Abstract
The African swine fever virus (ASFV) causes a fatal hemorrhagic disease in domestic swine, and at present no treatment or vaccine is available. Natural and gene-deleted, live attenuated strains protect against closely related virulent strains; however, they are yet to be deployed and evaluated in the field to rule out chronic persistence and a potential for reversion to virulence. Previous studies suggest that antibodies play a role in protection, but induction of cytotoxic T lymphocytes (CTLs) could be the key to complete protection. Hence, generation of an efficacious subunit vaccine depends on identification of CTL targets along with a suitable delivery method that will elicit effector CTLs capable of eliminating ASFV-infected host cells and confer long-term protection. To this end, we evaluated the safety and immunogenicity of an adenovirus-vectored ASFV (Ad-ASFV) multiantigen cocktail formulated in two different adjuvants and at two immunizing doses in swine. Immunization with the cocktail rapidly induced unprecedented ASFV antigen-specific antibody and cellular immune responses against all of the antigens. The robust antibody responses underwent rapid isotype switching within 1 week postpriming, steadily increased over a 2-month period, and underwent rapid recall upon boost. Importantly, the primed antibodies strongly recognized the parental ASFV (Georgia 2007/1) by indirect fluorescence antibody (IFA) assay and Western blotting. Significant antigen-specific gamma interferon-positive (IFN-γ+) responses were detected postpriming and postboosting. Furthermore, this study is the first to demonstrate induction of ASFV antigen-specific CTL responses in commercial swine using Ad-ASFV multiantigens. The relevance of the induced immune responses in regard to protection needs to be evaluated in a challenge study.
INTRODUCTION
African swine fever (ASF) is a highly contagious and fatal hemorrhagic swine disease. It has case morbidity and mortality rates that approach 100% (1). Swine that recover become carriers and shed the virus for up to 70 days (2). There is no treatment or vaccine available, and the only control strategy in case of an outbreak is quarantine and removal of infected and in-contact animals. The ASF causes economic losses worldwide and severely affects the pork industry in sub-Saharan Africa where it is endemic (3).
The pathogen, African swine fever virus (ASFV), is a double-stranded DNA enveloped icosahedral arbovirus belonging to the genus Asfivirus and the only member in the family Asfarviridae (4). ASFV has a 170- to 190-kb nonsegmented genome containing 150 to 167 open reading frames (ORFs) (5, 6). The ASFV has a natural sylvatic transmission cycle between Ornithodoros tick species and wild suids such as warthogs (3). Infections in wild suids are asymptomatic and persistent, leading to a carrier state and transmission to domestic pigs, which hinders eradication.
Although an effective ASFV vaccine has not yet been developed, the fact that swine exposed to less virulent isolates (naturally or experimentally attenuated) are protected when challenged with homologous or closely related virulent isolates suggests that vaccine development is possible (7–9). Published data suggest that antibodies and T cells play critical roles in virus control (9–16). ASFV-infected convalescent swine serum can neutralize the infectivity of homologous and some heterologous strains in vitro and in vivo, possibly by inhibiting virus attachment and internalization (16–19). Generally, anti-ASFV antibodies are detectable from about 6 days postinfection, and if the animal survives, antibodies may persist for long periods. However, despite the presence of antibodies, virus neutralization may not occur. Thus, the specific role of antibodies in ASFV protection is not yet fully understood (20). The importance of cytotoxic T lymphocytes (CTLs) in protection against ASFV has been demonstrated in a number of studies. Importantly, in vivo depletion of CD8+ T cells decreases protection against ASFV in swine, and in vitro studies indicate that there is preferential proliferation of CD8+ T cells in the presence of live virus, whereas both CD4+ and CD8+ T cells are stimulated by UV-inactivated virus (14, 21). In addition, ASFV-specific CTL activity is detected in swine infected with nonlethal ASFV isolates (9, 10, 12, 13). The requirement for CTLs in protection is further supported by the observation that adjuvant-formulated killed ASFV and recombinant vaccine candidate antigens that induce high antibody responses do not confer solid protection, and these outcomes strongly suggest that major histocompatibility complex (MHC) class I presentation of ASFV antigens is critical (15, 22–25). In addition, it has been observed that swine that generate high antibody titers but low cellular responses following immunization with a live attenuated virus develop chronic disease (26).
Although a vaccine using live attenuated ASFV can protect swine against the disease, it is not an ideal vaccine due to the potential risk of vaccine virus persistence and reversion to virulence. Additionally, a live, naturally attenuated ASFV vaccine used in Portugal in the 1960s resulted in severe immune-mediated postvaccination reactions in immunized animals, precluding any further use during outbreaks (27). Subunit vaccines based on some of the most extensively studied ASFV antigens, such as p32, p54, and p72 envelope protein, have shown some promise. These antigens, among others, have been tested as vaccine candidates either as baculovirus-expressed recombinant proteins or via DNA plasmid delivery (15, 22–25). Delayed onset of viremia, delayed mortality, and partial protection have been observed in most of these studies, which suggest that these antigens do play a role in protection but are not capable of conferring complete protection when they are used singly or in combination. Thus, it is envisaged that development of an efficacious vaccine requires empirical identification of multiple ASFV antigens formulated in a suitable delivery system that can successfully induce robust immunity.
Given that one or a combination of a few subunit antigens has not been able to confer complete protection so far, we set out to test the ability of a live-vectored ASFV multiantigen cocktail to elicit strong CTL, gamma interferon (IFN-γ)-secreting T cell, and B cell responses. We selected a replication-deficient human adenovirus (Ad5) vector as the antigen delivery platform for several reasons, such as safety, high transgene expression, and scalability (28–32). Additionally, adenovirus-vectored vaccines have been shown to induce stronger CTL responses than vaccinia virus, plasmid DNA, or a combination of these two (33). To test our approach, we used p32, p54, and p72 antigens since they are well characterized. Furthermore, antigens p32 and p72 have been previously identified as CTL targets (10, 12). We also included polyprotein pp62, which is a major component of the core shell, is essential for viral core development, and is very strongly recognized by ASFV-specific convalescent-phase serum (34). We tested this multiantigen (four-way) cocktail in a prime-boost regimen using two different adjuvant formulations and at two different immunizing doses.
MATERIALS AND METHODS
Generation of plasmid constructs encoding ASFV antigens.
The ASFV p32, p54, pp62 polyprotein (pp62), and p72 amino acid sequences based on the epidemiologically relevant Georgia 2007/1 isolate (GenBank accession no. FR682468) were modified to add, in frame, a FLAG and hemagglutinin (HA) tag at the N and C termini, respectively. This allowed the use of one primer pair to move the expression cassettes of all antigens across multiple expression vectors, in addition to the use of the tags for tracking protein expression and affinity purification of recombinant proteins. The resultant amino acid sequences of the ASFV antigens were used to design synthetic genes codon optimized for protein expression in the swine host. Codon optimization, gene synthesis, cloning into pUC57, and gene sequence validation were outsourced (GenScript). Each gene was then subcloned into pcDNA3.3-TOPO TA, pAd/CMV/V5-DESTGateway, and pFastBac HBM TOPO vectors (Invitrogen) to generate DNA plasmid constructs for protein expression in mammalian cells, generation of recombinant adenoviruses, and generation of recombinant baculoviruses, respectively, using the manufacturer's protocols. The constructs generated were validated by DNA sequencing.
Generation of virus constructs encoding ASFV antigens.
The pAd constructs were used to generate recombinant replication-incompetent adenoviruses using a ViraPower Adenoviral Expression System (Invitrogen). Following validation of protein expression by immunocytometric analysis, the recombinant adenoviruses were scaled up to generate virus for immunizations. Virus titers (infectious focus units [IFU]) were determined using a QuickTiter Adenovirus Titer Immunoassay kit (VPK-109; Cell Biolabs) with a minor modification. We used purified rabbit anti-adenovirus polyclonal IgGs (1:500 dilution) (made in-house) as the primary antibody, followed by an alkaline phosphatase-conjugated anti-rabbit IgG (1:1,000) (catalog number 711-055-152; Jackson ImmunoResearch) as the secondary antibody and Fast Red TR/Naphthol AS-MX as the substrate (F4523; Sigma). A recombinant adenovirus expressing luciferase (Ad-Luc) was similarly scaled up and titrated to serve as the negative-control immunogen. To generate recombinant baculoviruses, pFastBac constructs were used to generate bacmids which were subsequently transfected into Sf-9 cells. One clone of each baculovirus was scaled up, the titer was determined, and then the clone was used to infect High-Five cells (Invitrogen) to generate FLAG-tagged recombinant proteins, which were affinity purified with anti-FLAG M2 affinity gel (A2220; Sigma). Recombinant pp62 was generated using an HEK 293 Freestyle Expression system (Invitrogen).
