Abstract
CALR mutations are identified in about 30% of JAK2/MPL-unmutated myeloproliferative neoplasms (MPNs) including essential thrombocythemia (ET) and primary myelofibrosis. Although the molecular pathogenesis of CALR mutations leading to MPNs has been studied using in vitro cell lines models, how mutant CALR may affect developmental hematopoiesis remains unknown. Here we took advantage of the zebrafish model to examine the effects of mutant CALR on early hematopoiesis and model human CALR-mutated MPNs. We identified three zebrafish genes orthologous to human CALR, referred to as calr, calr3a and calr3b. The expression of CALR-del52 and CALR-ins5 mutants caused an increase in the hematopoietic stem/progenitor cells followed by thrombocytosis without affecting normal angiogenesis. The expression of CALR mutants also perturbed early developmental hematopoiesis in zebrafish. Importantly, morpholino knockdown of mpl but not epor or csf3r could significantly attenuate the effects of mutant CALR. Furthermore, the expression of mutant CALR caused jak-stat signaling activation in zebrafish that could be blocked by JAK inhibitors (ruxolitinib and fedratinib). These findings showed that mutant CALR activates jak-stat signaling through an mpl-dependent mechanism to mediate pathogenic thrombopoiesis in zebrafish, and illustrated that the signaling machinery related to mutant CALR tumorigenesis are conserved between human and zebrafish.
Introduction
The BCR-ABL-negative classic myeloproliferative neoplasms (MPNs) are clonal hematopoietic stem cell disorders including polycythemia vera, essential thrombocythemia (ET) and primary myelofibrosis (PMF).1 The JAK2V617F and MPL exon 10 mutations are two important driver mutations in MPNs and cause the activation of the JAK-signal transducer and activator of transcription (STAT) signaling that is central to the pathogenesis of MPNs.2 Calreticulin (CALR) is a 46-kDa highly conserved, multicompartmental and multifunctional protein.3 CALR has its role as a Ca2+-binding chaperone protein and acts in concert with calnexin to ensure proper protein and glycoprotein folding in the endoplasmic reticulum (ER).4 Recently, two research groups discovered CALR mutations in about 30% of JAK2 and MPL-unmutated ET and PMF patients.5, 6 All CALR mutations are indels mutations in exon 9 and cause +1 base frameshift generating a novel C-terminus characterized by the loss of the ER retention signal KDEL and the change from acidic to basic amino-acid sequence. Although there are >50 CALR mutants identified in MPNs, the most prevalent types of CALR mutations are a 52 bp deletion (L367fs*46, type 1 mutation, CALR-del52) and a 5 bp insertion of TTGTC (K385fs*47, type 2 mutation, CALR-ins5) accounting for >80% of all patients with mutant CALR.5, 6 Most CALR mutations are mutually exclusive with the JAK2 and MPL mutations, but some patients were found to have JAK2 and CALR co-mutations.7 ET and PMF patients with CALR mutations have been found to have different clinical characteristics such as younger age and higher platelet count and to carry a better prognosis than those patients with JAK2V617F mutation.7, 8, 9, 10
Recent studies have focused on the underlying mechanism of CALR mutations in the pathophysiology of MPNs. With the use of in vitro cell lines and retroviral mouse models, CALR mutants were found to activate the JAK-STAT signaling in an MPL-dependent manner.11, 12, 13, 14, 15 Although the expression of CALR mutants resulted in pathogenic thrombocytosis in adult mice, whether CALR mutants may disrupt normal hematopoiesis during early development remains unknown. The zebrafish is a useful disease model system and has been successfully utilized in studying hematopoiesis and leukemogenesis.16, 17, 18, 19, 20 The early hematopoietic system in zebrafish involves two distinct primitive and definitive waves of development that is rapidly established within a few days after fertilization.18 The developmental hematopoiesis of zebrafish also shows broad conservation with mammalian species and is regulated by conserved molecular pathways.18 The transparency of zebrafish at the embryonic and larval stages has made it suitable for direct observation of the hematopoietic process. In addition, zebrafish can be used for in vivo high throughput screening due to its good permeability to chemical added to water during early developmental stages.21, 22, 23 Here we aimed to evaluate the pathophysiologic effects of mutant CALR during embryonic hematopoietic development and to test the therapeutic effects of JAK inhibitors on mutant CALR using the in vivo zebrafish model.
Materials and methods
Zebrafish husbandry
Wild-type AB strain of zebrafish (Danio rerio) and the transgenic lines Tg(cd41:GFP)24 and Tg(fli1:EGFP)25 were maintained and manipulated with standard measure as previously described.26 The stages of embryonic development were determined based on Kimmel et al.27 Pigmentation was blocked by using 0.003% 1-phenyl-2-thiourea in some experiments. For pharmacologic inhibition, embryos were incubated with ruxolitinib (Abmole Bioscience, Houston, TX, USA) or fedratinib (Abmole Bioscience) from 1–2 cells stage to 5 days post fertilization (d.p.f.) with or without microinjection of CALR mRNA. The zebrafish experiments were approved by the MacKay Memorial Hospital Animal Care and Use Committee.
Identification of zebrafish ortholog of human CALR
Human genes located in 19p13.11-13.2 were identified using the National Center for Biotechnology Information (NCBI) Map Viewer.28 Genes surrounding the three zebrafish calr genomic regions were identified using Ensembl29 and Synteny database.30 Human CALR protein sequence was used to BLASTP against zebrafish GRCz10 using the Ensembl platform (Ensembl release 82).29 Alignment and comparative analysis between protein sequences was performed using the Clustal Omega algorithm31 and edited by GeneDoc.32
Human and zebrafish CALR cDNAs cloning and mRNA synthesis
Full-length CALR cDNAs were subcloned in the pCS2+ vector and into a bicistronic pSYC-102 T2A vector (a gift from Dr Seok-Yong Choi) replacing the mCherry-CAAX reporter gene using the In-Fusion Cloning Kit (Clontech, Mountain View, CA, USA) (Supplementary Figure S1).33 All vector sequences were verified by sequencing. The mMessage mMachine SP6 kit (Ambion, Austin, TX, USA) was used for in vitro transcription of capped mRNAs from vectors according to the manufacturer's protocol. mRNAs from the bicistronic pSYC-102-CALR vectors were only used to express EGFP and CALR concurrently in wild-type zebrafish embryos and only embryos expressing green fluorescence were collected under fluorescence microscope for use in the reverse transcription and real-time PCR.
