Abstract
Lefty is a member of transforming growth factor-beta (TGF-β) superfamily and a potent antagonist of the TGF-β/Nodal/Activin signaling pathway. Lefty is critical in sustaining self-renewal/pluripotency status, and implicated in the differentiation of embryonic stem cells (ESCs). However, emerging studies depict Lefty as a multifaceted protein involved in myriad cellular events. Lefty proteins (human Lefty A and B) are secreted glycoproteins, but their mode of secretion and the significance of their “glycan” moiety remain mostly unexplored. By employing an in vitro system of human ESCs (hESCs), we observed that Lefty protein(s) are encased in exosomes for extracellular release. The exosomal- and cell-associated Lefty diverge in their proteolytic processing, and possess N-glycan structures of high mannose and complex nature. Differentiation of hESCs to mesenchymal cells (MSCs) or neuronal progenitor cells (NPCs) entails distinct changes in the Lefty A/Lefty B gene(s), and protein expression. Specifically, the proteolytic cleavage and N-glycan composition of the cell-associated and exosomal Lefty differ in the differentiated progenies. These modifications affected Lefty's inhibitory effect on Nodal signaling in aggressive melanoma cells. The microheterogeneity in the processing and glycosylation of Lefty protein(s) between hESCs, MSCs, and NPCs could present efficient means of diversifying the endogenous functions of Lefty. Whether Lefty's diverse functions in embryonic patterning, as well as its diffusion range in the extracellular environment, are similarly affected remains to be determined. Our studies underscore the potential relevance of Lefty-packaged exosomes for combating debilitating diseases such as cancer.
Keywords: : Lefty A & B, embryonic stem cells, differentiation, N-glycosylation, post-translational modifications
Introduction
Human embryonic stem cells (hESCs) have the ability to self-renew and differentiate into many distinct cell types. However, the regulatory network directing such diverse processes is not fully resolved and remains the subject of intense scrutiny. Emerging studies indicate that beyond gene regulation, proteins with their diverse array of posttranslational modifications (PTMs) are critical in self-renewal/pluripotency status and differentiation of hESCs [1]. Glycosylation is the most common PTM in eukaryotic cells, and an altered glycosylation profile is noted in hESC cell surface membrane proteins upon differentiation [2].
Lefty, a member of transforming growth factor-beta (TGF-β) superfamily, encompasses two genes with the same transcriptional orientations: Lefty A and Lefty B in human [3] and their homologues Lefty 1 and 2 in mouse [4]. In humans, the protein products of these genes share 96% sequence homology [5], are abundantly expressed in embryonic stem cells (ESCs), and are considered a marker of stemness [6,7]. In addition, Lefty is an important regulator of very early ESC fate decisions [8,9], and critically involved in cell differentiation [10]. It has been suggested that Lefty resides at the crossroads of stemness and “differentiative events” [11], a premise supported by the observed changes in Lefty expression of hESCs upon differentiation along different pathways [12,13]. Lefty(s) is a secreted glycoprotein; its mode of secretion is unspecified, and the functional significance of its glycosylation is a matter for debate [14]. In brief, what regulates Lefty's diverse functional attributes has remained mostly unexplored.
The extracellular protein transport through the exosomal pathway, originally considered a waste disposal mechanism [15], has recently emerged as an important mediator of extracellular signaling [16–18]. Exosomes are nanovesicles (30–150 nm) encased in complex lipid membranes with integral surface proteins and interior cargo consisting of an assortment of proteins and nucleic acids [19]. Upon release from a cell, they can embed in the extracellular matrix, be engulfed by the neighboring cell or travel to distant sites, and fuse with the target cells eliciting functional changes [20–22].
We hypothesized that Lefty's route of extracellular release, the ratio of Lefty A and Lefty B gene, and the PTM of Lefty proteins are critical regulators of its function. Thus, an in vitro system was employed to prompt hESCs to differentiate along mesenchymal cell (MSC) or neuronal progenitor cell (NPC) pathways. Subsequently, LeftyA/LeftyB gene expression, protein profiles, and glycosylation status were collectively examined in the parental versus the differentiated progenies. In addition, exosomes were collected from hESC conditioned media (CM) and tested for the presence of Lefty proteins. Our data indicate, for the first time, the extracellular delivery of Lefty proteins through the exosomal pathway. In addition, the hESC current ratio of LeftyA/LeftyB alters upon differentiation along neuronal or mesenchymal pathways. Notably, the protein products of these two genes exhibit distinct proteolytic cleavage and glycosylation profiles compared to that of the parental hESCs. The implications of these PTMs in defining Lefty's functional significance in Nodal signaling in tumor cells and differentiation are discussed, and provide new insights into the exquisite regulation associated with this powerful antagonist.
Materials and Methods
Embryonic stem cell culture and differentiation
hESCs H7 and H9 were grown in the StemPro medium (Life Technologies) on a Matrigel® substrate (BD Bioscience). The cultures were passaged mechanically using the StemPro EZ Passage tool (Life Technologies).
Mesenchymal differentiation of hESCs (MSCs) was achieved as described [23]. Briefly, hESCs were grown in growth factor-depleted media (containing DMEM, knockout serum replacement, l-glutamine, and antibiotics) for 2 weeks. The media were replaced with the EGM-2 MV growth media (Lonza), and the cultures were maintained and passaged for an additional 25 days. FACS analysis using antibodies to CD73 (APC conjugated) and CD34 (FITC conjugated, Miltenyi Biotec), indicated that 97% of the cells were positive for CD73 with no expression of CD34.
Differentiation of hESCs to NPCs was attained as previously described [24]. Briefly, the neuronal rosettes were mechanically detached from the differentiated hESC colonies and plated on poly-ornithine/laminin-coated tissue culture dishes in the ENStem-A neural expansion medium (containing 20 ng/mL EGF/FGF2 [Millipore], 2 mM l-glutamine, and 1× PenStrep [Gibco]) at 37°C and 5% CO2 in a humidified atmosphere. Acutase (Millipore) was employed for cell detachment and passaging. The resulting NPCs expressed neuronal progenitor-specific markers, for example, nestin and Sox2.
Exosome purification and labeling
Exosomes were harvested from the CM by two different approaches: ultracentrifugation and using a commercially available exosome precipitation solution (Exoquick-TC™; System Biosciences).