Evaluation of protein expression.
Protein expression by the plasmid constructs and by the recombinant viruses encoding the ASFV antigens was validated by immunocytometric analysis as previously described (35). Briefly, HEK 293A cell monolayers transfected with the plasmid constructs or infected with the recombinant adenoviruses were probed with mouse anti-FLAG M2-alkaline phosphatase conjugate (Sigma, St. Louis, MO) diluted 1:1,000 in blocking buffer (phosphate-buffered saline [PBS] with 5% fetal bovine serum). Duplicate transfected or infected HEK 293A cell monolayers were first incubated with a 1:500 dilution of gamma-irradiated convalescent swine serum. (Several ASFV isolates were used to produce the convalescent-phase serum from a donor pig that was sequentially infected with a series of tissue culture-adapted and wild-type viruses from p72 genotypes I [DR11, Haiti 81, Lisbon 60, Malawi 83, and UG-61], VIII, and X. The serum was a kind gift from E. J. Kramer, Plum Island Animal Disease Center.) The cell monolayers were then probed with a 1:1,000 dilution of alkaline phosphatase-conjugated goat anti-porcine IgG (6050-04; Southern Biotech). The alkaline phosphatase activity was then detected using Fast Red TR/Naphthol AS-MX substrate (F4523; Sigma). Mock-transfected/infected cells served as negative controls. Protein expression by the generated recombinant baculoviruses was similarly evaluated by probing infected Sf-9 cells.
Immunization of swine.
Twenty weaned piglets (∼30 lb) were acquired, and during the quarantine period commercial vaccines were used against defined pathogens to meet institutional requirements. Three groups of piglets (n = 5) were immunized with a cocktail of the recombinant adenoviruses expressing ASFV antigens formulated in defined adjuvants (ENABL from BenchMark Biolabs and an experimental adjuvant from Zoetis) (Table 1). Sham-infected control piglets (n = 5) were inoculated with an equivalent amount of the Ad-Luc virus (Table 1). The inoculum was administered intramuscularly in the neck region behind the ears. Fourteen weeks postpriming, the animals were boosted with the cognate priming dose and adjuvant. The pigs were terminated at a rate of one group a week starting at 8 weeks postboost.
TABLE 1.
Immunization protocol
| Group (n = 5/group) | Immunogen | Dose/pig (IFU) | Adjuvant |
|---|---|---|---|
| T1 | Ad5-ASFV 4-way cocktail | 4 × 1010a | ENABLd |
| T2 | Ad5-ASFV 4-way cocktail | 4 × 1011b | ENABL |
| T3 | Ad5-ASFV 4-way cocktail | 4 × 1011b | Zoetise |
| T4 | Ad5-Lucc | 4 × 1011 | ENABL |
Pool of four Ad5-ASFV constructs, each at 1 × 1010 IFU (infectious focus units).
Pool of four Ad5-ASFV constructs, each at 1 × 1011 IFU.
Sham inoculation control.
ENABL adjuvant (Benchmark Biolabs catalog number 7010106-C6).
Experimental adjuvant (proprietary formulation).
ELISA.
A direct enzyme-linked immunosorbent assay (ELISA) was used to evaluate antigen-specific antibody responses as previously described (35). Briefly, microplates coated with 100 μl of 1 μg/ml of affinity-purified antigen in bicarbonate coating buffer were first incubated with 100 μl of serum (diluted at 1:100) in triplicates, followed by incubation with 100 μl of peroxidase-conjugated anti-swine IgG (114-035-003; Jackson ImmunoResearch) (1:5,000 dilution). The plates were developed using Sure Blue Reserve TMB (3,3′,5,5′-tetramethylbenzidine) substrate (53-00-02; KPL), and the reaction was stopped using 1 N hydrochloric acid. The optical density (OD) at 450 nm was then determined using a microplate reader. To determine antigen-specific IgM responses in serum from blood collected at week 1 postpriming, horseradish peroxidase (HRP)-conjugated anti-swine IgM (1:10,000) (A100-100P; Bethyl Laboratories) was used as the secondary antibody. Antigen-specific IgG endpoint titers were determined for serum from blood collected week 1 postboost by making a range of 2-fold serum dilutions starting at 1:1.6 × 104 to 1:1.6 × 107. Similarly diluted preimmunization sera served as cognate controls. The titer was then determined to be the dilution of the postboost serum for which the mean of the OD was higher than the mean plus 3 times the standard deviation of the cognate preimmunization serum. The significance of the difference in antigen-specific IgG titers among the groups was determined by analysis of variance (ANOVA), followed by Tukey's multiple-comparison test, and a P value of ≤0.05 was considered significant.
IFA assay.
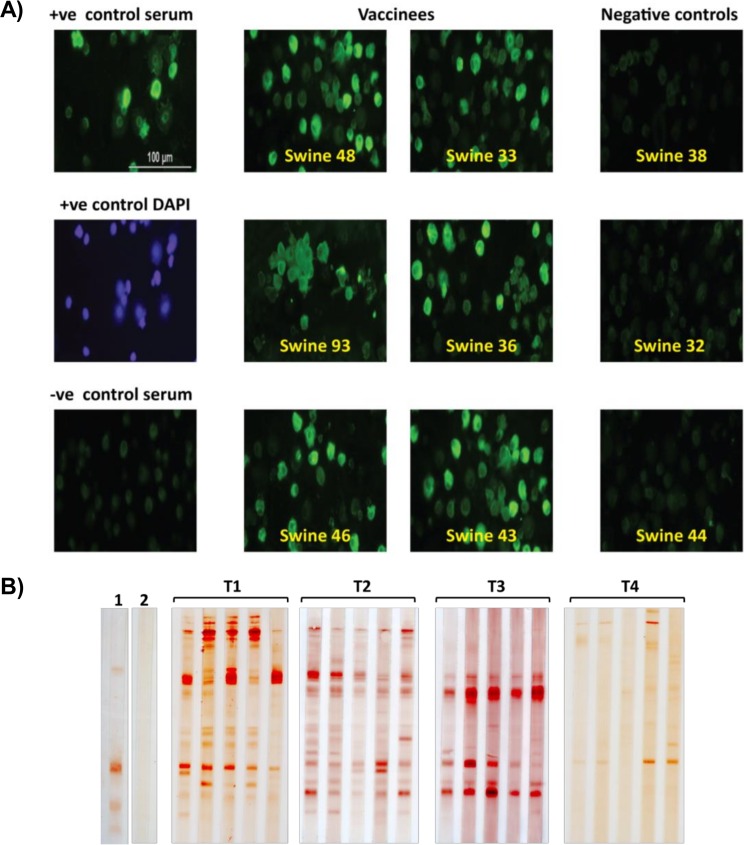
Teflon-coated slide wells (63425-05; Electron Microscopy Sciences) were pretreated by incubation with 300 μg/ml of rat tail collagen (354249; Corning) in Dulbecco's phosphate-buffered saline (DPBS) (14190-144; Invitrogen) for 1 h at 37°C, oven drying for 30 min, and incubation overnight in a biosafety cabinet (15 cm from UV light). Primary monocytes/macrophages were isolated from whole swine blood as previously described (36) and infected with ASFV (Georgia 2007/1) at a multiplicity of infection (MOI) of 1 for 1 h at 37°C. Approximately 4 × 105 infected cells and also mock-infected cells were then added to the wells of the pretreated Teflon slides (25 μl/well). The slides were incubated overnight at 37°C at 5% CO2, fixed with a chilled (−20°C) solution containing acetone and methanol (1:1) for 10 min, and stored at −70°C until required. To carry out the indirect fluorescence antibody (IFA) assay, the slides were incubated with blocking solution (5% nonfat dry milk, 2% horse serum, 2% calf serum, 2% fetal calf serum, and 5% bovine serum albumin [BSA] in DPBS) for 30 min in a humidified chamber at 37°C. After a blocking step, the infected and mock-infected wells were incubated with 1:20, 1:100, and 1:200 dilutions of serum (week 1 postboost) in blocking buffer for 1 h at 37°C. ASFV-specific convalescent-phase serum (1:500) was used as a positive control, and normal swine serum (Gibco) was used as a negative control. Following three rinses with DPBS, the wells were then incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-swine serum (02-14-02; Kirkegaard and Perry) for 45 min at 37°C. The wells were rinsed similarly again and mounted with Prolong Gold antifade reagent with 4′,6′-diamidino-2-phenylindole ([DAPI] PT389868; Invitrogen). The results observed at a 1:20 serum dilution are represented in Fig. 5A, and the results of 1:200 serum dilutions are summarized in Table 2. The IFA assays were conducted at Plum Island Animal Disease Center.
FIG 5.
