Abstract
Background
Circulating peripheral blood mononuclear cells (PBMCs) are exposed to metabolic and immunological stimuli that influence their functionality. We hypothesized that prevailing vitamin D status [25(OH)D] would modulate the bioenergetic profile of PBMCs derived from humans.
Materials and methods
38 participants (16 males, 22 females) ranging in body fat from 14–51% were studied. PBMCs were isolated from whole blood, counted and freshly seeded for bioenergetic analysis using the Seahorse XFe96 flux analyser. Whole body energy metabolism via indirect calorimetry, body composition by dual-energy X-ray absorptiometry, and relevant clinical biochemistry were measured. Data was analysed based on 25(OH)D cut-offs of <50 nmol/L (Group 1, n=12), 50–75 nmol/L (Group 2, n=15) and ≥75 nmol/L (Group 3, n=11). A multivariate general linear model adjusting for age, fat mass, fat-free mass, parathyroid hormone and insulin sensitivity was used.
Results
There were significant differences in cellular mitochondrial function between groups. Group 1 had significantly higher basal respiration (p=0.001), non-mitochondrial respiration (p=0.009), ATP production (p=0.001), proton leak (p=0.018), background glycolysis (p=0.023) and glycolytic reserve (p=0.039) relative to either Group 2 or Group 3; the latter two did not differ on any measures. There were no differences in bioenergetic health index (BHI), resting metabolic rates and systemic inflammatory markers between groups.
Conclusions
Inadequate vitamin D status adversely influenced bioenergetic parameters of PBMCs obtained from adults, in a pattern consistent with increased oxidative metabolism and activation of these cells.
Abbreviations: 2DG, 2-deoxyglucose; BHI, bioenergetic health index; FM, fat mass; FFM, fat free mass; PBMCs, peripheral blood mononuclear cells; PPR, proton production rate; OCR, oxygen consumption rate; RQ, respiratory quotient; RMR, resting metabolic rate; UCP, uncoupling proteins; VDR, vitamin D receptor
Keywords: Leukocytes, Peripheral Blood Mononuclear Cells, Bioenergetics, Vitamin D, 25(OH)D, Proton leak, Inflammation
Highlights
-
•
A model linking vitamin D status and the bioenergetic profile of PBMCs is proposed.
-
•
Adults with <50 nmol/L had greater oxidative and glycolytic metabolism.
-
•
Bioenergetic Health Index was positively related to fasting insulin sensitivity.
1. Introduction
Insufficient vitamin D status [25(OH)D] is commonly observed worldwide. Several epidemiological studies in the United States, Canada, United Kingdom, and New Zealand have reported a high prevalence of inadequate levels of 25(OH)D [1]. Currently, the importance of achieving and maintaining adequacy is limited to the prevention of rickets, osteoporosis and fractures [2]. However, many have argued that a causal link between 25(OH) D insufficiency, obesity and other diseases may exist [2], [3], [4], [5]. Consequently the concentration of circulating 25(OH)D that best represents sufficiency for extra-skeletal health, is currently debated. While some research groups argue that 50 nmol/L [6], [7] is sufficient, others have proposed that the value should be raised to 75 nmol/L [8], [9].
Cellular and animal models strongly argue for a role of vitamin D in immune function and energy metabolism. The vitamin D receptor (VDR), through which the majority of effects dependent on vitamin D are exerted, is found in most cells of the immune system, including activated lymphocytes, dendritic cells and macrophages [10]. The active metabolite, 1,25(OH)2D, is a well-known regulator of immune function and in a recent systematic review we showcased that the active metabolite was strongly associated with an anti-inflammatory cytokine profile in peripheral blood mononuclear cells (PBMCs) [11]. 1,25(OH)2D also enhanced the antimicrobial actions of macrophages, and promoted chemotaxis and phagocytic capabilities of innate immune cells [12]. The effect of 25(OH)D on cytokine release from immune cells is under-researched. One study has suggested that 25(OH)D had an anti-inflammatory effect as gauged from an increased IL-10 release with decreased concentrations of TNF-α levels [13], IL-6 and TNF-α mRNA, while another has observed a mixed profile [14]. The VDR has been identified in all key organs of energy metabolism including the pancreas, adipose, liver and skeletal muscle [15]. Low 25(OH)D, and the absence of vitamin D function in VDR knockout mice, resulted in an increase in energy expenditure [19], [20]. Prevailing 25(OH)D levels are crucial since they influence local tissue concentrations of the active metabolite [16]. Hence inadequate 25(OH)D may indirectly affect whole body energetics through promoting activation of the immune system, which typically increases resting metabolic rate by 30–50% [17].
As PBMCs circulate in the vasculature, they are exposed to various metabolic and immunological stimuli such as glucose, amino acids, free fatty acids and vitamins. There is growing evidence that circulating factors can mediate bioenergetic function of PBMCs [18]. Numerous studies have harnessed PBMC's as a potential tool to determine the inflammatory and metabolic status in a variety of different disease states, including sepsis [19], neurodegeneration [20], rheumatic disease [21], obesity [22], [23], cardiovascular disease [24], diabetes mellitus [25], [26], and anorexia nervosa [27], all of which have been linked to mitochondrial dysfunction or altered bioenergetics. These studies in immune cells are consistent with a large body of work suggesting altered mitochondrial respiratory capacity and function in tissues classically studied such as skeletal muscle, liver, islet cells, and the myocardium [28], [29], [30], [31]. Recently, the concept of a bioenergetic health index (BHI) has been proposed as a novel prognostic or diagnostic biomarker of disease [32].
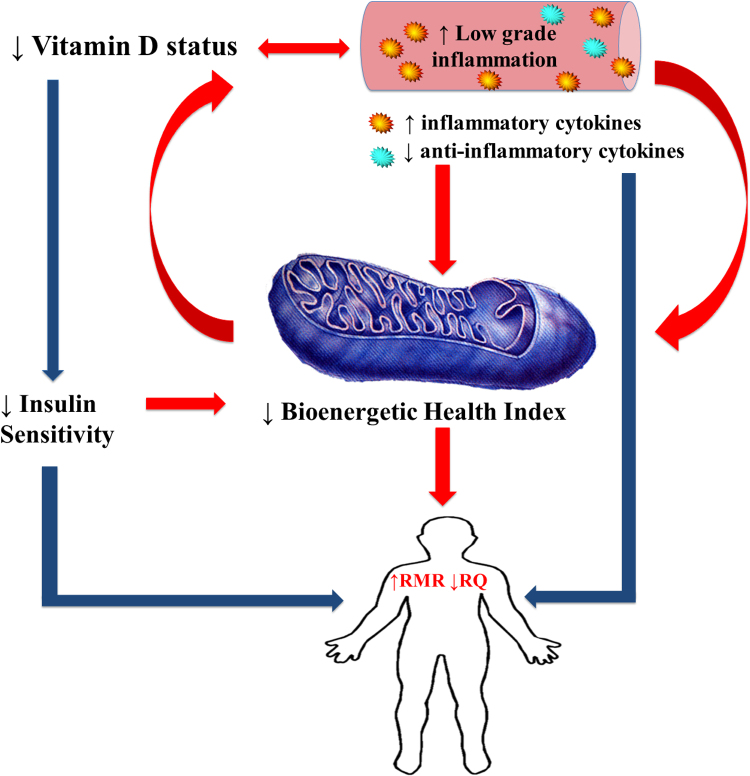
The aim of the current study was to determine whether prevailing 25(OH)D influenced the bioenergetics of circulating leukocytes. To the best of our knowledge, the impact of the prevailing 25(OH)D status on bioenergetics and the BHI has not been previously described. A secondary objective was to determine whether PBMC bioenergetic parameters were associated with whole body energy metabolism in the same person. We have presented our hypothesis through a schematic that links low vitamin D status to a decreased BHI (Fig. 1). Intermediates in this pathway are higher levels of inflammation and greater insulin resistance (lesser insulin sensitivity) that independently, or in combination, would affect BHI. Since a heightened inflammatory status is energetically expensive, we have also proposed a link to whole body resting metabolic rate (RMR) and whole body fuel oxidation, i.e. respiratory quotient (RQ). Finally, we have included the possibility that a lower BHI per se could increase the demand and utilisation of vitamin D in order to restore cell functioning, and hence act as a driver of the low vitamin status in a feed forward loop (Fig. 1).