Morpholinos and microinjection
Morpholinos (MOs) blocking splicing of mpl and epor, and translation (ATG/5′UTR) of csf3r were purchased from Gene Tools (Philomath, OR, USA; MO sequences are listed in Table 1).24, 34, 35 Standard control MO was used as negative control. Embryos at the 1–2 cells stage were injected with MO (1 ng) or mRNAs (100 pg). Co-injection of each MO and CALR mutant mRNA was performed in a subset of embryos.
Table 1. Morpholino sequences for mpl, epor and csf3r knockdown.
| Gene | Target | ZFIN identity | MO sequence |
|---|---|---|---|
| mpl | Intron1/exon2 boundary of exon 2 | ZDB-MRPHLNO-060421-1 | 5′-CAGAACTCTCACCCTTCAATTATAT-3′ |
| epor | Intron1/exon2 boundary of exon 2 | ZDB-MRPHLNO-080325-2 | 5′-AACTGGGCCACTGAACAATCAAATT-3′ |
| csf3r | ATG/5′UTR | ZDB-MRPHLNO-111213-1 | 5′-GAAGCACAAGCGAGACGGATGCCAT-3′ |
| Standard control | Human beta-globin intron mutation | NA | 5′-CCTCTTACCTCAGTTACAATTTATA-3′ |
Abbreviations: NA, not applicable; ZFIN, Zebrafish International Resource Center.
Reverse transcription and real-time PCR
Total RNA was extracted from embryos using miTotal Miniprep System (Viogene, New Taipei City, Taiwan) and reverse transcribed using a High Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA, USA). Primer sequences are listed in Supplementary Table 1. Fast SYBR Green Master Mix (Applied Biosystems) was used for real-time quantitative PCR according to the manufacturer's instructions.
Western blotting
Total proteins were extracted from zebrafish embryos at 24 h post fertilization (h.p.f.). Equal amounts of protein were denatured and electrophoresed. Membranes were immunoblotted with the following primary antibodies: CALR (Abcam, Cambridge, UK; recognizing N-terminal sequences of both human and zebrafish wild-type CALR proteins), gapdh and customized mutant CALR (GeneTex, Hsinchu City, Taiwan; specifically recognizing human CALR exon 9 indel mutant protein sequence), STAT5 (Santa Cruz, Dallas, TX, USA) and phospho-STAT5 (Cell Signaling, MA, USA).
Imaging
Live embryos were imaged using a Leica MSV269 fluorescence stereomicroscope and photographed using a Leica DFC425 C digital camera and Leica Application Suite software (Leica Microsystems, Wetzlar, Germany). GraphPad Prism 7 software and ImageJ (National Institutes of Health) were used to process images.
Statistical analysis
The Student t-test or analysis of variance test were used. Data were expressed as mean±s.e.m. Significance was determined at *P<0.05, **P<0.01 and ***P<0.001.
Results
Zebrafish ortholog of human CALR
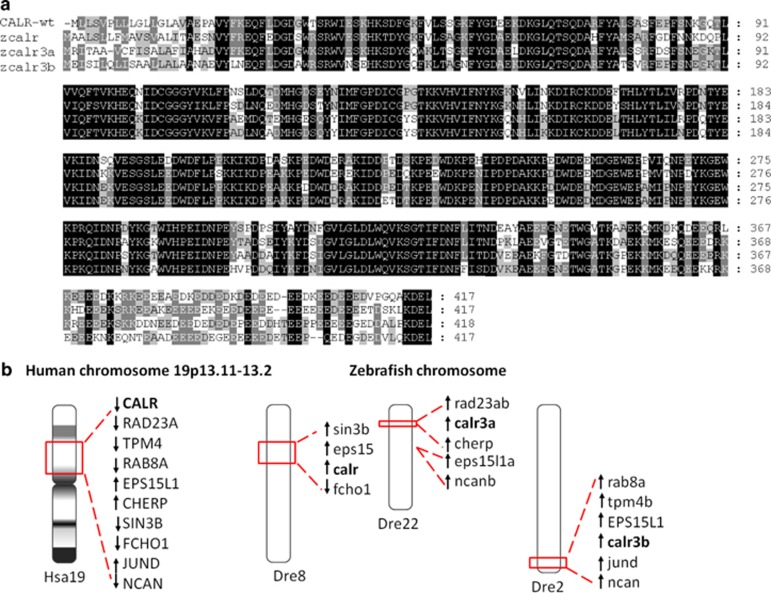
To search for the zebrafish ortholog of human CALR gene, human CALR protein sequence was used to BLASTP against zebrafish GRCz10 (Ensembl release 82). We identified three annotated zebrafish orthologs of the human CALR gene (ENSG000001792), calr (ENSDARG00000076290 at chromosome 8), calr3a (ENSDARG00000103979 at chromosome 22) and calr3b (ENSDARG00000102808 at chromosome 2). After comparative analysis using the Clustal Omega algorithm, the amino-acid sequence of zebrafish calr, calr3a and calr3b proteins shares an overall 75%, 71% and 70% identity to human CALR protein sequence, respectively. The three functional domains in CALR are conserved in all three zebrafish calr proteins, including the KDEL ER retention signal at the C-terminus (Figure 1a). In addition, the genomic loci surrounding human chromosome 19p13.2 containing the CALR gene are syntenic with the regions of zebrafish calr on chromosome 8, calr3a on chromosome 22 and calr3b on chromosome 2 based on the search in NCBI Map Viewer, Ensembl database and Synteny database (Figure 1b). These results indicated that the three zebrafish calr genes are likely true orthologs of human CALR.
Figure 1.
Identification of three zebrafish calr genes. (a) Alignment of human CALR (top row), zebrafish calr (second row), calr3a (third row) and calr3b (bottom row) protein sequences. The regions of sequence identity in the four proteins are shaded. (b) The genomic loci surrounding human CALR on chromosome 19p13.2 (Hsa19) are syntenic with the regions where zebrafish calr (on chromosome 8, Dre8), calr3a (on chromosome 22, Dre22) and calr3b (on chromosome 2, Dre2) are located in the zebrafish genome, respectively.