The Ad-ASFV multiantigen cocktail induced authentic ASFV-specific antibody responses. Indirect immunofluorescence antibody (IFA) assay and Western blotting using serum from week 1 postboost were used to confirm whether antibodies induced by the experimental Ad-ASFV multiantigen cocktail recognized ASFV-infected cells and ASFV-derived antigens. (A) Primary swine macrophages infected with the ASFV Georgia 2007/1 isolate and probed with individual, representative sera from six vaccinated and three control animals. ASFV-specific convalescent-phase serum was used as the positive (+ve) control, whereas normal pig serum was used as the negative control. The overall results are summarized in Table 2. (B) Western blots of lysates from Vero cells infected with the ASFV Georgia 2007/1 isolates, probed with sera from all animals. Lane 1, ASFV-specific convalescent-phase serum; lane 2, normal swine serum. The serum was also tested on mock-infected Vero cell lysates to check for background reactivity against host cell antigens (see Fig. S4 in the supplemental material).
TABLE 2.
Summary of IFA assay data
| Group and swine no. or control | Reactivity in ASFV-infected macrophagesa |
|---|---|
| T1 | |
| 33 | ++ |
| 35 | +++ |
| 36 | +++ |
| 40 | ++ |
| 42 | ++ |
| T2 | |
| 34 | +++ |
| 41 | ++ |
| 43 | ++++ |
| 46 | +++ |
| 48 | +++ |
| T3 | |
| 31 | +++ |
| 37 | ++ |
| 93 | ++++ |
| 94 | +++ |
| 96 | +++ |
| T4 | |
| 32 | − |
| 38 | − |
| 39 | − |
| 44 | − |
| 45 | − |
| Anti-ASFV convalescent-phase serumb | ++++ |
| Normal serumc | − |
The reactivity of the serum from each animal was compared to the reactivity of the positive-control serum (ASFV-specific convalescent-phase serum). The number of plus signs represents the intensity of the reaction, as follows: ++++, as strong as the positive-control serum; ++, weak but positive signal. The minus sign (−) indicates the absence of signal. The best response is indicated in boldface. Mock-infected cells showed no reactivity.
Positive control.
Negative control.
Western blotting.
Swine serum from week 1 postboost were blotted against cell lysates prepared from Georgia 2007/1 ASFV (Vero cell adapted)-infected Vero cells (ATCC CCL-81). Briefly, ASFV-infected Vero cells exhibiting cytopathic effect (CPE) at 72 h postinfection were harvested by centrifugation, lysed in mammalian protein extraction reagent (M-PER) (78501; Thermo Scientific), mixed 1:1 with 2× NuPAGE lithium dodecyl sulfate (LDS) sample buffer, boiled, electrophoresed on a NuPAGE 4 to 12% bis-Tris gel for 35 min, and transferred to 0.2-μm-pore-size polyvinylidene difluoride (PVDF) membranes (LC2002; Invitrogen). Following blocking in phosphate-buffered saline with Tween 20 (PBST) containing 5% nonfat dry milk, membranes were transferred to a Protean II Slot-Blotter and incubated with serum diluted 1:50 in blocking buffer in individual wells. The membranes were then removed from the blotting apparatus and incubated with goat anti-swine HRP (14-14-06; KPL) diluted 1:2,000 in blocking buffer for 1 h. The blots were developed by exposure to diaminobenzidine (DAB) substrate (D4293; Sigma). ASFV-specific convalescent-phase serum (1:10,000) was used as a positive control, and normal swine serum was used as a negative control (1:200). A similar blotting experiment was carried out using mock-infected cell lysates to gauge background reactivity to host cell antigens. The Western blotting was performed at Plum Island Animal Disease Center.
IFN-γ ELISPOT assays.
The frequencies of antigen-specific IFN-γ-secreting T cells were determined by an enzyme-linked immunospot (ELISPOT) assay performed biweekly postpriming and weekly postboost. The assay was conducted in triplicate wells of MultiScreen-HA plates (Millipore) using a Mabtech kit (catalog number 3130-2A), as per the manufacturer's instructions and as described previously (35). Briefly, 0.25 × 106 whole-blood-derived peripheral blood mononuclear cells (PBMCs) or splenocytes were incubated with affinity-purified antigens (2.5 μg/ml) in 100 μl per well of complete RPMI 1640 medium. Phytohemagglutinin (PHA) mitogen (5 μg/ml) was used as a positive control, and medium alone served as a negative control. The spots were quantified with an ELISPOT reader and AID software (version 3.4; AutoImmun Diagnostica, Strasburg, Germany). The results were presented as the mean number of antigen-specific IFN-γ spot-forming cells per 106 PBMCs after background medium counts were subtracted. The significance of the differences in IFN-γ-positive (IFN-γ+) PBMC responses between each vaccinated group (T1, T2, and T3) and control group (T4) was analyzed by ANOVA, followed by Bonferroni's multiple-comparison test, and a P value of ≤0.05 was considered significant.
CTL assays.
A standard chromium (51Cr) release assay was used to measure antigen-specific T cell cytotoxicity as previously described (37).
(i) Generation of effector cells.
PBMCs isolated from blood collected 4 weeks postboost were resuspended in RPMI 1640 medium (Lonza) containing 45% Click's medium (9195; Irvine Scientific), 10% fetal bovine serum (FBS), 1× β-mercaptoethanol, 1× GlutaMAX, 50 μg/ml gentamicin, and 1× penicillin-streptomycin (Pen/Strep; GIBCO) at a cell density of 4 × 106 cells/ml and distributed in aliquots of 1 ml/well of a 24-well culture plate. The PBMCs were infected with each of the recombinant adenoviruses at an MOI of 1,000 for in vitro stimulation of the T cells. After 10 days, the cells were harvested and centrifuged on a Ficoll gradient to remove dead cells. The live cells were then washed with PBS, resuspended in complete RPMI 1640 medium, and counted to serve as effectors for the CTL assay.
(ii) Generation of target cells.
Prior to immunization, skin biopsy specimens were taken from each piglet using 4-mm biopsy specimen punches (3785707; American Screening Corp.). Primary skin fibroblast cultures were established from these skin tissues as previously described (38). Briefly, the skin tissues were cut into small pieces under sterile conditions and cultured in 12-well plates containing 1 ml of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 1× GlutaMAX, 50 μg/ml gentamicin, and 1× Pen/Strep (Gibco). When the fibroblasts reached confluence, they were passaged and frozen regularly until sufficient stocks were generated. Target cells were generated 24 h prior to the CTL assay by transfecting autologous fibroblasts with the pcDNA plasmid constructs using Gene-In transfection reagent (GST-1000; MTI-Global Stem) as per the manufacturer's instructions. Transfection efficiencies of about 20 to 30% were achieved (predetermined by immunocytometric analysis). On the day the assay was conducted, the cells were detached using Accutase, washed with DMEM containing 10% FBS, labeled with 100 μCi per 106 cells of Na251CrO4 (PerkinElmer) for 1 h at 37°C in 5% CO2, washed three times, and resuspended in complete RPMI 1640 medium for use as targets in the assay.
(iii) Chromium release assay.
The effectors and targets were added at effector/target (E:T) ratios of 50:1 and 25:1 in duplicate wells of 96-well round-bottom microtiter plates in final volumes of 100 μl per well and incubated at 37°C in 5% CO2 for 6 h. The plate was then centrifuged at 1,000 rpm for 4 min, and supernatants were harvested to measure chromium release in a MicroBeta counter (1450 Liquid Scintillation Counter and Luminescence Counter; PerkinElmer). Spontaneous release of the label was measured from supernatants of targets incubated without effectors, and maximum release was measured from targets lysed with 5% Triton-X. Percent specific lysis was calculated as described previously (37). Fibroblasts transfected with a construct expressing a chimera of VP1 and 3D polymerase antigens of the foot and mouth disease virus (FMDV) served as a negative control to assess background lytic activity.
Statistical analysis.
All analyses were performed with GraphPad Prism, version 6.05, and the significance level used was a P value of <0.05. The antigen-specific IgG titers among the treatment groups were compared using one-way ANOVA, followed by Tukey's multiple-comparison test. For all the IFN-γ ELISPOT assays, the mean IFN-γ response of treatment groups (T1 to T3) was compared to the mean response of the sham-treated control group (T4) using one-way ANOVA, followed by Bonferroni's multiple-comparison test.
Ethics statement.
All animal procedures were conducted in accordance with Animal Use Protocol 2012-59, reviewed and approved by the Texas A&M University Institutional Animal Care and Use Committee (IACUC) (permit 2009067), which adheres to the regulations, policies, and guidelines outlined in the Animal Welfare Act (AWA), USDA Animal Care Resource Guide, and the PHS Policy on Humane Care and Use of Laboratory Animals. Pigs were monitored twice daily for any clinical signs and to document any localized and or systemic adverse effects. The animals were euthanized with an overdose of sodium pentobarbital. A lack of heartbeat was then confirmed by a stethoscope.
