Abstract
Pertussis is a severe respiratory disease caused by infection with the bacterial pathogen Bordetella pertussis. The disease affects individuals of all ages but is particularly severe and sometimes fatal in unvaccinated young infants. Other Bordetella species cause diseases in humans, animals, and birds. Scientific, clinical, public health, vaccine company, and regulatory agency experts on these pathogens and diseases gathered in Buenos Aires, Argentina from 5 to 8 April 2016 for the 11th International Bordetella Symposium to discuss recent advances in our understanding of the biology of these organisms, the diseases they cause, and the development of new vaccines and other strategies to prevent these diseases. Highlights of the meeting included pertussis epidemiology in developing nations, genomic analysis of Bordetella biology and evolution, regulation of virulence factor expression, new model systems to study Bordetella biology and disease, effects of different vaccines on immune responses, maternal immunization as a strategy to prevent newborn disease, and novel vaccine development for pertussis. In addition, the group approved the formation of an International Bordetella Society to promote research and information exchange on bordetellae and to organize future meetings. A new Bordetella.org website will also be developed to facilitate these goals.
INTRODUCTION
Bordetella pertussis infects the human respiratory tract and causes the disease pertussis, or whooping cough, which is characterized by severe and prolonged bouts of coughing. The disease affects individuals of all ages but is particularly severe in young infants, especially of prevaccination age, in which the disease can be fatal (1). Pertussis is reemerging in epidemics in several countries, including the United States, where the number of reported cases in 2012 was the highest since the 1950s. Previously used whole-cell pertussis (wP) vaccines have been replaced in much of the developed world by less reactogenic acellular pertussis (aP) vaccines, but the use of these new vaccines may have contributed to the changing epidemiology of disease and evolution of the organism (2). There is general consensus in the field that novel vaccines or novel immunization strategies are needed to combat the increasing epidemics and to protect newborns from fatal disease. Bordetella parapertussis and Bordetella holmesii are related pathogens that can also cause pertussislike disease in humans, but the incidence of disease caused by these pathogens is lower than that caused by B. pertussis and these organisms are still relatively poorly understood. Bordetella bronchiseptica is another related and more complex pathogen that causes respiratory disease in a number of different animal species, as well as infections in immunocompromised humans. Much is still unclear about the basic biology of these organisms, the pathogenesis of the diseases they cause, and the most effective strategies to treat and prevent these infections and diseases. Many members of the Bordetella community, including experts in scientific, clinical, public health, vaccine manufacturing, and regulatory fields, met in Buenos Aires, Argentina in April 2016 to discuss recent advances in these areas. As well as oral presentations, there were lively poster sessions each day with over a hundred poster presentations on a wide variety of topics. The following sections describe the highlights of the oral presentations in the various sessions of the symposium.
EPIDEMIOLOGY AND SURVEILLANCE
Session 1 focused on epidemiology, surveillance, and vaccine effectiveness. It was organized to give an overview of surveillance methods for pertussis, two examples of pertussis epidemiology, and factors relating to fatal pertussis in young infants. The vaccine section included overviews of methods for estimating vaccine effectiveness and results from maternal immunization in the United Kingdom, and a more rational use of acellular vaccines in the United States was considered. Nicole Guiso (Institut Pasteur, Paris, France) explained that although pertussis vaccines have been widely used for more than 50 years and have led to a significant reduction of morbidity and mortality, surveillance of pertussis is still required worldwide. Surveillance of pertussis can be done by statutory notification, by hospital-based surveillance, by sentinel-based surveillance, or by seroepidemiology. Irrespective of the method of surveillance, a specific biological diagnosis is needed, as other infections can mimic pertussis symptoms. Furthermore, it is also important to monitor the circulating bacterial isolates. Dr. Guiso presented examples of surveillance methods from different countries that may be used as a blueprint when setting up a surveillance program or analyzing an outbreak. Surveillance thus can define the burden of disease, facilitate the adaptation of vaccine strategies according to the type of pertussis vaccine used, and follow the evolution of the bacteria, allowing timely detection of escape mutants. Rudzani Muloiwa (Cape Town, South Africa) showed that 20 to 40 million cases of pertussis occur worldwide, resulting in 300,000 deaths, of which 90% are in low- and middle-income countries. From these countries, however, surveillance data are largely missing, and without these data, it is difficult if not impossible to review and amend pertussis control policies. He showed data from a systematic review of pertussis trends in these countries over a 40-year period since the inception of the Expanded Program on Immunization (EPI) in 1974 (3). He also discussed factors that have an impact on surveillance and burden reduction, as well as the role of diagnostics and choice of vaccine schedule, and he pointed to variables that are specific to the epidemiology in low- and middle-income countries. Maria Lucia Tondella (CDC, Atlanta, GA, USA) reported on the improved understanding of the burden of pertussis in Latin America from strengthening and standardizing laboratory-based surveillance in these countries. To achieve this, an in-country surveillance system with extensive laboratory capacities has been established. She presented the primary achievements of this study but also highlighted challenges to the implementation and sustainability of surveillance in Latin America. James Cherry (UCLA, Los Angeles, CA, USA) showed data about four studies on factors relating to infant deaths from pertussis. High and rapidly rising white blood cell counts, high and rapidly rising pulse and respiratory rates, and pneumonia with early onset were risk factors for infant death. The fatal cases also had lower birth weight, younger gestational age, and younger age at the onset of symptoms. Fatal cases mostly had shock/hypotension, and some had organ failure. Fatal cases were also less likely to have received macrolides but more likely to have received corticosteroids and/or nitric oxide. The role of exchange transfusion to prevent infants dying from pertussis is currently being evaluated.
