Abstract
Objective
To investigate the cerebellar inhibitory influence on the primary motor cortex in patients with focal dystonia using a cerebellar continuous theta-burst stimulation protocol (cTBS) and to evaluate any relationship with movement abnormalities.
Methods
Thirteen patients with focal hand dystonia, 13 patients with cervical dystonia and 13 healthy subjects underwent two sessions: (i) cTBS over the cerebellar hemisphere (real cTBS) and (ii) cTBS over the neck muscles (sham cTBS). The effects of cerebellar cTBS were quantified as excitability changes in the contralateral primary motor cortex, as well as possible changes in arm and neck movements in patients.
Results
Real cerebellar cTBS reduced the excitability in the contralateral primary motor cortex in healthy subjects and in patients with cervical dystonia, though not in patients with focal hand dystonia. There was no correlation between changes in primary motor cortex excitability and arm and neck movement kinematics in patients. There were no changes in clinical scores or in kinematic measures, after either real or sham cerebellar cTBS in patients.
Conclusions
The reduced cerebellar inhibitory modulation of primary motor cortex excitability in focal dystonia may be related to the body areas affected by dystonia as opposed to being a widespread pathophysiological abnormality.
Significance
The present study yields information on the differential role played by the cerebellum in the pathophysiology of different focal dystonias.
Keywords: Transcranial magnetic stimulation, Dystonia, Cerebellum, Motor control
1. Introduction
Adult-onset focal dystonia is clinically characterized by involuntary muscle contractions and abnormal postures that can affect different body regions, including the upper limb and neck (Defazio et al., 2007; Albanese et al., 2013; Jinnah et al., 2013). The pathophysiology of focal dystonia is still not entirely clear. Although dystonia is considered a basal ganglia disorder (Bhatia and Marsden 1994; DeLong and Wichmann 2007), recent studies indicate that the cerebellum may also be involved in this condition (Sadnicka et al., 2012; Prudente et al., 2014; Malone et al., 2014).
The results of animal studies show that abnormal cerebellar signalling may produce dystonia-like movements (Pizoli et al., 2002). Neuropathological examinations in post-mortem brain tissue of patients with cervical dystonia (CD) reveal Purkinje cell degeneration, areas of focal gliosis and torpedo bodies (Prudente et al., 2013). Clinical observations also indicate that focal dystonia may be associated with structural lesions of the cerebellum and its afferent pathways (LeDoux and Brady 2003; Batla et al., 2015). Moreover, neuroimaging studies using various techniques have provided evidence of cerebellar grey matter changes and altered cerebello-thalamo-cortical pathways in patients with focal hand dystonia (FHD) or CD (Draganski et al., 2003; Delmaire et al., 2007). Functional neuroimaging investigations have demonstrated abnormal resting state cerebello-thalamo-cortical connectivity in FHD patients (Dresel et al., 2014; Bharath et al., 2015).
Neurophysiological studies have also provided evidence of several cerebellar abnormalities in focal dystonia (Sadnicka et al., 2012). Eyeblink classical conditioning, a form of associative learning mediated by cerebellar circuits, is abnormally reduced in focal dystonia (Teo et al., 2009; Hoffland et al., 2013). Studies based on repetitive transcranial magnetic stimulation (TMS) techniques have shown that cerebellar stimulation in patients with FHD does not influence primary motor cortex (M1) excitability (Brighina et al., 2009; Hubsch et al., 2013).
Recent findings have raised a number of issues regarding the pathophysiological role of the cerebellum in focal dystonia that deserve further investigation. The abnormalities of the cerebellar influence on M1, as tested by repetitive TMS techniques, have been reported in FHD, whereas no data are available for CD. It is therefore unknown whether the abnormalities of the cerebellar inhibitory modulation of M1 are a common feature of the various forms of focal dystonia. In addition, no study has yet specifically addressed a possible relationship between abnormalities of the cerebellar inhibitory modulation of M1 and movement abnormalities in patients with focal dystonia. This information might provide further insight on the pathophysiological role of the cerebellum in focal dystonia.
In the present study, we first investigated the effects of cerebellar cTBS in patients with FHD and CD, as indexed by M1 excitability changes in the contralateral hemisphere. We then explored the relationships between individual M1 excitability changes following cerebellar cTBS with arm and neck movements as evaluated by a clinical assessment and kinematic analysis. Data from FHD and CD patients were compared with those observed in healthy controls.
2. Methods
2.1. Participants
Thirteen patients with FHD (2 women; mean age ±1 standard deviation: 48.5 ± 15.0) and 13 patients with CD (8 women; mean age ±1 standard deviation: 46.7 ± 14.5) were enrolled in the study (Table 1). A control group of thirteen healthy subjects (HS) (6 women; mean age ±1 standard deviation: 49.9 ± 11.3; Table 1) was also included in the study. The diagnosis of FHD and CD was based on clinical criteria (Albanese et al., 2013). The clinical assessment included the Wissel scale for FHD patients (Wissel et al., 1996) and the Toronto Western Spasmodic Torticollis Rating Scale-TWSTRS for CD patients (Comella et al., 1997). All the patients were right-handed and all patients with FHD had right arm dystonia. None of the patients exhibited upper limb tremor or neck pain that might interfere with the kinematic recordings. All the patients were studied three months after their last botulinum toxin injection and none of them were taking other medications active at the central nervous system level at the time of the experiments. The experimental procedures were approved by the local institutional review board and all the subjects gave their written informed consent to participation in the study. The experiments adhered to Declaration of Helsinki regulations.
Table 1.
Demographic and clinical data.
| FHD patients | CD patients | HS | |
|---|---|---|---|
| Gender | 11M/2F | 5M/8F | 9M/6F |
| Age (years) | 48.5 ± 15.0 | 46.7 ± 14.5 | 49.9 ± 11.3 |
| Disease duration (years) | 6.2 ± 6.6 | 6.4 ± 6.5 | – |
| Clinical Score | 11.6 ± 5.3 | 20.5 ± 9.8 | – |
Gender (M = male; F = female). FHD = focal hand dystonia; CD = cervical dystonia, HS = healthy subjects. The clinical score (at baseline) in FHD patients refers to the Wissel Scale writing movement score (ranging from 0 to 28). The clinical score (at baseline) in CD patients refers to the Toronto Western Spasmodic Torticollis Rating Scale-TWSTRS (ranging from 0 to 85). Plus and minus values are means ±1 standard deviation.
2.2. TMS and electromyographic techniques
TMS was delivered through two Magstim magnetic stimulators (Magstim Company, Withland, UK) connected with a figure-eight coil placed tangentially to the scalp with the handle pointing towards the back and approximately 45° away from the midline.
