Abstract
Succinate dehydrogenase (SDH) is a heterotetrameric complex, among which the catalytic core SDHB loss-of-function mutations lead to mitochondrial enzyme SDH dysfunction and are associated with cancer formation. However, the impact of SDHB loss on colorectal carcinoma and the underlying mechanisms are largely unknown. In this study, we found a coherent decreased SDHB expression both in human colorectal cancer (CRC) samples and CRC cell lines. Combined clinical analysis in a cohort of 43 CRC patients demonstrated a correlation between reduced SDHB activity and a more advanced clinical phenotype regarding lymphatic and distant metastasis. Applying genetic interference and cellular function approaches, we found that knocking down SDHB promoted cell migration and invasion through enabling epithelial-mesenchymal transition (EMT), and inverse results of SDHB overexpression further confirmed our theory. Mechanical exploration revealed that SDHB knockdown could activate TGFβ signaling pathway, more precisely through up-regulation of a tight-junction transcriptional repression complex SNAIL1-SMAD3/SMAD4, thus contributed to the increase in metastasis. In conclusion by identifying SNAIL1-SMAD3/SMAD4 as essential for the TGFβ-mediated tumorigenic capacity in SDHB-deficient CRC cells, this study revealed a critical mechanical vulnerability for potential future therapeutic target of SDHB-associated CRC.
Introduction
Colorectal cancer (CRC) is the third most common malignant disease in men and the second in women worldwide and its prevalence and mortality continued to climb up across the Asia-Pacific region [1]. Although neoadjuvant treatments kept on developing in last decade, stacking evidences have shown that high financial cost, chemoresistance and high toxicity severely constrained the therapeutic efficacy [2]. Therefore it is always welcoming for scientists to identify new biomarkers of advanced CRC and set up therapeutic strategy rooted from inspiring researches. Recent discoveries have brought forward that altered metabolism in malignant cells could serve as an underlying hallmark of neoplastic transformation [3].
The tricarboxylic acid (TCA) cycle is a central metabolic pathway that supplies pivotal source for mitochondrial NADH and many anabolic precursors [4]. Oncogenic mutations in TCA-cycle-related enzymes such as SDH have been identified to be a predisposing factor in various cancers [5]. Succinate dehydrogenase (SDH) is a mitochondrial metabolic enzyme responsible for the oxidation of succinate to fumarate in the citric acid cycle and its associated electron transport [6]. SDHB is the catalytic core component of heterotetrameric complex SDH, of which loss-of-function mutations lead to enzymatic dysfunction and are associated with cancer formation mostly reported in hereditary paragangliomas to date [7]. Although SDH generally displayed tumor suppressor properties to some extent [8], the impact of enzymatic subunit SDHB on colorectal tumors and how impaired bioenergetic and anabolic synthesis influenced cellular function remained largely unresolved.
SDHB dysfunction results in abnormal SDH enzyme activity, which causes accumulation of succinate, acting as a competitive inhibitor of the hypoxia-inducible factor (HIF) prolyl-hydroxylases [9]. This stabilization of HIF-alpha elicits a pseudohypoxic phenotype, activating genes that facilitate angiogenesis and anaerobic metabolism. So far, HIFs are the only mechanism reported linking SDHB-mutation to carcinogenesis, which have also been described insufficient comprehensively. Therefore a more profound understanding of SDHB-related tumorigenesis is crucial.
In this study, we discovered a ubiquitously low SDHB activity in colorectal carcinoma. By generating SDHB-knockdown and overexpression stable CRC cell lines and using analytical functional approaches to identify various levels of adaptations subsequent to SDHB loss. We found that lack of SDHB activity commits cells to epithelial-mesenchymal transition (EMT), which sustains CRC cell invasion and metastasis. Clinical assessment revealed a correlation between low SDHB expression and more advanced phenotype with poorer prognosis, corroborating our theory. We further demonstrated that SDHB deficiency activates TGFβ signaling [10] and thus triggers a transcriptional repression complex SNAIL1-SMAD3/SMAD4, previously documented to bind to several cell tight-junction related genes promoter area, consequently mobilizing malignant CRC cells [11]. By unraveling that SNAIL1-SMAD3/SMAD4 complex is critical for the TGFβ-dependent invasion in SDHB-deficient CRC cells, we revealed a new essential signal pathway other than HIFs, which could offer new therapeutic targets for SDHB mutation driven metabolically abnormal late-stage and metastatic CRC patients.
Materials and Methods
Cell Culture
Colorectal cancer cell line HT-29, SW480, SW620, SW1116 and normal colorectal epithelial cell line NCM460 were purchased from Cell Bank of Chinese Academy of Sciences. HT-29 and NCM460 cells were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml) and were incubated at 37 °C in a humidified incubator under 5% CO2 condition.