Fig. 1.
Vitamin D status, inflammation and bioenergetic health. Legend: Inadequate vitamin D status promotes an inflammatory state. Together they reduce whole body insulin sensitivity and the bioenergetics of peripheral blood mononuclear cells. The resultant lower BHI, an indicator of mitochondrial health status, will possibly drive an increase in whole body resting metabolism (RMR). A heightened inflammatory state is also energetically expensive and account for a greater whole body energy requirement, while a lower insulin sensitivity will increase RMR and decrease respiratory quotient (RQ). Finally a low BHI per se has the potential to increase the demand for vitamin D in an attempt to maintain cellular function, hence leading to a further lowering of vitamin D status. Improvement of vitamin D status (increased sun exposure or supplementation) will act to reverse this dysmetabolism through lesser inflammation and greater BHI. ↑, increased; ↓, decreased; red arrows indicate pathways tested in this paper and blue arrows indicate what is known. BHI, bioenergetic health index; RMR, resting metabolic rate. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2. Materials and methods
2.1. Participant recruitment
Participants were recruited via flyer advertisement, radio advertisement, community newspapers and social media websites. Interested participants were assessed for eligibility through a short screening questionnaire. Inclusion criteria were as follows: Australians of European origin; aged between 20 and 70 years; body mass index (BMI) ≥18.5 kg/m2, weight stable (±3 kg over the last six months); not suffering from any medical conditions involving the thyroid, liver, kidney or heart; absence of pregnancy; no history of cigarette smoking within a year prior to the study; not suffering from any current illness or infection requiring antibiotics; no gastrointestinal problems or history of gastrointestinal surgeries; no history of blood disorders; no history of mitochondrial disease; not on any medications that influence mitochondrial function (insulin, HMG-CoA reductase inhibitors, thiazolidinedione's), not on anti-convulsants (increased catabolism of vitamin D), not taking the following: parathyroid hormone (PTH) or its derivatives, calcitonin, HRT, corticosteroids, vitamin D supplements or any special or commercial diet programs that may affect the body's metabolism. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human volunteers were approved by the Human Research Ethics Committee of Curtin University, Perth, Western Australia (Ethics approval number RDHS-13-15). Written informed consent was obtained from all participants.
2.2. Intervention
Participants arrived at the laboratory after an overnight fast of 10–12 h, after refraining from strenuous physical activity 24 h prior to, and on the morning before the study day.
2.2.1. Physical characteristics and body composition
Weight was measured using an electronic platform balance (CW-11, Precision Balances Pty Ltd). Height was measured using a stadiometer fixed to a wall (Seca, Hamburg, Germany). Waist circumference was measured at the umbilicus. Body composition including fat mass (FM) and fat free mass (FFM) was assessed by dual energy x-ray absorptiometry (Prodigy, Lunar Corp USA).
2.2.2. Resting metabolic rate
Resting metabolic rate (RMR) was measured in the environmental chamber housed at the School of Public Health, Curtin University, Western Australia. The chamber is a purpose built structure within a large room of the building. It has a volume of 57.75 m3, insulated walls and roof and is independently controlled for temperature range from 4 °C to 50 °C. Participants first rested in the supine position for 30 min to equilibrate with the temperature of the environmental chamber that was set at 25 °C the day before. A trial RMR measurement was made to accustom participants to the canopy of the TrueOne indirect calorimetry system (Parvo Medics, USA). RMR was then measured for 30 min, with the first 10 min excluded from the calculations. RMR in kJ/d was derived from CO2 production and O2 consumption according to the Weir's formula, neglecting protein oxidation in the fasting state. Fasting respiratory quotient was calculated as volume of CO2 produced/volume O2 consumed. The TrueOne system was calibrated with gas mixtures of known composition before each measurement session and performance of the system was regularly checked through 30 min ethanol burn tests. A mean±SD for six tests gave an RQ of 0.67±0.01.
2.2.3. Blood collection and analysis
A fasting venous sample was obtained after 90 min exposure to the set temperature by experienced phlebotomists. Briefly, fasting blood glucose were determined using routine automated procedures on an Architect c16000 analyser that used specific enzyme-based colorimetric reagents (Abbott Diagnostics; CV <2%). Fasting insulin was determined by PathWest Laboratories Perth Australia using an Architect i2000SR Analyser (Abbott Diagnostics; CV<3%). PTH was determined using a Cobas e601 Analyser (Roche Diagnostics). Quantitative insulin sensitivity check index (QUICKI) was determined from fasting glucose and insulin [33]. 25(OH)D was measured by chemiluminescent micro particle immunoassay (Architect 25-OH Vitamin D assay, Abbott Diagnostics). Inflammatory cytokines IL-6, IL-8, IL-10, IL-12p70 and TNF-α were measured using Human High Sensitivity T Cell kits and run on a MAGPIX® system (Merck Millipore, Germany). All samples were measured in duplicate, and the average of the two values was used for data analyses. CRP was measured using QuickRead go CRP kits from Orian Diagnostica (Espoo, Finland).
2.2.4. Immune cell isolation and population determination
Eighteen millilitres of whole blood was drawn by venepuncture into commercially available EDTA-citrate vacutainers, from patients fasting overnight and diluted 1:1 in PBS-EDTA (2 mM). The diluted blood was transferred to a fresh tube containing an equivalent volume of Histopaque 1077 (Sigma-Aldrich, St Louis, USA) and centrifuged at 600×g for 20 min with minimum acceleration and no braking. Autologous plasma samples (3mls) were taken from the upper layer, while immune cells were isolated from the “buffy coat” and washed with EDTA-free PBS. Washing and centrifugation was repeated at 300, 200 and 100 g for 10 min each, to remove contaminating platelets. The cell pellet was re-suspended in 0.5 ml of warm RPMI-1640 (10% FBS, 2 mM glutamine, 100 U/ml penicillin & 0.1 mg/ml streptomycin), and an aliquot taken to determine the cell number and percentage proportion of immune cells (lymphocytes, monocytes and granulocytes) using the automatic Mindray BC2800 haematological analyser. The cell suspension was seeded into the Seahorse assay XFe96 culture plate. All samples were processed within 5 h of blood collection.