In the first approach, the CM was subjected to successive centrifugation at 2,000 g (10 min) and 10,000 g (30 min) to remove dead cells and apoptotic bodies. The supernatant was further centrifuged at 100,000 g (120 min) to pellet a crude exosome preparation (with contaminating proteins). The contaminating proteins were removed by proceeding with the phosphate-buffered saline (PBS) wash and exosomes were collected by a final centrifugation at 100,000 g (90 min) [25]. In the second approach, the CM (after removal of cell debris and apoptotic bodies) was concentrated 5× using centrifugal filters with 3K molecular weight cutoff (Amicon Ultra; Millpore). To 5 mL of the concentrated solution, 1 mL Exoquick-TC was added, the solution was mixed and left overnight at 4°C, and then centrifuged at 16,000 g for 30 min. The pellet was cleared of the excess Exoquick by washing in PBS, followed by centrifugation (100,000 g, 60 min). The exosomes were reconstituted in PBS and their concentration measured using the BCA protein assay.
To examine the cellular intake of hESC exosomes, the purified exosomes were labeled with PKH67 Fluorescent Cell Linker (Sigma) according to the manufacturer's specifications. Briefly, diluent C (1 mL) was added to a suspension of exosomes (400 μg total protein/25 μL of PBS) with gentle mixing. One mL of a 2× dye solution in diluent C was then added to the exosome suspension and the solution was mixed by pipetting and incubated for 3 min at room temperature (with periodic mixing). The excess dye was quenched by addition of 2 mL of serum and the solution was diluted with PBS and centrifuged at 100,000 g (60 min) to pellet the labeled exosomes.
Deglycosylation
For initial screening of glycan moieties of Lefty, we employed two glycosidases: Endoglycosidase H (Endo-H) and Peptide:N-glycosidase F (PNGase F) (New England BioLabs). Briefly, 30 μg cytosolic or exosomal protein from hESCs and its differentiated progenies was denatured and digested with Endo-H or PNGase F for 3 h at 37°C according to the manufacturer's instructions. The reaction products were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis using a monoclonal rabbit antibody to Lefty (rabbit monoclonal; Epitomics) to identify changes in the molecular mass of the digested products. For comparative purpose, recombinant human Lefty A and B (HumanZyme), were deglycosylated similarly.
Functional analysis of exosomal lefty from hESCs and differentiated progenies
For this purpose, we employed the aggressive melanoma cell line C8161, known to express Nodal, but deficient in Lefty proteins due to Lefty gene methylation [26]. Initially, the possible effect of hESC exosomal Lefty on Nodal signaling was examined by treating C8161 cells with or without hESC exosomes (5 μg/mL) for 72 h. Cell lysates were prepared and subjected to western blot analysis for Nodal expression. Smad-2 phosphorylation was assessed using an ELISA kit for pSmad-2 (S465/467) according to the manufacturer's instructions (Cell Signaling Technology). Flow cytometry was performed on a Guava Easycyte HT Flow Cytometer (Millipore) using Guava ViaCount Reagent for viability and cell number, and Nexin reagent for analysis of apoptotic cells according to the manufacturer's instructions. Two different Lefty antibodies, a goat polyclonal (Santa Cruz) and rabbit monoclonal (Epitomics), were used to demonstrate that the effects were Lefty mediated. Analysis was performed on triplicate samples with untreated and calf serum exosome-treated cells as controls.
The differential effect of exosomal Lefty from hESCs versus MSCs and NPCs was examined in C8161 cells grown in eight-well Chamber Slides (Nunc) to ∼65% confluency and treated with exosomes (at 5 μg/mL exosomal suspension) from hESCs (H7 or H9), or their differentiated progenies (MSCs and NPCs). Changes in morphology and cell growth were monitored for 72 to 96 h and documented. Controls comprised exosomal suspension pretreated with antibody to Lefty (Santa Cruz Biotechnology) or IgG control (4 μg/mL, 2 h RT). Recombinant Lefty (r-Lefty) at a concentration calculated to match that of exosomal Lefty served for comparative purpose.
Real-time polymerase chain reaction
Total RNA was isolated from cells using the PerfectPure RNA Cell Kit (5Prime) according to manufacturer's specifications. Reverse transcription of the total RNA was performed in a Veriti Thermal Cycler (Applied Biosystem). Polymerase chain reaction (PCR) was performed on a 7500 Real-Time PCR System (Life Technologies) using TaqMan® gene expression primer/probe sets [(Lefty1[B]:Hs00764128_S1, Lefty2[A]:Hs00745761_S1 (Life Technologies), Nodal: (Integrated DNA Technology)]. Briefly, 5 μL cDNA, 1.25 μL Gene Expression primer/probe, and 12.5 μL 2× PCR Master Mix in a total of 25 μL were amplified with the following thermocycler protocol: 1 cycle at 50°C for 2 min; 1 cycle at 95°C for 10 min; and 33 cycles at 95°C for 15 s; 60°C for 1 min. All data were analyzed with the 7500 System Software (version 1.2.3; Life Technologies). The expression of each target gene was normalized to an endogenous control gene, RPLPO large ribosomal protein (4333761F; Life Technologies). Each sample testing was performed in triplicates.
Results and Discussion
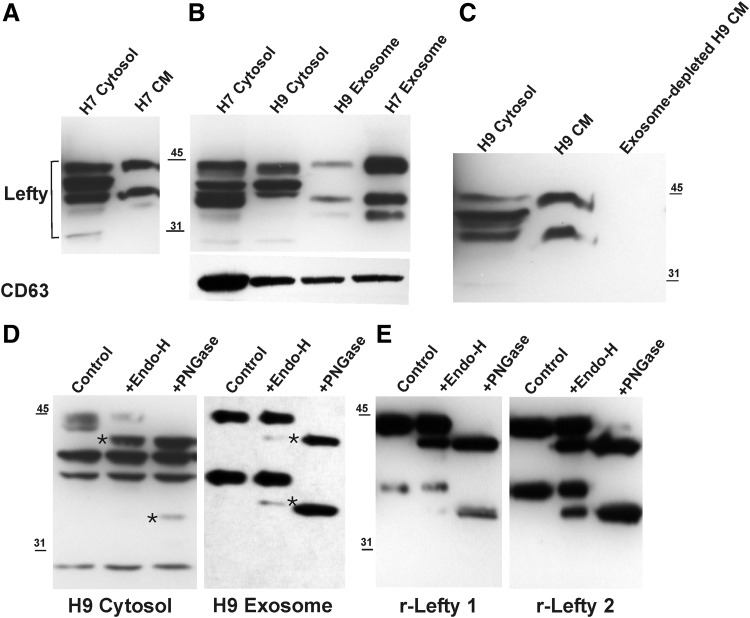
Lefty gene expression examined by reverse transcription-ploymerase chain reaction (RT-PCR) revealed abundant expression of both Lefty A and B in the hESCs tested. At the protein level, western blot analyses of the cell-associated Lefty and secreted Lefty in the CM indicated major bands of ≈44, 40, 38, and 28 kDa (and occasionally a minor band of ≈34 kDa) in the cell lysates compared to two major bands (≈45 and 38 kDa, and a minor band of ≈34 kDa) in the CM. The ≈40 and 28 kDa cell-associated Lefty were not detected in the CM (Fig. 1A). Based on the amino acid composition of Lefty and its glycosylated nature, ≈44 kDa equates to the whole molecule (on SDS-PAGE glycosylated proteins run with apparent molecular mass higher than their formula weight). The ≈40 kDa is most probably the proform without the signal peptide, while ≈38 and 28 kDa bands are the cleavage products at amino acid sequences RGKR (74–77) and RHGR (132–135)—by members of the convertase family of endopeptidases (SPCs) (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/scd) [27]. Based on the published studies mostly performed on mouse Lefty, SPC1, SPC4, SPC5, SPC6A, and SPC6B cleave Lefty 1 at both sites, but Lefty 2 is cleaved only at the first site (74–77) by SPC1 and SPC6A [28]. These enzymes are localized in the intracellular secretory network and extracellular matrix, and are functional at both sites. Clearly, the differential presence of processed forms in cell-associated versus secreted Lefty could signify specific functions for the cleaved products.