SW480, SW620, SW1116 cells were cultured in L-15 medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml) and were incubated at 37 °C in a humidified incubator under 0.038% CO2 condition.
Immunohistochemical Staining (IHC)
We have obtained paraffin-embedded CRC and adjacent normal tissue specimens from the Division of General Surgery at Zhongshan hospital. Formalin-fixed paraffin-embedded (FFPE) 6 mm sections were used for IHC. SDHB primary antibody (proteintech, cat# 10,620–1-AP, 1:50 dilution) was incubated overnight at 4 °C. Immunostaining was performed using diaminobenzidine reaction and controlled under microscope. Hematoxylin was used for counterstaining.
Quantitative Real-Time PCR
Total RNA was extracted from cultured cells using Trizol reagent (Invitrogen, Carlsbad, CA) and dissolved in diethylpyrocarbonate treated (DEPC) water. cDNA was synthesized using the Takara Reverse Transcription System Kit (Takara Biotechnology Co. Ltd., Japan) according to the manufacturer's instruction. Real time quantitative RT-PCR was performed using the Sybr green premix kit (BioRad, Hercules, CA, USA). GAPDH was used as a housekeeping gene. The following primers are used in this assay: SDHB: sense GACACCAACCTCAATAAGGTCTC, and anti-sense GGCTCAATGGATTTGTACTGTGC; Snail1: sense TCGGAAGCCTAACTACAGCGA, and anti-sense AGATGAGCATTGGCAGCGAG; Smad3: sense CCATCTCCTACTACGAGCTGAA, and anti-sense CACTGCTGCATTCCTGTTGAC; Smad4: sense CTCATGTGATCTATGCCCGTC, and anti-sense AGGTGATACAACTCGTTCGTAGT; ZO1: sense ACCAGTAAGTCGTCCTGATCC, and anti-sense TCGGCCAAATCTTCTCACTCC; E-cadherin: sense CGAGAGCTACACGTTCACGG, and anti-sense GGGTGTCGAGGGAAAAATAGG; N-cadherin: sense TGCGGTACAGTGTAACTGGG, and anti-sense GAAACCGGGCTATCTGCTCG; Fibronectin: sense CGGTGGCTGTCAGTCAAAG, and anti-sense AAACCTCGGCTTCCTCCATAA; GAPDH: sense ACAACTTTGGTATCGTGGAAGG, and anti-sense GCCATCACGCCACAGTTTC.
SDHB shRNA Transfection
SDHB shRNAs were obtained from Institute of Biochemistry and Cell Biology, SIBS, CAS. A scrambled sequence was also purchased as control. Cell transfection was performed with PEI reagent (Sigma-Aldrich, MO, USA).
Matrigel Invasion Assay
For transwell invasion assay, 50 μl matrigel (BD Bioscience, Franklin Lakes, NJ, USA) was added into top chamber for 30 minutes at 37°C. After starvation in serum free medium for 24 hours and cells were seeded at 1 × 104 cells/well to the top chambers. The bottom chambers were filled with completed medium. Any non-invading cells remaining in the top chamber were removed carefully with a swab in 48 hours culturing. Cells adhering to the lower membrane of the well were fixed in methanol and stained with crystal violet, and finally counted under 200× magnification. Crystal violet staining was dissolved in 33% acetic acid and optical density was detected at 570 nm.
Western Blotting
Equal amounts of protein were separated by 10% SDS polyacrylamide gel electrophoresis and transferred to PVDF membranes. After blocking with 5% non-fat milk in TBST for 1 hour, membranes were incubated overnight at 4 °C with SDHB , GAPDH, SNAIL1, SMAD3, SMAD4, E-cadherin, N-cadherin primary antibodies followed by incubation with HRP-conjugated secondary antibodies. Immunoreactive bands were developed with enhanced chemiluminescent HRP substrate (Millipore, Bollerica, MA, USA).
Luciferase Assay
Transfections were performed using LipofectAMINE-2000 (Invitrogen, Carlsbad, CA, USA) as per the manufacturer's instructions. Relative luciferase activity was detected with dual luciferase kit (Promega E1906) as per the manufacturer's instructions and calculated as the ratio of luciferase/renilla activity.
Succinate Detection
Succinate level was detected using Succinate Colorimetric Assay Kit (Sigma-Aldrich, MO) as per the manufacturer's instructions. Succinate level is measured as nmol/mg protein.
Statistical Analysis
Statistical analyses were performed using SPSS 21.0 (IBM, Chicago, IL, USA). chi-Squared test was used for analyzing relations between clinical parameters and SDHB expression levels. Student's t test was used to evaluate in vitro studies. P value was considered statistically significant under .05.