2.2.5. Seahorse XFe96 measurements
As per our previously established protocol [34], cells were seeded at a density of 3.5×105 cells/well into 96 well plates previously coated with poly-d-lysine (50 μg/mL) to maximise adherence and allowed to adhere overnight. After recording of basal measurements, the Mito Stress Test injection strategy consisted of oligomycin (5 µM), FCCP (1.5 µM), and rotenone/antimycin A in combination (5 µM). The Glycolytic Stress Test injection strategy consisted of glucose (25 mM), oligomycin (5 µM), followed by 200 mM 2-deoxyglucose (2DG). Oxygen consumption rate (OCR) and proton production rate (PPR) was measured using five 2 min cycles of mix and measurement following each injection.
2.2.6. Seahorse data analysis
Basal respiration was calculated by subtracting the minimum OCR following addition of rotenone/antimycin A (non-mitochondrial respiration) from the last OCR measurement recorded prior to addition of oligomycin. Proton leak was calculated by subtracting the minimum OCR following addition of rotenone/antimycin A (non-mitochondrial respiration) from the minimum OCR measurement recorded after addition of oligomycin. OCR related to ATP production (turnover) was calculated by the difference between the proton leak and basal respiration. Coupling efficiency percentage was calculated by dividing the ATP production dependent OCR by the basal respiration and multiplying by 100. Maximal respiration was determined by subtracting the non-mitochondrial respiration OCR from the maximum OCR in response to FCCP, while reserve capacity was the difference between the basal respiration and the calculated maximal respiration. Basal glycolysis in the presence of 0 mM glucose was determined by the last PPR measurement recorded prior to addition of 25 mM glucose. Glycolytic response to 25 mM glucose was determined by subtracting the maximum PPR following addition of glucose from the last PPR measurement prior to addition of glucose. Glycolytic capacity was measured by subtracting the minimum PPR following 2DG addition from the maximum PPR after injection of oligomycin. Finally, Glycolytic reserve was determined from the difference between the glycolytic capacity and the glycolytic response to 25 mM glucose. Each treatment was measured in at least triplicate wells.
2.2.7. Calculation of Bioenergetic Health Index (BHI)
The BHI of each sample was calculated as previously defined [35], [36] and is presented below. No power function was applied to these parameters [35].
2.3. Statistical analysis
We categorized our study sample into three groups based on cut offs for 25(OH)D of 50 nmol/L and 75 nmol/L. Participants with 25(OH)D <50 nmol/L formed Group 1, 50–75 nmol/L were Group 2 and those with status ≥75 nmol/L were defined as Group 3. Normally distributed data are presented as mean (SD) and skewed data are presented as median (IQR). Skewed data were transformed and statistical analyses comparing the three independent groups were performed using multivariate GLM. Multivariate regression was used to adjust for effects of age, fat mass (kg), fat-free mass (kg), PTH (pmol/L), and QUICKI (quantitative insulin sensitivity check index) on all bioenergetics parameters. These covariates were selected by a parsimonious backward approach that tested for many potential variables. Pearson's partial correlation coefficients were used to determine correlations for BHI, RQ and RMR with inflammatory cytokines and QUICKI. All statistical calculations were performed using SPSS version 22 and graphics were generated using GraphPad Prism software v. 6.0.
3. Results
3.1. Demographics, body composition and inflammatory profile of participants
The participant cohort consisted of 16 males and 22 females, ranging in percent body fat from 14–51% and aged between 19 and 69 years. There were no differences between groups in gender distribution (M/F) (Group 1: 7/5, Group 2: 5/10, Group 3: 4/7, p=0.420), age [Group 1: 43.25 (16.65) years, Group 2: 42.00 (20.09) years, Group 3: 40.18 (18.78) years, p=0.925] and BMI [Group 1: 26.32 (3.77) kg/m2, Group 2: 26.68 (4.04) kg/m2, Group 3: 23.6418 (3.39) kg/m2, p=0.115]. Further details of their body composition are provided in Table 1. We did not detect differences in CRP (p=0.174), TNF-α (p=0.952), IL-6 (p=0.883), IL-8 (p=0.986), IL-10 (p=0.499), or IL-12p70 (p=0.09) between the three groups whether unadjusted or adjusted for age, FM, FFM, PTH and QUICKI. Percentage of lymphocytes (p=0.217) and monocytes (p=0.424) also did not differ between vitamin D status groups.
Table 1.
Body composition, whole body energy metabolism and bioenergetic profiles of participants, according to vitamin D status group†.
| Characteristic | Whole group (n=38) | <50 nmol/L (n=12) | 50–75 nmol/L (n=15) | ≥75 nmol/L (n=11) | P value |
|---|---|---|---|---|---|
| Body composition & whole body energy metabolism | |||||
| Fat mass (kg) | 22.15 (12.84) | 26.41 (19.89) | 25.56 (16.07) | 19.69 (5.01) | 0.113 |
| Fat free mass (kg) | 52.14 (11.76) | 54.93(10.97) | 51.67 (12.74) | 49.75 (11.66) | 0.574 |
| PTH (pmol/L) | 3.04 (1.38) | 4.27 (2.52)a | 2.8 (0.84)b,c | 3.04 (0.86)c | 0.065 |
| QUICKI | 0.37 (0.04) | 0.37 (0.04) | 0.37 (0.04)a | 0.39 (0.02)b | 0.078 |
| RMR (kJ/d) | 6136 (1415) | 6535 (1444) | 6333 (1513) | 5433 (1058) | 0.138 |
| RQ | 0.83 (0.03) | 0.82 (0.04) | 0.84 (0.03) | 0.84 (0.03) | 0.260 |
| Mito stress test parameters | |||||
| Basal respiration (pmol O2/min) | 53.88(21.84) | 69.59(22.22)a | 43.69 (13.85)b,c | 50.64 (22.13)c | 0.005 |
| Non mitochondrial respiration (pmol O2/min)# | 3.5667 (0.32) | 3.7389 (0.33) | 3.4729 (0.23) | 3.507 (0.37) | 0.074 |
| ATP production (pmol O2/min)# | 6.16 (1.52) | 7.27 (1.37)a | 5.52 (1.05)b,c | 5.82 (1.67)c | 0.005 |
| Proton leak (pmol O2/min) | 15.06 (4.58) | 16.78 (5.04) | 13.75 (4.67) | 14.96 (3.62) | 0.239 |
| Maximal respiration (pmol O2/min) | 207.89(106.95) | 240.57(106.58) | 183.98 (76.31) | 204.84(139.74) | 0.402 |
| Coupling efficiency (%) | 72.46 (7.20) | 76.37 (6.05)a | 69.68 (7.42)b | 71.93 (6.57) | 0.050 |
| Reserve capacity (pmol O2/min) | 157.03(88.54) | 178.08 (83.67) | 140.91 (72.66) | 156.05(113.98) | 0.568 |
| BHI# | 2.29 (0.70) | 2.45 (0.68) | 2.14 (0.72) | 2.31 (0.73) | 0.534 |
| Glycolysis stress test parameters | |||||
| Background glycolysis (pmol H+/min)# | 4.52 (1.05) | 5.03 (0.92) | 4.27 (1.03) | 4.30 (1.10) | 0.127 |
| 25 mM Glucose response (pmol H+/min)# | 0.03 (0.01) | 0.03 (0.01) | 0.04 (0.01) | 0.04 (0.01) | 0.321 |
| Glycolytic reserve (pmol H+/min)# | 5.49 (1.40) | 6.03 (1.38) | 5.27 (1.40) | 5.16 (1.36) | 0.257 |
| Glycolytic capacity (pmol H+/min)# | 7.83 (1.51) | 8.55 (1.38) | 7.64 (1.73) | 7.29 (1.09) | 0.110 |
Values not sharing the same superscript are significantly different from each other.