Effects of mutant CALR expression on thrombopoiesis and angiogenesis in zebrafish
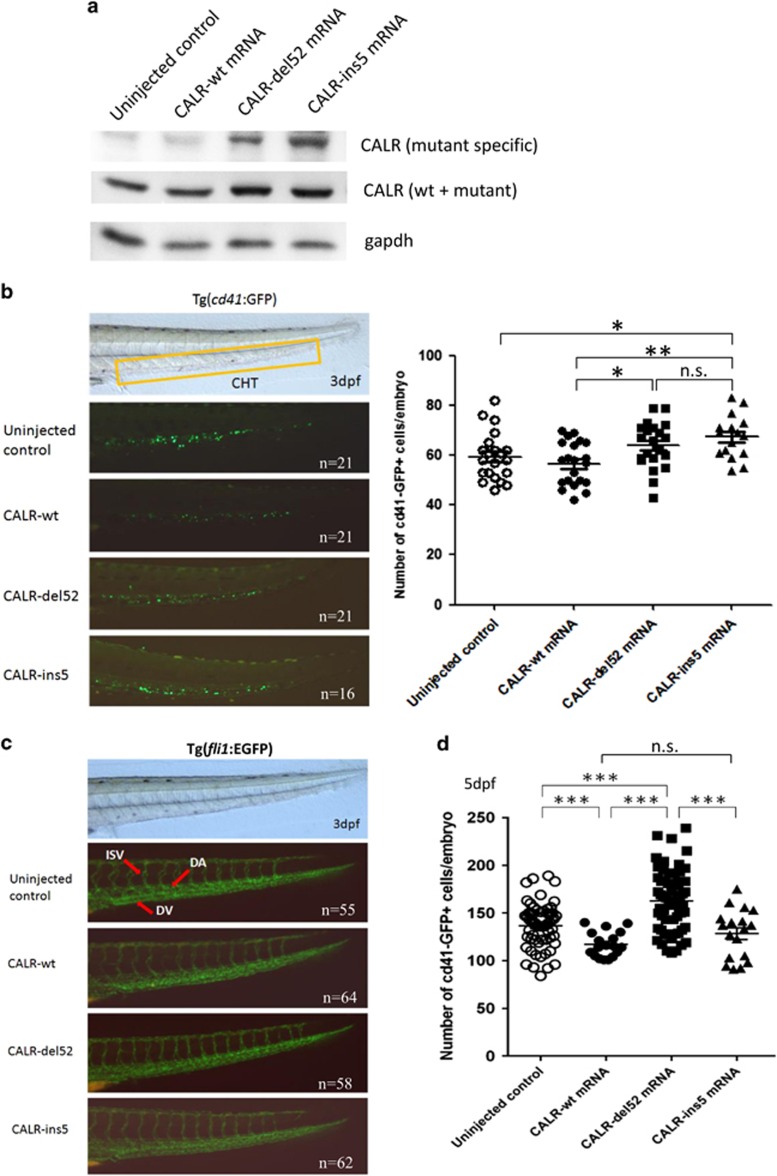
For the expression of mRNA in zebrafish embryo, we first performed a dose-finding study ranging from 50 to 200 pg CALR mRNA. Phenotype could be observed at dose of 100 pg mRNA per embryo which was compatible with normal development for most embryos. All CALR proteins were adequately expressed at comparable amount at dose of 100 pg (Figure 2a, middle panel). The expression of CALR-del52 and CALR-ins5 mutant proteins was also confirmed by mutant CALR-specific antibody (Figure 2a, top panel). Therefore, 100 pg mRNA was injected throughout the study. To determine whether mutant CALR had an effect on hematopoietic stem and progenitor cells (HSPCs) in zebrafish, we injected the three mRNAs encoding CALR wild-type (CALR-wt), CALR-del52, and CALR-ins5 into 1–2 cells stage embryos of the cd41:GFP line, and the numbers of CD41+ cells in the caudal hematopoietic tissue (CHT) at 3 d.p.f. indicating the HSPCs were counted.24 Expression of both CALR-del52 and CALR-ins5 mutant mRNA significantly increased the numbers of HSPCs in the CHT when compared with CALR-wt mRNA (Figure 2b). However, the numbers of HSPCs did not have statistically significant difference between CALR-del52 and CALR-ins5 mutant groups at this developmental stage. To ascertain that the increase of HSPCs was not affected by the change in angiogenesis during early development, mRNAs encoding CALR-wt, CALR-del52, and CALR-ins5 were injected into 1–2 cells stage embryos of the fli1:EGFP line. No obvious changes in the angiogenesis were visualized in CALR-wt and mutant CALR expressing embryos at 3 d.p.f. when compared with uninjected control (Figure 2c). To determine whether CALR had an effect on mature thrombocyte, the numbers of CD41+ thrombocytes in the cd41:GFP line were counted at 5 d.p.f. after injection. Mutant CALR-del52 significantly increased the number of CD41+ thrombocytes (mean 162.5±4.1 per embryo) when compared with CALR-wt (mean 117.1±3.1 per embryo, P<0.001), mutant CALR-ins5 (mean 128.3±6.1 per embryo, P<0.001) and uninjected control (mean 136.7±3.0 per embryo, P<0.001; Figure 2d). Although mutant CALR-ins5 slightly increased the number of CD41+ thrombocytes when compared with CALR-wt, there was no statistically significant difference. Altogether, our data demonstrated that the effect of mutant CALR on thrombopoiesis in zebrafish is dependent on the presence of the novel C-terminus and is also related to specific CALR mutant protein sequences.
Figure 2.
Effects of the expression of mutant CALR on the number of hematopoietic stem/progenitor cells and angiogenesis. (a) CALR protein expression in uninjected, CALR-wt-, CALR-del52- or CALR-ins5-injected embryos at 24 h.p.f. gapdh was used to normalize the total amount of protein in each sample. The expression of human CALR mutant proteins was confirmed in CALR-del52- and CALR-ins5-injected embryos by a customized human CALR mutant-specific polyclonal antibody (top panel). (b) Brightfield image (left panel) of a 3 d.p.f. embryo with a box area indicating caudal hematopoietic tissue where CD41+ cells were counted. Green cells in the darkfield images (left panel) indicated expression of GFP under the control of the cd41 promoter, and were counted and showed on the right panel. (c) The development of dorsal aorta (DA), dorsal vein (DV) and intersegmental vessel (ISV) as indicated by the red arrows (top panel) in uninjected, CALR-wt-, CALR-del52- or CALR-ins5-injected embryos of the Tg(fli1:EGFP) line at 3 d.p.f. (d) The total numbers of CD41+ thrombocytes were counted in uninjected, CALR-wt-, CALR-del52- or CALR-ins5-injected embryos of the Tg(cd41:GFP) line at 5 d.p.f. (the direction of embryos was anterior to the left, dorsal upwards, lateral view; n.s., not significant; *P<0.05, **P<0.01, ***P<0.001; Student t-test). The number of embryos used in each experiment is indicated by ‘n' in figures.