Results
SDHB Expression is Ubiquitously Low in CRC Cell Lines and in the Samples of CRC Patients
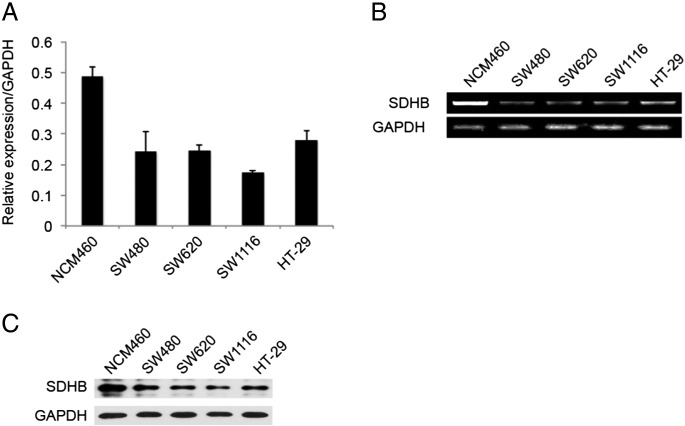
SDHB is the key enzymatic component of mitochondrial enzyme SDH constructively expressed across species, which oxidizing succinate to fumarate in the TCA cycle and feeding electrons into the mitochondrial respiratory chain for ATP production. We examined SDHB mRNA and protein level in four CRC cell lines and normal colorectal epithelial cell line NCM460. As shown in Figure 1 both SDHB mRNA level and protein level was relatively low in all four CRC cell lines (SW480, SW620, SW1116, and HT-29), compared to non-CRC epithelial NCM460 cells. For further confirmation, we detected SDHB mRNA expression in 29 pairs of CRC and adjacent noncancerous tissues by quantitative RT-PCR. As shown in Figure 2A, CRC tissues exhibited remarkably reduced SDHB mRNA level compared to corresponding normal tissues. Immunohistochemistry assay displayed similar results (Figure 2B). And as expected, reduced SDHB level in CRC tissues led to impaired succinate activity and subsequent succinate accumulating (Figure 2C). Conclusively, SDHB expression is generally down regulated in CRC and we have noticed a certain extent of heterogeneity among patients, which could indicate specific biological functions under pathological settings.
Figure 1.
SDHB expression is low in CRC cell lines. (A) Quantitative RT-PCR analysis of SDHB expression in four CRC cell lines and normal colorectal epithelial cell line NCM460. (B) Semi- quantitative RT-PCR analysis of SDHB expression in four CRC cell lines and normal colorectal epithelial cell line NCM460. (C) Western blotting analysis of SDHB protein expression in four CRC cell lines and normal colorectal epithelial cell line NCM460.
Figure 2.
SDHB expression is low in CRC tissues. (A) Quantitative RT-PCR analysis of SDHB expression in 29 pairs of colorectal tumor and its corresponding normal tissues. (B) Representative immunohistochemistry images showed varied but unanimously low expression of SDHB in 43 pair of CRC samples. (C) Succinate level was detected with colorimetric assay at 450 nm in 29 pairs of colorectal tumor and its corresponding normal tissues.
SDHB Knockdown Promotes Invasion of CRC Cells
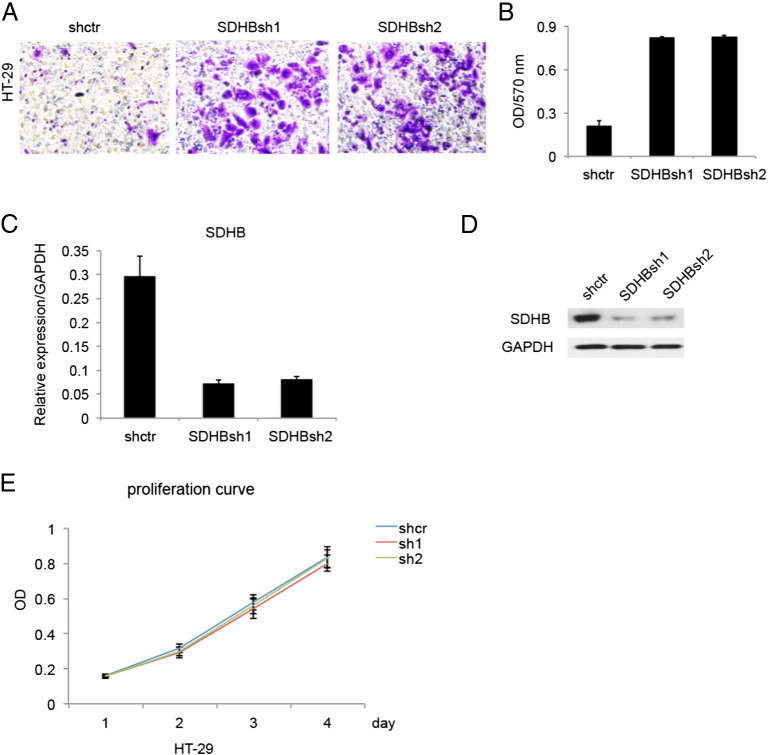
Recent studies have shown that SDHB deletion completely truncates TCA cycle and diverts cells to glycolysis to fulfill energetic needs. We wondered that how this major metabolic rewire affect cellular performance functionally. Based on previous finding that HT-29 harbored relative higher expression of SDHB, we constructed SDHB knockdown stable cell line through lentiviral infection. Once we excluded the possible effects that SDHB knockdown exerted on CRC cells proliferation (Figure 3E), which was not apparent, it became crystal clear that decreased expression of SDHB enhanced invasive capabilities in HT-29 cells (Figure 3, A and B), illustrated by matrigel assay. SDHB knockdown efficiency was confirmed on both mRNA and protein level (Figure 3, C and D).