ATP, adenosine triphosphate; BHI, Bioenergetic Health Index; RMR, resting metabolic rate; RQ, respiratory quotient; QUICKI, quantitative insulin sensitivity check index.
Data are mean (SD) following unadjusted multivariate ANOVA.
Variables were transformed.
Thirty one participants had no contamination by platelets in their samples. Seven individuals showed marginal contamination ranging from 1000 platelets/µL to 8000 platelets/µL. Based on the total volume aliquoted into each well, the sample with the highest contamination would have had ~1.22 million platelets/well. The latter value is 1/20th the number of cells needed to detect a change in oxygen consumption of platelets [32].
3.2. Bioenergetic parameters
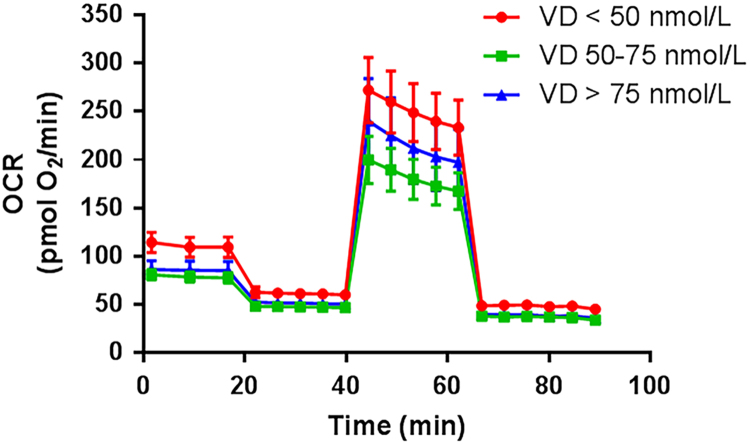
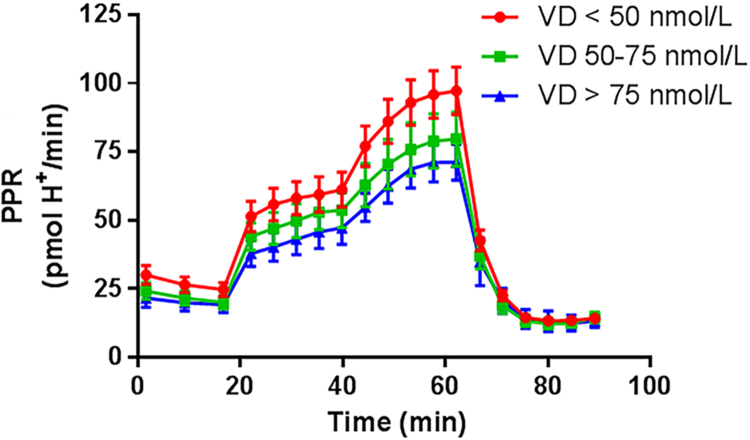
Insulin sensitivity was marginally different between vitamin D status groups, while RMR and RQ were similar among groups (Table 1). The Mito Stress Test trace and Glycolysis Stress Test bioenergetics responses are shown in Figs. 2 and 3, respectively. The unadjusted bioenergetic profile indicated significant group differences in basal respiration and ATP production. Basal respiration and ATP production were highest in the lowest vitamin D group (Group 1; <50 nmol/L) with trends towards a greater non-mitochondrial respiration, and coupling efficiency (Table 1). After adjustment for age, FM, FFM, PTH and QUICKI, group differences were accentuated for basal respiration, non-mitochondrial respiration, ATP production, and proton leak (Table 2) with Group 1 having the highest values compared to the other two groups. Glycolytic stress test parameters also indicated a greater background glycolysis and glycolytic capacity (Table 2), with a trend for higher glycolytic reserve as well. There were no differences in adjusted parameters between Group 2 and Group 3 (Table 2). Analysis of the data without the 7 individuals where contamination by platelets was detected, is presented in Table S1. Those outcomes were similar in direction and statistical significance to outcomes of the complete dataset in Table 2.
Fig. 2.
Effect of vitamin D status on oxygen consumption rate during the Mito Stress Test. Legend: 25(OH)D, 25-hydroxy vitamin D; OCR, oxygen consumption rate.
Fig. 3.
Effect of vitamin D status on proton production rate during the glycolysis stress test. Legend: 25(OH)D, 25-hydroxy vitamin D; PPR, proton production rate.
Table 2.
Adjusted bioenergetic measurements compared across three groups varying in vitamin D status†.
| Characteristic | <50 nmol/L (n=12) | 50–75 nmol/L (n=15) | >75 nmol/L (n=11) | P value |
|---|---|---|---|---|
| Whole body energy metabolism | ||||
| RMR (kJ/d) | 6251 (785) | 6294 (766) | 5796 (755) | 0.236 |
| RQ | 0.82 (0.03)a | 0.84 (0.04) | 0.85 (0.03)b | 0.100 |
| Mito stress test parameters | ||||
| Basal respiration (pmol O2/min) | 75.14 (19.94)a | 40.74 (19.46)b,c | 48.61 (19.18)c | 0.001 |
| Non mitochondrial respiration (pmol O2/min)# | 3.84 (0.33)a | 3.41 (0.32)b,c | 3.48 (0.32)c | 0.009 |
| ATP production (pmol O2/min)# | 7.59 (1.4)a | 5.35 (1.37)b,c | 5.70 (1.35)c | 0.001 |
| Proton leak (pmol O2/min) | 18.23 (4.61)a | 12.56 (4.49)b | 15.00 (4.43) | 0.018 |
| Maximal respiration (pmol O2/min) | 248.83 (109.58) | 189.15 (106.94) | 188.77 (105.43) | 0.337 |
| Coupling efficiency (%) | 76.23 (7.76) | 70.17(7.55) | 71.36 (7.49) | 0.156 |
| Reserve capacity (pmol O2/min) | 182.78 (90.55) | 146.81 (88.37) | 142.87 (87.12) | 0.522 |
| BHI# | 2.39 (0.69) | 2.25 (0.67) | 2.23 (0.66) | 0.839 |
| Glycolysis stress test parameters | ||||
| Background glycolysis (pmol H+/min)# | 5.27 (1.04)a | 4.07 (1.01)b,c | 4.31 (0.99)c | 0.023 |
| 25 mM Glucose response (pmol H+/min)# | 0.03 (0.01) | 0.04 (0.01) | 0.04 (0.01) | 0.253 |
| Glycolytic reserve (pmol H+/min)# | 6.31 (1.39) | 5.14 (1.46) | 5.03 (1.43) | 0.094 |
| Glycolytic capacity (pmol H+/min)# | 8.79 (1.45)a | 7.44 (1.43)b,c | 7.30 (1.39)c | 0.039 |
Values not sharing the same superscript are significantly different from each other.