RESULTS AND DISCUSSION
Protein expression by constructs encoding ASFV antigens.
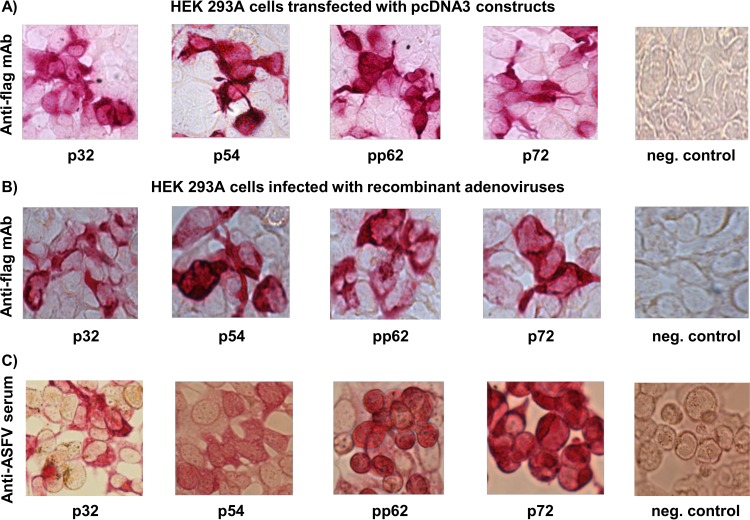
Codon-optimized synthetic genes encoding p32, p54, pp62, and p72 ASFV antigens fused in frame to FLAG tag were used to generate pcDNA3 constructs, recombinant adenoviruses, and recombinant baculoviruses. Immunocytometric analysis of HEK 293A cells transfected with the pcDNA3 constructs and probed with an anti-FLAG monoclonal antibody (MAb) confirmed expression of each antigen (Fig. 1A). Similarly, HEK 293A cells infected with the recombinant adenoviruses and probed with anti-FLAG MAb confirmed protein expression (Fig. 1B), and in addition, infected cells probed with the ASFV-specific convalescent-phase serum validated that the expressed antigens were authentic (Fig. 1C). The recombinant baculoviruses were used to generate affinity-purified recombinant ASFV proteins, which were used for ELISA and IFN-γ ELISPOT assays. We did not generate recombinant baculovirus for antigen pp62 since transfection of 293 Freestyle cells with the pcDNA construct and subsequent purification yielded sufficient protein for in vitro analyses. The affinity-purified proteins were shown to be authentic by Western blotting using ASFV-specific convalescent-phase serum (see Fig. S1 in the supplemental material).
FIG 1.
Protein expression by ASFV constructs. Protein expression by the constructs encoding ASFV antigens was evaluated by immunocytometric analysis of HEK 293A cells. (A) Cells transfected with pcDNA3 constructs and probed with anti-FLAG MAb. (B) Cells infected with recombinant adenoviruses and probed with anti-FLAG MAb. (C) Cells infected with recombinant adenoviruses and probed with gamma-irradiated ASFV-specific convalescent-phase serum. Negative controls are mock-transfected (A) or mock-infected (B and C) HEK 293A cells.
Ad-ASFV multiantigen cocktail rapidly induced robust antibody responses. (i) Postprime response.
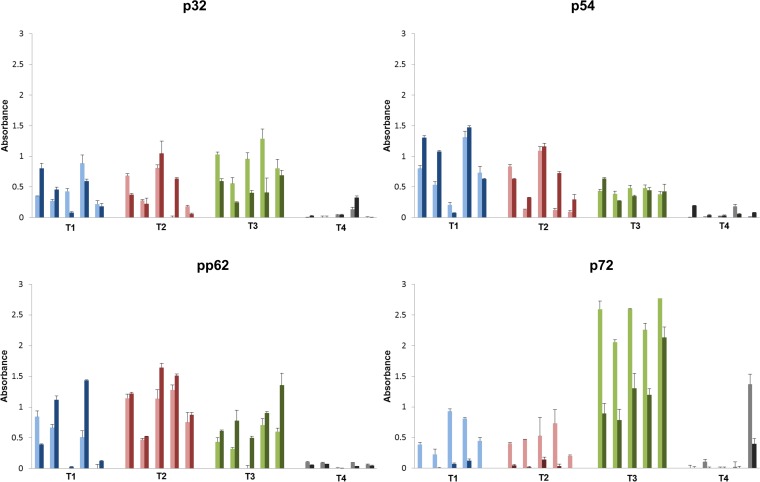
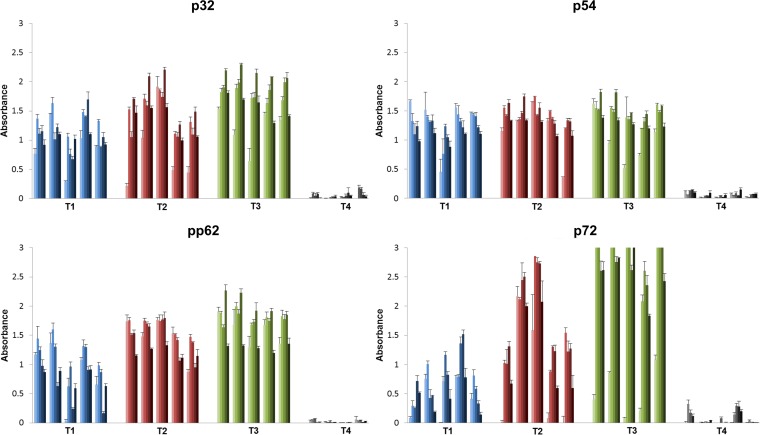
ELISA evaluation of antigen-specific IgM and IgG antibody responses in serum from blood collected at 1 week postpriming showed that all the pigs inoculated with either the 1010 (T1) or 1011 (T2 and T3) Ad-ASFV multivalent cocktail dose, but not the negative controls (T4), had seroconverted and mounted robust ASFV antigen-specific antibodies (Fig. 2). More importantly, most pigs underwent isotype switching within 1 week, based on relatively higher antigen-specific IgG than IgM antibody responses (Fig. 2). The IgM and IgG profiles were similar for p32, p54, and pp62 antigens, with no notable difference between the three treatment groups. However, compared to the pigs immunized with the ENABL adjuvant (T1 and T2), the pigs immunized using the Zoetis adjuvant (T3) clearly had higher p72 antibody responses, which were IgM dominant. Biweekly monitoring of antigen-specific IgG responses in each animal showed that the postprime antibody responses peaked at any time between weeks 2 to 8 and gradually declined by week 10 in most animals for all antigens (Fig. 3). Not much difference was detected in the p32-, p54-, and pp62-specific IgG responses among the treatment groups (Fig. 3A to C). In contrast, p72-specific IgG responses were highest in T3 animals, slightly lower in T2 animals, and the lowest in T1 vaccinees (Fig. 3D). One animal in the control group, T4, had high anti-p72 IgM and IgG responses at week 1, but these responses were not detected in the subsequent weeks, suggesting that the response at week 1 was nonspecific and not necessarily primed by the immunization (Fig. 2D and 3D). In addition, adenovirus vector-specific IgG responses were generally consistent with the ASFV antigen-specific IgG responses (see Fig. S2 in the supplemental material). Overall, postprime antibody response data clearly showed the ability of the vaccine cocktail to rapidly induce ASFV-specific IgM and IgG responses in all vaccinees following single-dose inoculation (Fig. 2 and 3).
FIG 2.
Ad5-ASFV multiantigen cocktail rapidly primed antibody responses. Antigen-specific IgMs (light shades) and IgGs (dark shades) in serum from week 1 postprime were evaluated by ELISA. The individual animal response to each antigen was evaluated in triplicate and is depicted as the mean of the absorbance values at 450 nm minus the mean absorbance of cognate preimmune serum. The error bars represent the standard deviation between triplicates. T4, negative control.
FIG 3.
Antigen-specific serum IgG profiles postpriming. Antigen-specific IgGs were monitored biweekly postprime up to week 10 by ELISA. The absorbance values at 450 nm across weeks 2, 4, 6, 8, and 10 postpriming for each animal are depicted using a color gradient where the lightest shade (first bar) represents week 2 and the darkest shade (last bar) represents week 10. Error bars show standard deviations among triplicate absorbance values. T4, negative control.
(ii) Postboost response.