FIG. 1.
Protein profile and glycan composition of cellular and exosomal Lefty in hESCs. (A) Depicts Lefty protein profile in the cytosolic extract and CM from hESCs (H7) as determined by SDS-PAGE (4%–20% acrylamide gels) and western analysis. The same approach was employed to examine Lefty's presence in the purified exosomal fractions from H7 and H9 CM and is presented in (B). For comparative purpose, the cytosolic extracts from H7 and H9 cells are included, with CD63 serving as exosomal marker. (C) The exosome-depleted CM was further tested for the presence of Lefty proteins. (D) Glycoform analysis of cellular versus exosomal Lefty in H9 hESCs. Thirty micrograms of cytosolic extracts and 5 μg of exosomal fractions were subjected to Endo-H and PNGase F treatment and analyzed by SDS-PAGE on a 10% acrylamide gel. (E) Recombinant Lefty 1 and 2 were included as control and subjected to identical digestion protocol. Cleavage products are indicated by ‘*’. CM, conditioned media; Endo-H, Endoglycosidase H; hESCs, human embryonic stem cells; PNGase F, peptide:N-glycosidase F; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Due to the generic nature of commercial Lefty antibodies, clear distinction of Lefty A and B in the cell lysates or the CM was not possible. However, based on the proteomic profiling of the mouse ESC secretome, only Lefty 1 (equivalent to Lefty B in humans) was detected in mouse ESC secretome. Whether this is mirrored in hESCs remains to be determined [29].
Lefty is secreted through an exosomal pathway
The observed difference in the processing of the cell-associated and secreted Lefty prompted the hypothesis that Lefty might be released through an exosomal pathway. Thus, exosomes were collected from the concentrated CM and examined by western blot analyses for the presence of exosomal marker(s) CD63 and/or CD81, and Lefty protein(s). Analogous to the CM, two major bands (≈45 and 38 kDa, with occasional presence of the ≈34 kDa band) were detected in the exosomes (Fig. 1B), with no detectable presence of Lefty in the exosome-depleted CM (Fig. 1C). To our knowledge, this is the first report of Lefty proteins using an exosomal pathway for extracellular release, and it extends the growing list of proteins/growth factors employing this transport route [30–33]. Undoubtedly, proteins marked for exosomal delivery constitute part of an elaborated “exsosomal code” unique to the cells of their origin, which is thus far incompletely deciphered.
Lefty is N-glycosylated on asparagine residue 158 [14] located within the specific glycosylation motif Asn-Arg-Thr [34]. Depending on their carbohydrate composition, N-glycans fall into the three categories of high mannose, hybrid, and complex glycans with a common core structure constitutive in most cell types (Supplementary Fig. S2A). However, further structural diversification of glycan chains occurs in a tissue- or cell lineage-specific manner by additional branching and/or terminal sugars [35] (Supplementary Fig. S2B). Initial screening of the N-glycan composition of cell- and exosome-associated Lefty was implemented using two specific glycosidases: Endo-H [cleaves the chitobiose core (-GlcNAc-GlcNAc) of high mannose and some hybrid glycans of the N-linked glycoproteins] and PNGase F (cleaves between Asn-GlcNAc bond of all three N-glycan categories) (Supplementary Fig. S2A). Based on western blot analyses of hESC cell lysates and exosomes, the ≈44 and ≈38 kDa Lefty bands contained a mixture of Endo-H sensitive (comprising high mannose and/or hybrid saccharide structures) with apparent molecular mass of ≈2.3–2.4 kDa, and PNGase F sensitive (consisting high mannose, hybrid, and complex glycans) of ≈2.8 kDa (shown in Fig. 1D). Three different hESCs (H7, H9, and H14) rendered similar results and presented with comparable cleavage products. Of interest, r-Lefty A and B included as control, and subjected to digestion with Endo-H and PNGase F, also showed a similar glycoform profile, but with slightly different molecular mass for the released glycans (Fig. 1E)—not surprising since the origin of r-Lefty A and B used in these experiments is from HEK cells.