ATP, adenosine triphosphate; BHI, Bioenergetic Health Index; RMR, resting metabolic rate; RQ, respiratory quotient; QUICKI, quantitative insulin sensitivity check index.
Data are mean (SD) following multivariate ANCOVA with adjustment for fat mass (kg), fat-free mass (kg), PTH (pmol/L), and QUICKI.
Transformed variables.
3.3. Correlations between bioenergetics and whole body measurements
After adjustment for age, FM, FFM and PTH, BHI was positively related to QUICKI (r=0.527, p=0.002) and demonstrated inverse trends with RMR (r=−0.335, p=0.061), IL-6 (r=−0.312, p=0.082) and TNF-α (r=−0.325, p=0.07). A similar analysis demonstrated RMR to be positively associated with TNF-α (r=0.396, p=0.025), while RQ was inversely correlated with QUICKI (r=−0.433, p=0.013) and CRP (r=−0.477, p=0.006). QUICKI demonstrated a trend whereby values were inversely correlated with MCP-1 (r=−0.305, p=0.09). Upon further adjustment for QUICKI, all associations with BHI disappeared, however RMR remained directly related to TNF-α levels (r=0.375, p=0.038), while RQ was inversely related to CRP (r=−0.40, p=0.024).
4. Discussion
Bioenergetic dysfunction has been demonstrated in various disease states including Alzheimer's disease [37], type 2 diabetes [38] and anorexia nervosa [39]. Each of these disorders has also been associated with low vitamin D status [40], [41], [42]. There are few studies investigating the impact of vitamin D status on energetic parameters of PBMCs, and whether prevailing 25(OH)D, whole body energy metabolism, inflammatory markers and cellular energetics are interrelated in the same individual was, up to now, unknown.
4.1. Main findings
Our results suggest that in vivo circulating 25(OH)D, a proxy for vitamin D status, was associated with ex-vivo PBMC cell bioenergetics resulting in significantly greater basal respiration, ATP production, proton leak, and non-mitochondrial respiration in those with 25(OH)D<50 nmol/L (Table 2). Concomitantly, we also uncovered significantly greater background glycolysis and glycolytic capacity in the same vitamin D inadequate group (Table 2). These outcomes were consistent whether we used the entire dataset or restricted our analysis to those with no platelet contamination (Table S1). Overall, a lower 25(OH)D was associated with heightened PBMC bioenergetics, both oxidative phosphorylation and glycolysis. It appears from our data that this effect may plateau above 50 nmol/L, since the group >75 nmol/L showed no further change in these bioenergetic parameters (Tables 2 and S1).
To the best of our knowledge, this is the first study to suggest there may be an effect of circulating 25(OH)D on PBMC bioenergetics. Other studies have investigated the effect of cholecalciferol±calcium on skeletal muscle bioenergetics using P Magnetic resonance spectroscopy [43], [44], [45]. No overt abnormalities of skeletal muscle mitochondrial oxidative function in vitamin d-deficient subjects compared with healthy controls were found [43], which are in contrast to the present findings. Reasons for the discrepancy may include the different tissues/cells studied, and the use of small sample sizes that may not overcome the biological variability in measurement of mitochondrial function.
Previous animal and cellular studies suggested that vitamin D modulated energy metabolism. Mouse models demonstrate that vitamin D deficiency or impaired VDR signalling (through VDR knockout), results in increased energy expenditure. In global VDR null mice, increased energy expenditure measured by indirect calorimetry is seen [46]. These VDR null mice fed a high-fat diet displayed reduced lipid accumulation in the liver through greater fatty acid oxidation and increased expression of uncoupling proteins (UCPs), UCP-1, UCP-2, which increases energy expenditure [46]. Dietary induced vitamin D deficiency also alleviates hepatic lipid accumulation, upregulates key enzymes involved in fatty acid oxidation and uncoupling protein 3 (UCP-3) [47]. UCP-1 is an important regulator of proton flux, and can allow dissipation of the proton gradient across the mitochondrial inner membrane in specialised tissues such as brown adipose tissue [46]. While UCP-2 and UCP-3, will transport protons and increase the net proton conductance of mitochondria in the presence of specific activators. Such observations would support our findings of increased proton leak and ATP production with low 25(OH)D, which may reflect increased energy demand, increased energy expenditure, and/or dysfunctional energy utilisation. Interestingly, recent molecular studies have shown that the silencing of VDR signalling and impairment of VDR translocation to the mitochondria in cancer cells, promoted elevated mitochondrial respiration and electron transport chain activity through upregulation of cytochrome oxidase enzymes (COX II and IV) [48]. In another study, treatment with 1,25(OH)2D reduced glycolytic and citric acid cycle metabolic flux by decreasing the concentration of key intermediates [49]. Collectively, these cellular studies demonstrate that VDR signalling is a regulator of glycolytic and oxidative metabolism, and appears to function by restraining metabolic flux under normal physiological conditions.
The increased bioenergetic profile associated with 25(OH)D <50 nmol/L is consistent with enhanced oxidative stress and activation of PBMCs. Others have previously demonstrated that oxidative stress increases ATP-linked oxygen consumption and proton leak [50]. Chacko et al. hypothesized that oxidative stress induces increased non-mitochondrial respiration (e.g. ROS generation), which leads to increased proton leak and greater ATP demand; together this is reflected by an increased basal respiration [35]. In leukocytes, non-mitochondrial respiration is attributed to enzymes associated with inflammation, such as cyclooxygenases, lipoxygenases and NADPH oxidases and possibly intra-mitochondrial sources of ROS [35], [36]. It is well-recognized that activation of leukocytes increases metabolic rate; with the increase depending on the condition that activates the immune system. RMR was positively associated with TNF-α, after accounting for several confounders including insulin sensitivity, while RQ was inversely related to CRP. Such observations mimic the increased energetic cost of an activated immune system [51]. However, immune cell fuel utilisation during low grade inflammation is an area which requires further investigation as no consensus exists regarding fuel choice by immune cells and the impact of circulating hormones and cytokines associated with stress responses.
We found that BHI, a proposed indicator of mitochondrial health status [32], was directly related to insulin sensitivity but inversely to RMR, and some markers of systemic inflammation. This potentially validates a scenario where attenuation of systemic inflammation, improved insulin sensitivity, reduced whole body energetic demand and BHI are inter-linked (Fig. 1). Given that proton leak is accountable for ~25% of RMR [52] and proton leak is associated with inefficient ETC activity, this would further enhance the conceptual arguments presented. That these relationships were not obvious once the data were adjusted for QUICKI, could indicate the primacy of whole body insulin sensitivity in the derangement of bioenergetics associated with a poor vitamin D status.