Figure 3.
SDHB knockdown promoted invasion in CRC cell line HT-29. (A) Matrigel invasion assay showed that SDHB knockdown strengthened invasive capacity. (B) Absorbance at 570 nm showed quantitative matrigel invasion data after 24 hours. P < .05. (C) Quantitative RT-PCR analysis of knockdown efficiency in SDHB shRNA transfected stable cell lines. (D) Western blotting analysis of knockdown efficiency in SDHB shRNA transfected stable cell lines. (E) MTT assay showed SDHB knockdown had no remarkable effect on cell proliferation.
SDHB Overexpression Inhibits Invasion of CRC Cells
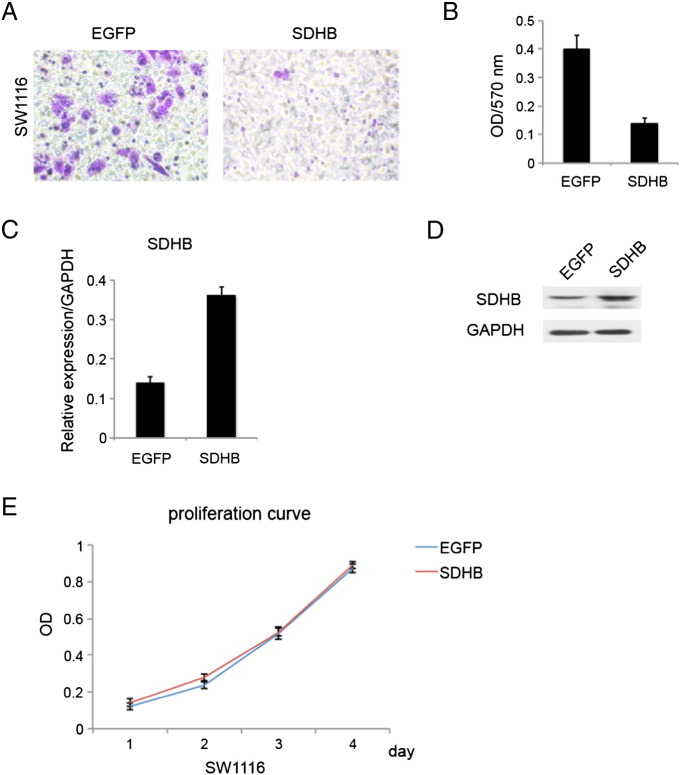
For further validation, we also constructed SDHB overexpression stable cell line in SW1116 and performed corresponding experiments. Results came in coherent with our previous loss-of-function data (Figure 4), that SDHB overexpression suppressed CRC cells invasive and migratory capability (Figure 4, A and B). All in all, that SDHB had a fundamental role in cellular motility and adhesion during CRC oncogenesis in vitro.
Figure 4.
SDHB overexpression inhibited invasion in CRC cell line SW1116. (A) Matrigel invasion assay showed that SDHB overexpression decreased invasive capacity. (B) Absorbance at 570 nm showed quantitative matrigel invasion data after 24 hours. P < .05. (C) Quantitative RT-PCR analysis of overexpression efficiency in SDHB virus transfected stable cell lines. (D) Western blotting analysis of overexpression efficiency in SDHB virus transfected stable cell lines. (E) MTT assay showed SDHB overexpression had no remarkable effect on cell proliferation.