In the vaccine section of this session, Natasha Crowcroft (Public Health Ontario, Toronto, ON, Canada) discussed the issue of how to measure pertussis vaccine effectiveness. Study designs include case-control studies using a variety of controls, cohort- and household-based approaches, and the screening method. Each method and data source has different associated strengths, degrees of feasibility, weaknesses, and biases. Studies are often conducted in specific contexts, such as during epidemic cycle peaks or outbreaks in specific settings, which may bias estimates. A partnership among clinicians, public health practitioners, mathematical modelers, microbiologists, and immunologists would help in determining methods and definitions and in designing future studies to improve the understanding of how well and how long pertussis vaccines protect in the field. Gayatri Amirthalingam (Public Health England, London, United Kingdom) presented the latest evaluation of the maternal pertussis immunization program in England, which was introduced in response to a national outbreak. This program continues to provide high levels of protection (>90%) for infants whose mothers received pertussis vaccine at least 1 week prior to delivery, and the levels of effectiveness of the two vaccine products (3-component and 5-component acellular vaccines) used in the national program are similarly high. Evaluation of the impact of blunting of the infant's immune response is ongoing. In 2016, updated advice on the timing of maternal vaccination has been published to recommend offering women pertussis vaccine from 16 weeks of gestation onwards. Nicola Klein (Kaiser Permanente Vaccine Study Center, Oakland, CA, USA) used data from recent outbreaks of pertussis in California to advocate for a more effective use of acellular pertussis (aP) vaccines. She showed that the number of pertussis cases was higher in adolescents who received primary immunization with aP vaccines in infancy than in those who were immunized with whole-cell pertussis (wP) vaccines. More than 90% coverage with aP vaccines (tetanus-diphtheria-acellular pertussis [Tdap], which has reduced antigen content, versus diphtheria-tetanus-acellular pertussis [DTaP], used in infants) in adolescents did not prevent outbreaks, and protection waned relatively rapidly. Thus, aP vaccines with reduced antigen content provide only modest benefit for 2 to 3 years after vaccination, and universal adolescent vaccination is unlikely to have a major impact on pertussis outbreaks or the burden of disease. Given these findings, it should be considered whether a new strategy for the use of aP vaccines can be deployed to more effectively contain pertussis.
BIOLOGY, GENOMICS, AND EVOLUTION
The session on biology, genomics, and evolution encompassed functional genomics, evolutionary genomics, and vaccine antigen deficiency of B. pertussis isolates that probably resulted from selection pressure from vaccine-induced immunity (4–14). New transcriptomics data expanded our understanding of gene regulation and its complexity in Bordetella biology. Michael Weigand (CDC, Atlanta, GA, USA) presented the CDC's massive effort of sequencing over 260 isolates to produce fully closed genomes using PacBio and Illumina sequencing and optical mapping. The advantage of fully completed genomes over draft genomes is that genome structural changes can be detected and used for phylogenetic inference. The data showed that the B. pertussis genome is structurally fluid, with 62 unique genome architectures identified among the 260 isolates. Interestingly, certain structural types are predominant and correlate with pulsed-field gel electrophoresis (PFGE) patterns, confirming that PFGE predominantly detects genome structural changes, as expected. Although considerable structural diversity was seen, some structural changes are fixed in certain lineages, which may shed light on adaptation.
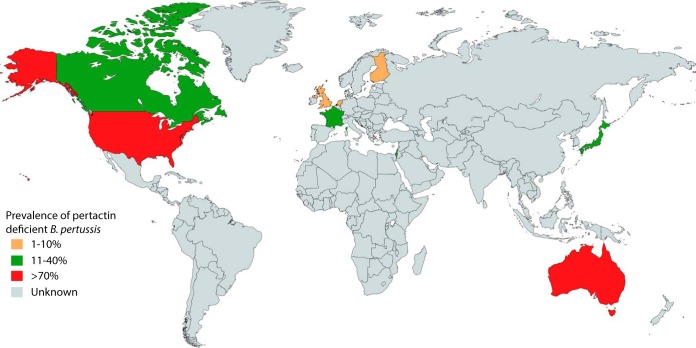
Andrew Preston (University of Bath) presented new work on functional genomic analysis of B. pertussis metabolism, which gave good insight into the biology of infection. B. pertussis uses amino acids, particularly glutamate, as carbon sources. Using transposon-directed insertion sequencing (TraDIS) and transcriptome sequencing (RNA-seq) analysis, his group identified genes essential for growth and genes that are differentially expressed in Bvg+ (virulent) and Bvg− (avirulent) phases. A flux balance analysis-based metabolic model was presented. These approaches identified differences in the levels of activity of the tricarboxylic acid (TCA) cycle between Bvg phases that point to a mechanistic basis for the difference in growth between Bvg+ and Bvg− phase B. pertussis. Marjolein van Gent (RIVM, the Netherlands) presented studies from her group on the molecular characteristics and epidemiology of B. pertussis populations in the Netherlands, particularly the emergence of strains not expressing the vaccine antigens pertactin (Prn) and filamentous hemagglutinin (Fha). They used a multiplex bead-based assay to detect the expression of the vaccine antigens Prn, Fha, and pertussis toxin (Ptx). The Netherlands introduced an acellular vaccine in 2005, and the Prn-deficient isolates seem to be on the rise, from 4% in 2004 to 16% in 2015. Analysis of whole-genome data showed that B. pertussis can use different mechanisms to inactivate Prn expression, and a phylogenetic tree showed that the Prn-deficient isolates were found in different clusters, which suggests positive selection. Her group also identified Fha-negative isolates that result from phase variation due to a homopolymeric G tract in the coding region of fhaB that changes from 10 Gs to either 9 or 11 Gs to turn off expression. Although the frequency of Fha-negative isolates was low, this demonstrated that B. pertussis can potentially escape Fha-targeted immunity. Valerie Bouchez (Institut Pasteur, Paris, France) presented a thorough review on vaccine antigen-deficient B. pertussis and B. parapertussis isolates. B. pertussis isolates deficient for the production of the three key vaccine antigens, Ptx, Prn, and Fha, have been reported in different areas of the world (Fig. 1 shows data for Prn-deficient isolates). Ptx- and Fha-deficient isolates existed well before the introduction of aP vaccines, but their incidence has always been very low. In contrast, the prevalence of Prn-deficient isolates has increased in several areas of the world that use aP vaccines in recent years, reaching very high levels in some states of the United States and in Australia in particular. A review of functional consequences of vaccine antigen deficiency was also presented. If Prn-deficient B. pertussis isolates are not more virulent, as evidenced in humans or using in vitro models (15), they present better fitness than Prn-producing isolates. In addition, Prn-deficient B. parapertussis isolates have been reported: in France, they have replaced Prn-producing isolates since 2007. The talk emphasized the need for very close surveillance of vaccine antigen deficiency all around the world, not only in countries where aP vaccine is used.
FIG 1.
World map showing prevalence of Prn-deficient B. pertussis strains in countries where assessments have been made (colored according to the key). The map was created using mapchart.net (http://mapchart.net/).