Mutant CALR requires mpl to cause thrombocytosis in zebrafish
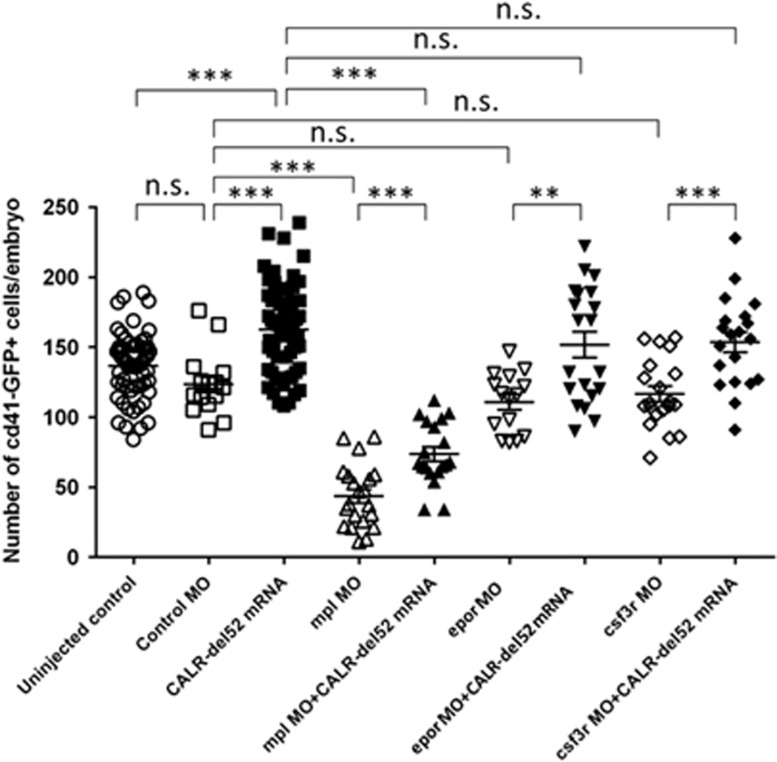
To test whether cytokine receptors are involved in the pathogenesis of thrombocytosis caused by mutant CALR in zebrafish, mpl, epor and csf3r MOs (each with 1 ng) were injected in 1–2 cells stage embryos of cd41:GFP line and assayed for their effects on the number of CD41+ thrombocytes at 5 d.p.f. Co-injection of CALR-del52 mutant mRNA (100 pg) with each MO was also performed in a subset of embryos. At 5 d.p.f., the number of CD41+ thrombocytes significantly decreased upon mpl knockdown (mean 43.6±4.9 per embryo) when compared with the control MO group (mean 123.5±5.9 per embryo, P<0.001) and the mutant CALR-del52 group (P<0.001; Figure 3). Importantly, co-injection of CALR-del52 mutant mRNA (mean 73.7±5.1 per embryo) can only partially reverse the knockdown effect of mpl MO. In contrast, the numbers of CD41+ thrombocytes did not decrease significantly upon epor MO (mean 110.6±5.5 per embryo) or csf3r MO (mean 116.6±5.6 per embryo) knocked down compared with the control MO group. When CALR-del52 mutant mRNA was co-injected with epor (mean 151.7±9.2 per embryo) or csf3r (mean 153.6±7.2 per embryo) MOs, the numbers of CD41+ thrombocytes were comparable to those of CALR-del52–injected embryos (both P=0.3). Collectively, these findings indicated that the expression of mutant CALR causes thrombocytosis through an mpl-dependent mechanism in zebrafish.
Figure 3.
Mutant CALR requires mpl to cause thrombocytosis in zebrafish. Morpholinos (MOs) targeting mpl, epor or csf3r (1 ng per embryo) were injected into 1–2 cells stage embryos of the Tg(cd41:GFP) line with or without co-injection of CLAR-del52 mRNA (100 pg). Standard control MO was used as negative control. The total numbers of CD41+ thrombocytes were counted at 5 d.p.f. and compared as indicated. The number of CD41+ thrombocytes significantly decreases upon mpl knockdown when compared with the control MO group as well as the CALR-del52 group. Co-injection of CALR-del52 mRNA can only partially reverse the knockdown effect of mpl MO. When CALR-del52 mRNA was co-injected with epor MO or csf3r MO, the numbers of CD41+ thrombocytes were comparable to those of CALR-del52-injected embryos (n.s., not significant; **P<0.01, ***P<0.001; Student t-test).
Effects of CALR mutants on lineage-specific and cytokine gene expression
The increase in thrombopoiesis upon expression of mutant CALR prompted us to evaluate their effects on hematopoietic lineage-specific, thrombopoiesis,36 cytokine and cytokine receptor gene expression in zebrafish embryos at 3 d.p.f. The expression of HSC gene runx1 was significantly upregulated in CALR-ins5 group but was modestly downregulated in CALR-del52 group (Table 2). Also, the expression of c-myb and scl was only downregulated in CALR-del52 group. Although gata1 was modestly downregulated in mutant CALR groups, the expression of α-eHb and β-eHb was not affected by both CALR mutants. The expression of early (spi1b) and late myeloid (mpo: granulocytic; l-plastin: macrophage) lineage genes, epo and epor showed no significant changes. However, the expression of lymphoid lineage genes (rag1, rag2 and lck) was modestly downregulated in mutant CALR groups. Although the expression of mpl was significantly downregulated in both mutant CALR groups, both tpo and csf3r expressions were only downregulated in CALR-del52 group. In the group of genes related to thrombopoiesis, only the expression of nbeal2 was significantly downregulated in CALR-del52 group.