SDHB Knockdown Encourages Epithelial-Mesenchymal Transition (EMT) in CRC Cells
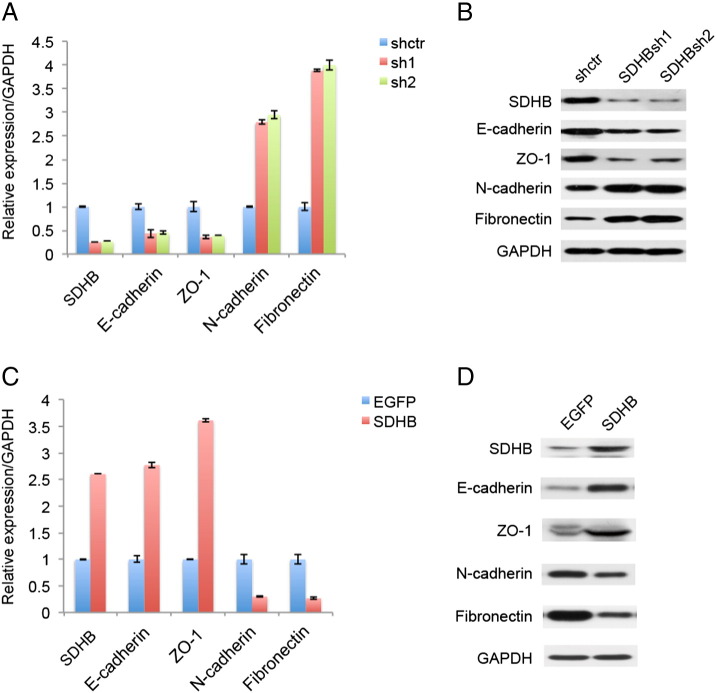
EMT is a biological process that converts epithelial cells to motile mesenchymal cells, which was utilized by cancer cells to enhance invasive and migratory ability, and to acquire stem-like characteristics as chemoresistance. EMT is defined by the loss of epithelial marker E-cadherin and the gain of mesenchymal marker N-cadherin alongside the morphology conversion. Therefore, we examined the influence of SDHB expression level imposing on EMT process. As shown in Figure 5, A and B, SDHB knockdown decreased E-cadherin, ZO-1 expression and increased N-cadherin, Fibronectin expression, otherwise stated that SDHB knockdown greatly promoted EMT. Coherently, SDHB overexpression had opposing effects (Figure 5, C and D).
Figure 5.
SDHB expression level regulated epithelial-mesenchymal transition (EMT) in CRC cell line. (A) Quantitative RT-PCR analysis showed that SDHB knockdown advanced EMT, as in down-regulation of epithelial markers and simultaneously up-regulation of mesenchymal markers. (B) Western blotting analysis showed that SDHB knockdown advanced EMT. (C) Quantitative RT-PCR analysis showed that SDHB overexpression suppressed EMT, as in up-regulation of epithelial markers and simultaneously down-regulation of mesenchymal markers. (D) Western blotting analysis showed that SDHB overexpression suppressed EMT.
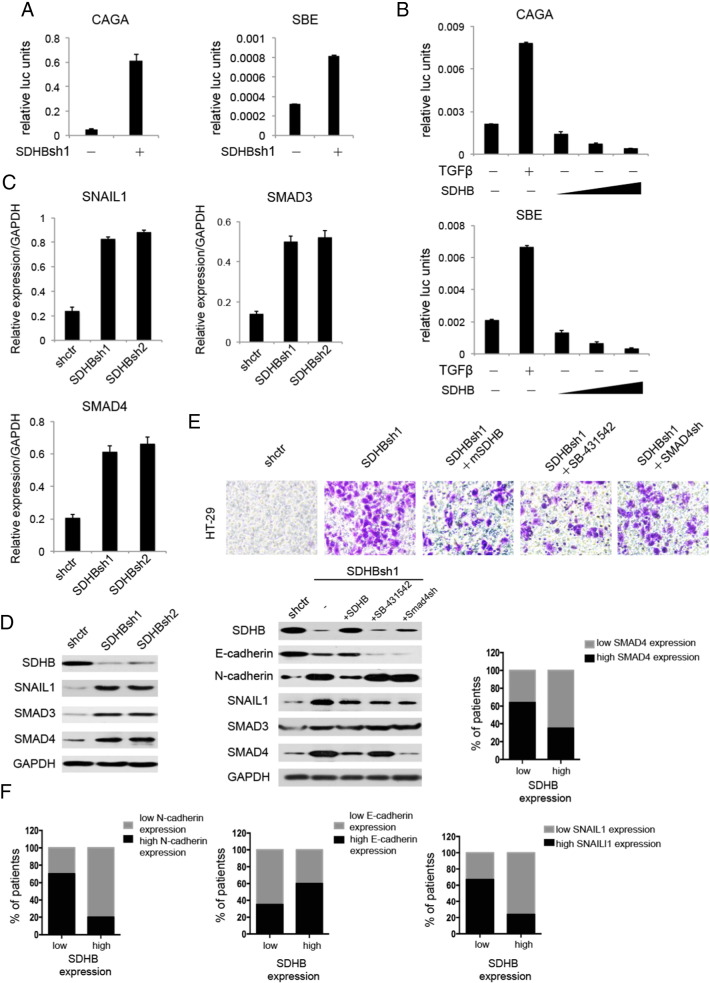
SDHB Knockdown Cctivated TGFβ Signaling Pathway and its Subsequent up Regulation of Transcriptional Repression Complex SNAIL1-SMAD3/4 Induced EMT
Despite the fact that various levels of EMT regulations have been subjected to intense research, the role of metabolic enzymes in the maintenance of EMT has not been extensively studied. Utilizing a dual luciferase screening system, we discovered a hyperactivated TGFβ signaling pathway upon SDHB knockdown (Figure 6A), which was significantly repressed when SDHB is overexpressed (Figure 6B). In light of this, we proceeded to identify a more precise mechanism linking the lack of SDHB activity to EMT enhancement. It has been reported that SNAIL1-SMAD3/4 could bind to several cellular tight-junction genes promoter region including E-cadherin to transcriptionally repress genetic expression thus facilitating invasiveness, which we sought to verify in this context. As expected, knockdown of SDHB resulted in a drastic increase in SNAIL1-SMAD3/4 mRNA and protein level (Figure 6, C and D). Matrigel transwell assay was performed in the case of adding mouse SDHB, TGFβ inhibitor SB-431,542 and SMAD4shrna. Results demonstrated that adding mouse SDHB, TGFβ inhibitor SB-431,542 and SMAD4shrna could all partially restored SDHB-deficient impelled