Vitamin D is recognized as an anti-inflammatory agent [11], thus vitamin D may influence bioenergetics through inflammatory mechanisms. However, our study did not find any group differences in circulating inflammatory markers. Many randomized controlled trials report no difference in inflammatory markers following vitamin D supplementation [53], [54], [55], [56], however cellular studies convincingly demonstrate an anti-inflammatory benefit following exposure to 1,25(OH)2D(11). Although the majority of cellular studies have tested the effects of 1, 25(OH)2D, there is close relationship between circulating 25(OH)D and its active metabolite. This is especially crucial for tissues that can convert 25OHD to 1,25(OH)2D such as the immune cells [16]. In support of this, observations that the concentration of serum vitamin D influences cytokine secretion from peripheral blood mononuclear immune cells has been reported [57]. We acknowledge that measuring systemic cytokine concentrations does not provide information on cytokine release at the local tissue level [58]. It is also likely that we could not detect group differences between study groups because we excluded participants with acute inflammatory conditions.
We also observed that insulin sensitivity tended to be highest in Group 3 (≥75 nmol/L) (Table 1). This positive association between vitamin D status and insulin sensitivity has been found in many cross-sectional studies [59], [60], [61]. Furthermore in this study QUICKI trended towards an inverse association with MCP-1, and MCP-1 has been repeatedly demonstrated to induce insulin resistance in obese mice models [62], [63]. It is well-accepted that the insulin resistance that accompanies obesity is attributable, at least in part, to changes in the secretion of adipokines [64]. Overall, as the links between BHI, inflammatory markers and whole body energy expenditure disappeared on adjustment for QUICKI, insulin sensitivity may also be key to the relationship of bioenergetics and systemic inflammation.
4.2. Study limitations, strengths and future directions
As a cross-sectional design we cannot confirm a causal effect of 25(OH)D on PBMC bioenergetics but the results strongly support the examination of correcting inadequate vitamin D status on bioenergetics parameters. The samples used contained a heterogeneous population of immune cells, each of which have unique bioenergetic profiles, as eloquently discussed by Chacko et al. [32] and Kramer et al. [65]. Since immune cells in vivo interact with one another, it is likely that the metabolic state of the whole human system is better reflected through a heterogeneous sample, rather than just one immune cell type. We do however acknowledge the value in determining the influence of vitamin D on bioenergetic parameters in isolated and purified populations of immune cells. Future studies may investigate whether this explains the relationship between vitamin D status and bioenergetics that we have observed. Despite finding significantly increased ATP production and non-significant increased reserve capacity [numerator terms of the BHI equation], we also uncovered an increased proton leak and non-mitochondrial respiration [denominator terms]. It would appear such effects approximately cancelled out as we found no significant differences between vitamin D groups in BHI. While BHI was 7% marginally higher in those with <50 nmol/L compared to those with status >75 nmol/L, our study was not powered to detect such a difference. It is also possible that the BHI equation as originally proposed may need modification, however developing such an equation was outside the scope of this manuscript given its cross sectional design.
The major strength of our study is its holistic approach that combined metabolic profiling of PBMCs, whole body energy metabolism parameters, markers of whole body insulin sensitivity, body composition and systemic inflammatory profile. This allowed a broad overview of bioenergetics across the range of prevailing 25(OH)D seen in this study. We believe such studies are better equipped to allow translation of key cellular and molecular events to a clinical scenario of disease. However they necessarily have to overcome the complexity and inherent larger biological variability associated with the whole systems approach, relative to isolated cell systems. There is a requirement for well-designed randomized controlled trials to extend such findings towards a causal role for the vitamin in human energy metabolism and bioenergetics of leukocytes in chronic disease states.
In conclusion, this study documents for the first time the potential influence of vitamin D status on bioenergetics in freshly isolated peripheral blood mononuclear cells. Taken together, these data indicate a relationship between Vitamin D and immune cell bioenergetic responses. Specifically we propose that low vitamin D status engenders a pattern consistent with increased oxidative metabolism and inflammatory activation that is reflected in altered bioenergetics of PBMCs. Future studies need to validate whether vitamin D has a causal role in cellular function and bioenergetic health.
Author Contributorship
The present work was designed by KNK, MJS, PN and EKC. Initial manuscript preparation and draft was undertaken by EKC and revised by KNK, MJS, PN, and JR. Patients were recruited by EKC and body composition was assessed by EKC. Immune cell isolation was performed by JR, KNK and EKC. Bioenergetic parameters were measured by EKC. Data analysis and statistical analysis were made by EKC and KNK. Figure preparation was made by EKC, MJS and KNK. Supervision of the manuscript was made by PN and MJS. All authors approved the final version of the paper.
Funding
The present work was supported by competitive research funds provided by the Schools of Biomedical Sciences and Public Health in the Faculty of Health Sciences, Curtin University.
Acknowledgments
The authors thank Curtin University Schools of Biomedical Sciences and Public Health in the Faculty of Health Sciences for research support. We would also like to thank the Curtin Health Innovation Research Institute for providing excellent facilities. EKC is the recipient of an Australian postgraduate scholarship.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2016.10.007.
Contributor Information
Mario J. Soares, Email: m.soares@curtin.edu.au.
Philip Newsholme, Email: philip.newsholme@curtin.edu.au.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Daly R.M., Gagnon C., Lu Z.X., Magliano D.J., Dunstan D.W., Sikaris K.A., Zimmet P.Z., Ebeling P.R., Shaw J.E. Prevalence of vitamin D deficiency and its determinants in Australian adults aged 25 years and older: a national, population-based study. Clin. Endocrinol. (Oxf.) 2012;77(1):26–35. doi: 10.1111/j.1365-2265.2011.04320.x. [DOI] [PubMed] [Google Scholar]
- 2.Tran B., Armstrong B.K., McGeechan K. Predicting vitamin D deficiency in older Australian adults. Clin. Endocrinol. (Oxf.) 2013;79(5):631–640. doi: 10.1111/cen.12203. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y., Leung D.Y., Richers B.N., Liu Y., Remigio L.K., Riches D.W., Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012;188(5):2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badenhoop K., Kahles H., Penna-Martinez M. Vitamin D, immune tolerance, and prevention of type 1 diabetes. Curr. Diab Rep. 2012;12(6):635–642. doi: 10.1007/s11892-012-0322-3. [DOI] [PubMed] [Google Scholar]