We assessed M1 excitability using single pulse TMS. For this purpose we first measured the resting motor threshold (RMT), i.e., the intensity of M1 stimulation able to elicit motor-evoked potential (MEP) of ~50 μV peak-to-peak amplitude in the resting first dorsal interosseous (FDI) muscle, as shown by surface electromyography (EMG). After the RMT assessment we collected the MEP input–output (I/O) curve using stimulation intensities of 100%, 110%, 120%, 130%, 140% and 150% of the RMT in random order. Traces with background EMG activity ≥50 μV were rejected online (less than 1% of trials).
We delivered cerebellar cTBS (ipsilateral to the affected side of the body in FHD patients) at intensities of 80% of the active motor threshold (AMT), i.e., the intensity of M1 stimulation able to elicit motor-evoked potential (MEP) of ~200 μV peak-to-peak amplitude in the tonically active FDI muscle, using a biphasic magnetic stimulator. The cTBS protocol consists of high frequency (50 Hz) burst of three stimuli, repeated at 5 Hz for an overall duration of 40 s. Cerebellar real cTBS was delivered with the coil positioned over the cerebellar hemisphere, i.e., 3 cm laterally to and 1 cm below the inion, while cerebellar sham cTBS consisted of the stimulation of neck muscles. Sham cTBS does not stimulate the cerebellum but does induce slight twitches in the neck muscle similar to those induced by real cTBS (Koch et al., 2008; Hoffland et al., 2012, 2013; Li Voti et al., 2014; Schirinzi et al., 2016).
Surface EMG was recorded from the FDI muscle ipsilateral to cerebellar cTBS using silver chloride electrode. EMG signals were amplified and band-pass filtered (20 Hz-1 kHz) using Digitimer D 360 (Digitimer, UK). EMG recordings were sampled at 5 kHz and stored on a PC using an analog–digital converter (AD 1401 plus Cambridge Electronic Design, UK). Off-line analysis was then performed using dedicated software (Signal® version 4.00, Cambridge Electronic Design, UK).
2.3. Kinematic recordings and analysis of arm and neck movements
During the experiments, FHD, CD patients and HS were seated in a chair with their limbs resting on a table. The arm and neck movements were assessed using a dedicated optoelectronic device (SMART, BTS, Milan, ltaly) consisting of three infrared cameras (120 Hz sampling rate) following the displacement in the tri-dimensional space of reflective markers taped on the upper arm and on the head. To record arm movements a reflective marker was placed on the wrist (Bologna et al., 2015). To record head movements two reflective markers were placed over the frontal orbital processes (bilaterally) and one over the nasion (Gregori et al., 2008). Three additional reflective markers were placed on the trunk to define a reference plane which allowed automatic exclusion of possible contamination due to trunk movements from the upper arm and head movement recordings. The kinematic analysis of the upper arm and head movements was performed using dedicated software (SMART Analyzer, BTS, Milan, ltaly) that runs an automatic algorithm to assess kinematic measures.
Subjects were instructed to reach and grasp with their index finger and thumb a 2 cm diameter, 15 cm long cylinder, firmly attached to the table at a distance of two thirds of their own arm’s length. The movement duration was defined as the time elapsing between the times at which the arm velocity exceeded and remained above, or fell and remained below, 5% of the velocity peak. We measured movement duration, velocity peak and acceleration peak. We also measured the trajectory straightness, as determined by the index of curvature, (calculated as the percentage ratio between the arm average path length during reaching movements and the length of a straight line joining the initial and final positions), the smoothness of the arm velocity curves (determined as the movement units, i.e. a local velocity peak preceded and followed respectively by increasing and decreasing values for at least 20 ms), and target overshooting (Bologna et al., 2015).
For head movement recordings, the participants were asked to perform fast head rotations and to move “as fast and widely as possible” (Gregori et al., 2008; Shaikh et al., 2015). As the CD patients were most commonly affected by torticollis, other movements such as flexion and extension movements were not recorded. The angular amplitude and peak angular velocity of rotational head movement were analysed. For the FHD patients and HS we analysed the data of fast neck movements toward the right side. In CD patients we analysed “pro-dystonic” movements (toward the side of the dystonic head movements) (Gregori et al., 2008; Shaikh et al., 2015).
2.4. Experimental design
FHD and CD patients and HS underwent two experimental sessions (real and sham cerebellar cTBS). The two sessions were randomly performed at least 1 week apart. The MEP I/O curve and the kinematic recordings of arm and neck movements were collected in each session before cerebellar cTBS (baseline) and 5 min (Post 1) and 45 min (Post 2) after. Ten MEPs were collected at intensities of 100%, 110%, 120%, 130%, 140% and 150% of the RMT in the three measurement time point; for each subject a total of 150 MEP were collected (the MEP testing lasted approximately 5–7 min in each measurement time point). For each participant, two trials of 5 reaching arm movements were recorded in each measurement time point (30 movements overall). Similarly, two trials of five consecutive head movements were recorded in each measurement (30 movements overall). In each session and measurement time point the I/O curves were collected before the kinematic recordings. During the kinematic recordings the arm and head movements were alternated during the same session.
2.5. Statistics
Age differences between FHD and CD patients and HS were assessed using Kruskar-Wallis analysis of variance (ANOVA). Differences in gender ratio between the three groups were evaluated using the X2 test. The MEP I/O curve and kinematic data were analysed by repeated measures ANOVAs using the factors GROUP (FHD, CD and HS), SESSION (real and sham stimulation), TIME (baseline, Post 1 and Post 2) and STIMULATION INTENSITIES (100%, 110%, 120%, 130%, 140% and 150% of the RMT). The clinical scores of FHD and CD severity were assessed using separate Friedman’s ANOVAs, with factors TIME (baseline, Post1 and Post2). Post hoc analysis was performed using Tukey honest test. Pearson’s correlation was used to investigate possible relationship between individual M1 excitability changes (i.e., the average MEP amplitude at Post 1/baseline) and individual percentage changes in arm and head movement kinematics (Post 1/baseline measurements) after the real cerebellar cTBS. Unless otherwise stated results are reported as mean values ±1 standard error of the mean (SEM) with the statistical significance threshold set at P < 0.05.
3. Results
No difference in age was observed between FHD patients, CD patients and HS. Their RMT and AMT values were also similar between FHD patients, CD patients and HS in the two experimental sessions (Supplementary Table S1). No adverse effects were observed during the experimental procedures.