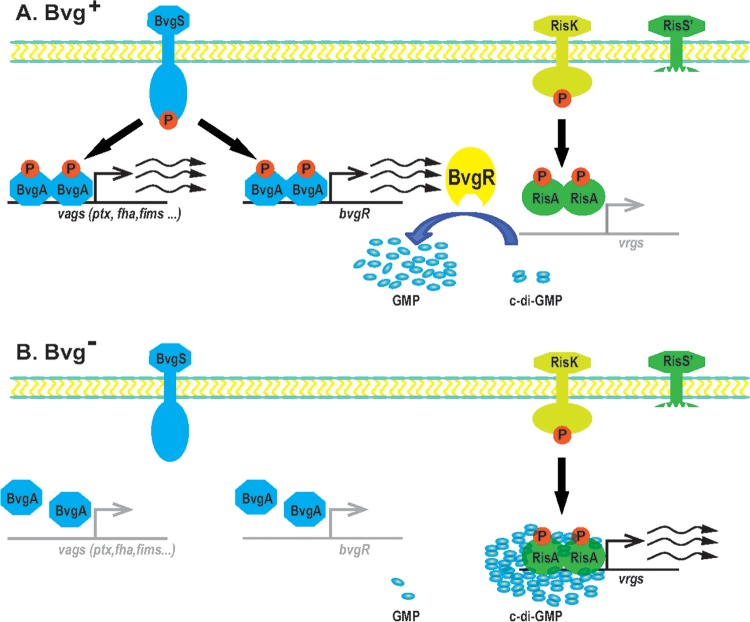
Two short talks were both on gene regulation in B. pertussis, with fascinating insights elucidated through transcriptomic analysis using RNA-seq. Kyung Moon (NIDDK, Bethesda, MD, USA) presented new findings on Bvg-dependent regulation. Using RNA-seq, her group showed that about 500 genes had a twofold or greater difference in gene expression under Bvg regulation, and over 300 were newly identified genes. Thus, more than 10% of the genes in the B. pertussis genome may be regulated by the Bvg system. Newly identified genes included other regulatory genes/systems, such as kdpE and vrgX, and metabolic genes, including those for glycolate oxidase (glcFED) and malate synthase (glcB). Loic Coutte (INSERM, Lille, France) presented new work on a less well known two-component regulator, RisA, which adds a new layer of complexity to the regulation of B. pertussis gene expression (Fig. 2). The RisAS system was first identified in B. bronchiseptica, but in B. pertussis, risS is a pseudogene that encodes a truncated RisS sensor. His group identified RisK (EnvZ) as the cognate kinase of RisA, and most RisA-regulated genes are RisK dependent. The genes regulated by RisA and Bvg overlap, and the modulation of some genes by the Bvg system is dependent on RisA.
FIG 2.
Model showing Bvg regulation of gene expression in B. pertussis and its relationship to the RisK/RisA regulatory system (Copyright Scott Stibitz and Qing Chen). (A) In the Bvg+ phase, BvgA is phosphorylated by BvgS and activates the transcription of virulence factor genes (vags) and also of the bvgR gene. BvgR reduces the level of c-di-GMP in the cell, repressing the activity of the RisA transcriptional activator (which is constitutively phosphorylated by RisK in the presence of truncated RisS). (B) In the Bvg− phase, BvgA is not phosphorylated and so no BvgR is produced. High levels of c-di-GMP activate phosphorylated RisA, which then activates transcription of the vir-repressed genes (vrgs).
VIRULENCE FACTORS AND PATHOGENESIS
Maria Rodriguez (University of La Plata, La Plata, Argentina) described findings on intracellular B. pertussis. The bacteria survive the innate interaction with human macrophages and replicate in this niche after 48 h, persisting in a compartment with characteristics of early endosomes, where they have access to recycling vesicles. Shotgun proteomic analyses of intracellular bacteria revealed increased production of stress proteins and iron uptake systems and large changes in the abundance of proteins involved in metabolism, transcription regulation and virulence. The data suggest that Ptx and adenylate cyclase toxin (Act) contribute to intracellular survival by manipulating the host cell inflammatory and microbicidal responses. Intracellular survival of B. pertussis potentially represents a strategy to increase persistence within hosts.
Several talks unveiled some of the complexity of gene regulation in B. pertussis. Scott Stibitz (FDA, USA) presented data focusing on the regulation of BvgAS-repressed genes, collectively called vrgs (virulence-repressed genes) (Fig. 2). Perplexingly, several studies have identified fim3, encoding FIM3 serotype fimbriae, which are produced only in the Bvg+ phase, as a vrg. They identified a strong Bvg-repressed promoter (BRP) upstream from Pfim3. Although the BRP is highly expressed in the Bvg– phase, the assembly of FIM3 fimbriae is only possible in the Bvg+ phase when fimbrial biogenesis proteins are produced. Thus, while the biological relevance of the BRP is unknown, it serves as a useful reporter for studying the control of vrg expression. Stibitz found that transcription from BRP under modulating conditions requires the response regulator RisA in a phosphorylated form and alternative sigma factors. RisA is phosphorylated by an unlinked sensor kinase, RisK (Fig. 2). Although risK can be mutated, the response regulator encoded adjacent to RisK (RisR) appears to be essential. Adding to the complexity of the regulatory network, risR can be mutated if the bvgS gene contains a mutation that renders BvgS constitutively active. Further insight into the complexity of virulence gene regulation in Bordetella was provided by Steve Julio (Westmont College, Santa Barbara, CA, USA), who identified another two-component regulatory system, PlrSR. He showed that the bacterial response to CO2 in vitro, which includes increased production of Act and Fha and higher adherence to host cells, requires PlrSR. A plrS knockout mutant is defective for colonization of mouse lungs but not of the nose. Using a reporter system of promoter expression in vivo, he showed that ΔplrS mutants (but not wild-type bacteria) switched to the Bvg– phase in the lungs, while all bacteria remained Bvg+ in the nose. By combining the ΔplrS deletion mutation with a constitutive BvgS variant, he showed that lack of expression of BvgAS-dependent virulence factors was not the (only) reason that ΔplrS mutants were cleared from the lower respiratory tract, demonstrating that PlrSR has both Bvg-dependent and Bvg-independent functions. PlrS may sense a drop in oxygen level to trigger an appropriate response, and BvgS may in turn detect a metabolite whose level depends on the activity of the PlrSR regulon. Umesh Ahuja (UCLA, USA) described a regulatory node responsible for the differential regulation of type III secretion among Bordetella species. In B. bronchiseptica, a type III secretion system (T3SS) causes cytotoxicity and contributes to persistence in vivo. B. pertussis strains do not display T3SS-dependent phenotypes, although their T3SS-encoding genes are intact. Transcription of T3SS-encoding genes depends on the extracytoplasmic function (ECF) sigma factor BtrS, whose gene is Bvg regulated. The secreted BtrA protein antagonizes BtrS and positively regulates many genes, including some virulence factors. Deletion of btrA in B. pertussis derepresses T3SS genes, leading to the expression of T3SS phenotypes. Rudy Antoine (Institut Pasteur of Lille, France) presented a structure/function study of BvgS. A combination of in silico analyses, mutagenesis, and cysteine-scanning experiments revealed that a predicted 2-helix coiled-coil between the cytoplasmic PAS domain and the dimerization and histidine phosphoryl transfer (DHp) domain of the kinase mechanically determines BvgS activity. In the kinase mode, the coiled-coil is in a state of great rotational dynamics, and it shifts to a more rigid conformation upon negative modulation. Notably, a similar mode of control appears to occur in sensor kinases homologous to BvgS but devoid of a PAS domain. This study corroborates the importance of protein dynamics for the regulation of BvgS activity, as already revealed in a study on its periplasmic Venus flytrap domains (16).