- 5.Jayaratne N., Hughes M.C., Ibiebele T.I., van den Akker S., van der Pols J.C. Vitamin D intake in Australian adults and the modeled effects of milk and breakfast cereal fortification. Nutrition. 2013;29(7–8):1048–1053. doi: 10.1016/j.nut.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Ross A.C., Manson J.E., Abrams S.A. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J. Clin. Endocrinol. Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterlik M. Vitamin D insufficiency and chronic diseases: hype and reality. Food Funct. 2012;3(8):784–794. doi: 10.1039/c2fo10262e. [DOI] [PubMed] [Google Scholar]
- 8.Heaney R.P. Vitamin D in health and disease. Clin. J. Am. Soc. Nephrol. 2008;3(5):1535–1541. doi: 10.2215/CJN.01160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vieth R. Why the minimum desirable serum 25-hydroxyvitamin D level should be 75 nmol/L (30 ng/ml) Best. Pr. Res Clin. Endocrinol. Metab. 2011;25(4):681–691. doi: 10.1016/j.beem.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Nagy L., Szanto A., Szatmari I., Szeles L. Nuclear hormone receptors enable macrophages and dendritic cells to sense their lipid environment and shape their immune response. Physiol. Rev. 2012;92(2):739–789. doi: 10.1152/physrev.00004.2011. [DOI] [PubMed] [Google Scholar]
- 11.Calton E.K., Keane K.N., Newsholme P., Soares M.J. The impact of Vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One. 2015;10(11):e0141770. doi: 10.1371/journal.pone.0141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prietl B., Treiber G., Pieber T.R., Amrein K. Vitamin D and immune function. Nutrients. 2013;5(7):2502–2521. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakdash G., van Capel T.M., Mason L.M., Kapsenberg M.L., de Jong E.C. Vitamin D3 metabolite calcidiol primes human dendritic cells to promote the development of immunomodulatory IL-10-producing T cells. Vaccine. 2014;32(47):6294–6302. doi: 10.1016/j.vaccine.2014.08.075. [DOI] [PubMed] [Google Scholar]
- 14.Bartels L.E., Hvas C.L., Agnholt J., Dahlerup J.F., Agger R. Human dendritic cell antigen presentation and chemotaxis are inhibited by intrinsic 25-hydroxy vitamin D activation. Int. Immunopharmacol. 2010;10(8):922–928. doi: 10.1016/j.intimp.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Fraser D.R. Vitamin D deficiency and energy metabolism. Endocrinology. 2015;156(6):1933–1935. doi: 10.1210/en.2015-1298. [DOI] [PubMed] [Google Scholar]
- 16.Hewison M. Antibacterial effects of Vitamin D. Nat. Rev. Endocrinol. 2011;7(6):337–345. doi: 10.1038/nrendo.2010.226. [DOI] [PubMed] [Google Scholar]
- 17.Straub R.H., Cutolo M., Buttgereit F., Pongratz G. Energy regulation and neuroendocrine–immune control in chronic inflammatory diseases. J. Intern. Med. 2010;267(6):543–560. doi: 10.1111/j.1365-2796.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 18.Tyrrell D.J., Bharadwaj M.S., Van Horn C.G., Marsh A.P., Nicklas B.J., Molina A.J. Blood-cell bioenergetics are associated with physical function and inflammation in overweight/obese older adults. Exp. Gerontol. 2015;70:84–91. doi: 10.1016/j.exger.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belikova I., Lukaszewicz A.C., Faivre V., Damoisel C., Singer M., Payen D. Oxygen consumption of human peripheral blood mononuclear cells in severe human sepsis. Crit. Care Med. 2007;35(12):2702–2708. doi: 10.1097/01.ccm.0000295593.25106.c4. [DOI] [PubMed] [Google Scholar]
- 20.Hanagasi H.A., Ayribas D., Baysal K., Emre M. Mitochondrial complex I, II/III, and IV activities in familial and sporadic Parkinson’s disease. Int J. Neurosci. 2005;115(4):479–493. doi: 10.1080/00207450590523017. [DOI] [PubMed] [Google Scholar]
- 21.Kuhnke A., Burmester G.R., Krauss S., Buttgereit F. Bioenergetics of immune cells to assess rheumatic disease activity and efficacy of glucocorticoid treatment. Ann. Rheum. Dis. 2003;62(2):133–139. doi: 10.1136/ard.62.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samara A., Pfister M., Marie B., Visvikis-Siest S. Visfatin. Low-grade inflammation and body mass index (BMI) Clin. Endocrinol. (Oxf.) 2008;69(4):568–574. doi: 10.1111/j.1365-2265.2008.03205.x. [DOI] [PubMed] [Google Scholar]
- 23.Rogacev K.S., Ulrich C., Blomer L. Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur. Heart J. 2010;31(3):369–376. doi: 10.1093/eurheartj/ehp308. [DOI] [PubMed] [Google Scholar]
- 24.Visvikis-Siest S., Marteau J.B., Samara A., Berrahmoune H., Marie B., Pfister M. Peripheral blood mononuclear cells (PBMCs): a possible model for studying cardiovascular biology systems. Clin. Chem. Lab Med. 2007;45(9):1154–1168. doi: 10.1515/CCLM.2007.255. [DOI] [PubMed] [Google Scholar]
- 25.Widlansky M.E., Wang J., Shenouda S.M. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl. Res. 2010;156(1):15–25. doi: 10.1016/j.trsl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartman M.L., Shirihai O.S., Holbrook M. Relation of mitochondrial oxygen consumption in peripheral blood mononuclear cells to vascular function in type 2 diabetes mellitus. Vasc. Med. 2014;19(1):67–74. doi: 10.1177/1358863X14521315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omodei D., Pucino V., Labruna G. Immune-metabolic profiling of anorexic patients reveals an anti-oxidant and anti-inflammatory phenotype. Metabolism. 2015;64(3):396–405. doi: 10.1016/j.metabol.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 28.Lowell B.B., Shulman G.I. Mitochondrial dysfunction and Type 2 diabetes. Science. 2005;307(5708):384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 29.Petersen K.F., Dufour S., Befroy D., Garcia R., Shulman G.I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. New Engl. J. Med. 2004;350(7):664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fink B., Herlein J., O'Malley Y., Sivitz W. Endothelial cell and platelet bioenergetics: effect of glucose and nutrient composition. PLoS One. 2012;7(6):e39430. doi: 10.1371/journal.pone.0039430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen K.F., Dufour S., Shulman G.I. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med. 2005;2(9):e233. doi: 10.1371/journal.pmed.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chacko B.K., Kramer P.A., Ravi S., Johnson M.S., Hardy R.W., Ballinger S.W., Darley-Usmar V.M. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab Invest. 2013;93(6):690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenzo C., Haffner S.M., Stancakova A., Laakso M. Relation of direct and surrogate measures of insulin resistance to cardiovascular risk factors in nondiabetic finnish offspring of type 2 diabetic individuals. J. Clin. Endocrinol. Metab. 2010;95(11):5082–5090. doi: 10.1210/jc.2010-1144. [DOI] [PubMed] [Google Scholar]
- 34.Keane K.N., Calton E.K., Cruzat V.F., Soares M.J., Newsholme P. The impact of cryopreservation on human peripheral blood leucocyte bioenergetics. Clin. Sci. (Lond.) 2015;128(10):723–733. doi: 10.1042/CS20140725. [DOI] [PubMed] [Google Scholar]
