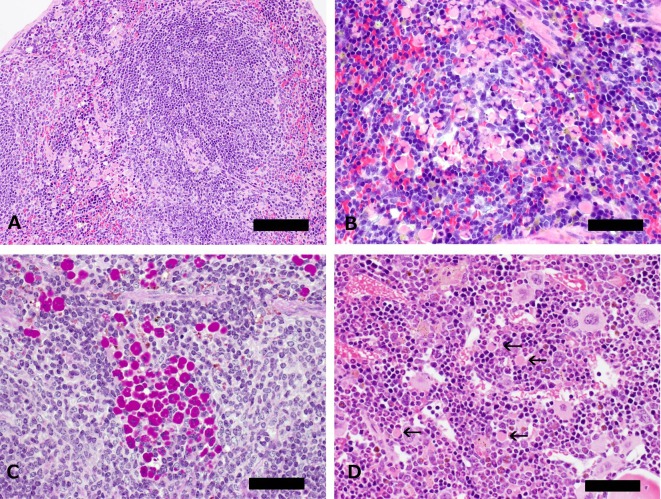
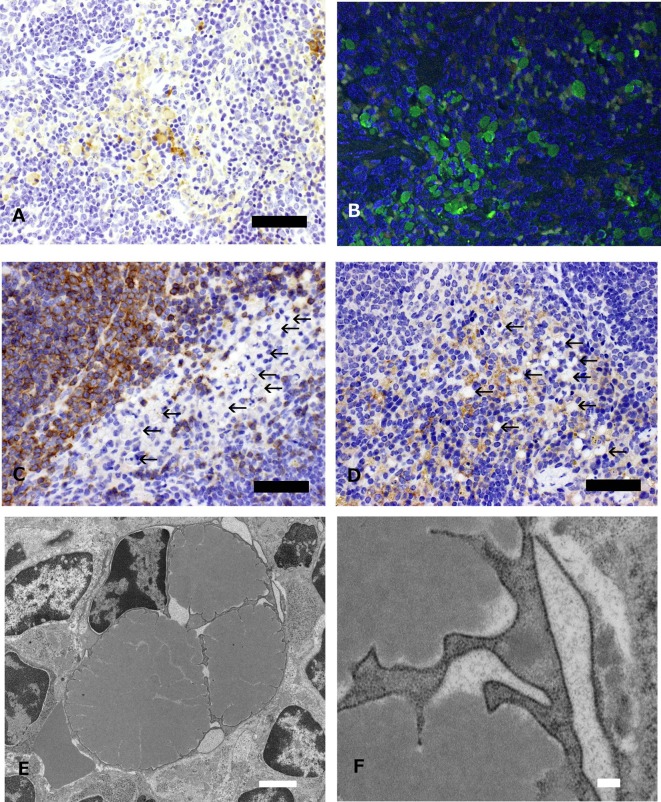
Microscopically, a large number of round cells with abundant cytoplasm containing various sizes of eosinophilic globules were distributed in the red pulp adjacent to the marginal zone (Figs. 1A and 1B), and these eosinophilic globules were positive for PAS stain (Fig. 1C). Smaller numbers of them were evident in the submandibular lymph node and bone marrow but not in the mesenteric lymph node and Peyer’s patch (Fig. 1D). In addition, there were no inflammatory changes in any tissues, and no hematopoietic proliferative lesions were observed in this animal. In immunohistochemistry, the cytoplasmic eosinophilic globules were reactive with mouse IgG and the fluorescent labeling mouse Igs (Figs. 2A and 2B), but negative for CD45R/B220 and F4/80, which are B cell and macrophage markers, respectively (Figs. 2C and 2D). Ultrastructurally, these cells have a nucleus with peripherally clumped chromatin, and the rough endoplasmic reticulum (rER) was markedly dilated and contained a large amount of a homogeneous substance with an intermediate electron density. The dilated rER compressed the nucleus, and its contour often appeared distorted (Figs. 2E and 2F).
Fig. 1.
Spleen and bone marrow from a 33-week-old Tg rasH2 mouse. A: The normal architecture of the spleen is well preserved, and a large number of round cells with abundant cytoplasm containing eosinophilic globules are distributed in the red pulp adjacent to the marginal zone (bar: 200 μm). B: A large number of round cells have a nucleus with peripherally clumped chromatin and variable sizes of eosinophilic globules in the cytoplasm (bar: 100 μm). C: Eosinophilic globules were positive for PAS stain (bar: 100 μm). D: Smaller numbers of round cells with abundant cytoplasm containing eosinophilic globules are evident in the bone marrow (arrows, bar: 100 μm).
Fig. 2.