Table 2. Effects of CALR mutant mRNA injection on the expression of genes in zebrafish embryo genes involved in lineage-specific hematopoiesis, thrombopoiesis, cytokines and cytokine receptors were examined based on real-time quantitative PCR of zebrafish embryos at 3 days post fertilization, with reference to that of CALR wild-type mRNA injection.
| Category | Gene | CALR-wta | CALR-del52 Mean±s.e.m. | CALR-ins5 Mean±s.e.m. | CALR-wt vs CALR-del52 vs CALR-ins5 P-valueb | CALR-wt vs CALR-del52 P-valuec | CALR-wt vs CALR-ins5 P-valuec | CALR-del52 vs CALR-ins5 P-valuec |
|---|---|---|---|---|---|---|---|---|
| Hematopoietic stem cells | cmyb | 1.00 | 0.73±0.04 | 1.07±0.02 | <0.001 | 0.02 | NS | 0.001 |
| runx1 | 1.00 | 0.78±0.06 | 1.20±0.06 | 0.003 | NS | 0.029 | 0.008 | |
| Hemangioblast | scl | 1.00 | 0.75±0.08 | 0.91±0.05 | 0.043 | NS | NS | NS |
| lmo2 | 1.00 | 0.85±0.06 | 0.99±0.09 | NS | NS | NS | NS | |
| Erythropoiesis | gata1 | 1.00 | 0.87±0.01 | 0.89±0.02 | 0.002 | 0.012 | 0.036 | NS |
| α eHb | 1.00 | 0.82±0.11 | 0.89±0.11 | NS | NS | NS | NS | |
| β eHb | 1.00 | 0.73±0.12 | 0.96±0.22 | NS | NS | NS | NS | |
| Vasculature | fli1 | 1.00 | 0.76±0.06 | 0.84±0.08 | NS | 0.017 | NS | NS |
| Early myelomonocytic lineage | spi1b | 1.00 | 1.13±0.10 | 1.01±0.10 | NS | NS | NS | NS |
| Late myelomonocytic lineage | L-plastin | 1.00 | 0.99±0.17 | 0.94±0.09 | NS | NS | NS | NS |
| Lymphoid lineage | rag1 | 1.00 | 0.56±0.07 | 1.01±0.12 | 0.012 | 0.003 | NS | 0.033 |
| rag2 | 1.00 | 0.96±0.07 | 0.84±0.03 | NS | NS | 0.01 | NS | |
| lck | 1.00 | 0.50±0.06 | 1.08±0.13 | 0.006 | 0.015 | NS | 0.017 | |
| Thrombopoiesis | arhgef3 | 1.00 | 0.80±0.09 | 0.90±0.07 | NS | NS | NS | NS |
| emilin1a | 1.00 | 0.90±0.03 | 0.91±0.04 | NS | 0.036 | NS | NS | |
| nbeal2 | 1.00 | 0.64±0.10 | 1.08±0.06 | 0.007 | NS | NS | 0.019 | |
| max | 1.00 | 0.88±0.03 | 0.87±0.06 | NS | 0.021 | NS | NS | |
| Cytokines and cytokine receptors | tpo | 1.00 | 0.88±0.05 | 1.12±0.05 | 0.016 | NS | NS | 0.026 |
| mpl | 1.00 | 0.57±0.05 | 0.52±0.06 | 0.001 | 0.014 | 0.002 | NS | |
| epo | 1.00 | 0.99±0.10 | 1.12±0.23 | NS | NS | NS | NS | |
| epor | 1.00 | 0.80±0.10 | 0.96±0.09 | NS | NS | NS | NS | |
| mpo | 1.00 | 0.85±0.09 | 0.84±0.06 | NS | NS | NS | NS | |
| csf3r | 1.00 | 0.62±0.04 | 1.15±0.14 | 0.01 | 0.001 | NS | 0.021 |
Abbreviations: ANOVA, analysis of variance; NS, not significant; wt, wild-type.
Data are from triplicate results.
Data of CALR-wt was arbitrarily set to 1.00 in all cases.
ANOVA test.
Student t-test.
Effects of mutant CALR on jak-stat signaling in zebrafish
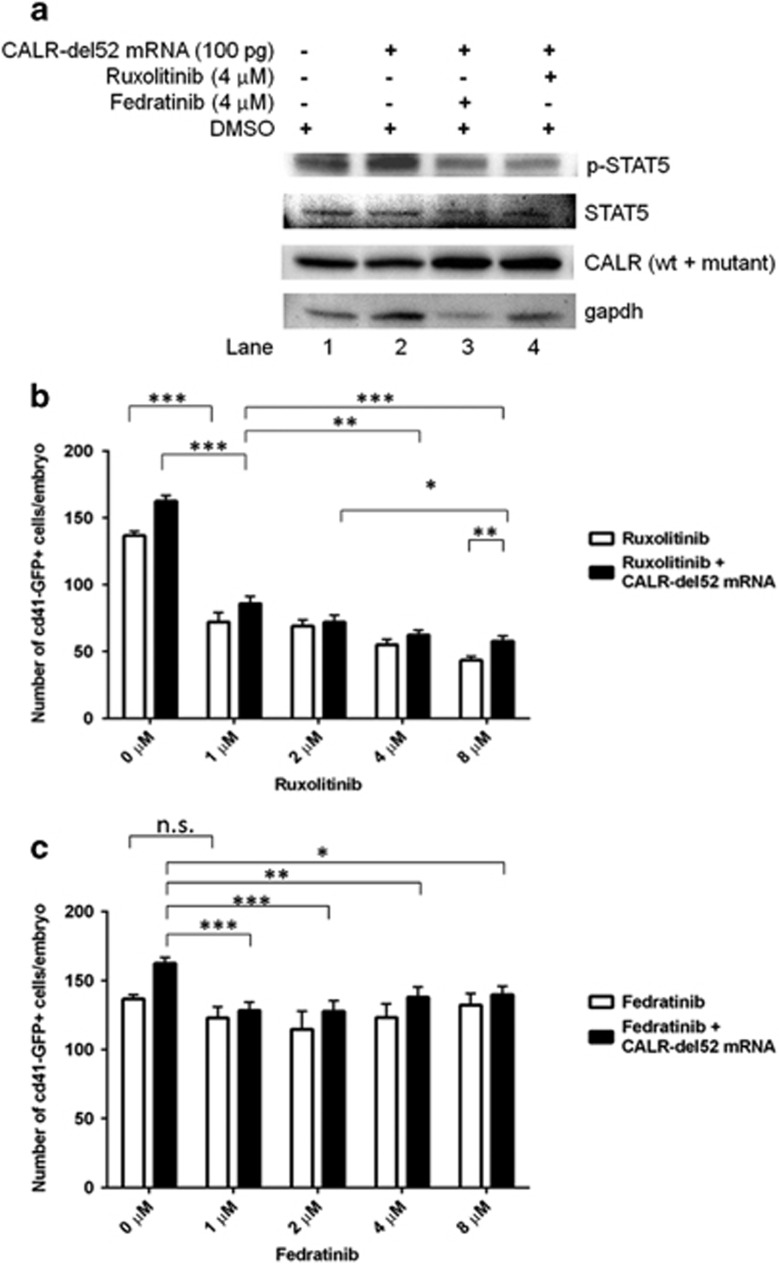
We then investigated whether the expression of mutant CALR can activate the jak-stat signaling in zebrafish. The expression of CALR-del52 mRNA significantly increased stat5 phosphorylation (Figure 4a, lane 2). Furthermore, treatment with ruxolitinib and fedratinib significantly ameliorated the enhanced stat5 phosphorylation induced by CALR-del52 mRNA (Figure 4a, lane 3 and 4). In addition, treatment with ruxolitinib significantly decreased the numbers of CD41+ thrombocytes in uninjected control as well as CALR-del52-injected embryos in a dose-dependent manner (Figure 4b). Whereas treatment with fedratinib only had minimal inhibitory effect on the number of CD41+ thrombocytes in uninjected control embryos, and had a modest and significant dose-independent inhibitory effect on mutant CALR-induced thrombocytosis (Figure 4c). Our results demonstrated that mutant CALR-mediated pathogenic thrombopoiesis involves jak-stat activation that can be blocked by JAK inhibitors.