Lefty A/Lefty B ratio and glycosylation status are altered upon differentiation of hESCs
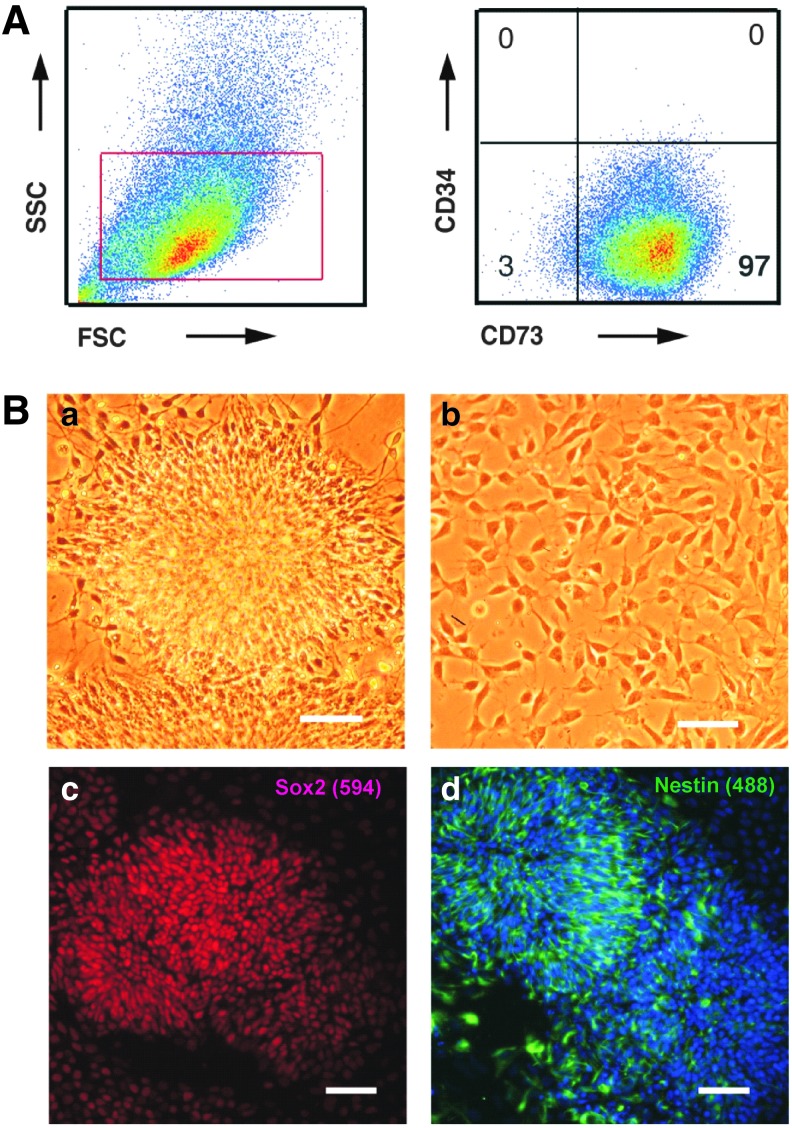
It has been suggested that Lefty resides at the crossroads of stemness and “differentiative events” [11], and emerging studies indicate considerable changes in Lefty expression of hESCs upon differentiation along different pathways [13,14,36]. Thus, we embarked on examining the possible variations in Lefty mRNA and protein expression as hESCs differentiated into NPCs and MSCs. Initially, both progenies were screened for their specific markers: FACS analysis revealed that ∼97% of MSCs were positive for CD73, with no expression of CD34 (Fig. 2A), and immunofluorescence imaging of NPCs revealed the presence of markers specific to neuronal progenitors (eg, Nestin and Sox2) (Fig. 2B). Subsequent experiments revealed that at the mRNA level, Lefty B mRNA is downregulated and Lefty A is minimally detected in MSCs, while both Lefty A and B are elevated in NPCs (Fig. 3A). These findings support the variability noted in the Lefty 1 and 2 ratio in the pluripotent differentiation of mouse ESCs, and during zebra fish embryo development [37,38].
FIG. 2.
Characterization of differentiated progenies of hESCs by FACS and immunofluorescence analysis. (A) FACS analysis of MSCs using antibody to CD73 and CD34 indicated ∼97% cells were positive for CD73, with no detectable presence of CD34. (B) Neuronal rosettes (a) and NPCs (b) were examined by phase contrast microscopy for their morphology and immunofluorescence analysis for their expression of markers specific to neuronal progenitors, Sox2 (c) and Nestin (d) The bar corresponds to 50 microns and nucleus is stained with DAPI (blue). MSCs, mesenchymal cells; NPCs, neuronal progenitor cells.
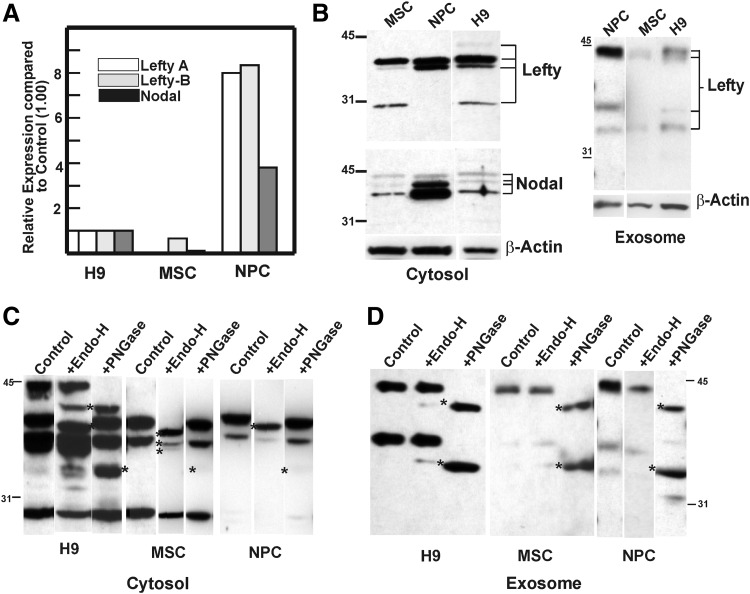
FIG. 3.
Differentiation of hESCs to mesenchymal cells (MSCs) and neuronal progenitor cells (NPCs) entails changes in Lefty A and B mRNA and protein expression, proteolytic processing, and glycoform composition. (A) Depicts relative expression of Lefty (A, B) mRNA in MSCs and NPCs compared to control parental hESC H9 (set at 1.0). (B) Represents the corresponding protein profile for cell- and exsosome-associated Lefty. The cytosolic extract is resolved on a 4%–20% acrylamide gel, and includes Nodal immunostaining and β-actin loading control. The exsosomal proteins were resolved on a 10% gel for better resolution. (C, D) Glycoform profile of cellular- (C) and exosome-associated (D) Lefty proteins differ in hESC, MSC, and NPC progenies. Cytosolic extract (30 μg), and exosomal proteins (5 μg) were denatured and subjected to digestion with Endo-H and PNGase F for 3 h, 37°C. The products were resolved on 10% SDS-PAGE, followed by western blot analysis for Lefty proteins. Cleavage products are indicated by ‘*’.
Western blot analyses of the cell lysates indicated differences in Lefty protein profiles. Specifically, the ≈44 kDa band was absent in both MSCs and NPCs, the ≈38 kDa band was marginally detected in MSCs, and the cleavage of Lefty to ≈28 kDa product was not noted in NPCs (Fig. 3B). The absence of the ≈28 kDa product in the NPCs might be due to the lack of appropriate convertases in these progenitor cells and indicative of a specific function for the cleavage products. It is plausible to speculate that the cleavage to the ≈40 kDa molecular form occurs as the hESCs commit to differentiate, and the latter differences signify lineage-specific PTMs of Lefty. Our observed increase in Lefty A and B in NPCs further confirms those reported by [3], indicating that an increased expression of Lefty 1 or 2 in animal cap explants of Xenopus laevis leads to neuralization as evidenced by the expression of neural markers, including neural cell adhesion molecule. NPCs also presented with elevated Nodal expression both at the mRNA and protein levels compared with the hESCs or MSCs.