3.1. M1 excitability measurements
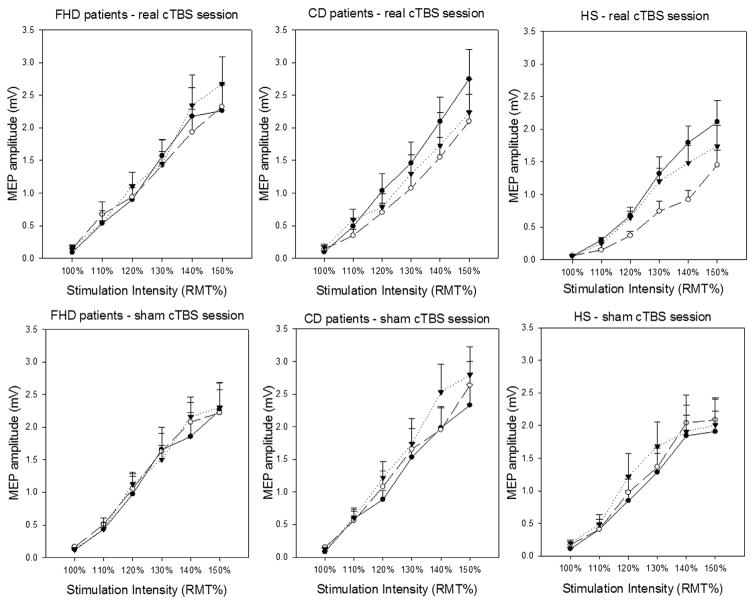
As expected, the factor STIMULATION INTENSITY was significant (F5,180 = 107.18, P < 0.001), thereby indicating increasing MEP amplitudes with higher stimulation intensities. No significant interactions were observed for GROUP × STIMULATION INTENSITY (F10,180 = 0.62, P = 0.79) and GROUP × STIMULATION INTENSITY × SESSION (F10,180 = 0.66, P = 0.75), demonstrating a similar I/O curve of the MEP, i.e. similar baseline M1 excitability, in all three groups of participants (Fig. 1).
Fig. 1.
MEP input–output curve in FHD and CD patients and HS in the real and sham cerebellar cTBS sessions. Y axis indicates MEP amplitudes (mV); X axis indicates the stimulation intensities (from 100% to 150% resting motor threshold – RMT) in the two experimental sessions (real cerebellar cTBS – upper panels; sham cerebellar cTBS – lower panels) in patients with focal hand dystonia – FHD (left panels), in patients with cervical dystonia – CD (central panels) and in healthy subjects (HS) (right panels) at baseline (before cTBS), circular black symbols (continuous lines), at Post 1 (5 min after cTBS), circular white symbols (dashed lines), and at Post 2 (45 min after cTBS), triangular black symbols (dotted lines).
Repeated measure ANOVA also revealed a significant effect for the main factor TIME (F2,72 = 3.45, P = 0.04) and for the interactions SESSION × TIME (F2,72 = 7.08, P = 0.002) and SESSION × TIME × STIMULATION INTENSITY (F10,360 = 2.99, P = 0.001). Most importantly, however, repeated-measures ANOVA showed a significant effect for the interactions GROUP × SESSION × TIME × STIMULATION INTENSITY (F20,360 = 1.61, P = 0.04) which indicates differences in the effect of real and sham cerebellar cTBS on the MEP I/O curve in FHD patients, CD patients and HS. The post hoc analysis showed that real cerebellar cTBS reduced the excitability of the contralateral M1 in CD and in HS, but not in FHD. Lower MEP amplitude values were observed 5 min after cTBS while no MEP amplitude changes were detected after sham cerebellar cTBS in any of the three groups of participants (all, P > 0.05). Lastly, no significant effects were observed for the main factors GROUP (F2,36 = 0.67, P = 0.52), SESSION (F1,36 = 3.67, P = 0.06) and for the interactions GROUP × SESSION (F2,36 = 1.48, P = 0.24), GROUP × TIME (F4,52 = 0.58, P = 0.72), GROUP × SESSION × TIME (F4,72 = 1.77, P = 0.14), SESSION × STIMULATION INTENSITY (F5,180 = 1.99, P = 0.08), TIME × STIMULATION INTENSITY (F10,360 = 1.68, P = 0.08) and GROUP × TIME × STIMULATION INTENSITY (F20,360 = 0.85, P = 0.65). Further analysis performed on separate groups are provided in Supplementary Results.
3.2. Clinical scores and arm and neck movement kinematics
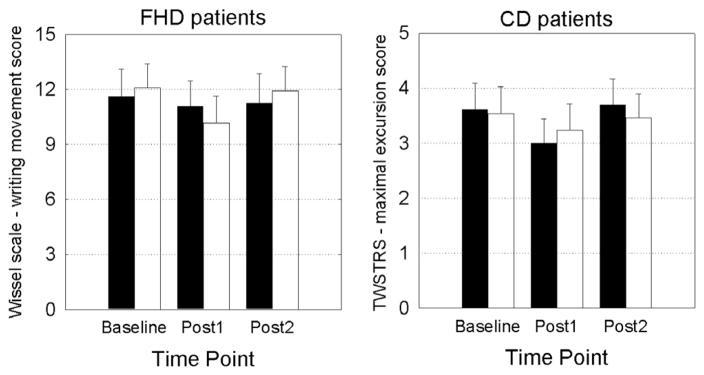
Friedman’s ANOVA showed that cerebellar cTBS did not significantly modify the clinical scores, i.e. the Wissel scale – writing movement score in FHD (real cerebellar cTBS: , P = 0.97; sham cerebellar cTBS: , P = 0.33, Fig. 2), or the TWSTRS – maximal excursion score in CD (real cerebellar cTBS: , P = 0.13; sham cerebellar cTBS: , P = 0.35, Fig. 2).
Fig. 2.
Clinical scores in FHD and CD patients in the real and sham cerebellar cTBS sessions. Clinical rating of FHD patients (left panel) and CD patients (right panel) during the three measurement time points (baseline, Post1 – 5 min after cTBS and Post 2 – 45 min after cTBS). Black histograms indicate the real cerebellar cTBS session. White histograms indicate the sham cerebellar cTBS session.
The kinematic variables of reaching movements are shown in Table 2. There was no significant effect of GROUP, SESSION and TIME POINT or any significant interaction for the kinematic parameters analysed, as assessed by repeated measures ANOVA (all P > 0.05). The analysis suggests that duration, velocity peak, acceleration peak, straightness, smoothness and overshooting of reaching movements did not differ between FHD, CD patients and HS; there were no significant change of the reaching movement kinematics in the three groups after real or sham cerebellar cTBS (Supplementary Table S2).
Table 2.