Sandra Armstrong (University of Minnesota, USA) described nutrient acquisition in the host. Bordetellae can use heme as an iron source, and they also chelate iron using alcaligin and xenosiderophores. The expression of each system is repressed by iron and activated by the cognate source, enabling the bacteria to adapt to changes in their environment. Niacin is also required for Bordetella growth, and genes for its utilization are upregulated under modulating conditions. Intriguingly, B. bronchiseptica can grow on nicotinic acid and sulfate ions, two molecules known to modulate BvgS activity, as sole carbon and sulfur sources. Finally, Nicholas Carbonetti (University of Maryland, Baltimore, MD, USA) described the upregulation of pendrin in the lungs of B. pertussis-infected mice and baboons. An epithelial HCO3−/Cl− exchanger, pendrin is involved in asthma pathology in humans (17). B. pertussis-infected pendrin knockout mice showed reduced inflammatory pathology but higher bacterial loads in the lungs. Treatment with a pendrin inhibitor already in clinical use (acetazolamide) decreases lung inflammatory pathology in infected mice. It also reduces cough in human volunteers (18), suggesting that it may be used as a novel therapy for pertussis disease.
INVERTEBRATE AND VERTEBRATE MODELS
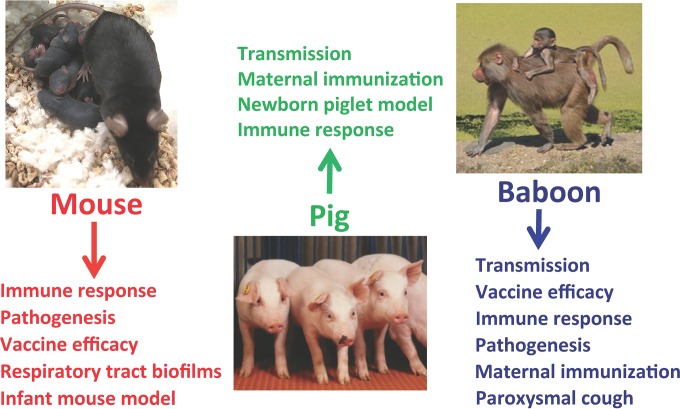
Investigators from the United States and Argentina presented their work on invertebrate and vertebrate models (Fig. 3), covering several areas related to Bordetella infection, pathogenesis, transmission, and disease as examined in amoeba, mouse, pig, and baboon models. Eric Harvill (University of Georgia, Athens, GA, USA) demonstrated that Dictyostelium discoideum could theoretically serve as an environmental reservoir for B. bronchiseptica. D. discoideum, a unicellular amoebal protozoan, feeds on bacteria by utilizing phagosomal and endolysosomal mechanisms similar to those observed in mammalian phagocytes. Harvill presented data showing that, by facilitating a symbiotic relationship between the amoeba and the bacteria, the Bvg− phase allows bacterial growth in the amoeba.
FIG 3.
Animal models of Bordetella infection and disease. Mouse, pig, and baboon models are shown, along with the categories of studies that can be performed in each of these models.
Karen Scanlon (University of Maryland, Baltimore, MD, USA) described a neonatal mouse infection model for B. pertussis. She compared B. pertussis pathogenesis and the role of Ptx in this model and in the well-established adult mouse model. Unlike infection of adult mice, neonatal infection resulted in dose-dependent mortality, which was dependent on the expression of Ptx but, surprisingly, had reduced Ptx-dependent lung inflammatory pathology. Infected neonates displayed disseminated infection and had Ptx-associated reduction in integrin surface expression on lymphocytes, white pulp depletion in the spleen, liver pathology, and disruption of the blood-brain barrier. Together, these data suggest that impaired immune responses in neonates permit bacterial dissemination, systemic Ptx intoxication, and ultimately, death. The neonatal mouse model represents a powerful tool to examine the causes of B. pertussis pathogenesis in human infants and to develop and test future therapeutics.
Rajendar Deora (Wake Forest School of Medicine, Winston Salem, NC, USA) presented his research on the development of mouse models of respiratory tract biofilms of Bordetella. Biofilms are largely defined as multicellular surface-adherent microbial communities which are encased in a self-produced or host-derived matrix. He presented data that showed the development of sessile bacterial communities on the nasal and tracheal epithelium up to 19 days after bacterial inoculation. These respiratory tract biofilms are encased in a matrix composed of the Bps polysaccharide and are disrupted upon ex vivo treatment of respiratory tract tissues with DNase I. He concluded that this mouse model of respiratory tract biofilm corresponds well with experimental observations on abiotic surface biofilms and represents an appropriate animal model for studying bacterial biofilm infections (19).
Continuing the story on Bordetella biofilms, Natalia Cattelan (University of La Plata, La Plata, Argentina) presented her research on biofilm formation by recently isolated strains of B. pertussis from Argentina and the United States. She demonstrated that a large number of these isolates displayed a hyperbiofilm phenotype. Enhanced biofilm formation in many of these strains correlated with enhanced bacterial autoaggregation and Fha production (a protein previously shown to promote biofilm formation in B. pertussis). Cattelan demonstrated further that some of these strains demonstrated hyperadherence to lung epithelial cells and colonized at higher numbers in the mouse nose, trachea, and lungs. Cattelan proposed that enhanced biofilm formation by recent clinical isolates may explain the continued circulation of B. pertussis and the resurgence of pertussis infection and disease.