Figure 4.
The expression of mutant CALR activates jak-stat signaling in zebrafish. (a) Western blotting showing embryos of wild-type zebrafish injected with CALR-del52 mRNA (100 pg) have significantly increased signal transducer and activation of transcription (stat) 5 phosphorylation (lane 2) as compared with uninjected control embryos from the same batch at 24 h.p.f. (lane 1). Pharmacologic treatment of embryos with a JAK2-selective inhibitor (fedratinib) and a dual JAK1/JAK2 inhibitor (ruxolitinib) significantly attenuated the enhanced stat5 phosphorylation induced by CALR-del52 mRNA stat5 phosphorylation (lane 3 and 4, respectively). Total amount of stat5 protein was not affected in all experiments. (b and c) Effects of the pharmacologic treatment with ruxolitinib and fedratinib (from 1 μM to 8 μM) on the numbers of CD41+ thrombocytes at 5 d.p.f. with or without the injection of CALR-del52 mRNA. Treatment with ruxolitinib significantly decreases the numbers of CD41+ thrombocytes in uninjected control as well as CALR-del52-injected embryos in a dose-dependent manner. Whereas treatment with fedratinib has minimal and insignificant inhibitory effect on the number of CD41+ thrombocyte in uninjected control embryos, and has a modest and significant dose-independent inhibitory effect on mutant CALR-induced thrombocytosis (n.s., not significant; *P<0.05, **P<0.01, ***P<0.001; Student t-test).
Discussion
In this study, we have used the zebrafish animal model to examine the pathogenesis of mutant CALR in MPNs. We first identified three zebrafish orthologs for human CALR gene. We have shown that expression of the CALR-del52 mutant disturbs thrombopoiesis and increases the number of HSPCs in the CHT followed by significant thrombocytosis in the zebrafish embryo. These findings are consistent with the myeloproliferative phenotype in retroviral mouse bone marrow transplantation models elicited by mutant CALR expression characterized by thrombocytosis and megakaryocytic hyperplasia recapitulating those seen in patients with ET and myelofibrosis.12, 14
The highly conserve protein sequences between human CALR and the three zebrafish calr genes suggested functional conservation between human and zebrafish CALR. Ma et al.37 recently reported that MO knockdown of calr perturbs myeloid and HSCs lineages during zebrafish embryonic development including a decrease in the expression of genes associated with myeloid lineages at 24 h.p.f. and an increase in the expression of cmyb at 48, 72 and 96 h.p.f. We have also shown that the expression of genes involved in lineage-specific hematopoiesis, thrombopoiesis, cytokines and cytokine receptors could be perturbed by the expression of mutant CALR in zebrafish during early development. These data suggested that zebrafish calr genes have an important role in the regulation of vertebrae hematopoiesis. In addition, our data suggested that mutant CALR does not promote thrombopoiesis through the upregulation of mpl and tpo levels. Rather, the downregulation of mpl and tpo might represent a negative-feedback mechanism related to increased thrombopoiesis due to mutant CALR expression.