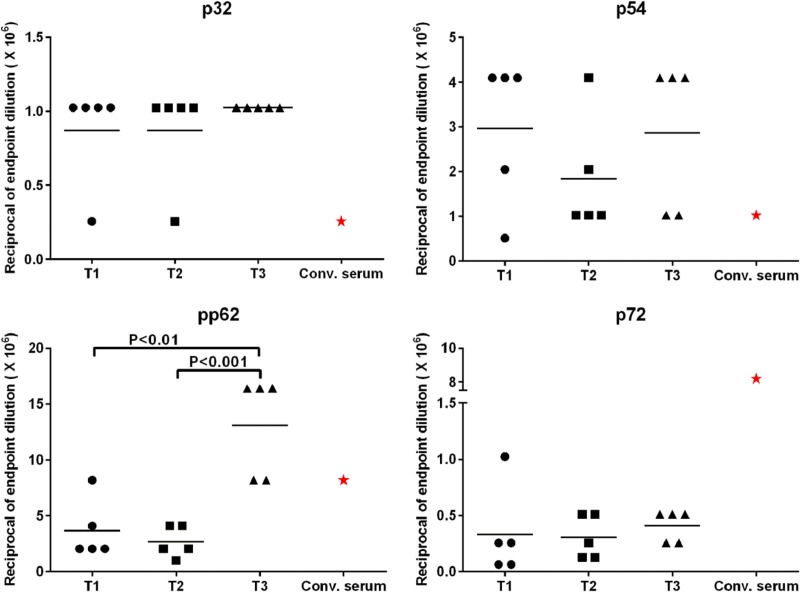
The gradual increase in antigen- and vector-specific antibody titers up to 8 weeks postpriming was an unexpected result and was a critical determinant with respect to the administration of the boost dose at week 14 in order to reduce any impact due to existing adenovirus-specific antibodies. Following boosting, robust antigen-specific recall IgG responses against all four antigens were detected in serum collected at weeks 1 to 4 postboost (see Fig. S3 in the supplemental material). Evaluation of antigen-specific endpoint titers by ELISA in serum collected at week 1 postboost showed that all vaccinees (groups T1 to T3), but none of the sham-treated controls (group T4), had high antibody titers against each antigen (Fig. 4). Among the four ASFV antigens, pp62-specific titers were the highest, and the p72-specific titers were the lowest (approximately 103 times lower) (Fig. 4). Convalescent-phase serum ASFV antigen-specific titers were also evaluated. The majority of vaccinees had p32- and p54-specific titers that were equivalent to or higher than those in convalescent-phase serum (Fig. 4A and B), whereas for pp62 only T3 animals had titers equal to or higher than the convalescent-phase serum titer (Fig. 4C). However, in contrast, p72-specific titers in convalescent-phase serum were higher than those of the vaccinees in groups T1 to T3 (Fig. 4D). A multiple comparison of antigen-specific titers between the three treatment groups showed a significant difference only for pp62, with T3 titers significantly higher than those of both T1 (P < 0.01) and T2 (P < 0.001) (Fig. 4C). A comparison of preboost and postboost ASFV antigen-specific antibody responses showed that boosting with the cognate priming dose and adjuvant effectively amplified the primary response, resulting in high antibody titers postboost (Fig. 4; see also Fig. S3). Importantly, that the two-dose immunization with the Ad-ASFV multiantigen cocktail induced titers comparable to those of the ASFV-specific convalescent-phase serum clearly demonstrates the ability of the multiantigen cocktail formulations to elicit very strong immune responses.
FIG 4.
ASFV-specific endpoint antibody titers. Antigen-specific endpoint titers of serum collected week 1 postboost were determined by ELISA. The endpoint dilution was determined to be the dilution at which the sample OD was higher than the OD of cognate prebleed plus 3 standard deviations. The lowest titer was 1:64 × 103 against p72, and some of the highest titers were as high as 1:16 × 106 against pp62. The ASFV-specific convalescent-phase serum was also titrated, and the titer against each antigen is indicated by a red star. Sera from T4 animals showed no reactivity above background to any of the antigens (see Fig. S3 in the supplemental material). The antigen-specific titers among the treatment groups were compared using ANOVA, followed by Tukey's multiple-comparison test.
A critical role for antibodies in protection against ASFV has not been clearly established. Partial to complete protection has been reported following immunization of pigs with a combination of recombinant subunit p30 and p54 antigens (23). In addition, complete protection was reported in another study in which swine were immunized with recombinant CD2v (HA) and then challenged with wild-type ASFV (39). However, in a separate study, antibodies induced following a combination of recombinant subunit p22, p30, p54, and p72 antigens did not provide sufficient protection (24). Furthermore, an immunization strategy to avoid ASFV-specific antibody responses by genetic fusion of recombinant subunit p30, p54, and CD2v antigens to ubiquitin conferred protection against lethal challenge in a proportion of vaccinees (15). These disparate findings have not allowed the protective role of host antibodies, if any, to be clearly defined during virulent ASF infection. A protective role for antibodies in ASFV is strongly supported by the observation that passive immunization with anti-ASFV serum confers complete protection against a subsequent lethal challenge (40). Results in the present study using an Ad-ASFV multivalent cocktail formulated in two different adjuvants and administered in a prime-boost regimen induced detectable antibody titers in 100% of immunized pigs and against each of the four ASFV antigens.
Ad-ASFV multiantigen cocktail-primed antibodies recognized ASF virus.
Indirect fluorescence antibody (IFA) assay and Western blot analysis using serum from week 1 postboost confirmed that antibodies induced by the experimental Ad-ASFV multiantigen cocktail recognized intact, native ASFV. All Ad-ASFV multiantigen cocktail-immunized swine, but none of the sham-treated controls, had a strong IFA signal against primary swine macrophages infected with the ASFV Georgia 2007/1 isolate (Fig. 5A). Overall IFA results strongly demonstrated that the Ad-ASFV multiantigen cocktail induced authentic ASFV-specific antibody responses (Table 2). This outcome was also confirmed by Western blotting using lysates from Vero cells infected with the ASFV Georgia 2007/1 isolate. Sera from all the three treatment groups (T1 to T3), but not the control group (T4), strongly recognized the ASFV antigens (Fig. 5B). A control Western blot performed using mock-infected Vero cell lysate showed no background reactivity against host cell antigens (see Fig. S4 in the supplemental material). It is important to note that these results do not suggest that the primed antibodies can neutralize ASFV; however, they do confirm that the synthetic genes used to generate the Ad-ASFV constructs expressed authentic antigens.
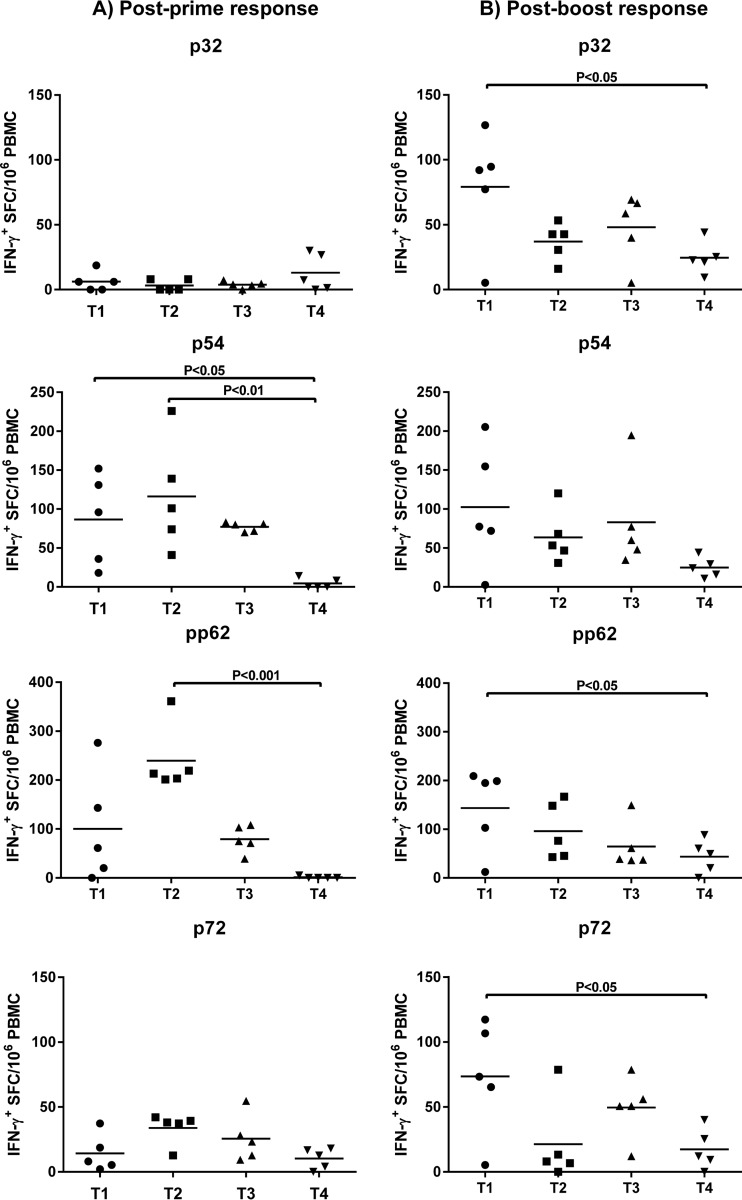
Ad5-ASFV multiantigen cocktail-primed antigen-specific IFN-γ-secreting cells.