Of interest, despite the absence of the ≈44 kDa band in the cell lysates of MSCs and NPCs, the exosome-associated Lefty had similar protein profiles in the parental and differentiated progenies (with the exception of low abundance and slightly smaller molecular mass noted in Lefty proteins of MSC exosomes) (Fig. 3B). This might signify a distinct role for the unprocessed full-length Lefty protein in hESCs (ie, self-renewal/pluripotency) and advocates independent functions for cell-associated versus exosomal Lefty by nominal PTM. Whether exosomal Lefty undergoes further proteolytic cleavage at its destination site remains to be determined.
In addition to the distinct differences in proteolytic processing, considerable heterogeneity exists between the parental and differentiated progenies in their cell- and exosome-associated Lefty glycoforms. Under the experimental conditions used, the ≈40 kDa cell-associated band in both MSCs and NPCs had Endo-H-sensitive glycans of smaller molecular mass (≈1.5 compared to ≈2.3–2.4 kDa; Fig 3C). Exosomal Lefty from MSCs was devoid of any Endo-H-sensitive glycans (which supports the lower molecular mass noted for 44 kDa Lefty), while that of NPCs contained Endo-H sensitive glycans, but of smaller molecular mass (Fig. 3C, D). The microheterogeneity in the protein processing and glycoform composition of Lefty protein(s) is a distinct feature of the differentiation along these lineages. Based on the studies in frog and zebra fish embryo, secreted Lefty displays a range of diffusion [28,39], regulated by interactions with ECM components [40]. Similarly, Lefty antagonizes Nodal signaling via EGF-CFC coreceptors (Criptic or Cripto) [41]. Processes that might be mediated by Lefty's observed PTMs remain to be further elucidated.
The observed differences in Lefty processing and glycan composition between parental and differentiated progenies could signify that beyond gene regulation, PTM of proteins could play critical role in the differentiation process. Differential processing or glycosylation per se could provide fine-tuning of protein properties in a cell- or tissue-specific manner without any changes in the amino acid sequence of the protein. Support for such a premise is emerging from studies that depict differential glycosylation as a key regulator of inter- and specifically extracellular signaling [42–44], and in directing pluripotency and differentiation of pluripotent stem cells [45].
Functional implication of changes in Lefty
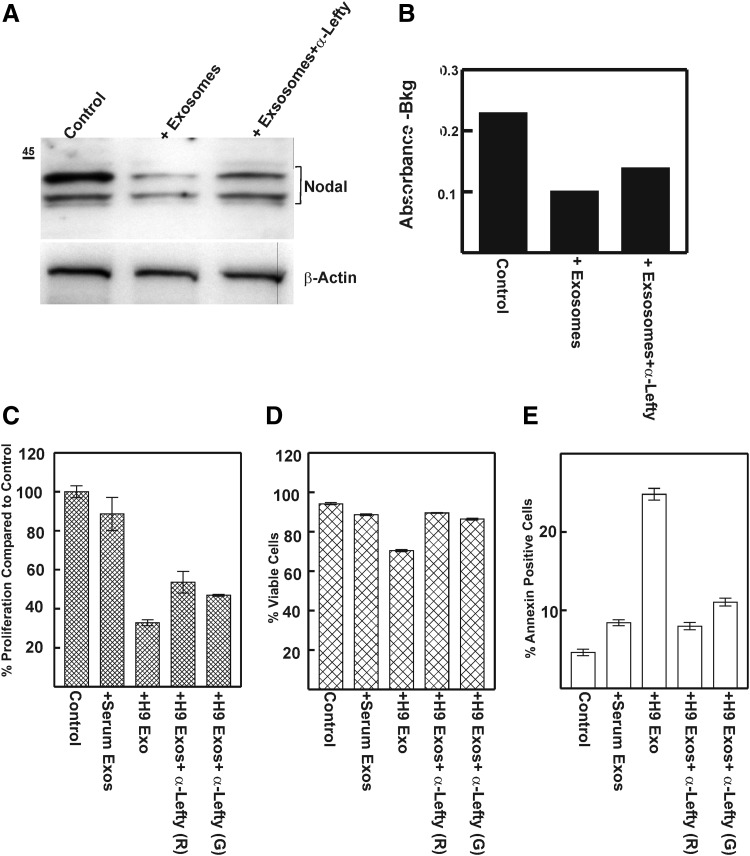
Based on the model primarily unveiled by developmental biology studies, Lefty antagonizes TGF-β/Nodal/Activin signaling through Smad2/3 [41,46,47]. This signaling pathway, which is believed to maintain stem cell pluripotency, is also a major contributor to the aggressive and metastatic phenotype of many cancers [48], and inhibition of Nodal signaling in aggressive melanoma cells reduces cell viability and proliferation considerably [49]. To assess whether Lefty encased in the exosomes was competent in antagonizing Nodal signaling, we treated cultures of aggressive melanoma cells with hESC exosomes. Nodal expression and Smad-2 phosphorylation were attenuated (Fig. 4A, B) and this effect was partially restored by prior treatment of exosomes with antibody to Lefty (goat polyclonal) (Fig. 4A, B). In addition, cell viability and apoptosis assays evaluated on a Guava Easycyte HT Flow Cytometer indicated that exosome-treated melanoma cells had reduced proliferation rate and viability compared to untreated controls (Fig. 4C, D). Notably, ∼25% of the cells were Annexin positive, indicative of apoptotic cells (Fig. 4E). These effects were partially restored with prior treatment with two different antibodies to Lefty (rabbit monoclonal and goat polyclonal).
FIG. 4.
Effect of hESC exsosomes on Nodal expression and signaling in aggressive melanoma cells. (A) Cultures of aggressive melanoma cells C8161 were treated with 5 μg total exsosomal protein/mL, with or without preincubation with 4 μg/mL antibody to Lefty (goat polyclonal). After 72 h, cell lysates were prepared and subjected to western blot analysis for Nodal expression. (B) For Smad-2 phosphorylation assay, the cultures were only treated overnight and cell lysates were prepared and used for pSmad-2 estimation by sandwich ELISA according to the manufacturer's instructions. (C–E) C8161 cells treated as described in (A) were also subjected to flow cytometry on Guava Easycyte HT Flow Cytometer using Guava ViaCount Reagent for the analysis of proliferation (C) and viability (D), and apoptosis (E, using Nexin reagent) according to the manufacturer's instructions. Analysis was performed on triplicate samples with untreated and calf serum exosome-treated cells as control. Exos, exosomes; G, goat polyclonal; R, rabbit monoclonal.