- 35.Chacko B.K., Kramer P.A., Ravi S. The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clin. Sci. (Lond.) 2014;127(6):367–373. doi: 10.1042/CS20140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chacko B.K., Zhi D., Darley-Usmar V.M., Mitchell T. The Bioenergetic Health Index is a sensitive measure of oxidative stress in human monocytes. Redox Biol. 2016;8:43–50. doi: 10.1016/j.redox.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maynard S., Keijzers G., Gram M. Relationships between human vitality and mitochondrial respiratory parameters, reactive oxygen species production and dNTP levels in peripheral blood mononuclear cells. Aging (Albany NY) 2013;5(11):850–864. doi: 10.18632/aging.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartman M.-L., Shirihai O.S., Holbrook M. Relation of mitochondrial oxygen consumption in peripheral blood mononuclear cells to vascular function in type 2 diabetes mellitus. Vasc. Med. 2014;19(1):67–74. doi: 10.1177/1358863X14521315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omodei D., Pucino V., Labruna G. Immune-metabolic profiling of anorexic patients reveals an anti-oxidant and anti-inflammatory phenotype. Metab. - Clin. Exp. 2015;64(3):396–405. doi: 10.1016/j.metabol.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 40.Allen K.L., Byrne S.M., Kusel M.M., Hart P.H., Whitehouse A.J. Maternal vitamin D levels during pregnancy and offspring eating disorder risk in adolescence. Int J. Eat. Disord. 2013;46(7):669–676. doi: 10.1002/eat.22147. [DOI] [PubMed] [Google Scholar]
- 41.Shen L., Ji H.F. Vitamin D deficiency is associated with increased risk of Alzheimer’s disease and dementia: evidence from meta-analysis. Nutr. J. 2015;14:76. doi: 10.1186/s12937-015-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitri J., Muraru M.D., Pittas A.G. Vitamin D and type 2 diabetes: a systematic review. Eur. J. Clin. Nutr. 2011;65(9):1005–1015. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinha A., Hollingsworth K.G., Ball S., Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 2013;98(3):E509–E513. doi: 10.1210/jc.2012-3592. [DOI] [PubMed] [Google Scholar]
- 44.Gupta R., Sharma U., Gupta N., Kalaivani M., Singh U., Guleria R., Jagannathan N.R., Goswami R. Effect of cholecalciferol and calcium supplementation on muscle strength and energy metabolism in vitamin d-deficient Asian Indians: a randomized, controlled trial. Clin. Endocrinol. (Oxf.) 2010;73(4):445–451. doi: 10.1111/j.1365-2265.2010.03816.x. [DOI] [PubMed] [Google Scholar]
- 45.Rana P., Marwaha R.K., Kumar P., Narang A., Devi M.M., Tripathi R.P., Khushu S. Effect of vitamin D supplementation on muscle energy phospho-metabolites: a (3)(1)P magnetic resonance spectroscopy-based pilot study. Endocr. Res. 2014;39(4):152–156. doi: 10.3109/07435800.2013.865210. [DOI] [PubMed] [Google Scholar]
- 46.Wong K.E., Szeto F.L., Zhang W., Ye H., Kong J., Zhang Z., Sun X.J., Li Y.C. Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. Am. J. Physiol. Endocrinol. Metab. 2009;296(4):E820–E828. doi: 10.1152/ajpendo.90763.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X.-J., Wang B.-W., Zhang C., Xia M.-Z., Chen Y.-H., Hu C.-Q., Wang H., Chen X., Xu D.-X. Vitamin D deficiency attenuates high-fat diet-induced hyperinsulinemia and hepatic lipid accumulation in male mice. Endocrinology. 2015;156(6):2103–2113. doi: 10.1210/en.2014-2037. [DOI] [PubMed] [Google Scholar]
- 48.Consiglio M., Destefanis M., Morena D., Foglizzo V., Forneris M., Pescarmona G., Silvagno F. The vitamin D receptor inhibits the respiratory chain, contributing to the metabolic switch that is essential for cancer cell proliferation. PLoS One. 2014;9(12):e115816. doi: 10.1371/journal.pone.0115816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng W., Tayyari F., Gowda G.A. 1,25-Dihydroxyvitamin D regulation of glucose metabolism in Harvey-ras transformed MCF10A human breast epithelial cells. J. Steroid Biochem. Mol. Biol. 2013;138:81–89. doi: 10.1016/j.jsbmb.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill B.G., Benavides G.A., Lancaster J.R., Jr., Ballinger S., Dell’Italia L., Jianhua Z., Darley-Usmar V.M. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393(12):1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Straub R.H. Interaction of the endocrine system with inflammation: a function of energy and volume regulation. Arthritis Res. Ther. 2014;16(1):1–15. doi: 10.1186/ar4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brookes P.S. Mitochondrial H+ leak and ROS generation: an odd couple. Free Radic. Biol. Med. 2005;38(1):12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 53.Sollid S.T., Hutchinson M.Y., Fuskevag O.M. No effect of high-dose vitamin D supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care. 2014;37(8):2123–2131. doi: 10.2337/dc14-0218. [DOI] [PubMed] [Google Scholar]
- 54.Kampmann U., Mosekilde L., Juhl C., Moller N., Christensen B., Rejnmark L., Wamberg L., Orskov L. Effects of 12 weeks high dose vitamin D3 treatment on insulin sensitivity, beta cell function, and metabolic markers in patients with type 2 diabetes and vitamin D insufficiency - a double-blind, randomized, placebo-controlled trial. Metabolism. 2014;63(9):1115–1124. doi: 10.1016/j.metabol.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Yiu Y.F., Yiu K.H., Siu C.W. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis. 2013;227(1):140–146. doi: 10.1016/j.atherosclerosis.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Gepner A.D., Ramamurthy R., Krueger D.C., Korcarz C.E., Binkley N., Stein J.H. A prospective randomized controlled trial of the effects of vitamin D supplementation on cardiovascular disease risk. PLoS One. 2012;7(5):e36617. doi: 10.1371/journal.pone.0036617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ojaimi S., Skinner N.A., Strauss B.J., Sundararajan V., Woolley I., Visvanathan K. Vitamin d deficiency impacts on expression of toll-like receptor-2 and cytokine profile: a pilot study. J. Transl. Med. 2013;11:176. doi: 10.1186/1479-5876-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Albers R., Antoine J.M., Bourdet-Sicard R. Markers to measure immunomodulation in human nutrition intervention studies. Br. J. Nutr. 2005;94(3):452–481. doi: 10.1079/bjn20051469. [DOI] [PubMed] [Google Scholar]
- 59.Chiu K.C., Chu A., Go V.L., Saad M.F. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004;79(5):820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 60.Liu E., Meigs J.B., Pittas A.G., McKeown N.M., Economos C.D., Booth S.L., Jacques P.F. Plasma 25-Hydroxyvitamin D is associated with markers of the insulin resistant phenotype in nondiabetic adults. J. Nutr. 2009;139(2):329–334. doi: 10.3945/jn.108.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kayaniyil S., Vieth R., Retnakaran R. Association of vitamin D with insulin resistance and β-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care. 2010;33(6):1379–1381. doi: 10.2337/dc09-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanda H., Tateya S., Tamori Y. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tateya S., Tamori Y., Kawaguchi T., Kanda H., Kasuga M. An increase in the circulating concentration of monocyte chemoattractant protein-1 elicits systemic insulin resistance irrespective of adipose tissue inflammation in mice. Endocrinology. 2010;151(3):971–979. doi: 10.1210/en.2009-0926. [DOI] [PubMed] [Google Scholar]
- 64.Marette A. Mediators of cytokine-induced insulin resistance in obesity and other inflammatory settings. Curr. Opin. Clin. Nutr. Metab. Care. 2002;5(4):377–383. doi: 10.1097/00075197-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Kramer P.A., Ravi S., Chacko B., Johnson M.S., Darley-Usmar V.M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–210. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material