On the basis of the data from the murine and zebrafish animal models, the causative relationship between CALR mutations and thrombocytosis can be confirmed, and CALR mutations have been established as one of the driver mutations in MPNs. Furthermore, we demonstrated that expression of CALR-del52 (type 1 mutation) causes higher thrombocyte count than CALR-ins5 (type 2 mutation) at 5 d.p.f. in zebrafish embryo. Similar finding has been reported in murine model that marked thrombocytosis was rapidly induced in CALR-del52-expressing mice and then progressed to myelofibrosis, and CALR-ins5-expressing mice only developed modest thrombocytosis resembling mild ET phenotype.12 This is consistent with the clinical finding that CALR-del52 mutation is more frequently detected in PMF than in ET,5 and also confirms the differential effects of CALR variants on thrombopoiesis and clinical phenotypes.10, 38, 39
To further elucidate the molecular pathogenesis of mutant CALR in our zebrafish model, we have used MO knockdown experiments to show that only the mpl MO can significantly attenuate the effect of mutant CALR on thrombopoiesis. Both epor and csf3r MOs were not able to inhibit the effect of mutant CALR. These findings indicated that mpl has an essential and specific role required by mutant CALR to cause thrombocytosis in zebrafish. Because CALR is physiologically functioning as a chaperone for MPL, it is reasonable to speculate that mutant CALR may interact directly with mpl to cause thrombocytosis in zebrafish. Our data are consistent with those recently reported by several groups of researchers using in vitro cell line models.11, 12, 13, 14, 15 In these studies, both the novel C-terminus of CALR mutants and the direct interaction of mutant CALR with MPL receptor are required to activate MPL and the downstream JAK-STAT signaling, which in turn is responsible for cytokine-independent growth of Ba/F3-MPL and UT-7/TPO cell lines. Chachoua et al.13 reported that the specific activation of MPL receptor by mutant CALR required both the presence of extracellular N-glycosylation residues of MPL and the glycan-binding site at the novel C-terminus of mutant CALR. In addition, Elf et al.14 reported that the physical interaction between mutant CALR and MPL is dependent on the positive electrostatic charge of the C-terminus of the mutant CALR but not dependent on specific novel C-terminal sequence. Recently, Balligand et al.40 reported similar finding that highly similar but not identical murine Calr exon 9 frameshift mutants also require Mpl interaction to activate the JAK-STAT signaling. Moreover, the positive charge predominant novel C-terminus of the mutant CALR results in different calcium-binding capacity, which may alter calcium homeostasis and signaling processes in mutant cells. All these structural differences and changes will likely contribute to the different clinical phenotypes seen in different CALR variants.10, 38, 39, 41
We have also demonstrated that the expression of human CALR mutant is able to activate jak-stat signaling in zebrafish. In addition, jak-stat signaling in zebrafish can also be inhibited by JAK2 inhibitors used in clinical trials illustrating that the conserved signaling machinery in human and zebrafish.42, 43, 44 Our data showed that ruxolitinib treatment results in a dose-dependent inhibitory effect on both normal thrombopoiesis and thrombocytosis caused by mutant CALR in zebrafish. By contrast, JAK2-selective inhibitor fedratinib has only minimal inhibitory effects on normal thrombopoiesis but has modest and dose-independent inhibitory effect on thrombocytosis caused by mutant CALR. Our data suggested that fedratinib can normalize the thrombocytosis caused by the expression of mutant CALR and does not cause significant thrombocytopenia in zebrafish model. These observations are comparable to the findings that both ruxolitinib and fedratinib have been demonstrated to have clinical responses in MPN patients harboring CALR mutations.45, 46, 47 However, fedratinib has less hematological toxicities than ruxolitinib especially thrombocytopenia, which is a dose-limiting toxicity of ruxolitinib.42, 43, 44 Despite both JAK inhibitors are effective in the reduction of splenomegaly and the relief of clinical symptoms, they are not likely to substantially modify the natural history of the BCR-ABL-negative classic MPNs including CALR-mutated PMF. Importantly, these JAK inhibitors are not specifically designed for JAK2V617F mutation. However, the unique pathogenic mechanism of mutant CALR in MPNs has led to the possibility of new therapeutic approach targeting the interaction and binding between mutant CALR and MPL. In this regard, our results highlight the advantage and support the use of zebrafish as a relevant in vivo whole-organism model for the testing and screening of therapeutic compounds targeting mutant CALR.22
In conclusion, we have used the zebrafish model to show that mutant CALR promotes the activation of jak-stat signaling through an mpl-dependent mechanism to mediate pathogenic thrombopoiesis during zebrafish early hematopoiesis. These findings are consistent with those observed in in vitro cell line and mouse models and illustrated that the signaling machinery related to mutant CALR tumorigenesis are conserved between human and zebrafish. Zebrafish has also been shown to be a relevant in vivo model for the development of novel therapeutic compounds targeting mutant CALR. Future studies using stable mutant CALR transgenic or knock-in zebrafish models for this purpose will be warranted.
Acknowledgments
The present study was supported by grants from the Ministry of Science and Technology of Taiwan to KHL (grant numbers: MOST 102-2314-B-195-015-MY2 and 104-2314-B-195-017-MY3) and YYK (grant numbers: MOST 103-2314-B-002-168 and 105-2314-B-002-185-MY2), and the intramural grant from the Department of Medical Research of MacKay Memorial Hospital to KHL (grant number: MMH-105-09). We acknowledge that the transgenic lines Tg(cd41:GFP) and Tg(fli1:EGFP) were obtained from Zebrafish International Resource Center through the support from Taiwan Zebrafish Core Facility at Academia Sinica (supported by grant MOST 103-2321-B-001-050) and National Health Research Institutes Zhunan branch (supported by grant MOST 104-2321-B-001-045), respectively. We thank the staff at MacKay Memorial Hospital animal core facility for their assistance with zebrafish husbandry as well as Dr Chung-Der Hsiao and Dr Ching-Hung Chen for helpful discussions.
Author contributions
KHL and YYK conceived the project. KHL, YCC, YHC, HCL, CGC, YYK and WCC participated in its design, results discussion and edited the manuscript. KHL, CYC, CSL, LH, WTW and TYL performed the experiments and analyzed the data. KHL and LH drafted the manuscript. All authors approved the manuscript.