Reaching movement kinematics in patients with focal hand dystonia (FHD), cervical dystonia (CD) and in healthy subjects (HS), before (baseline) and after (POST1 and POST2) real and sham cerebellar cTBS.
| Real cTBS
|
Sham cTBS
|
|||||
|---|---|---|---|---|---|---|
| baseline | Post1 | Post2 | baseline | Post1 | Post2 | |
| FHD patients | ||||||
| Duration | 485.44 ± 25.31 | 486.92 ± 25.52 | 482.11 ± 23.49 | 506.69 ± 30.84 | 498.65 ± 27.49 | 492.76 ± 25.86 |
| Velocity peak | 1.42 ± 0.09 | 1.44 ± 0.10 | 1.41 ± 0.10 | 1.32 ± 0.08 | 1.39 ± 0.09 | 1.41 ± 0.09 |
| Acceleration peak | 8.95 ± 0.78 | 9.29 ± 0.94 | 9.02 ± 0.88 | 7.97 ± 0.73 | 8.66 ± 0.85 | 8.84 ± 0.90 |
| Straightness | 105.16 ± 0.63 | 104.90 ± 0.64 | 104.08 ± 0.56 | 104.85 ± 0.61 | 104.91 ± 0.62 | 104.41 ± 0.55 |
| Smoothness | 1.30 ± 0.17 | 1.35 ± 0.27 | 1.12 ± 0.07 | 1.61 ± 0.27 | 1.28 ± 0.10 | 1.15 ± 0.06 |
| Overshooting | 7.62 ± 1.35 | 7.93 ± 1.06 | 7.45 ± 1.43 | 5.48 ± 0.93 | 6.12 ± 0.07 | 5.88 ± 0.97 |
| CD patients | ||||||
| Duration | 512.13 ± 27.81 | 521.96 ± 25.18 | 519.97 ± 23.90 | 524.38 ± 23.04 | 509.07 ± 22.72 | 509.73 ± 21.70 |
| Velocity peak | 1.39 ± 0.08 | 1.38 ± 0.08 | 1.37 ± 0.08 | 1.37 ± 0.10 | 1.39 ± 0.08 | 1.39 ± 0.09 |
| Acceleration peak | 8.28 ± 0.76 | 8.14 ± 0.85 | 8.19 ± 0.89 | 7.43 ± 0.64 | 8.09 ± 0.78 | 8.30 ± 0.89 |
| Straightness | 105.17 ± 0.84 | 105.85 ± 1.07 | 105.29 ± 1.07 | 104.00 ± 0.49 | 104.25 ± 0.79 | 104.10 ± 0.73 |
| Smoothness | 1.19 ± 0.08 | 1.10 ± 0.03 | 1.08 ± 0.04 | 1.30 ± 0.16 | 1.14 ± 0.05 | 1.16 ± 0.04 |
| Overshooting | 6.01 ± 1.46 | 4.59 ± 0.75 | 4.46 ± 0.70 | 5.36 ± 0.96 | 5.98 ± 1.40 | 4.77 ± 0.82 |
| HS | ||||||
| Duration | 470.47 ± 19.96 | 482.50 ± 20.10 | 476.50 ± 20.98 | 458.83 ± 19.71 | 475.56 ± 19.82 | 479.58 ± 23.30 |
| Velocity peak | 1.50 ± 0.07 | 1.49 ± 0.09 | 1.50 ± 0.08 | 1.56 ± 0.010 | 1.48 ± 0.08 | 1.49 ± 0.09 |
| Acceleration peak | 9.34 ± 0.67 | 9.22 ± 0.78 | 9.52 ± 0.75 | 9.36 ± 0.73 | 9.38 ± 0.80 | 9.43 ± 0.73 |
| Straightness | 104.64 ± 0.29 | 104.61 ± 0.45 | 104.70 ± 0.50 | 105.27 ± 0.45 | 105.72 ± 0.59 | 105.25 ± 0.43 |
| Smoothness | 1.32 ± 0.14 | 1.39 ± 0.19 | 1.23 ± 0.13 | 1.19 ± 0.10 | 1.10 ± 0.05 | 1.16 ± 0.10 |
| Overshooting | 7.21 ± 1.45 | 7.42 ± 1.04 | 6.23 ± 1.11 | 6.52 ± 1.59 | 7.58 ± 1.23 | 6.26 ± 1.07 |
Duration is expressed in ms. Velocity peak is expressed in m/s. Acceleration peak is expressed in m/s2. Straightness refers to the index of curvature, i.e. the percentage ratio between the arm average path length and the length of a straight line joining the initial and final positions. Smoothness refers to the number of units of the reaching movement velocity curves. Overshooting is expressed in mm. Values are means ±1 standard error of the mean.
The kinematic variables of neck movements are shown in Table 3. The analysis showed a significant effect for the main factor GROUP for both the angular amplitude (F2,36 = 4.59, P = 0.02) and peak angular velocity (F2,36 = 8.66, P = 0.001), post hoc analysis showed that the angular amplitude and peak angular velocity of fast neck movements were lower in CD patients than in FHD patients and HS (all P ≤ 0.05), whereas no difference emerged between patients with FHD and HS. Finally, the analysis showed no significant effect of SESSION and TIME POINT or any significant interaction for the kinematic variables considered (all P > 0.05), thus indicating that neither real nor sham cerebellar cTBS changed the kinematic variables of fast neck movements in FHD, CD patients or HS (Supplementary Table S3).
Table 3.
Neck rotational movements in patients with focal hand dystonia (FHD), cervical dystonia (CD) and in healthy subjects (HS), before (baseline) and after (POST1 and POST2) real and sham cerebellar cTBS.
| Real cTBS
|
Sham cTBS
|
|||||
|---|---|---|---|---|---|---|
| baseline | Post1 | Post2 | baseline | Post1 | Post2 | |
| FHD patients | ||||||
| Angular amplitude | 64.49 ± 2.70 | 64.31 ± 2.48 | 64.47 ± 2.62 | 63.27 ± 3.33 | 61.30 ± 3.47 | 62.23 ± 3.60 |
| Peak angular velocity | 214.89 ± 29.68 | 225.30 ± 28.66 | 245.19 ± 24.23 | 214.16 ± 33.17 | 211.98 ± 30.57 | 207.25 ± 34.28 |
| CD patients | ||||||
| Angular amplitude | 55.50 ± 2.89 | 58.44 ± 3.38 | 57.71 ± 3.44 | 55.33 ± 2.33 | 57.23 ± 2.53 | 55.71 ± 2.38 |
| Peak angular velocity | 111.31 ± 12.15 | 114.79 ± 13.23 | 129.32 ± 13.30 | 129.48 ± 12.70 | 130.99 ± 12.66 | 139.24 ± 17.32 |
| HS | ||||||
| Angular amplitude | 66.04 ± 3.59 | 63.96 ± 3.40 | 66.22 ± 3.38 | 68.42 ± 2.35 | 67.94 ± 2.67 | 68.69 ± 3.42 |
| Peak angular velocity | 296.53 ± 41.90 | 297.56 ± 41.87 | 294.98 ± 39.08 | 265.31 ± 36.17 | 266.80 ± 38.05 | 283.57 ± 41.48 |
Angular amplitude is expressed in degrees, peak angular velocity is expressed in degrees/s. Values are means ±1 standard error of the mean.
