Abstract
Nociceptin/Orphanin FQ (N/OFQ) is a 17 amino acid peptide that was deorphanized in 1995. The generation of specific agonists, antagonists and receptor deficient mice and rats has enabled progress in elucidating the biological functions of nociceptin. In addition, radio-imaging technologies have been advanced for investigation of this system in animals and humans. Together with traditional neurobehavioral techniques, these tools have been utilized to identify the biological significance of the N/OFQ system and its interacting partners. The present commentary focuses on the role of N/OFQ in the regulation of feeding and body weight homeostasis, stress and the stress-related psychiatric disorders of depression and anxiety, and in drug and alcohol dependence. Critical evaluation of the current scientific preclinical literature suggests that small molecule modulators of nociceptin opioid peptide receptors (NOP) might be useful in the treatment of diseases related to these biological functions. In particular, the literature data suggest that antagonism of NOP receptors will produce anti-obesity and antidepressant activities in humans. However, there are also contradictory data discussed. The current literature on the role of N/OFQ in anxiety and addiction, on the other hand points primarily to a role of agonist modulation being potentially therapeutic. Some drug-like molecules that function either as agonists or antagonists of NOP receptors have been optimized for human clinical study to test some of these hypotheses. The discovery of PET ligands for NOP receptors, combined with the pharmacological tools and burgeoning preclinical data set discussed here bodes well for a rapid advancement of clinical understanding and potential therapeutic benefit.
Keywords: Nociceptin/Orphanin FQ, N/OFQ, ORL1, NOP, depression, anxiety, stress, obesity, drug dependence, alcohol dependence
Introduction
Nociceptin/Orphanin FQ (N/OFQ) is a 17 amino acid peptide, and was the first neuropeptide discovered by screening brain extracts as a natural ligand for the orphan G protein-coupled receptor (GPCR) Opioid Receptor Like-1 (ORL1) also known as NOP, OP4, or LC132 (Lachowicz et al., 1995; Meunier et al. 1995; Reinscheid et al. 1995). Both N/OFQ and the ORL1 receptor (NOP) exhibit a high degree of sequence identity to dynorphin and the kappa opioid receptor, respectively. However, it is important to note that N/OFQ does not activate any of the classical opioid receptors (mu, delta, kappa) nor do classical opioid receptor ligands (such as naloxone) bind to NOP.
One of the first scrutinized areas of the involvement of N/OFQ in biological systems was that of pain. Indeed the name nociceptin (Meunier et al. 1995) was derived from the observations of pro-nociceptive behaviors following administration of the peptide. Subsequent studies have revealed that the modulation of pain pathways by N/OFQ is complex. The general consensus is that N/OFQ produces anti-opioid hyperalgesic effects in supraspinal pain pathways, while exerting analgesic properties in spinal pain pathways. Several reviews on the analgesic and hyperalgesic effects of N/OFQ have been published (Fioravanti and Vanderah, 2008; Chiou et al., 2007; Mika et al., 2011). Since its discovery, numerous other physiological process appear to be modulated by N/OFQ. In addition to pain, other pathological CNS effects of N/OFQ include anxiety, depression, hyperphagia and obesity, addiction, Parkinson’s disease, and cognition (Jenck et al, 2000; Lambert, 2008; Matsushita et al., 2009; Nabeshima et al., 1999; Pomonis et al., 1996; Olszewski et al., 2002; Volta et al., 2011; Martin-Fardon et al., 2010). The effects of N/OFQ are not limited to the CNS. In peripheral tissues N/OFQ produces antitussive effects, negative chronotropic and ionotripic effects on heart, vasodilation, inhibition of gastrointestinal motility, inflammation, and sepsis (Lambert, 2008; Armstead, 2011; Leggett et al., 2009; Serrano-Gomez et al., 2011). However, at present a dearth of data from human clinical studies exists that have explored modification of these disease processes following manipulation of the N/OFQ system. Therefore, development of potent, selective and safe agonists and antagonists of NOP are needed to facilitate the clinical study of N/OFQ; in turn clinical scrutiny of such tools will provide the definitive understanding regarding NOP and human disease.