ASFV antigen-specific IFN-γ-secreting cells were detected in whole peripheral blood mononuclear cells (PBMCs) postpriming and postboost by IFN-γ ELISPOT assays (Fig. 6). Postpriming, the majority of pigs in the three treatment groups (T1 to T3) had higher IFN-γ responses against p54 and pp62 antigens than to p32 and p72 antigens. Notably, the p54-specific IFN-γ responses were significantly higher in T1 pigs (P < 0.05) and in T2 pigs (P < 0.01) than in the T4 sham-treated controls (Fig. 6A). In addition, pp62-specific IFN-γ responses were significantly higher in T2 pigs (P < 0.001) than in the T4 controls (Fig. 6A). Antigen-specific IFN-γ recall responses observed in PBMCs at 3 weeks postboost, most notably the p32- and p72-specific responses, highlighted the booster dose effect (Fig. 6B). Compared to the T4 sham-treated controls, significantly higher (P < 0.05) IFN-γ responses against p32, pp62, and p72, but not p54, were observed in T1 but not in T2 and T3 animals (Fig. 6B). Postboost, T1 pigs had higher, detectable IFN-γ responses against all four antigens tested than pigs in the other two treatment groups. This result differs from the postprime results in which T2 immunized pigs were overall the best responders (Fig. 6A and B). One possibility for the discordant postprime and postboost results may be the relatively high anti-adenovirus titers in T2 versus those of T1 at the time of boost, which reduced the overall effectiveness of the booster dose in T2.
FIG 6.
ASFV antigen-specific IFN-γ responses postprime and postboost. The frequencies of antigen-specific IFN-γ-secreting cells in PBMCs induced at 2 weeks postprime (A) and 3 weeks postboost (B) were evaluated by IFN-γ ELISPOT assay. The mean response of treatment groups T1 to T3 was compared to the mean response of the sham-treated control group (T4) using ANOVA, followed by Bonferroni's multiple-comparison test. SFC, spot-forming cells.
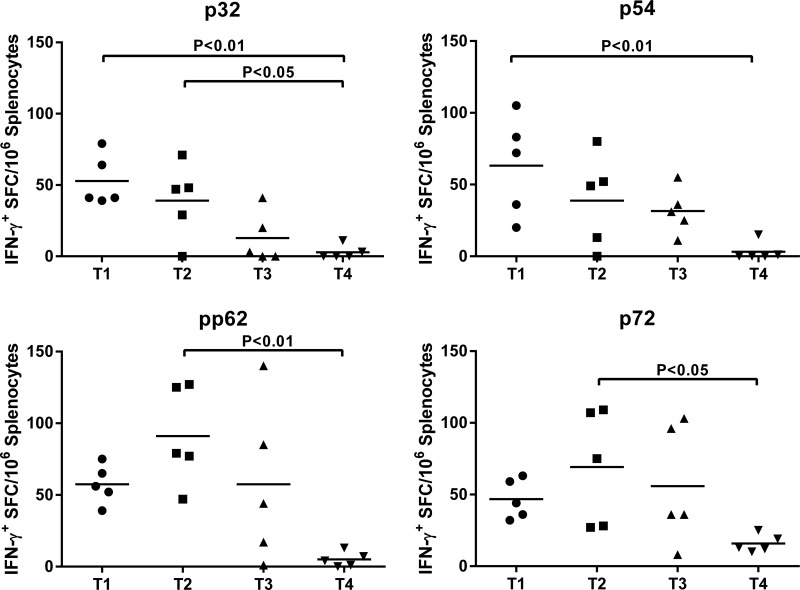
ELISPOT assays performed at study termination (week 8 postboost) using isolated splenocytes confirmed the presence of antigen-specific IFN-γ-secreting memory cells. Notably, strong IFN-γ+ responses against the four ASFV antigens were detected (Fig. 7). Significantly higher p32-specific IFN-γ+ responses were detected in T1 (P < 0.01) and T2 (P < 0.05) swine (Fig. 7) than in the T4 controls. Significant (P < 0.01) p54-specific IFN-γ+ responses were detected only in T1 animals, whereas significant (P < 0.01) pp62-specific IFN-γ+ responses and (P < 0.05) p72-specific IFN-γ+ responses were detected only in T2 animals (Fig. 7).
FIG 7.
ASFV antigen-specific IFN-γ recall responses in spleen. The presence of antigen-specific IFN-γ-secreting memory T cells in the spleen at study termination (week 8 postboost) was evaluated by IFN-γ ELISPOT assay. Statistical analysis was done as described in the legend of Fig. 6.
A recent study using a BacMam system to express a p30-p54-CD2v chimera reported partial protection upon sublethal challenge and a direct correlation between protection and induction of ASFV-specific IFN-γ+ T cells (41). In this study, strong IFN-γ+ peripheral and splenic tissue responses were elicited against each antigen in the Ad-ASFV multiantigen cocktail in the majority of immunized swine (groups T1 to T3) following prime-boost. Taken together, the ASFV antigen-specific IFN-γ responses observed in this immunogenicity study are promising and support the need to evaluate their potential to confer protection in a challenge study.
Ad5-ASFV cocktail-primed antigen-specific CTLs.
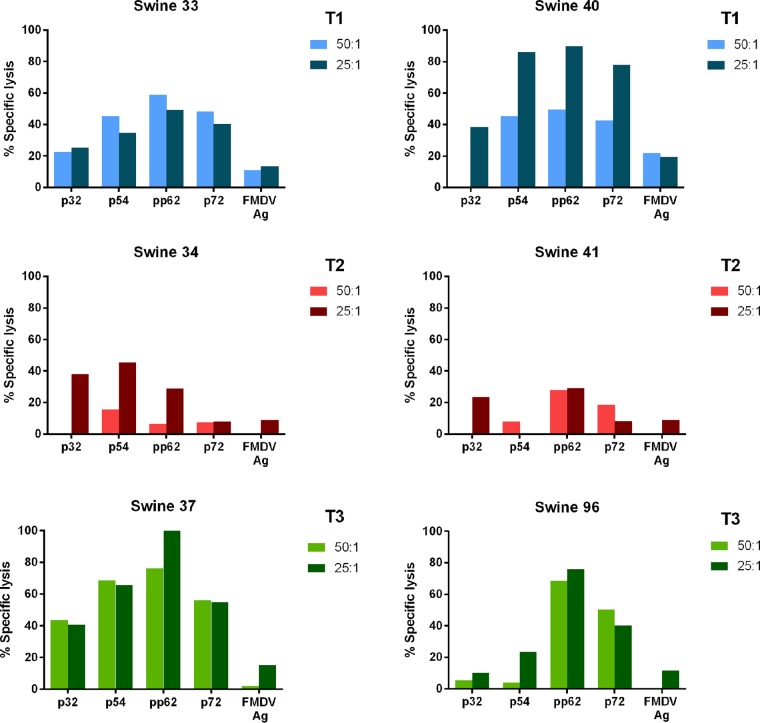
One round of in vitro-restimulated PBMCs (T1 to T3) isolated 4 weeks postboost was shown to effectively lyse autologous skin fibroblast transfectants in an ASFV antigen-specific manner at defined effector/target (E:T) ratios in 51Cr release assays (Fig. 8). Lytic activity against autologous skin fibroblasts transfected with a construct expressing an FMDV negative-control antigen remained at 20% or less for all animals, thus validating that the lytic activity observed against ASFV antigens can be attributed to ASFV antigen-specific cytotoxic T lymphocytes (CTLs) and not nonspecific NK cells (Fig. 8). Stimulation of the PBMCs for an additional round to further enrich CTLs failed to increase CTL activity, possibly due to activation-induced death of effectors. Among the immunized swine in groups T1 to T3, the level of antigen-specific lysis was equivalent in the T1 and T3 groups and lower in the T2 group. This result is consistent with the postboost observation discussed earlier in which relatively higher anti-adenovirus titers in T2 than in T1 at the time of boost may have reduced the overall effectiveness to amplify the primed CD8+ T cell responses. The heterogeneous CTL responses observed within the context of the study and assay designs are consistent with expected outcomes from the outbred commercial pigs used. This study is the first to demonstrate induction of ASFV antigen-specific CTL responses in commercial swine using an Ad-ASFV multiantigen cocktail, but further evaluation in subsequent studies using ASFV-infected target cells will be required.