invasive phenotype, among which the last had the modest effect. This phenotypic rescue was accompanied with an up regulation of epithelial marker E-cadherin and down regulation of mesenchymal marker N-cadherin as well as SNAIL1-SMAD3/4, confirming our hypothesis (Figure 6E). Clinical research also substantiated our findings by elaborating a close correlation between SDHB expression level and EMT marker and SNAIL1-SMAD3/4 expression in a cohort of 29 CRC patient samples (Figure 6F). In conclusion, these results showed that mitochondrial enzyme SDHB could emerge as critical regulators of tumor aggressiveness, colligating energy metabolism and biological functions in malignant cells, which was at least partly enabled by an aberrantly active TGFβ signaling pathway.
Figure 6.
SDHB knockdown promoted TGFβ signal pathway in CRC cells, which activated EMT through positive modulation of SNAIL1-SMAD3/4 complex. (A) Dual luciferase assay showed that SDHB knockdown promoted TGFβ signaling in HT-29 cells. CAGA and SBE are the promoter plasmids commonly used representing TGFβ signal pathway activity. (B) Dual luciferase assay showed that SDHB overexpression attenuated TGFβ signaling in HT-29 cells. (C) Quantitative RT-PCR assay showed that SDHB knockdown enhanced transcription factor SNAIL1 expression as well as TGFβ signaling marker gene SMAD3/4 expression in HT-29 cells. (D) Western blotting showed that SDHB knockdown enhanced transcription factor SNAIL1 expression as well as TGFβ signaling marker gene SMAD3/4 expression in HT-29 cells. (E) Matrigel invasion assay showed that the addition of murine SDHB, TGFβR1 inhibitor SB-431,542 and SMAD4shRNA could all rescue human SDHB knockdown phenotype, among which the last had the modest effect. Western blotting showed expression levels of SDHB and EMT marker genes along with SNAIL1-SMAD3/4 complex in the process accordingly. (F) Quantitative RT-PCR analysis showed that the expression of SDHB is significantly correlated with that of EMT marker genes and SNAIL1-SMAD3/4 complex in 29 pair of CRC tissues (P < .05, Student's t test).
SDHB Expression is Negatively Correlated With Metastasis and Prognosis in CRC
Although our studies have verified lower level of SDHB in CRC samples compared to adjacent non-tumorous tissues, its relevance with clinical outcomes remained elusive. Combining tissue microarray data of 43 pairs of CRC specimens including corresponding normal colorectal mucosal tissues with accurate clinical records analysis, we were able to unveil that SDHB expression level was negatively correlated with CRC metastasis and advanced pathological TNM stage (Table 1). Conclusively, SDHB could function as a potential biomarker and even an independent prognostic factor for metastatic CRC.
Table 1.