3.3. Correlations
There was no relationship between individual M1 excitability changes and arm or neck movements kinematics in patients (all Ps > 0.05).
4. Discussion
In the present study, we found that real cerebellar cTBS reduced M1 excitability in HS. Our results are consistent with previous observations showing that it is possible to modulate the motor cortex from the cerebellum using cerebellar cTBS (Koch et al., 2008; Li Voti et al., 2014; Schirinzi et al., 2016). The novel finding of this study is that cerebellar cTBS reduced the M1 excitability in patients with CD though not in those with FHD. There was no relationship between individual inhibitory effects evoked by cerebellar cTBS on M1 excitability, clinical scores and arm and neck movements kinematics, in patients. Lastly, there was no significant change in arm and neck movements as evaluated by a clinical assessment and kinematic analysis following cerebellar cTBS in patients.
In keeping with the results of previous studies, no differences were observed in the resting motor threshold at baseline between FHD and CD patients and HS (Kojovic et al., 2013; Hubsch et al., 2013). As the I/O curves of the MEP did not differ between FHD and CD patients and HS, we ruled out the possibility that the differential effects of cerebellar cTBS over M1 in the three groups of participants reflect differences in baseline M1 excitability. In this regard, Ikoma et al. (1996) observed that M1 excitability is increased in dystonia, whereas according to more recent observations M1 excitability at rest is normal in FHD patients (Tinazzi et al., 2005) and in primary dystonia with arm involvement (Kojovic et al., 2013). These contrasting results concerning M1 excitability in FHD are likely to reflect differences in the methodology used and in the clinical features of the patients enrolled in the studies cited (Tinazzi et al., 2009). Since FHD and CD patients were studied at least three months after their last botulinum toxin injection, we believe that the effects of cerebellar cTBS on M1 excitability are unlikely to have been confounded by the effects of botulinum toxin (Abruzzese and Berardelli, 2006). Changes in corticomotor excitability have been described after voluntary muscle contraction, including exhaustive exercise with muscle fatigue and non-exhaustive contraction (Teo et al., 2012). Thus, it could be possible that changes in I/O curves are due to movement itself and not to cerebellar stimulation. However, since we did not observe any M1 excitability change during the sham session in all three groups of subjects enrolled in this study, we exclude this possibility. Finally the number of arm and neck movements performed were limited, also making unlikely that movement itself may have influenced the M1 excitability.
To the best of our knowledge, this is the first study that has compared the influence of the cerebellar cTBS on M1 excitability in CD and FHD patients. The observation that cerebellar cTBS inhibited M1 excitability in CD though not in FHD patients, indicates that the influence of the cerebellum on M1 in the various forms of focal dystonia may vary. The lack of M1 inhibition following cerebellar cTBS in FHD patients, though not in CD patients, indicates that cerebellar modulation of M1 excitability, as tested by cTBS, is reduced in FHD. Our findings are in agreement with those of previous studies that were based on different TMS techniques, i.e., cerebellar-brain inhibition (Brighina et al., 2009) and cerebellar cTBS (Hubsch et al., 2013). The lack of cerebellar inhibitory modulation of M1 in FHD and the hypothesis of a pathophysiological role of cerebellum in FHD is also in line with recent evidence showing a significant reduction in resting state functional connectivity in patients, compared with HS, involving the cerebellum, thalamus, basal ganglia and frontal areas (Bharath et al., 2015) or the therapeutic benefit of cerebellar transcranial direct current stimulation in patients with FHD (Bradnam et al., 2015). The mechanism of action of cerebellar repetitive TMS, including cTBS, is still matter of debate. Studies using 1 Hz rTMS applied over the cerebellum led to an increase of MEP amplitude (Oliveri et al., 2005; Fierro et al., 2007; Popa et al., 2010), thus suggesting a decrease of the Purkinje output to dentate nucleus and as a consequence disinhibition of the dentate-cortical drive. Harrington and Hammond (2015), however, have recently demonstrated that cTBS (delivered at low intensity, i.e., 80% of AMT) decreased the N100 waveform of the TMS-evoked potential, an indirect measure of cortical inhibition, and the resting MEP amplitude in the contralateral M1. These authors thus concluded that the effects of cTBS are likely exerted by inhibition of the superficial layer of the cerebellum (which has an inhibitory role on Purkinje cell activity). As a consequence cerebellar cTBS facilitates the Purkinje cells (inhibitory) activity and decreases the resting MEP amplitude in the contralateral M1 through the dentate-thalamo-cortical pathway. The results of the various studies, therefore, suggest that cerebellar 1 Hz rTMS and cTBS target different neuronal populations (the Purkinje cells vs cells of the superficial layer of the cerebellar cortex). The lack of any inhibitory cerebellar effect on the contralateral M1, as tested by cerebellar cTBS, in FHD patients may contribute to the loss of M1 inhibition, altered M1 plasticity and the development of incorrect motor programs and maladaptive behaviours (Hubsch et al., 2013). Alternatively, as FHD patients are asymptomatic at rest, reduced cerebellar inhibitory modulation of M1 might reflect compensatory changes in this disorder (Dresel et al., 2014).