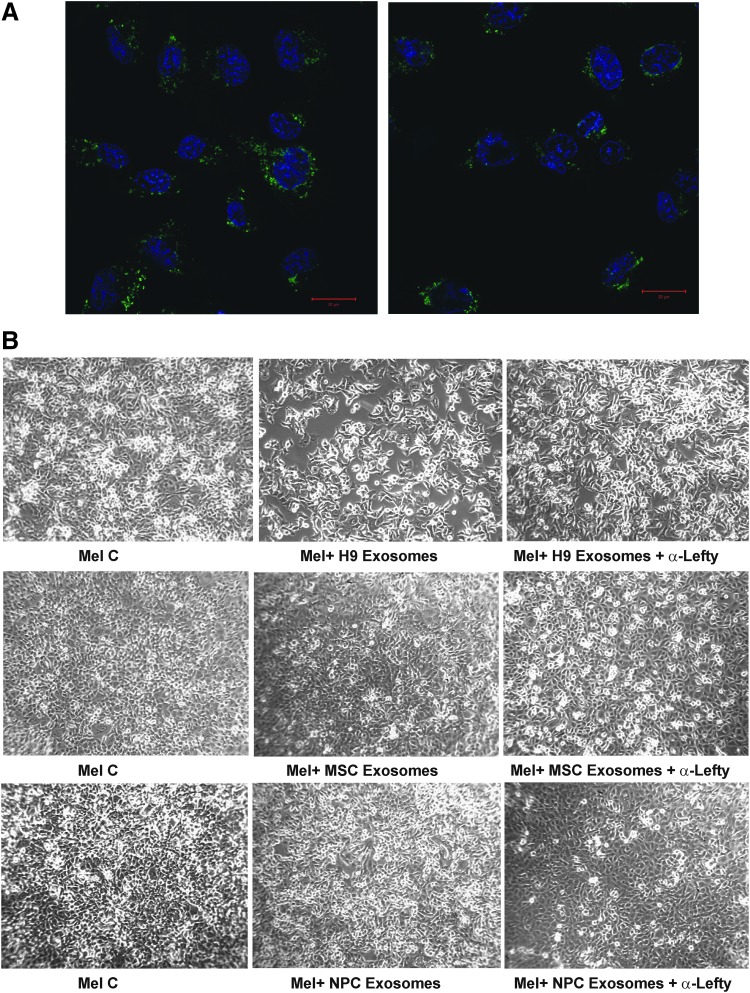
These studies were further extended to examine the functional significance of Lefty's differential processing and N-glycan moiety on aggressive melanoma cells. Initially, the cellular uptake of exosomes was verified by confocal microscopy of aggressive melanoma cells cocultured with PKH67-tagged exosomes. Within 48 h, vesicular association of tagged exosomes was clearly apparent and captured by confocal microscopy (Fig. 5A). As noted before, C8161 cells cocultured with hESC exosomes sustained substantial cell death (Fig. 5B). Pretreatment of hESC exosomes with Lefty antibody reduced their cytotoxicity considerably, but did not fully abrogate it (Fig. 5B). MSC exosomes had minimal effect on melanoma cell viability and/or proliferation, which did not change when the exosomes were preincubated with a Lefty antibody (Fig. 5B). Surprisingly, NPC exosomes induced apparent cell death and altered the morphology of melanoma cells from their “cobble stone” epithelial appearance to a more mesenchymal phenotype. Both cell death and morphology were somewhat reduced by prior treatment of exosomes with the Lefty antibody (Fig. 5B). Interestingly, r-Lefty at concentrations comparable to that of hESC H9 exosomes was considerably less effective in inhibiting melanoma cell proliferation (Supplementary Fig. S3).
FIG. 5.
Uptake and functional analysis of exosomal Lefty collected from parental hESCs and differentiated progenies (MSCs and NPCs) using cultures of aggressive melanoma cells. (A) Exosomes from H9 hESCs and MSCs were tagged with fluorescent PHK67 and incubated with aggressive melanoma cells (4 μg/mL). Images were obtained 48 h later using confocal microscopy. Nuclei are stained with DAPI (blue), and the bar corresponds to 20 microns. (B) Melanoma cells (Mel) were plated on eight-well culture slides and allowed to reach ∼75% confluency. They were then treated with 5 μg/mL of exosomal proteins from H9 hESCs, MSCs, and NPCs for 72 h. The media were replaced every 24 h and cultures were monitored by phase contrast microscopy, and images were captured at 72 h after incubation. Original magnification: 10×.
These findings raise the question of how the exosomal Lefty can access Nodal and its signaling components on the cell surface. Based on Fig. 5A, exosomes are mostly localized to the vesicular (most probably endosomal/lysosomal) compartments of melanoma cells, where the exosomal membrane could be digested and the content released. Studies have indicated that certain proteins processed in the lysosomes are transported back to the cell surface. Alternatively, upon attachment of exosomes to the cell membrane, specific protease(s) are released, leading to dissolution of the exsosomal membrane and release of Lefty at sight.
The functional differences in exosomal Lefty between the parental and differentiated progenies relate primarily to their PTMs. Differential processing of Lefty and distinct glycoform heterogeneity in both NPC and MSC progenies are discerning features. Specifically, MSC exosomes contained Lefty proteins lacking Endo-H-sensitive glycans, while exosomal Lefty from NPCs contained Endo-H-sensitive glycans—although of smaller apparent molecular mass. Clearly, further experiments are required to dissect the precise glycan composition of Lefty in parental versus the progenies to rule out the possibility that glycosylation differences could be coincidental, resulting from different localization of the protein or the differentiation status of the cells—and not vice versa.
Notably, Lefty proteins were far less abundant in MSCs compared to NPCs. Whether the NPC's exosomal Lefty is cleaved in a similar manner to its cellular counterpart at its destination site (which would reinforce the observed proteolytic divergence in cell-associated Lefty between the two progenies) remains to be determined. In addition, based on our mRNA data, Lefty A was minimally detected in MSCs, which suggests that the exosomal Lefty protein is likely Lefty B alone. However, in the absence of antibodies specific to Lefty A or B, this was not established by western blot analysis. Molecular approaches are required to transfect Lefty A or B tagged with specific probes to establish the Lefty isoforms present in the exosomes. It is also important to note that Lefty is not the only protein in the exosome vesicles. Based on published data and our observations presented in this article, exosomes contain myriad microRNAs, DNA, and other proteins capable of affecting cell viability and morphology.