Disclaimer
All the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114: 937–951. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Kralovics R. From Janus kinase 2 to calreticulin: the clinically relevant genomic landscape of myeloproliferative neoplasms. Blood 2014; 123: 3714–3719. [DOI] [PubMed] [Google Scholar]
- Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J 2009; 417: 651–666. [DOI] [PubMed] [Google Scholar]
- Caramelo JJ, Parodi AJ. Getting in and out from calnexin/calreticulin cycles. J Biol Chem 2008; 283: 10221–10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013; 369: 2379–2390. [DOI] [PubMed] [Google Scholar]
- Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013; 369: 2391–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KH, Chang YC, Gon-Shen Chen C, Lin HC, Wang WT, Chiang YH et al. Frequent CALR exon 9 alterations in JAK2 V617F-mutated essential thrombocythemia detected by high-resolution melting analysis. Blood Cancer J 2015; 5: e295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia 2014; 28: 1472–1477. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Wassie EA, Lasho TL, Finke C, Belachew AA, Ketterling RP et al. Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia 2014; 28: 2300–2303. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Wassie EA, Guglielmelli P, Gangat N, Belachew AA, Lasho TL et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol 2014; 89: E121–E124. [DOI] [PubMed] [Google Scholar]
- Araki M, Yang Y, Masubuchi N, Hironaka Y, Takei H, Morishita S et al. Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms. Blood 2016; 127: 1307–1316. [DOI] [PubMed] [Google Scholar]
- Marty C, Pecquet C, Nivarthi H, El-Khoury M, Chachoua I, Tulliez M et al. Calreticulin mutants in mice induce an MPL-dependent thrombocytosis with frequent progression to myelofibrosis. Blood 2016; 127: 1317–1324. [DOI] [PubMed] [Google Scholar]
- Chachoua I, Pecquet C, El-Khoury M, Nivarthi H, Albu RI, Marty C et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood 2016; 127: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Elf S, Abdelfattah NS, Chen E, Perales-Paton J, Rosen EA, Ko A et al. Mutant calreticulin requires both its mutant C-terminus and the thrombopoietin receptor for oncogenic transformation. Cancer Discov 2016; 6: 368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivarthi H, Chen D, Cleary C, Kubesova B, Jager R, Bogner E et al. Thrombopoietin receptor is required for the oncogenic function of CALR mutants. Leukemia 2016; 30: 1759–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenau DM, Traver D, Ferrando AA, Kutok JL, Aster JC, Kanki JP et al. Myc-induced T cell leukemia in transgenic zebrafish. Science 2003; 299: 887–890. [DOI] [PubMed] [Google Scholar]
- Teittinen KJ, Gronroos T, Parikka M, Ramet M, Lohi O. The zebrafish as a tool in leukemia research. Leuk Res 2012; 36: 1082–1088. [DOI] [PubMed] [Google Scholar]
- Rasighaemi P, Basheer F, Liongue C, Ward AC. Zebrafish as a model for leukemia and other hematopoietic disorders. J Hematol Oncol 2015; 8: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnebo SM, Rasighaemi P, Kumar J, Liongue C, Ward AC. Alternative TEL-JAK2 fusions associated with T-cell acute lymphoblastic leukemia and atypical chronic myelogenous leukemia dissected in zebrafish. Haematologica 2012; 97: 1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JW, Hsieh MS, Liao HA, Yang YJ, Ho YJ, Lin LI. Zebrafish as a model for the study of human myeloid malignancies. Biomed Res Int 2015; 2015: 641475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Shimada Y, Kuroyanagi J, Nishimura Y, Umemoto N, Nomoto T et al. Zebrafish xenotransplantation model for cancer stem-like cell study and high-throughput screening of inhibitors. Tumour Biol 2014; 35: 11861–11869. [DOI] [PubMed] [Google Scholar]
- Ridges S, Heaton WL, Joshi D, Choi H, Eiring A, Batchelor L et al. Zebrafish screen identifies novel compound with selective toxicity against leukemia. Blood 2012; 119: 5621–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morash MG, Douglas SE, Robotham A, Ridley CM, Gallant JW, Soanes KH. The zebrafish embryo as a tool for screening and characterizing pleurocidin host-defense peptides as anti-cancer agents. Dis Model Mech 2011; 4: 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HF, Traver D, Zhu H, Dooley K, Paw BH, Zon LI et al. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood 2005; 106: 3803–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 2002; 248: 307–318. [DOI] [PubMed] [Google Scholar]
- Westerfield M (eds). The Zebrafish Book: a Guide for The Laboratory Use of Zebrafish (Danio rerio), 4th edn. University of Oregon Press Eugene: Oregon, USA, 2000. [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 1995; 203: 253–310. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information Map Viewer. Available at http://www.ncbi.nlm.nih.gov.ezp-prod1.hul.harvard.edu/mapview/ (accessed on March 2016).
- Flicek P, Amode MR, Barrell D, Beal K, Billis K, Brent S et al. Ensembl 2014. Nucleic Acids Res 2014; 42: D749–D755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchen JM, Conery JS, Postlethwait JH. Automated identification of conserved synteny after whole-genome duplication. Genome Res 2009; 19: 1497–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 2011; 7: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas KB, Nicholas HB GeneDoc: a tool for editing and annotating multiply sequence alignments. Pittsburgh Supercomputing Center's National Resource for Biomedical Supercomputing. 1997.
- Kim JH, Lee SR, Li LH, Park HJ, Park JH, Lee KY et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One 2011; 6: e18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffett-Lugassy N, Hsia N, Fraenkel PG, Paw B, Leshinsky I, Barut B et al. Functional conservation of erythropoietin signaling in zebrafish. Blood 2007; 110: 2718–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011; 117: e49–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandekar G, Kim S, Jagadeeswaran P. Zebrafish thrombocytes: functions and origins. Adv Hematol 2012; 2012: 857058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CH, Leung AYH, Man KF. The role of calreticulin (Calr) in vertebrate hematopoiesis. Annual Zebrafish Disease Models Conference, ZDM-8 2015, 24–27 August 2015; Boston, MA, USA. Available at http://hub.hku.hk/handle/10722/217507.
- Cabagnols X, Defour JP, Ugo V, Ianotto JC, Mossuz P, Mondet J et al. Differential association of calreticulin type 1 and type 2 mutations with myelofibrosis and essential thrombocytemia: relevance for disease evolution. Leukemia 2015; 29: 249–252. [DOI] [PubMed] [Google Scholar]
- Pietra D, Rumi E, Ferretti VV, Buduo CA, Milanesi C, Cavalloni C et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016; 30: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balligand T, Achouri Y, Pecquet C, Chachoua I, Nivarthi H, Marty C et al. Pathologic activation of thrombopoietin receptor and JAK2-STAT5 pathway by frameshift mutants of mouse calreticulin. Leukemia 2016; 30: 1775–1778. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Lasho TL, Finke C, Belachew AA, Wassie EA, Ketterling RP et al. Type 1 vs type 2 calreticulin mutations in primary myelofibrosis: differences in phenotype and prognostic impact. Leukemia 2014; 28: 1568–1570. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012; 366: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012; 366: 787–798. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Harrison C, Cortes JE, Cervantes F, Mesa RA, Milligan D et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: A Randomized Clinical Trial. JAMA Oncol 2015; 1: 643–651. [DOI] [PubMed] [Google Scholar]
- Patel KP, Newberry KJ, Luthra R, Jabbour E, Pierce S, Cortes J et al. Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood 2015; 126: 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmelli P, Rotunno G, Bogani C, Mannarelli C, Giunti L, Provenzano A et al. Ruxolitinib is an effective treatment for CALR-positive patients with myelofibrosis. Br J Haematol 2016; 173: 938–940. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Caramazza D, Maffioli M. JAK inhibitor in CALR-mutant myelofibrosis. N Engl J Med 2014; 370: 1168–1169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.