Tracy Nicholson (USDA, Ames, IA, USA) presented data highlighting the contribution of specific virulence factors to B. bronchiseptica disease and transmission in swine. Her group found that the T3SS was required for maximal persistence and airway disease but not for transmission by either a direct or indirect route, demonstrating that transmission can occur in spite of attenuated disease. Focusing on the role of phenotypic modulation, they were unable to recover a Bvg− phase-locked mutant from any respiratory tract site at any time point examined. A Bvg-intermediate (Bvgi) phase-locked mutant was reduced in respiratory tract colonization and caused limited disease in pigs. In contrast, the colonization, disease, and transmission caused by a Bvg+ phase-locked mutant were indistinguishable from those of the wild-type strain. These results indicate that the Bvg+ phase is sufficient for respiratory infection and transmission of B. bronchiseptica in swine and that swine serve as an exceptional natural model for exploring disease and transmission dynamics of bacterial respiratory pathogens. Tod Merkel (FDA, Silver Spring, MD, USA) presented his group's studies describing the pathogenesis of B. pertussis in the baboon model. The histopathology observed in the trachea and lungs of infant baboons that succumbed to B. pertussis infection closely matched the histopathology observed in human infant fatal pertussis cases (1). They observed a pulmonary infection dominated by necrotizing bronchiolitis, intra-alveolar hemorrhage, and fibrinous edema. All samples had marked leukocytosis and showed luminal aggregates of abundant leukocytes in the small pulmonary blood vessels. They also presented the results of a comprehensive histopathology study that examined disease progression in typical, nonfatal cases of pertussis, data that are not available from human pertussis infections. They observed increasingly severe inflammatory disease in the conducting airways and lungs with massive infiltration of neutrophils early, replaced by macrophages later in infection. The lung pathology included intra-alveolar hemorrhage, along with edema and fibrin deposits, as well as bronchopneumonia typified by acute necrotizing bronchiolitis. Large areas of collagen deposition in the lungs were observed late in infection. This extensive scarring may explain, at least in part, the long-term reduction in lung capacity observed in children following pertussis infection (20).
IMMUNE RESPONSES TO INFECTION AND IMMUNIZATION
Kingston Mills (Trinity College, Dublin, Ireland) discussed investigations into mechanisms of immunity induced by wP and aP vaccines in mouse models. He found that wP but not aP vaccine induces the development of T follicular helper (Tfh) cells and tissue-resident memory T and B cells in the lungs, which contribute to protection against B. pertussis infection. He also found that agonists that stimulate responses through Toll-like receptor 2 (TLR2) (BP1569 lipoprotein) or STING (cyclic di-GMP [c-di-GMP]), added as adjuvants to aP vaccines in both primary and booster vaccinations, promote protective Th1 immune responses equivalent to those stimulated by wP vaccination. However, boosting a previous aP vaccination with this novel adjuvanted aP vaccine was unable to shift the immune response to a Th1 profile. Giorgio Fedele (Istituto Superiore di Sanità, Rome, Italy) talked about T cell phenotype skewing by human monocyte-derived dendritic cells (MDDC) exposed to B. pertussis. He found that despite skewing to a Th1/Th17 profile, interleukin 12 (IL-12) production is low. IL-12p40 is induced through p38 mitogen-activated protein (MAP) kinase, but Act blocks IL-12p35 production by inhibiting the interferon regulatory factor 3 (IRF3)/beta interferon (IFN-β) pathway. He also showed that the live attenuated candidate vaccine strain BPZE1 induces proinflammatory cytokines similarly to B. pertussis but that it induces lower levels of immunopathological prostaglandin E2 (PGE2) through cyclooxygenase 2 (COX-2). This strain also induces suppressor T cells that are FoxP3 negative but express surface ectoenzymes that convert ATP or NAD to adenosine, which suppresses T cell activity.
Cécile van Els (RIVM, Bilthoven, the Netherlands) studied the dynamics of human B and T cell mechanisms against B. pertussis following clinical symptomatic infection in search of biomarkers of natural immunity. In general, specific antibody and B and T cell responses declined within 9 months, and yet, parallel mechanisms may wane differently. She also found that the extent to which specific lymphocyte populations expand after a pertussis episode depends on the age of the cases studied and differs for B and T lymphocytes. The expansion of memory B cells early in the response is more vigorous with increasing age, while the capacity of long-term memory T cells to proliferate to pertussis antigens deteriorates with age. To increase the knowledge of the antigenic breadth of postinfection immune mechanisms, novel antigens other than aP vaccine antigens also need to be investigated. Anne-Marie Buisman (RIVM, Bilthoven, the Netherlands) talked about differences identified in memory immune responses to B. pertussis in school children primed in infancy with aP or wP vaccines. In a series of clinical studies, she followed antibody, memory B cell, and T cell responses to aP vaccine antigens in these children around a set of subsequent aP booster immunizations. The levels of antibody and B cell responses remained higher in aP vaccine-primed children than in their wP vaccine-primed counterparts before and up to 5 years after a preschool aP booster vaccination at 4 years of age. However, after an additional aP vaccination at 9 years of age, aP vaccine-primed children responded more weakly. At the T cell level, she found significantly lower IFN-γ/IL-13 (Th1/Th2) ratios in the PBMC culture supernatants of aP vaccine-primed children than in those of wP vaccine-primed children around the extra aP vaccination at 9 years of age, highlighting the inferiority of aP vaccine priming for the maintenance of subsequent protective responses. Tod Merkel (FDA, USA) described immune responses to infection and vaccination in the baboon model. Infection induces multiple cytokines and chemokines, including IL-17, detected in nasopharyngeal washes, correlating with the airway neutrophil influx seen in histopathology samples. He summarized published data on responses to wP and aP vaccines and showed that aP vaccination does not induce IL-17 recall responses (in contrast to wP vaccine) and appears to delay clearance of the infection. Maternal immunization with aP vaccine protects newborns from pertussis, and anti-Ptx antibody responses are sufficient for this effect. He also described a three-pronged approach to new vaccine antigen discovery, using transposon mutant pool sequencing (Tn-seq) for gene discovery, RNA-seq for gene induction, and immunoproteomics for antibody responses.