In conclusion, our studies reveal, for the first time, that Lefty proteins use an exosomal pathway for extracellular release, and distinct differences exist in the cleavage products of cellular versus exosome-packaged Lefty, which suggests independent functions at two sites. In addition, differentiation of hESCs to neuronal progenitors or mesenchymal cells is associated with changes in LeftyA/LeftyB mRNA expression and PTM of Lefty protein(s), including differential protein cleavage and glycoforms. Collectively, these observations support the notion that specific changes in Lefty A and B, along with the generation of particular cleavage products and glycoforms, could provide a molecular model for dissecting Lefty's myriad functions.
From a clinical perspective, the increasing interest in the use of stem cells for treatment of debilitating diseases, such as cancer, diabetes, blindness, and neurodegeneration, has prompted the emergence of new therapies based on stem cell transplantation or use of endogenous stem cells. However, our findings related to the effects of hESC exosomes on aggressive melanoma cells could potentially provide an alternative and efficient exosome-based therapeutic approach in cancer treatment.
Supplementary Material
Acknowledgments
These studies were supported by NIH CA 121205 grant to MJCH. The authors are grateful to Sandra Clark (Cancer Biology and Epigenomics, Stanley Manne Children's Research Institute) for her generous help with the ultracentrifugation.
Author Disclosure Statement
M.J.C.H. and E.A.S. are the coinventors on a patent addressing the therapeutic utility of Lefty in cancer. The remaining authors declare no conflicts of interest.
References
- 1.Wang Y-C, Peterson SE. and Loring JF. (2014). Protein post-translatiomnal modificatios, and regulation of pluripotency in human stem cells. Cell Res 24:143–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson JK, Draper JS, Baillie HS, Fishel S, Thomson JA, Moore H. and Andrews PW. (2002). Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells 20:329–337 [DOI] [PubMed] [Google Scholar]
- 3.Meno C, Ito Y, Saijoh Y, Matsuda Y, Tashiro K, Kuhara S. and Hamada H. (1997). Two closely-related left-right asymmetrically expressed genes, lefty-1 and lefty-2: their distinct expression domains, chromosomal linkage and direct neuralizing activity in Xenopus embryos. Genes Cells 2:513–524 [DOI] [PubMed] [Google Scholar]
- 4.Kothapalli R, Buyuskal I, Wu S-Q, Chegini N. and Tabibzadeh S. (1997). Detection of ebaf a novel human gene of TGF-β superfamily: association of gene expression with endometrial bleeding. J Clin Invest 99:2342–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yashiro K, Saijoh Y, Sakuma R, Tada M, Tomita N, Amano K, Matsuda Y, Monden M, Okada S. and Hamadaet H. (2000). Distinct transcriptional regulation and phylogenetic divergence of human LEFTY genes. Genes Cells 5:343–357 [DOI] [PubMed] [Google Scholar]
- 6.Sperger JM, Chen X, Draper JS, Antosiewicz JE, Chon CH, Jones SB, Brooks JD, Andrews PW, Brown PO. and Thomson JA. (2003). Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Nat Acad Sci U S A 100:13350–13355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adjaye J, Huntriss J, Herwig R, BenKahla A, Brink TC, Wierling C, Hultschig C, Groth D, Yaspo ML, et al. (2005). Primary differentiation in the human blastocyst: comparative molecular portraits of inner cell mass and trophectoderm cells. Stem Cells 23:1514–1525 [DOI] [PubMed] [Google Scholar]
- 8.Vallier L, Alexander M. and Pedersen RA. (2005). Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci 118:4495–4509 [DOI] [PubMed] [Google Scholar]
- 9.James D, Levine AJ, Besser D. and Hemmati-Brivanlou A. (2005). TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 132:1273–1282 [DOI] [PubMed] [Google Scholar]
- 10.Dvash T, Sharon N, Yanuka O. and Benvenisty N. (2007). Molecular analysis of LEFTY-expressing cells in early human embryoid bodies. Stem Cells 25:465–472 [DOI] [PubMed] [Google Scholar]
- 11.Tabibzadeh S. and Hemmati-Brivanlou A. (2006). Lefty at the cossroads of “stemness” and differentiative events. Stem Cells 24:1998–2006 [DOI] [PubMed] [Google Scholar]
- 12.Fathi A, Hatami M, Hajihosseini V, Fattahi F, Kiani S, Baharvand H. and Salekdeh GH. (2011). Comprehensive gene expression analysis of human embryonic stem cells during differentiation into neural cells. PLoS One 6:e22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang M, Mikhailik A, Pauli I, Giudice LC, Fazelabas AT, Tulac S, Carson DD, Kaufman DG, Barbier C, Creemers JW. and Tabibzadeh S. (2005). Decidual differentiation of stromal cells promotes proprotein Convertase 5/6 expression and Lefty processing. Endocrinology 146:5313–5320 [DOI] [PubMed] [Google Scholar]
- 14.Westmoreland JJ, Takahashi S. and Wright CVE. (2007). Xenopus Lefty requires proprotein cleavage but not N-linked glycosylation to inhibit Nodal signaling. Dev Dynam 236:2050–2061 [DOI] [PubMed] [Google Scholar]
- 15.Pan BT, Teng K, Wu C, Adam M. and Johnstone RM. (1985). Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101:942–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tetta C, Ghigo E, Silengo L, Deregibus MC. and Camussi G. (2013). Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine 44:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangoda L, Boukouris S, Liem M, Kalra H. and Mathivanan S. (2015). Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics 15:260–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawson C, Vicencio JM, Yellon DM. and Davidson SM. (2016). Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol 228:R57–R71 [DOI] [PubMed] [Google Scholar]
- 19.Raposo G. and Stoorvogel W. (2013). Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, et al. (2001). Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7:297–303 [DOI] [PubMed] [Google Scholar]
- 21.Andre F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, et al. (2004). Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J. Immunol 172:2126–2136 [DOI] [PubMed] [Google Scholar]