Clinicopathologic correlation of Sdhb expression in human sporatic colorectal carcinoma
| Sdhb |
||||
|---|---|---|---|---|
| Feature | Low | High⁎ | χ2 | P value# |
| All cases | 21 | 22 | ||
| Gender | 0.239 | .625 | ||
| Male | 13 | 12 | ||
| Female | 8 | 10 | ||
| Age | 0.196 | .658 | ||
| >65 | 11 | 13 | ||
| ≤65 | 10 | 9 | ||
| Intravascular cancer embolus | 6.435 | .011 | ||
| Present | 9 | 2 | ||
| Absent | 12 | 20 | ||
| Perineuronal invasion | 5.880 | .015 | ||
| Present | 7 | 1 | ||
| Absent | 14 | 21 | ||
| Tumor stage | ||||
| I | 1 | 5 | 9.119 | .028 |
| II | 3 | 9 | ||
| III | 11 | 6 | ||
| IV | 6 | 2 | ||
The median expression level was used as the cutoff. Low expression of Sdhb in 21 patients was classified as values below the 50th percentile. High Sdhb expression in 22 patients was classified as values at or above the 50th percentile.# For analysis of correlation between Sdhb and clinical features, Pearson's chi-square tests were used. Results were considered statistically significant at P < 0.05.
Discussion
It has come to the senses of many scientists that metabolic reprogramming is an emerging hallmark during CRC, among which alterations in TCA were delineated first and the most widely known [12]. Documented 100 years ago, Warburg effect was characterized by enhanced glycolysis in the presence of oxygen to meet the substantially augmented energetic need in tumors [13]. In fact, discoveries that germline mutations of TCA intermediate enzymes namely fumarate hydratase and succinate dehydrogenase resulted in familial paraganglioma and pheochromocytoma syndrome have come forth in the past decade, supporting Warburg Effect [14]. SDH genes comprise four subunits (SDHA, B, C, D), also known as complex II of mitochondrial respiratory chain, located at inner mitochondrial membrane, oxidizing succinate to fumarate. SDHA and SDHB are the enzymatic cores of SDH heterotetrameric complex. SDHB mutation inactivates SDH enzyme and causes cellular succinate to build up, which is now considered oncometabolites, acting manifoldly including partly through the aberrant epigenetic reprogramming [15]. However, the role of SDHB in other tumors apart from reported endocrine-associated carcinomas, remained elusive, let alone how metabolic consequences of disabling SDH impacts on cellular function and the molecular mechanisms supporting it. We endeavored to fill up this void in CRC by demonstrating that SDHB expression is unanimously low in CRC patients, which is even lower in late-stage CRC patients. In vitro studies confirmed that knockdown SDHB mobilized CRC cells, whereas SDHB overexpression hindered its invasive capability. By supervising CRC cell phenotype comprehensively, we observed that SDHB deletion conferred a metastatic advantage in CRC, via significantly boosting EMT. In vitro experiments in accordance with our clinical analysis have clearly indicated an anti-metastatic role of SDHB in CRC, which represents a crucial link between glucose metabolism and tumorigenesis.
SDHB mutation has been recently reported to cause respiratory chain dysfunction and hypoxia inducible factors (HIFs) stabilization with a subsequent state of pseudohypoxia [16]. Although this could lead to enhanced angiogenesis and contribute to oncogenesis [17], it doesn't account for all the phenotypic transformations and the underlying mechanisms is open for further exploitation. In this study, with a dual luciferase screening system, we were able to reveal a critical role of TGFβ in SDHB-loss-associated EMT [18]. Following mechanism study depicted that SDHB knockdown significantly increased pivitol EMT transcription factor SNAIL1 [19] expression as well as classical TGFβ pathway modulators SMAD3/4 [20] expression in CRC cells. SNAIL1 has been previously reported in breast cancers to interact with SMAD3/4, which then targeted to the gene promoters of multiple tight-junction proteins namely E-cadherin and acted as co-repressors thus drive EMT. Our study proposed a similar role for transcriptional repressor complex SNAIL1-SMAD3/4 under SDHB-deficient circumstances in CRC. In consistent with this, either adding TGFβ inhibitor or SMAD4shRNA to SDHB-silenced CRC cells could partly inhibited the bolstered EMT process and the comparative milder effect of SMAD4shRNA may ascribe to its relative downstream position in TGFβ transduction conduit. Moreover, loss of SDHB correlated with epithelial marker and SNAIL1-SMAD3/4 in a cohort of CRC patients. On top of all above, rather than act solely and independently, it is also possible that TGFβ [21] and HIFs [22] synergize and interwind since them both has been implicated in chronic inflammatory microenvironment and could both produce cytokines like interleukin superfamily.