In short talks, Joshua Eby (University of Virginia, Charlottesville, VA, USA) described toxin neutralization assays and enzyme-linked immunosorbent assays (ELISAs) for anti-Act antibodies in human and baboon serum. Among serum samples from humans with pertussis, 72% were ELISA positive for anti-Act antibodies and 83% were positive for toxin-neutralizing activity. All sera with anti-Act antibodies detected by ELISA neutralized toxin activity, and no sera from control patients were positive for toxin-neutralizing activity. He proposed that the neutralization assay would be a useful tool for studying the antibody responses to Act following infection or immunization. Thomas Rice (Imperial College, London, United Kingdom) compared maternal and cord blood cellular (cytokine) responses to circulating B. pertussis strains and vaccine antigens in vaccinated versus nonvaccinated mother-infant pairs. Multiple Th1 and Th2 cytokine responses were higher in vaccinated pregnancies in both maternal and cord blood samples, though the responses to Prn-negative and Prn/Fha-negative strains were higher than the responses to the wild-type strain, suggesting a suppressive effect of these antigens. Similar responses were seen to the cocktail of vaccine antigens (Ptx/Fha/Prn). These data provide new insights into the effects of maternal vaccination beyond simple transfer of antibodies to the infant. Ciaran Skerry (University of Maryland, Baltimore, MD, USA) discussed the prophylactic and therapeutic use of sphingosine-1-phosphate (S1P) receptor agonists to reduce pertussis inflammatory pathology in the mouse model. He showed that S1P receptor 1 on myeloid cells is the relevant target for this effect. However, Ptx does not inhibit this activity, even though S1P receptors are G protein-coupled receptors that signal through Ptx-sensitive Gi proteins, and he speculated on an alternative mechanism involving signaling through β-arrestin and downregulation of type I interferon responses. Andrew Gorringe (Public Health England, Porton Down, United Kingdom) studied complement interactions with genome-sequenced B. pertussis isolates from the United Kingdom. He found that varying Vag8 expression correlates with C1-esterase inhibitor deposition, negatively impacting C3b deposition on the bacterial surface. He also found that recent clinical and vaccine strains differ widely in their levels of susceptibility to antibody-independent and antibody-mediated opsonophagocytosis (OP). A weaker correlation between C3b deposition and OP is found for B. pertussis than for Neisseria meningitidis, suggesting a role for other factors. In a special workshop, functional antibody assays such as OP were discussed in more detail.
VACCINE DESIGN
To explain the resurgence of pertussis disease in the last decades, several causes have been proposed, most of them associated with the vaccines currently in use: (i) the decrease of vaccine effectiveness over time (waning immunity) (21, 22), (ii) the selection of mutants able to escape immunity induced by the vaccine (23, 24) and/or (iii) failure of the vaccine to induce sterilizing immunity to the pathogen and to avoid transmission (25). With the massive use of wP vaccines since the 50s, the incidence and mortality associated with pertussis fell sharply (26). However, reports on safety concerns in the 1970s reduced the confidence in wP vaccines (27, 28). Adverse reactions to wP vaccines are partly attributed to the presence of lipopolysaccharides (LPS), the major constituents of the bacterial outer membranes. To improve the traditional wP vaccines, recently a wP vaccine with reduced amounts of LPS (Plow) was developed at the Butantan Institute (Brazil). During the Vaccine Design Session, Paulo Lee Ho from this institute presented results related to the biotechnological process of Plow production. From 2004 until 2006, the Butantan Institute has produced several lots at a pilot scale, and since 2007, various processes to scale up the production have been evaluated. He showed that the Plow vaccine produced by industrial centrifugation had an 80% reduction in endotoxin activity compared to that of the traditional wP vaccine. The main protective immunogens and the integrity of the bacteria were maintained at the end of that process. The goal of Butantan is to produce consistent lots under good manufacturing practice conditions by the centrifugation method in the next few years. Martine Caroff (LPS-BioSciences, Université de Paris Sud, France) described a nontoxic-LPS extraction methodology and mass spectrometry microanalysis strategies for strain characterization. She proposed that these tools could also be useful for strain characterization and prediction of biological activities.
The adverse events associated with traditional wP vaccines, which have led to a decrease in acceptance of these vaccines in several countries (28, 29), prompted the development of aP vaccines that contain purified protein antigens of B. pertussis. Although there is no conclusive evidence that wP vaccines cause infant deaths, brain damage, or severe neurological disorders, the aP vaccines have gradually replaced wP vaccines in most industrialized countries, and currently, most European countries, Japan, Australia, and the United States only use aP vaccines in their vaccination schedules. However, several recent outbreaks were reported in these countries, indicating faster waning of immunity induced by aP vaccines compared to that induced by wP vaccines. Accordingly, children primed with wP vaccines had longer lasting immunity than those primed with aP vaccines (30, 31). Thus, much interest is now being devoted to the development of new, alternative options for antipertussis immunization. Data from animal models and human studies indicate that although antibodies may mediate protection, cellular Th1 and Th17 responses are responsible for long-lasting protection (32). Since current acellular vaccines elicit mainly a Th2 response, several solutions have been proposed to improve the Th1/Th17 responses. One possibility is to combine these vaccines with Th1-driving adjuvants, at least for the priming doses (33). Scott Halperin (IWK Health Center, Halifax, Canada) described the development of such a candidate vaccine, based on a single-immunization platform consisting of three immune stimulators, namely, host defense peptides, polyphosphazenes, and poly(I · C)/CpG oligodeoxynucleotides (ODN). He showed that a single immunization with this vaccine candidate induced humoral immune responses (IgG, IgG1, and IgG2a) in neonatal mice and piglets that were 100- to 1,000-fold stronger than those induced by the currently licensed vaccine. In addition, a much earlier onset of immunity, a clear shift toward a more balanced or Th1 response, and duration of immunity of more than 2 years were found. Furthermore, the adjuvant platform was highly effective in the presence of maternal antibodies and against other pathogens, including respiratory syncytial virus (RSV).
Watanalai Panbangred (Mahidol University, Thailand) described results obtained with new genetically engineered B. pertussis strains for recombinant pertussis vaccine production, and Punnee Pitisuttithum (Mahidol University, Thailand) presented results of the clinical study performed to assess noninferiority of an aP vaccine containing genetically detoxified Ptx versus Adacel vaccine in Thai adolescents. She reported that the new vaccine was tolerated and as safe as the Adacel vaccine but induced higher levels of neutralizing anti-Ptx antibodies than Adacel. A different alternative was the acellular vaccine candidate presented by Daniela Hozbor (La Plata National University, Argentina). Her team has developed a new vaccine based on outer membrane vesicles (OMVs), which have been shown to be safe and to induce protection in mice. Furthermore, it elicits an immune response with a mixed Th1, Th2, and Th17 profile and also induces a robust antibody response. Interestingly, this OMV-based vaccine induced antibodies similar to those induced by commercial wP and aP vaccines, which contribute to protection against B. pertussis infection in mice. This OMV-based vaccine was shown to protect against different B. pertussis strains with different genetic backgrounds, including those not expressing Prn. The presence of a high number of immunogens in the vaccine formulation was presented as an important advantage, since this formulation may avoid the selective pressure conferred by vaccines containing only a single or a few protective antigens.