Immunohistochemical staining and electron micrographs of Mott cells in a 33-week-old Tg rasH2 mouse. A: The cells with eosinophilic globules are positive for IgG (bar: 100 μm). B: The cells with eosinophilic globules are positive for fluorescent immunoglobulins (green: Igs-FITC; blue, nuclei, DAPI (4’,6-diamidino-2-phenylindole); bar: 100 μm). C: The cells with eosinophilic globules are negative for CD45R/B220 (arrows, bar: 100 μm), whereas marginal zone lymphocytes are positive for CD45R/B220. D: The cells with eosinophilic globules are negative for F4/80 (arrows, bar: 100 μm), whereas macrophages in the red pulp are positive for F4/80. E: A homogeneous substance with an intermediate electron density is observed in the cisternae of the rER in Mott cells, and most of the nuclei show apparent distortion of the outline or are compressed because of the dilated rER (bar: 2 μm). F: Higher magnification of Fig. 2E (bar: 200 nm).
Based on the above findings, the cells that had a dilated rER containing immunoglobulins and characteristic nuclei were considered Mott cells distributed in the hematopoietic tissues, especially in the spleen.
Plasma cells in the spleen are thought to develop from marginal zone B cells and follicular B cells, from germinal center B cells, and from memory B cells5. When marginal zone B cells encountered an antigen in the spleen, they were able to rapidly differentiate into plasma cells, and marginal zone B cells are also known to migrate into red pulp and form foci of plasmablasts5. Follicular B cells are also known to develop into plasma cells or to move to the germinal center, and differentiate from memory B cells or plasmablasts to plasma cells, which can migrate into bone marrow5. Considering the distribution of Mott cells in the spleen and their presence in the bone marrow, both marginal zone and follicular B cells could be a possible source of the Mott cells in the present case5.
Mott cell formation is a consequence of a disorder of abnormal synthesis, degradation, and/or secretion of Ig and is related to various pathological conditions including autoimmune diseases, reactive plasmacytosis, and several types of hematolymphoid malignancies1, 2, 6; however, neither inflammatory changes nor hematopoietic tumors were observed in this case. In Ig light chain-deficient mice, it was postulated that the mechanism of Mott cell formation was lack of Ig light chains, which prevents antibody aggregation, leading to Ig heavy chain aggregation, which is known as Russell body formation7.
On the basis of the histopathologic and electron microscopic findings, the present lesion is thought to be an unusual appearance of Mott cells. To the best of our knowledge, this is the first case showing Mott cell accumulation without any pathological conditions in mice. The etiology of this change in Tg rasH2 mice remains unclear, but we believe this case will provide useful information regarding spontaneous background findings in Tg rasH2 mice.
Acknowledgments
The authors would like to thank Ms. Naoko Awasaki and Ms. Naomi Inui for their excellent technical support during this work.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare no conflict of interest associated with this paper.
References
- 1.Jiang Y, Hirose S, Hamano Y, Kodera S, Tsurui H, Abe M, Terashima K, Ishikawa S, and Shirai T. Mapping of a gene for the increased susceptibility of B1 cells to Mott cell formation in murine autoimmune disease. J Immunol. 158: 992–997. 1997. [PubMed] [Google Scholar]
- 2.Honjo K, Kubagawa Y, Suzuki Y, Takagi M, Ohno H, Bucy RP, Izui S, and Kubagawa H. Enhanced auto-antibody production and Mott cell formation in FcμR-deficient autoimmune mice. Int Immunol. 26: 659–672. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamaoki N. The rasH2 transgenic mouse: nature of the model and mechanistic studies on tumorigenesis. Toxicol Pathol. 29(Suppl): 81–89. 2001. [DOI] [PubMed] [Google Scholar]
- 4.Paranjpe MG, Shah SA, Denton MD, and Elbekai RH. Incidence of spontaneous non-neoplastic lesions in transgenic CBYB6F1-Tg(HRAS)2Jic mice. Toxicol Pathol. 41: 1137–1145. 2013. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro-Shelef M, and Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 5: 230–242. 2005. [DOI] [PubMed] [Google Scholar]
- 6.Rampisela D, and Donner LR. An unusual self-limited clonal Mott cell proliferation with lymphoplasmacytic lymphoma-like features in a child with the Wiskott-Aldrich syndrome and Von Recklinghausen’s neurofibromatosis. Pathol Res Pract. 206: 467–471. 2010. [DOI] [PubMed] [Google Scholar]
- 7.Corcos D, Osborn MJ, Matheson LS, Santos F, Zou X, Smith JA, Morgan G, Hutchings A, Hamon M, Oxley D, and Brüggemann M. Immunoglobulin aggregation leading to Russell body formation is prevented by the antibody light chain. Blood. 115: 282–288. 2010. [DOI] [PubMed] [Google Scholar]