The second aim of the study was to assess whether individual changes in M1 excitability, following cerebellar cTBS, correlate with the disease severity, as evaluated by clinical scores, or movement abnormalities, as assessed by kinematic techniques. The results indicate no relationship between individual M1 excitability changes after cerebellar cTBS and the patients’ clinical rating scale scores. The results of the present study also indicate that reaching movement kinematics, i.e., duration, velocity and acceleration, are normal in FHD and CD patients. Moreover, we found no significant group difference in terms of the quality of the movement, i.e., straightness, smoothness and overshooting. Our kinematic results are in contrast to those of previous studies showing the slowness of movement in patients with dystonia (Agostino et al., 1992; Curra et al., 2000; Prodoehl et al., 2008). Differences in results, however, may reflect the different movements analysed or the clinical heterogeneity of patients enrolled in the various studies. There is also evidence showing that reaching movements are impaired in patients with idiopathic dystonia of the upper limb (Inzelberg et al., 1995) as well as to those of more recent investigations in patients with CD (Pelosin et al., 2009). However, in their study, Inzelberg et al. (1995) investigated patients with dystonia involving other body segments besides the upper limbs, whereas in our study we only enrolled patients with an isolated FHD. The abnormal findings reported by Pelosin et al. (2009) might have depended on the clinical heterogeneity (i.e. subtle involvement of shoulder region or associated features like tremor of the upper limbs or neck pain which often occur in CD and can possibly impair reaching arm movements) or other methodological differences. Finally, Katschnig-Winter et al. (2014) found that the motor performance of the upper limb in patients with CD was similar to HC, apart from a significantly higher peak velocities in patients. Thus further studies should address the issue whether that the integration of proprioceptive input, which is involved in the internal models of limb dynamics, is altered in focal dystonia. Our results indicate that FHD may only involve a specific motor program, such as writing, whereas other motor tasks may well not be affected.
Taken as a whole, the results of the present study indicate that cerebellar dysfunction patterns vary in the different forms of primary focal dystonia and that the abnormally reduced cerebellar inhibitory outflow observed in FHD patients is not a characteristic feature of CD. This hypothesis is supported by a number of recent studies based on various neurophysiological techniques, in which the cerebellum was found not to be affected in CD. For example, it has been recently reported that CD patients did not differ from HS in the adaptation of the walking parameter, including speed, step width, step length symmetry and swing/stance ratio (Hoffland et al., 2014). Using a visuomotor task, Sadnicka et al. (2014) tested the hypothesis that cerebellar abnormalities in CD patients would translate into motor adaptation deficits. However, not only were adaptation rates (learning) in CD patients found to be similar to those of HS, but the ability to adapt had no relationship with the clinical features of CD. The only reports of a possible involvement of the cerebellum in CD patients is based on evidence indicating that the EBCC paradigm is abnormally reduced (Teo et al., 2009; Hoffland et al., 2013). We therefore conclude that reduced cerebellar inhibitory modulation over M1 is likely to be related to the body areas affected by dystonia as opposed to being a widespread pathophysiological abnormality of the disease. An alternative interpretation is that CD and FHD may differ in terms of pathophysiological mechanisms with the inhibitory pathways between the cerebellum and M1 being involved in FHD but not in CD. Recently, Koch et al. (2014) demonstrated that 2 weeks of cerebellar cTBS induced a mild clinical improvement and a normalization of physiological abnormalities of M1 (including altered plasticity) in CD. The results of the present study, and those of Koch et al. (2014) thus suggest that the therapeutic effects of cerebellar cTBS may possibly depend on a normalization of abnormal M1 mechanisms rather than of normalization of abnormal cerebellar activity per se. Finally, the lack of any correlation between individual M1 excitability changes and clinical scores of dystonia severity is line with the hypothesis that dystonia is a network disorder that affects multiple brain regions (Prudente et al., 2014).
The present study has certain limitations. We found a significant inhibitory effect of cerebellar cTBS on M1 excitability at 5–10 min after stimulation but it should be acknowledged that synaptic plasticity in the cerebello-thalamo-cortical circuit may require a longer time to allow biological changes as shown by experimental recordings in vitro (Aumann et al., 2000) and neurophysiological studies in humans (Schirinzi et al., 2016). Since we only assessed CD and FHD patients we cannot easily generalize our findings to patients with other forms of focal dystonia. Additionally, we did not examine M1 excitability of the hand muscles after stimulation of the cerebellar hemisphere corresponding to the unaffected body segment in FHD patients. Moreover, since we did not test M1 excitability of neck muscles after cerebellar stimulation in CD patients, we did not further investigate the possibility that cerebellar influence on M1 connectivity is abnormal in CD patients exclusively in circuits that control the neck muscles thus strengthening the hypothesis that the cerebellar inhibitory modulation of M1 excitability in focal dystonia may be related to the body areas affected by dystonia. However, techniques for evaluating M1 excitability in the cortical representation of the neck muscles are still technically challenging since the M1 projection to both the ipsilateral and contralateral sternocleidomastoid muscles arises from an area of cortex on the cerebral convexity close to the trunk representation (Berardelli et al., 1991; Thompson et al., 1997).
5. Conclusions
The present study yields information on the possible role played by the cerebellum in the pathophysiological mechanisms underlying dystonia. Unlike M1 plasticity mechanism abnormalities, which are present throughout the cortical sensorimotor areas (Quartarone et al., 2008), the abnormal cerebellar influence is only found in cortical areas that control the hand muscles. The reasons for these differences are as yet unknown. One possibility is that cerebellar changes only occur in the motor circuits corresponding to the affected body segments. Alternatively, the cerebellum may be involved in the pathophysiology of FHD though not in that of CD. Indeed, although the cerebellum is known to regulate the movements of various body segments, the cerebellar representation of the hand muscles prevails over that of the axial muscles, including the neck muscles (Mottolese et al., 2013). If true, the cerebellum may consequently be considered an important node in the network that is responsible for FHD but not for CD.
Supplementary Material
HIGHLIGHTS.
Cerebellar cTBS reduced the M1 excitability in cervical dystonia, but not in focal hand dystonia.
Cerebellar cTBS had no effect on movement kinematics in either cervical dystonia or focal hand dystonia.
The data indicate that the pathophysiological role of the cerebellum is not identical in all types of dystonia.
Acknowledgments
This study was funded in part by grants to the Dystonia Coalition from the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences (NCATS U54 TR01456) and the National Institute of Neurological Disorders and Stroke (NINDS U54 NS065701) from the NIH. Dr. Hallett is supported by the NINDS Intramural Program.
Conflicts of interest: None.
Abbreviations
- AMT
active motor threshold
- ANOVA
analysis of variance
- CD
cervical dystonia
- cTBS
continuous theta-burst stimulation
- EMG
electromyographic
- FDI
first dorsal interosseous
- FHD
focal hand dystonia
- HS
healthy subjects
- IC
index of curvature
- I/O
input–output
- MSO
maximal stimulator output
- MEP
motor-evoked potential
- M1
primary motor cortex
- RMT
resting motor threshold
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.clinph.2016.09.008.
Footnotes
The abstract has been published as a poster presentation at the 20th International Congress of Parkinson’s Disease and Movement Disorders in June 2016, Berlin.