Finally, an entirely different approach was taken by Camille Locht (Inserm, Institut Pasteur de Lille, France). He presented the recent progress on a live attenuated pertussis vaccine (BPZE1) to be delivered by the nasal route. This vaccine is expected to protect not only against pertussis disease but also against B. pertussis infection and, therefore, against transmission. Work on baboon models has shown that, in contrast to wP and aP vaccines, prior infection protects against both disease and infection (25). Based on this observation, it was felt that the best way to protect against infection is nasal vaccination with a vaccine that mimics infection as much as possible without causing disease. Preclinical data on the live vaccine BPZE1 were presented, showing that the vaccine induced long-lasting protection after a single nasal administration and that it was safe even in severely immunocompromised animals. A first-in-human clinical trial showed that BPZE1 is safe in human adults and is able to transiently colonize the human nasopharynx and to induce immune responses to all tested antigens in all colonized subjects. A dose optimization study is now ongoing, the results of which are expected in approximately 1 year.
SUMMARY
It became clear from the presentations in the Vaccine Design session and in a collective roundtable discussion on the future of pertussis vaccines that after 20 years of relative silence, a new momentum in pertussis vaccine development has emerged. Several complementary approaches are being undertaken in various parts of the world, both in academia and in industry. Significant hurdles still remain, including overcoming the adverse immunological priming by aP vaccines and the uncertainty associated with completely new vaccine strategies, but it is hoped that at least some of these vaccine developments will be successful in reaching the populations at need and help to provide improved tools ultimately to control pertussis disease and transmission. Many aspects of the basic biology, infection, and disease pathogenesis associated with B. pertussis and other bordetellae are not sufficiently well understood yet, but the knowledge that is progressively being acquired and shared at these symposia will without doubt be of great benefit for optimized vaccination and therapeutic strategies against whooping cough.
ACKNOWLEDGMENTS
We thank all presenters who contributed or checked summaries of their talks and Marcela Pasetti for helpful information and suggestions. Thanks also to Scott Stibitz and Qing Chen for providing Fig. 2.
Work in the laboratories of these authors was supported by the following grants: NIH R01AI101055, R21AI119566, and R21AI117095 to N.H.C., NIH R01AI125560 and R21AI126147 and contract no. HHSN272201200005C to R.D., and NIH R01AI094991 to P.A.C.
Funding Statement
The symposium was funded in part by conference grant RC 2015 0031 from the National Agency for Scientific and Technological Promotion (MINCyT) (to Maria Eugenia Rodriguez) and by an R13 conference grant from the National Institute of Allergy and Infectious Diseases, NIH (to Eric Hewlett).
REFERENCES
- 1.Paddock CD, Sanden GN, Cherry JD, Gal AA, Langston C, Tatti KM, Wu KH, Goldsmith CS, Greer PW, Montague JL, Eliason MT, Holman RC, Guarner J, Shieh WJ, Zaki SR. 2008. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis 47:328–338. doi: 10.1086/589753. [DOI] [PubMed] [Google Scholar]
- 2.Mooi FR, Van Der Maas NA, De Melker HE. 2014. Pertussis resurgence: waning immunity and pathogen adaptation—two sides of the same coin. Epidemiol Infect 142:685–694. doi: 10.1017/S0950268813000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muloiwa R, Kagina BM, Engel ME, Hussey GD. 2015. The burden of pertussis in low- and middle-income countries since the inception of the Expanded Programme on Immunization (EPI) in 1974: a systematic review protocol. Syst Rev 4:62. doi: 10.1186/s13643-015-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam C, Octavia S, Ricafort L, Sintchenko V, Gilbert GL, Wood N, McIntyre P, Marshall H, Guiso N, Keil AD, Lawrence A, Robson J, Hogg G, Lan R. 2014. Rapid increase in pertactin-deficient Bordetella pertussis isolates, Australia. Emerg Infect Dis 20:626–633. doi: 10.3201/eid2004.131478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otsuka N, Han HJ, Toyoizumi-Ajisaka H, Nakamura Y, Arakawa Y, Shibayama K, Kamachi K. 2012. Prevalence and genetic characterization of pertactin-deficient Bordetella pertussis in Japan. PLoS One 7:e31985. doi: 10.1371/journal.pone.0031985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawloski LC, Queenan AM, Cassiday PK, Lynch AS, Harrison MJ, Shang W, Williams MM, Bowden KE, Burgos-Rivera B, Qin X, Messonnier N, Tondella ML. 2014. Prevalence and molecular characterization of pertactin-deficient Bordetella pertussis in the United States. Clin Vaccine Immunol 21:119–125. doi: 10.1128/CVI.00717-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeddeman A, van Gent M, Heuvelman C, van der Heide H, Bart M, Advani A, Hallander H, Wirsing von Konig C, Riffelman M, Storsaeter J, Vestrheim D, Dalby T, Krogfelt K, Fry N, Barkoff A, Mertsola J, He Q, Mooi F. 2014. Investigations into the emergence of pertactin-deficient Bordetella pertussis isolates in six European countries, 1996 to 2012. Euro Surveill 19:pii20881 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20881. [DOI] [PubMed] [Google Scholar]
- 8.Bamberger E, Abu Raya B, Cohen L, Golan-Shany O, Davidson S, Geffen Y, Srugo I. 2015. Pertussis resurgence associated with pertactin-deficient and genetically divergent Bordetella pertussis isolates in Israel. Pediatr Infect Dis J 34:898–900. doi: 10.1097/INF.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 9.Hegerle N, Dore G, Guiso N. 2014. Pertactin deficient Bordetella pertussis present a better fitness in mice immunized with an acellular pertussis vaccine. Vaccine 32:6597–6600. doi: 10.1016/j.vaccine.2014.09.068. [DOI] [PubMed] [Google Scholar]