FIG 8.
Ad5-ASFV multiantigen cocktail-primed ASFV antigen-specific cytotoxic T lymphocyte (CTL) responses. Antigen-specific CTL responses in PBMCs collected postboost (week 4) were evaluated at effector-to-target ratios of 50:1 and 25:1 using a standard 51Cr release assay. Data are represented as the percent specific lysis against each ASFV antigen and an FMDV negative-control antigen. Representative data for 2 animals from treatment groups T1, T2, and T3 are shown. Assays were not conducted for animals from the control group, T4. Ag, antigen.
Substantial evidence in published literature emphasizes the importance of CTLs in protection against ASFV. Studies have shown that ASFV-specific CTLs can be induced in swine infected with live attenuated ASFV, and the primed CTLs were shown to be responsible for clearance of infected cells (13). Higher proportions of ASFV-specific CTLs expressing CD4 and high levels of CD8 (CD4+ CD8high) have been detected in immune swine that are protected from clinical disease than in immune but clinically diseased pigs, suggesting that these CTLs are required for disease control (9). Furthermore, depletion of CD8+ T cells in swine results in loss of protective immunity to ASFV infections (14). Within this context, the CTL results reported here are noteworthy and clearly demonstrate the ability of a replication-deficient virus-vectored ASFV multiantigen cocktail to induce antigen-specific CTLs that are capable of recognizing and lysing autologous ASFV antigen-presenting fibroblasts.
Ad5-ASFV cocktail was well tolerated.
Following inoculation of the Ad-ASFV multiantigen cocktail, both the 1010 and the 1011 doses and adjuvant formulations (Table 1) were reasonably well tolerated in all the swine. Although no adverse systemic effects or injection site reactions were observed, some pigs in T1, T2, and T4 that received the ENABL formulation had mild injection site swelling, were transiently depressed, and had reduced appetite for 2 days following the booster dose. T3 vaccinees (Zoetis adjuvant) were active, but all had a pink discoloration at the injection site. However, by the third day postboost, all the swine were active and healthy with a good appetite and remained so for the rest of the study period. Thus, overall, the Ad-ASFV multiantigen cocktails formulated at both doses and with both adjuvants were safe and well tolerated by all the swine.
Conclusions.
The ASFV is a large complex DNA virus encoding >150 proteins. Experimental subunit vaccines based on a few of these antigens have generated different protective outcomes, demonstrating that these antigens do play some role in host protection. Immunization of animals with an expression library of restriction enzyme-digested ASFV genome fragments protected 60% of the animals (42). This outcome suggests that protection through subunit vaccines is feasible but is unlikely to be highly efficacious using a single or only a few antigens. Empirical identification of antigens necessary for inducing a protective response, along with a suitable antigen delivery system that elicits strong cellular as well as humoral responses, may be a reasonable strategy to develop an efficacious, prototype ASFV vaccine. The immunogenicity data generated from this proof-of-concept study showed that the replication-deficient adenovirus vector, dose, adjuvant formulation, and immunization regimen effectively induced strong antibody (with unprecedented rapid isotype switching) and cellular responses against four ASFV antigens. An analysis of the overall differences in antibody and T cell immune responses observed across the three different treatment groups revealed some interesting outcomes. In the case of the humoral responses, the T3 animals (immunized with the Zoetis adjuvant) had a slightly higher antibody response; however, the endpoint titration data (Fig. 4) failed to demonstrate any significant differences in the titers among the three treatment groups for three of the four ASFV antigens tested (pp62 was the only exception). With respect to T cell-mediated immune responses, the postprime antigen-specific IFN-γ response clearly showed that T2 (high dose; ENABL adjuvant) animals were the best overall responders. However, the postboost data suggest that the low-dose prime and low-dose boost group (T1) had the highest recall response. Based on this outcome, it may be useful in future immunogenicity and efficacy studies to test whether priming with the low dose (1010 IFU/Ad-ASFV construct) and boosting with the high dose (1011 IFU/Ad-ASFV construct) will elicit better immune responses. In addition, there is merit to testing both adjuvants in future efficacy studies to better understand the relevance of the varied immune responses induced in the context of the protection conferred.
In conclusion, an Ad-ASFV multiantigen cocktail two-dose formulation was immunogenic and safe when administered in a prime-boost regimen. Results showed evidence of rapid postprime antibody class switching, induction of robust antibody responses which recognize ASFV-infected cells, and the generation of antigen-specific IFN-γ and antigen-specific CTL responses to all four ASFV antigens. The immunogenicity data from this study validate our approach of using an adenovirus-vectored cocktail of ASFV antigens and set the stage for conducting future challenge studies using a cocktail of the above antigens as well as other novel ASFV antigens. Collectively, these data validate a synthetic gene-based approach to generate ASFV antigen delivery constructs and provide a rational strategy for further screening of ASFV antigen targets toward development of a multiantigen, efficacious ASF vaccine that is able to differentiate infected from vaccinated animals (DIVA vaccine).
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00395-16.
REFERENCES
- 1.Scott GR. 1957. Notes on animal diseases. XI. Virus diseases of pigs. East Afr Agric J 22:168–174. [Google Scholar]
- 2.de Carvalho Ferreira HC, Weesendorp E, Elbers AR, Bouma A, Quak S, Stegeman JA, Loeffen WL. 2012. African swine fever virus excretion patterns in persistently infected animals: a quantitative approach. Vet Microbiol 160:327–340. doi: 10.1016/j.vetmic.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Costard S, Wieland B, de Glanville W, Jori F, Rowlands R, Vosloo W, Roger F, Pfeiffer DU, Dixon LK. 2009. African swine fever: how can global spread be prevented? Philos Trans R Soc Lond B Biol Sci 364:2683–2696. doi: 10.1098/rstb.2009.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed). 2011. Virus taxonomy: classification and nomenclature of viruses: ninth report of the International Committee on Taxonomy of Viruses. Academic Press, London, United Kingdom. [Google Scholar]
- 5.Dixon LK, Chapman DAG, Netherton CL, Upton C. 2013. African swine fever virus replication and genomics. Virus Res 173:3–14. doi: 10.1016/j.virusres.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Salas ML, Andrés G. 2013. African swine fever virus morphogenesis. Virus Res 173:29–41. doi: 10.1016/j.virusres.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Mulumba-Mfumu LK, Goatley LC, Saegerman C, Takamatsu HH, Dixon LK. 2016. Immunization of African indigenous pigs with attenuated genotype I African swine fever virus OURT88/3 induces protection against challenge with virulent strains of genotype I. Transbound Emerg Dis 63:e323–e327. doi: 10.1111/tbed.12303. [DOI] [PubMed] [Google Scholar]
- 8.Lewis T, Zsak L, Burrage TG, Lu Z, Kutish GF, Neilan JG, Rock DL. 2000. An African swine fever virus ERV1-ALR homologue, 9GL, affects virion maturation and viral growth in macrophages and viral virulence in swine. J Virol 74:1275–1285. doi: 10.1128/JVI.74.3.1275-1285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takamatsu HH, Denyer MS, Lacasta A, Stirling CM, Argilaguet JM, Netherton CL, Oura CA, Martins C, Rodriguez F. 2013. Cellular immunity in ASFV responses. Virus Res 173:110–121. doi: 10.1016/j.virusres.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Alonso F, Dominguez J, Vinuela E, Revilla Y. 1997. African swine fever virus-specific cytotoxic T lymphocytes recognize the 32 kDa immediate early protein (vp32). Virus Res 49:123–130. doi: 10.1016/S0168-1702(97)01459-7. [DOI] [PubMed] [Google Scholar]