References
- Abbruzzese G, Berardelli A. Neurophysiological effects of botulinum toxin type A. Neurotox Res. 2006;9:109–14. doi: 10.1007/BF03033927. [DOI] [PubMed] [Google Scholar]
- Agostino R, Berardelli A, Formica A, Accornero N, Manfredi M. Sequential arm movements in patients with Parkinson’s disease, Huntington’s disease and dystonia. Brain. 1992;115:1481–95. doi: 10.1093/brain/115.5.1481. [DOI] [PubMed] [Google Scholar]
- Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–73. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumann TD, Redman SJ, Horne MK. Long-term potentiation across rat cerebello-thalamic synapses in vitro. Neurosci Lett. 2000 Jun 23;287(2):151–5. doi: 10.1016/s0304-3940(00)01162-9. [DOI] [PubMed] [Google Scholar]
- Batla A, Sánchez MC, Erro R, Ganos C, Stamelou M, Balint B, et al. The role of cerebellum in patients with late onset cervical/segmental dystonia?-Evidence from the clinic. Parkinsonism Relat Disord. 2015;21:1317–22. doi: 10.1016/j.parkreldis.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Bharath RD, Biswal BB, Bhaskar MV, Bhaskar MV, Gohel S, Jhunjhunwala K, et al. Repetitive transcranial magnetic stimulation induced modulations of resting state motor connectivity in writer’s cramp. Eur J Neurol. 2015;22:796–805. doi: 10.1111/ene.12653. [DOI] [PubMed] [Google Scholar]
- Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117:859–76. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Priori A, Inghilleri M, Cruccu G, Mercuri B, Manfredi M. Corticobulbar and corticospinal projections to neck muscle motoneurons in man. A functional study with magnetic and electric transcranial brain stimulation. Exp Brain Res. 1991;87:402–6. doi: 10.1007/BF00231857. [DOI] [PubMed] [Google Scholar]
- Bologna M, Rocchi L, Leodori G, Paparella G, Conte A, Kahn N, et al. Cerebellar continuous theta burst stimulation in essential tremor. Cerebellum. 2015;14:133–41. doi: 10.1007/s12311-014-0621-0. [DOI] [PubMed] [Google Scholar]
- Bradnam LV, Graetz LJ, McDonnell MN, Ridding MC. Anodal transcranial direct current stimulation to the cerebellum improves handwriting and cyclic drawing kinematics in focal hand dystonia. Front Hum Neurosci. 2015;9:286. doi: 10.3389/fnhum.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighina F, Romano M, Giglia G, Saia V, Puma A, Giglia F, et al. Effects of cerebellar TMS on motor cortex of patients with focal dystonia: a preliminary report. Exp Brain Res. 2009;192:651–6. doi: 10.1007/s00221-008-1572-9. [DOI] [PubMed] [Google Scholar]
- Comella CL, Stebbins GT, Goetz CG, Chmura TA, Bressman SB, Lang AE. Teaching tape for the motor section of the Toronto Western Spasmodic Torticollis Scale. Mov Disord. 1997;12:570–5. doi: 10.1002/mds.870120414. [DOI] [PubMed] [Google Scholar]
- Currá A, Berardelli A, Agostino R, Giovannelli M, Koch G, Manfredi M. Movement cueing and motor execution in patients with dystonia: a kinematic study. Mov Disord. 2000;15:103–12. doi: 10.1002/1531-8257(200001)15:1<103::aid-mds1016>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;130:1183–93. doi: 10.1093/brain/awl355. [DOI] [PubMed] [Google Scholar]
- Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology. 2007;69:376–80. doi: 10.1212/01.wnl.0000266591.49624.1a. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–4. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Draganski B, Thun-Hohenstein C, Bogdahn U, Winkler J, May A. “Motor circuit” gray matter changes in idiopathic cervical dystonia. Neurology. 2003;61:1228–31. doi: 10.1212/01.wnl.0000094240.93745.83. [DOI] [PubMed] [Google Scholar]
- Dresel C, Li Y, Wilzeck V, Castrop F, Zimmer C, Haslinger B. Multiple changes of functional connectivity between sensorimotor areas in focal hand dystonia. J Neurol Neurosurg Psychiatry. 2014;85:1245–52. doi: 10.1136/jnnp-2013-307127. [DOI] [PubMed] [Google Scholar]
- Fierro B, Giglia G, Palermo A, Pecoraro C, Scalia S, Brighina F. Modulatory effects of 1 Hz rTMS over the cerebellum on motor cortex excitability. Exp Brain Res. 2007;176:440–7. doi: 10.1007/s00221-006-0628-y. [DOI] [PubMed] [Google Scholar]
- Gregori B, Agostino R, Bologna M, Dinapoli L, Colosimo C, Accornero N, et al. Fast voluntary neck movements in patients with cervical dystonia: a kinematic study before and after therapy with botulinum toxin type A. Clin Neurophysiol. 2008;119:273–80. doi: 10.1016/j.clinph.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Harrington A, Hammond-Tooke GD. Theta burst stimulation of the cerebellum modifies the TMS-evoked N100 potential, a marker of GABA inhibition. PLoS One. 2015;10:e0141284. doi: 10.1371/journal.pone.0141284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffland BS, Bologna M, Kassavetis P, Teo JT, Rothwell JC, Yeo CH, et al. Cerebellar theta burst stimulation impairs eyeblink classical conditioning. J Physiol. 2012;590:887–97. doi: 10.1113/jphysiol.2011.218537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffland BS, Kassavetis P, Bologna M, Teo JT, Rothwell JC, Yeo CH, et al. Cerebellum-dependent associative learning deficits in primary dystonia are normalized by rTMS and practice. Eur J Neurosci. 2013;38:2166–71. doi: 10.1111/ejn.12186. [DOI] [PubMed] [Google Scholar]
- Hoffland BS, Veugen LC, Janssen MM, Pasman JW, Weerdesteyn V, van de Warrenburg BP. A gait paradigm reveals different patterns of abnormal cerebellar motor learning in primary focal dystonias. Cerebellum. 2014;13:760–6. doi: 10.1007/s12311-014-0594-z. [DOI] [PubMed] [Google Scholar]
- Hubsch C, Roze E, Popa T, Russo M, Balachandran A, Pradeep S, et al. Defective cerebellar control of cortical plasticity in writer’s cramp. Brain. 2013;136:2050–62. doi: 10.1093/brain/awt147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma K, Samii A, Mercuri B, Wassermann EM, Hallett M. Abnormal cortical motor excitability in dystonia. Neurology. 1996;46:1371–6. doi: 10.1212/wnl.46.5.1371. [DOI] [PubMed] [Google Scholar]