- 10.Martin SW, Pawloski L, Williams M, Weening K, DeBolt C, Qin X, Reynolds L, Kenyon C, Giambrone G, Kudish K, Miller L, Selvage D, Lee A, Skoff TH, Kamiya H, Cassiday PK, Tondella ML, Clark TA. 2015. Pertactin-negative Bordetella pertussis strains: evidence for a possible selective advantage. Clin Infect Dis 60:223–227. doi: 10.1093/cid/ciu788. [DOI] [PubMed] [Google Scholar]
- 11.Queenan AM, Cassiday PK, Evangelista A. 2013. Pertactin-negative variants of Bordetella pertussis in the United States. N Engl J Med 368:583–584. doi: 10.1056/NEJMc1209369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safarchi A, Octavia S, Luu LD, Tay CY, Sintchenko V, Wood N, Marshall H, McIntyre P, Lan R. 2015. Pertactin negative Bordetella pertussis demonstrates higher fitness under vaccine selection pressure in a mixed infection model. Vaccine 33:6277–6281. doi: 10.1016/j.vaccine.2015.09.064. [DOI] [PubMed] [Google Scholar]
- 13.Safarchi A, Octavia S, Wu SZ, Kaur S, Sintchenko V, Gilbert GL, Wood N, McIntyre P, Marshall H, Keil AD, Lan R. 2016. Genomic dissection of Australian Bordetella pertussis isolates from the 2008-2012 epidemic. J Infect 72:468–477. doi: 10.1016/j.jinf.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Tsang RS, Shuel M, Jamieson FB, Drews S, Hoang L, Horsman G, Lefebvre B, Desai S, St-Laurent M. 2014. Pertactin-negative Bordetella pertussis strains in Canada: characterization of a dozen isolates based on a survey of 224 samples collected in different parts of the country over the last 20 years. Int J Infect Dis 28:65–69. doi: 10.1016/j.ijid.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Bouchez V, Brun D, Cantinelli T, Dore G, Njamkepo E, Guiso N. 2009. First report and detailed characterization of B. pertussis isolates not expressing pertussis toxin or pertactin. Vaccine 27:6034–6041. doi: 10.1016/j.vaccine.2009.07.074. [DOI] [PubMed] [Google Scholar]
- 16.Dupre E, Herrou J, Lensink MF, Wintjens R, Vagin A, Lebedev A, Crosson S, Villeret V, Locht C, Antoine R, Jacob-Dubuisson F. 2015. Virulence regulation with Venus flytrap domains: structure and function of the periplasmic moiety of the sensor-kinase BvgS. PLoS Pathog 11:e1004700. doi: 10.1371/journal.ppat.1004700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yick CY, Zwinderman AH, Kunst PW, Grunberg K, Mauad T, Dijkhuis A, Bel EH, Baas F, Lutter R, Sterk PJ. 2013. Transcriptome sequencing (RNA-Seq) of human endobronchial biopsies: asthma versus controls. Eur Respir J 42:662–670. doi: 10.1183/09031936.00115412. [DOI] [PubMed] [Google Scholar]
- 18.Foresi A, Cavigioli G, Pelucchi A, Mastropasqua B, Marazzini L. 1996. Effect of acetazolamide on cough induced by low-chloride-ion solutions in normal subjects: comparison with furosemide. J Allergy Clin Immunol 97:1093–1099. doi: 10.1016/S0091-6749(96)70263-4. [DOI] [PubMed] [Google Scholar]
- 19.Cattelan N, Dubey P, Arnal L, Yantorno OM, Deora R. 2016. Bordetella biofilms: a lifestyle leading to persistent infections. Pathog Dis 74:ftv108. doi: 10.1093/femspd/ftv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston ID, Strachan DP, Anderson HR. 1998. Effect of pneumonia and whooping cough in childhood on adult lung function. N Engl J Med 338:581–587. doi: 10.1056/NEJM199802263380904. [DOI] [PubMed] [Google Scholar]
- 21.Wendelboe AM, Van Rie A, Salmaso S, Englund JA. 2005. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J 24:S58–S61. doi: 10.1097/01.inf.0000160914.59160.41. [DOI] [PubMed] [Google Scholar]
- 22.McGirr A, Fisman DN. 2015. Duration of pertussis immunity after DTaP immunization: a meta-analysis. Pediatrics 135:331–343. doi: 10.1542/peds.2014-1729. [DOI] [PubMed] [Google Scholar]
- 23.Mooi FR, van Loo IH, King AJ. 2001. Adaptation of Bordetella pertussis to vaccination: a cause for its reemergence? Emerg Infect Dis 7:526–528. doi: 10.3201/eid0707.017708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bart MJ, Harris SR, Advani A, Arakawa Y, Bottero D, Bouchez V, Cassiday PK, Chiang CS, Dalby T, Fry NK, Gaillard ME, van Gent M, Guiso N, Hallander HO, Harvill ET, He Q, van der Heide HG, Heuvelman K, Hozbor DF, Kamachi K, Karataev GI, Lan R, Lutynska A, Maharjan RP, Mertsola J, Miyamura T, Octavia S, Preston A, Quail MA, Sintchenko V, Stefanelli P, Tondella ML, Tsang RS, Xu Y, Yao SM, Zhang S, Parkhill J, Mooi FR. 2014. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. mBio 5:e01074. doi: 10.1128/mBio.01074-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warfel JM, Zimmerman LI, Merkel TJ. 2014. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A 111:787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amirthalingam G. 2013. Strategies to control pertussis in infants. Arch Dis Child 98:552–555. doi: 10.1136/archdischild-2012-302968. [DOI] [PubMed] [Google Scholar]
- 27.Cody CL, Baraff LJ, Cherry JD, Marcy SM, Manclark CR. 1981. Nature and rates of adverse reactions associated with DTP and DT immunizations in infants and children. Pediatrics 68:650–660. [PubMed] [Google Scholar]
- 28.Jefferson T, Rudin M, DiPietrantonj C. 2003. Systematic review of the effects of pertussis vaccines in children. Vaccine 21:2003–2014. doi: 10.1016/S0264-410X(02)00770-3. [DOI] [PubMed] [Google Scholar]
- 29.Klein NP. 2014. Licensed pertussis vaccines in the United States. History and current state. Hum Vaccin Immunother 10:2684–2690. doi: 10.4161/hv.29576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witt MA, Katz PH, Witt DJ. 2012. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin Infect Dis 54:1730–1735. doi: 10.1093/cid/cis287. [DOI] [PubMed] [Google Scholar]
- 31.Klein NP, Bartlett J, Fireman B, Rowhani-Rahbar A, Baxter R. 2013. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics 131:e1716–e1722. doi: 10.1542/peds.2012-3836. [DOI] [PubMed] [Google Scholar]
- 32.Brummelman J, Wilk MM, Han WG, van Els CA, Mills KH. 2015. Roads to the development of improved pertussis vaccines paved by immunology. Pathog Dis 73:ftv067. doi: 10.1093/femspd/ftv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen AC, Mills KH. 2014. Improved pertussis vaccines based on adjuvants that induce cell-mediated immunity. Expert Rev Vaccines 13:1253–1264. doi: 10.1586/14760584.2014.936391. [DOI] [PubMed] [Google Scholar]