- 11.Dixon LK, Abrams CC, Bowick G, Goatley LC, Kay-Jackson PC, Chapman D, Liverani E, Nix R, Silk R, Zhang F. 2004. African swine fever virus proteins involved in evading host defence systems. Vet Immunol Immunopathol 100:117–134. doi: 10.1016/j.vetimm.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Leitao A, Malur A, Cornelis P, Martins CL. 1998. Identification of a 25-amino-acid sequence from the major African swine fever virus structural protein VP72 recognised by porcine cytotoxic T lymphocytes using a lipoprotein based expression system. J Virol Methods 75:113–119. doi: 10.1016/S0166-0934(98)00105-0. [DOI] [PubMed] [Google Scholar]
- 13.Martins CL, Lawman MJ, Scholl T, Mebus CA, Lunney JK. 1993. African swine fever virus specific porcine cytotoxic T cell activity. Arch Virol 129:211–225. doi: 10.1007/BF01316896. [DOI] [PubMed] [Google Scholar]
- 14.Oura CA, Denyer MS, Takamatsu H, Parkhouse RM. 2005. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J Gen Virol 86:2445–2450. doi: 10.1099/vir.0.81038-0. [DOI] [PubMed] [Google Scholar]
- 15.Argilaguet JM, Perez-Martin E, Nofrarias M, Gallardo C, Accensi F, Lacasta A, Mora M, Ballester M, Galindo-Cardiel I, Lopez-Soria S, Escribano JM, Reche PA, Rodriguez F. 2012. DNA vaccination partially protects against African swine fever virus lethal challenge in the absence of antibodies. PLoS One 7:e40942. doi: 10.1371/journal.pone.0040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escribano JM, Galindo I, Alonso C. 2013. Antibody-mediated neutralization of African swine fever virus: myths and facts. Virus Res 173:101–109. doi: 10.1016/j.virusres.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Zsak L, Onisk DV, Afonso CL, Rock DL. 1993. Virulent African swine fever virus isolates are neutralized by swine immune serum and by monoclonal antibodies recognizing a 72-kDa viral protein. Virology 196:596–602. doi: 10.1006/viro.1993.1515. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz Gonzalvo F, Caballero C, Martinez J, Carnero ME. 1986. Neutralization of African swine fever virus by sera from African swine fever-resistant pigs. Am J Vet Res 47:1858–1862. [PubMed] [Google Scholar]
- 19.Ruiz Gonzalvo F, Carnero ME, Caballero C, Martinez J. 1986. Inhibition of African swine fever infection in the presence of immune sera in vivo and in vitro. Am J Vet Res 47:1249–1252. [PubMed] [Google Scholar]
- 20.Arias ML, Sanchez-Vizcaino JM. 2002. African swine fever, p 119–139. In Morilla A, Yoon KJ, Zimmermann JF (ed), Trends in emerging viral infections of swine. Iowa State Press, Ames, IA. [Google Scholar]
- 21.Canals A, Alonso F, Tomillo J, Dominguez J. 1992. Analysis of T lymphocyte subsets proliferating in response to infective and UV-inactivated African swine fever viruses. Vet Microbiol 33:117–127. doi: 10.1016/0378-1135(92)90040-Z. [DOI] [PubMed] [Google Scholar]
- 22.Argilaguet JM, Pérez-Martín E, Gallardo C, Salguero FJ, Borrego B, Lacasta A, Accensi F, Díaz I, Nofrarías M, Pujols J, Blanco E, Pérez-Filgueira M, Escribano JM, Rodríguez F. 2011. Enhancing DNA immunization by targeting ASFV antigens to SLA-II bearing cells. Vaccine 29:5379–5385. doi: 10.1016/j.vaccine.2011.05.084. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Puertas P, Rodriguez F, Oviedo JM, Brun A, Alonso C, Escribano JM. 1998. The African swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology 243:461–471. doi: 10.1006/viro.1998.9068. [DOI] [PubMed] [Google Scholar]
- 24.Neilan JG, Zsak L, Lu Z, Burrage TG, Kutish GF, Rock DL. 2004. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology 319:337–342. doi: 10.1016/j.virol.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Barderas MG, Rodriguez F, Gomez-Puertas P, Aviles M, Beitia F, Alonso C, Escribano JM. 2001. Antigenic and immunogenic properties of a chimera of two immunodominant African swine fever virus proteins. Arch Virol 146:1681–1691. doi: 10.1007/s007050170056. [DOI] [PubMed] [Google Scholar]
- 26.Leitao A, Cartaxeiro C, Coelho R, Cruz B, Parkhouse RME, Portugal FC, Vigario JD, Martins CLV. 2001. The non-haemadsorbing African swine fever virus isolate ASFV/NH/P68 provides a model for defining the protective anti-virus immune response. J Gen Virol 82:513–523. doi: 10.1099/0022-1317-82-3-513. [DOI] [PubMed] [Google Scholar]
- 27.Manso-Ribeiro J, Nunes-Petisca JL, Lopes-Frazao F, Sobral M. 1963. Vaccination against ASF. Bull Off Int Epizoot 60:921–937. [Google Scholar]
- 28.Tatsis N, Ertl HC. 2004. Adenoviruses as vaccine vectors. Mol Ther 10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamen A, Henry O. 2004. Development and optimization of an adenovirus production process. J Gene Med 6(Suppl 1):S184–S192. doi: 10.1002/jgm.503. [DOI] [PubMed] [Google Scholar]
- 30.Gogev S, Vanderheijden N, Lemaire M, Schynts F, D'Offay J, Deprez I, Adam M, Eloit M, Thiry E. 2002. Induction of protective immunity to bovine herpesvirus type 1 in cattle by intranasal administration of replication-defective human adenovirus type 5 expressing glycoprotein gC or gD. Vaccine 20:1451–1465. doi: 10.1016/S0264-410X(01)00458-3. [DOI] [PubMed] [Google Scholar]
- 31.Zhu J, Huang X, Yang Y. 2007. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol 81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elahi SM, Shen SH, Talbot BG, Massie B, Harpin S, Elazhary Y. 1999. Recombinant adenoviruses expressing the E2 protein of bovine viral diarrhea virus induce humoral and cellular immune responses. FEMS Microbiol Lett 177:159–166. doi: 10.1111/j.1574-6968.1999.tb13727.x. [DOI] [PubMed] [Google Scholar]
- 33.Maeda K, West K, Hayasaka D, Ennis FA, Terajima M. 2005. Recombinant adenovirus vector vaccine induces stronger cytotoxic T-cell responses than recombinant vaccinia virus vector, plasmid DNA, or a combination of these. Viral Immunol 18:657–667. doi: 10.1089/vim.2005.18.657. [DOI] [PubMed] [Google Scholar]
- 34.Suarez C, Salas ML, Rodriguez JM. 2010. African swine fever virus polyprotein pp62 is essential for viral core development. J Virol 84:176–187. doi: 10.1128/JVI.01858-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mwangi W, Brown WC, Splitter GA, Davies CJ, Howard CJ, Hope JC, Aida Y, Zhuang Y, Hunter BJ, Palmer GH. 2007. DNA vaccine construct incorporating intercellular trafficking and intracellular targeting motifs effectively primes and induces memory B- and T-cell responses in outbred animals. Clin Vaccine Immunol 14:304–311. doi: 10.1128/CVI.00363-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genovesi EV, Villinger F, Gerstner DJ, Whyard TC, Knudsen RC. 1990. Effect of macrophage-specific colony-stimulating factor (CSF-1) on swine monocyte/macrophage susceptibility to in vitro infection by African swine fever virus. Vet Microbiol 25:153–176. doi: 10.1016/0378-1135(90)90074-6. [DOI] [PubMed] [Google Scholar]
- 37.Ceppi M, de Bruin MG, Seuberlich T, Balmelli C, Pascolo S, Ruggli N, Wienhold D, Tratschin JD, McCullough KC, Summerfield A. 2005. Identification of classical swine fever virus protein E2 as a target for cytotoxic T cells by using mRNA-transfected antigen-presenting cells. J Gen Virol 86:2525–2534. doi: 10.1099/vir.0.80907-0. [DOI] [PubMed] [Google Scholar]
- 38.Veelken H, Jesuiter H, Mackensen A, Kulmburg P, Schultze J, Rosenthal F, Mertelsmann R, Lindemann A. 1994. Primary fibroblasts from human adults as target cells for ex vivo transfection and gene therapy. Hum Gene Ther 5:1203–1210. doi: 10.1089/hum.1994.5.10-1203. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz-Gonzalvo F, Rodriguez F, Escribano JM. 1996. Functional and immunological properties of the baculovirus-expressed hemagglutinin of African swine fever virus. Virology 218:285–289. doi: 10.1006/viro.1996.0193. [DOI] [PubMed] [Google Scholar]
- 40.Onisk DV, Borca MV, Kutish G, Kramer E, Irusta P, Rock DL. 1994. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology 198:350–354. doi: 10.1006/viro.1994.1040. [DOI] [PubMed] [Google Scholar]
- 41.Argilaguet JM, Perez-Martin E, Lopez S, Goethe M, Escribano JM, Giesow K, Keil GM, Rodriguez F. 2013. BacMam immunization partially protects pigs against sublethal challenge with African swine fever virus. Antiviral Res 98:61–65. doi: 10.1016/j.antiviral.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Lacasta A, Ballester M, Monteagudo PL, Rodriguez JM, Salas ML, Accensi F, Pina-Pedrero S, Bensaid A, Argilaguet J, Lopez-Soria S, Hutet E, Le Potier MF, Rodriguez F. 2014. Expression library immunization can confer protection against lethal challenge with African swine fever virus. J Virol 88:13322–13332. doi: 10.1128/JVI.01893-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.