- Inzelberg R, Flash T, Schechtman E, Korczyn AD. Kinematic properties of upper limb trajectories in idiopathic torsion dystonia. J Neurol Neurosurg Psychiatry. 1995;58:312–9. doi: 10.1136/jnnp.58.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah HA, Berardelli A, Comella C, Defazio G, Delong MR, Factor S, et al. The focal dystonias: current views and challenges for future research. Mov Disord. 2013;28:926–43. doi: 10.1002/mds.25567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katschnig-Winter P, Schwingenschuh P, Davare M, Sadnicka A, Schmidt R, Rothwell JC, et al. Motor sequence learning and motor adaptation in primary cervical dystonia. J Clin Neurosci. 2014;21:934–8. doi: 10.1016/j.jocn.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Koch G, Mori F, Marconi B, Codecà C, Pecchioli C, Salerno S, et al. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin Neurophysiol. 2008;119:2559–69. doi: 10.1016/j.clinph.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Koch G, Porcacchia P, Ponzo V, Carrillo F, Cáceres-Redondo MT, Brusa L, et al. Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul. 2014;7:564–72. doi: 10.1016/j.brs.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Kojovic M, Pareés I, Kassavetis P, Palomar FJ, Mir P, Teo JT, et al. Secondary and primary dystonia: pathophysiological differences. Brain. 2013;136:2038–49. doi: 10.1093/brain/awt150. [DOI] [PubMed] [Google Scholar]
- LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. MovDisord. 2003;18:60–9. doi: 10.1002/mds.10301. [DOI] [PubMed] [Google Scholar]
- Li Voti P, Conte A, Rocchi L, Bologna M, Khan N, Leodori G, et al. Cerebellar continuous theta-burst stimulation affects motor learning of voluntary arm movements in humans. Eur J Neurosci. 2014;39:124–31. doi: 10.1111/ejn.12391. [DOI] [PubMed] [Google Scholar]
- Malone A, Manto M, Hass C. Dissecting the links between cerebellum and dystonia. Cerebellum. 2014;13:666–8. doi: 10.1007/s12311-014-0601-4. [DOI] [PubMed] [Google Scholar]
- Mottolese C, Richard N, Harquel S, Szathmari A, Sirigu A, Desmurget M. Mapping motor representations in the human cerebellum. Brain. 2013;136:330–42. doi: 10.1093/brain/aws186. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Koch G, Torriero S, Caltagirone C. Increased facilitation of the primary motor cortex following 1 Hz repetitive transcranial magnetic stimulation of the contralateral cerebellum in normal humans. Neurosci Lett. 2005;376:188–93. doi: 10.1016/j.neulet.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Pelosin E, Bove M, Marinelli L, Abbruzzese G, Ghilardi MF. Cervical dystonia affects aimed movements of nondystonic segments. Mov Disord. 2009;24:1955–61. doi: 10.1002/mds.22693. [DOI] [PubMed] [Google Scholar]
- Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22:7825–33. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa T, Russo M, Meunier S. Long-lasting inhibition of cerebellar output. Brain Stimul. 2010;3:161–9. doi: 10.1016/j.brs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Corcos DM, Leurgans S, Comella CL, Weis-McNulty A, MacKinnon CD. Changes in the relationship between movement velocity and movement distance in primary focal hand dystonia. J Mot Behav. 2008;40:301–13. doi: 10.3200/JMBR.40.4.301-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudente CN, Pardo CA, Xiao J, Hanfelt J, Hess EJ, Ledoux MS, Jinnah HA. Neuropathology of cervical dystonia. Exp Neurol. 2013;241:95–104. doi: 10.1016/j.expneurol.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience. 2014;260:23–35. doi: 10.1016/j.neuroscience.2013.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarone A, Morgante F, Sant’angelo A, Rizzo V, Bagnato S, Terranova C, et al. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79:985–90. doi: 10.1136/jnnp.2007.121632. [DOI] [PubMed] [Google Scholar]
- Sadnicka A, Hoffland BS, Bhatia KP, van de Warrenburg BP, Edwards MJ. The cerebellum in dystonia – help or hindrance? Clin Neurophysiol. 2012;123:65–70. doi: 10.1016/j.clinph.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Sadnicka A, Patani B, Saifee TA, Kassavetis P, Pareés I, Korlipara P, et al. Normal motor adaptation in cervical dystonia: a fundamental cerebellar computation is intact. Cerebellum. 2014;13:558–67. doi: 10.1007/s12311-014-0569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirinzi T, Di Lorenzo F, Ponzo V, Palmieri MG, Bentivoglio AR, Schillaci O, Pisani A, Koch G. Mild cerebello-thalamo-cortical impairment in patients with normal dopaminergic scans (SWEDD) Parkinsonism Relat Disord. 2016;28:23–8. doi: 10.1016/j.parkreldis.2016.03.023. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Wong A, Zee DS, Jinnah HA. Why are voluntary head movements in cervical dystonia slow? Parkinsonism Relat Disord. 2015;21:561–6. doi: 10.1016/j.parkreldis.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo JT, van de Warrenburg BP, Schneider SA, Rothwell JC, Bhatia KP. Neurophysiological evidence for cerebellar dysfunction in primary focal dystonia. J Neurol Neurosurg Psychiatry. 2009;80:80–3. doi: 10.1136/jnnp.2008.144626. [DOI] [PubMed] [Google Scholar]
- Teo WP, Rodrigues JP, Mastaglia FL, Thickbroom GW. Post-exercise depression in corticomotor excitability after dynamic movement: a general property of fatiguing and non-fatiguing exercise. Exp Brain Res. 2012;216:41–9. doi: 10.1007/s00221-011-2906-6. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Farina S, Edwards M, Moretto G, Restivo D, Fiaschi A, et al. Task-specific impairment of motor cortical excitation and inhibition in patients with writer’s cramp. Neurosci Lett. 2005;378:55–8. doi: 10.1016/j.neulet.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Thompson ML, Thickbroom GW, Mastaglia FL. Corticomotor representation of the sternocleidomastoid muscle. Brain. 1997 Feb;120:245–55. doi: 10.1093/brain/120.2.245. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Squintani G, Berardelli A. Does neurophysiological testing provide the information we need to improve the clinical management of primary dystonia? Clin Neurophysiol. 2009;120:1424–32. doi: 10.1016/j.clinph.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Wissel J, Kabus C, Wenzel R, Klepsch S, Schwarz U, Nebe A, et al. Botulinum toxin in writer’s cramp: objective response evaluation in 31 patients. J Neurol Neurosurg Psychiatry. 1996;61:172–5. doi: 10.1136/jnnp.61.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.