- 22.Melo SA, Luecke LB, Kahlert C, Fernandez AF. and Seth T. (2015). Glypican-1 identifies cancer exosomes and detects early pacreatic cancer. Nature 523:177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson DL, Wehman B, Galat Y, Sharma S, Mishra R, Galat V, Kaushal S. (2014). Engineering patient-specific valves using stem cells generated from skin biopsy specimens. Ann Thorac Surg 98:947–954 [DOI] [PubMed] [Google Scholar]
- 24.Malchenko S, Xie J, de Fatima Bonaldo M, Vanin EF, Bhattacharyya BJ, Belmadani A, Xi G, Galat V, Goossens W, et al. (2014). Onset of rosette formation during spontaneous neural differentiation of hESC and hiPSC colonies. Gene 534:400–407 [DOI] [PubMed] [Google Scholar]
- 25.Thery C, Clayton A, Amigorena S. and Raposo G. (2006). Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3:Unit 3.22 [DOI] [PubMed] [Google Scholar]
- 26.Costa FF, Seftor EA, Bischof JM, Kirschmann DA, Strizzi L, Arndt K, Bonaldo MF, Soares MB. and Hendrix MJC. (2009). Epigenetically reprogramming metastatic tumor cells with an embryonic microenvironment. Epigenomics 1:387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulloa L, Creemers JWM, Roy S, Liu S, Mason J. and Tabibzadeh S. (2001). Lefty proteins exhibit unique processing and activate the MAPK pathway. J Biol Chem 276:21387–21396 [DOI] [PubMed] [Google Scholar]
- 28.Sakuma R, Ohnishi Y, Meno C, Fujii H, Juan H, Takeuchi J, Ogura T, Li E, Miyazono K. and Hamada H. (2002). Inhibition of Nodal signaling by Lefty mediated through interaction with common receptors and efficient diffusion. Genes Cells 7:401–412 [DOI] [PubMed] [Google Scholar]
- 29.Farina A, D'Aniello C, Severino V, Hochstrasser DF, Parente A, Minchiotti G. and Chambery A. (2011). Temporal proteomic profiling of embryonic stem cell secretom during cardiac and neuronal differentiation. Proteomics 11:3972–3982 [DOI] [PubMed] [Google Scholar]
- 30.Zhang HG, Liu C, Su K, Zhang L, Zhang S, Wang J, Cao X, Grizzle W. and Kimberly RP. (2006). A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J Immunol 176:7385–7393 [DOI] [PubMed] [Google Scholar]
- 31.Sanderson MP, Keller S, Alonso A, Riedle S, Dempsey PJ. and Altevogt P. (2008). Generation of novel, secreted epidermal growth factor receptor (EGFR/ErbB1) isoforms via metalloprotease-dependent ectodomain shedding and exosome secretion. J Cell Biochem 103:1783–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seelenmeyer C, Stegmayer C. and Nickel W. (2008). Unconventional secretion of fibroblast growth factor 2 and galectin-1 does not require shedding of plasma membrane-derived vesicles. FEBS Lett 582:1362–1368 [DOI] [PubMed] [Google Scholar]
- 33.Webber J, Steadman R, Mason MD, Steadman R, Mason MD, Tabi Z. and Clayton A. (2010). Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res 70:1–10 [DOI] [PubMed] [Google Scholar]
- 34.Neuberger A. and Marshall. RD. (1968). Aspects of the structure of glycoproteins. In: Symposium on Foods—Carbohydrates and Their Roles. Schultz HW, Cain RF, Wrolstad RW, eds. Avi Publishing Co., Westport, CT, pp 115–132 [Google Scholar]
- 35.Kornfeld R. and Kornfeld S. (1985). Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem 54:631–664 [DOI] [PubMed] [Google Scholar]
- 36.Dvash T, Mayshar Y, Darr H, McElhaney M, Barker D. and Yanuka O. (2004). Temporal gene expression during differentiation of human embryonic stem cells and embryoid bodies. Human Repro 19:2875–2883 [DOI] [PubMed] [Google Scholar]
- 37.Kim DK, Cha Y, Ahn HJ, Kim G. and Park KS. (2014). Lefty1 and lefty2 control the balance between self-renewal and pluripotent differentiation of mouse embryonic stem cells. Stem Cells Dev 23:457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Boxtel AL, Chesebro JE, Heliot C, Ramel MC, Stone RK. and Hill CS. (2015). A temporal window for signal activation dictates the dimensions of a Nodal signaling domain. Dev Cell 35:175–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller P, Rogers KW, Jordan BM, Lee JS, Robson D, Ramanathan S. and Schier AF. (2012). Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science 336:721–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marjoram L. and Wright C. (2011). Rapid differential transport of Nodal and Lefty on sulfated proteoglycan-rich extracellular matrix regulates left-right asymmetry in Xenopus. Development 138:475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen C. and Shen MM. (2004). Two modes by which Lefty proteins inhibit nodal signaling. Cur Biol 14:618–624 [DOI] [PubMed] [Google Scholar]
- 42.Billington CJ, Jr, Fiebig JE, Forsman CL, Pham L, Burbach N, Sun M, Jaskoll T, Mansky K, Gopalakrishnan R, et al. (2011). Glycosylation of twisted gastrulation is required for BMP binding and activity during raniofacial development. Front Physiol 2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haines N. and Irvine KD. (2003). Glycosylation regulates Notch signaling. Nat Rev Mol Cell Biol 4:786–797 [DOI] [PubMed] [Google Scholar]
- 44.Varelas X, Bouchie MP. and Kukuruzinska MA. (2014). Protein N-glycosylation in oral cancer: dysregulated cellular networks among DPAGT1, E-cadherin adhesion and canonical Wnt signaling. Glycobiol 24:579–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasehira K, Tateno H, Onuma Y, Ito Y, Asashima M. and Hirabayashi J. (2012). Structural and quantitative evidence for dynamic glycome shift on production of induced pluripotent stem cells. Mol Cell Proteomics 11:1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, Ohishi S, Noji S, Kondoh H. and Hamada H. (1998). Lefty-1 is Required for Left-Right determination as a regulator of lefty-2 and nodal. Cell 94:287–297 [DOI] [PubMed] [Google Scholar]
- 47.Meno M, Takeuchi J, Sakuma R, Koshiba-Takeuchi K, Ohishi S, Saijoh Y, Miyazaki J, Ten Dijke P, Ogura T. and Hamada H. (2001) Diffusion of Nodal signaling activity in the absence of the feedback inhibitor Lefty2. Dev Cell 1:127–138 [DOI] [PubMed] [Google Scholar]
- 48.Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J. and Hendrix MJC. (2006). Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med 12:925–932 [DOI] [PubMed] [Google Scholar]
- 49.Hardy KM, Strizzi L, Margaryan NV, Gupta K, Murphy GF, Scolyer RA. and Hendrix MJC. (2015). Targeting nodal in conjunction with dacarbazine induces synergistic anticancer effects in metastatic melanoma. Mol Cancer Res 13:670–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.