Taken together, we demonstrated that SDHB knockdown conferred metastatic advantages on CRC cells leastways through positive modulation of transcriptional repressor complex SNAIL1-SMAD3/4. Most cancer-related deaths are caused by metastasis, the disseminated cancer cells relocated and colonized in distant organ sites, where our insights are still limited. Therefore, our SDHB finding linking de novo TCA to tumor motility is especially beneficial since SDHB is broadly expressed rather than confining to specific diseases, indicating an extensive application once further verified. Along the years, the biphasic nature of the TGF-β signaling pathway during tumorigenesis largely hinders its utilization in clinical treatment. Thus, disclosing specific downstream effectors of TGF-β tumor-progressing arm while avoiding the tumor-suppressing arm allows for accurate CRC treatment. This discovery holds the promise of resolving unsettled problems and yield innovative therapeutics when targeting colon cancer metabolic vulnerabilities.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China [grant number. 81372197].
Footnotes
Conflict of Interest: I state that all authors have no conflicts of interest to this work submitted.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2) doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein DA, Ahmad BB, Chen Q, Ayer T, Howard DH, Lipscomb J, El-Rayes BF, Flowers CR. Cost-Effectiveness Analysis of Regorafenib for Metastatic Colorectal Cancer. J Clin Oncol. 2015;33(32):3727–3732. doi: 10.1200/JCO.2015.61.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wishart DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov. 2016 doi: 10.1038/nrd.2016.32. [DOI] [PubMed] [Google Scholar]
- 4.Alderton GK. Tumour metabolism: Feeding the TCA cycle in vivo. Nat Rev Cancer. 2016;16(4):198. doi: 10.1038/nrc.2016.29. [DOI] [PubMed] [Google Scholar]
- 5.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoekstra AS, d. GM, Briaire-de Bruijn IH, Ras C, Seifar RM, van Minderhout I, Cornelisse CJ, Hogendoorn PC, Breuning MH, Suijker J. Inactivation of SDH and FH cause loss of 5hmC and increased H3K9me3 in paraganglioma pheochromocytoma and smooth muscle tumors. Oncotarget. 2015;6(36):38777–38788. doi: 10.18632/oncotarget.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao HX, K O, Schraders M, Dephoure N, Bayley JP, Kunst H, Devilee P, CW C, Schiffman JD, Bentz BG. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325(5944):1139–1142. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni Y, Zbuk KM, Sadler T, Patocs A, Lobo G, Edelman E, Platzer P, Orloff MS, Waite KA, Eng C. Germline mutations and variants in the succinate dehydrogenase genes in Cowden and Cowden-like syndromes. Am J Hum Genet. 2008;83(2):261–268. doi: 10.1016/j.ajhg.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7(1):77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Jiang L, Deberardinis R, Boothman DA. The cancer cell 'energy grid': TGF-beta1 signaling coordinates metabolism for migration. Mol Cell Oncol. 2015;2(3):e981994. doi: 10.4161/23723556.2014.981994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent T, N. E, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11(8):943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 13.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134(5):703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Eng C, Kiuru M, Fernandez MJ, Aaltonen LA. A role for mitochondrial enzymes in inherited neoplasia and beyond. Nat Rev Cancer. 2003;3(3):193–202. doi: 10.1038/nrc1013. [DOI] [PubMed] [Google Scholar]
- 15.Letouze E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C, Janin M, Menara M, Nguyen AT, Benit P. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23(6):739–752. doi: 10.1016/j.ccr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol. 2008;28(2):718–731. doi: 10.1128/MCB.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Liu T, Zhang S, Zhou J, Wang Y. Di, W, Succinate dehydrogenase subunit B inhibits the AMPK-HIF-1α pathway in human ovarian cancer in vitro. J Ovarian Res. 2014;7:115. doi: 10.1186/s13048-014-0115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Shi J, Luo Y, Liao Q, Niu Y, Zhang F, Shao Z, Ding Y, Zhao L. LIM and SH3 protein 1 induces TGFbeta-mediated epithelial-mesenchymal transition in human colorectal cancer by regulating S100 A4 expression. Clin Cancer Res. 2014;20(22):5835–5847. doi: 10.1158/1078-0432.CCR-14-0485. [DOI] [PubMed] [Google Scholar]
- 19.Kim NH, K H, Li XY, Lee I, Choi HS, Kang SE, Cha SY, Ryu JK, Yoon D, Fearon ER. A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J Cell Biol. 2011;195(3):417–433. doi: 10.1083/jcb.201103097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, F X, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature. 1998;394(6696):909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]
- 21.Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, Liu F, Pan W, Wang TT, Zhou CC. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25(5):666–681. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496(7444):238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]