Since the discovery of N/OFQ and de-orphanization of its receptor 18 years ago, there has been some progress in understanding the basic biological systems impacted by this neuropeptide system. The biological appreciation of the N/OFQ system has encouraged the generation of specific research tools (small molecule, peptide, and genetic) to enable preclinical investigation. Such tools have included NOP selective agonists and antagonists (Jenck et al., 2000; Röver et al., 2000; Wichmann et al., 2000; Fioravanti and Vanderah, 2008; Przydzial and Heisler, 2008; Chiou et al., 2010; Largent-Milnes and Vanderah, 2010; Zaveri, 2011), N/OFQ-deficient mice (Koster et al., 1999; Kuzmin et al., 2009), and NOP receptor-deficient mice (Nishi et al., 1997), and rats (Homberg et al., 2009; Rizzi et al., 2011). In contrast, few studies have explored the effects of modifying the synthesis and/or metabolic disposition of N/OFQ. Evaluation of the endogenous effects of N/OFQ has relied on the direct central application of nociceptin and on the generation and study of N/OFQ gene knockouts. Experimental data from these preparations support a role for endogenous N/OFQ in regulating stress responses, development of morphine tolerance, and alterations in pain thresholds (Reinscheid and Civelli, 2002; Chung et al., 2006). In addition, several studies have explored the post-translational processing and enzymatic metabolism of the N/OFQ (for review see Terenius et al., 2000; Hallberg and Nyberg, 2003). Collectively, the use of these various research tools has created the opportunity to explore the impact of manipulations of N/OFQ system on physiological function and integrated disease-related functional correlates (Largent-Milnes and Vanderah, 2010). Results of such investigations have led to increased understanding of the potential utility of employing small molecule modulators of NOP to help treat a number of disease states (Lambert, 2008).
The present commentary utilizes the current data in the scientific literature to focus on three potential disease areas in which N/OFQ receptor modulators might be impactful: obestity, psychiatric disorders related to stress including depression and anxiey, and drug dependence disorders. We will not address the potential interaction or pharmacology of releated peptides such as the opioid peptides or nocistatin, that is produced from prepro-nociceptin, the same precursor protein as N/OFQ (Okuda-Ashitaka et al., 1998).
N/OFQ Biology
The receptor for N/OFQ is NOP, a Class A GPCR that is widely expressed in several areas of the central nervous system (CNS) including several regions associated with mood disorders (cortex, hippocampus, amygdala, and dorsal raphe nucleus), food intake and metabolism (hypothalamus and nucleus of the solitary tract), locomotor activity (substantia nigra and striatum), cognition (cortex and hippocampus), and pain (spinal cord and periaquiductal gray area). Significant species differences in receptor distribution exist when comparing rodent and non-rodent species making consistent translation of physiological responses between species difficult (Fig. 1). Moreover, NOP receptors are expressed in the peripheral nervous system as well as in the GI tract, smooth muscles, and the immune system.
Figure 1.
Top: Autoradiographic binding of in striatal sections (12 μM) of (A) rat and (B) dog brain. Sections were incubated with [3H]nociceptin (200 pM) for 120 min at room temperature in buffer containing 50 mM Tris-HCl, 5 mM MgCl2, 1 mM EDTA, and 0.1% bovine serum albumin. Sections were washed 2X for 10 min in ice cold incubation buffer containing no bovine serum albumin or radioligand, followed by a rinse in ice cold distilled water. Sections were exposed for 3 days at room temperature. Color intensity denotes the degree of NOP receptor binding sites with red/orange and yellow colors representing areas of high receptor density and blue representing areas of low receptor density. Note the difference in [3H]nociceptin binding site density within the caudate nucleus (a) exemplifying profound species-related differences in NOP receptor expression.
Bottom: Autoradiographic binding of the NOP antagonist tracer [3H]NOP-1A (0.6 nM) in 12 μm coronal sections of wildtype 129/S6 and ORL1 knockout mouse brain according to Pike et al. (2011). Nonspecific binding was determined in the presence of 10 μM SB612111 (from an adjacent section of wildtype mouse). Wildtype 129/S6 mice exhibited high binding in numerous cortical regions (A), medial septum (B), dorsal endopiriform nucleus (C) hippocampus (D), vetromedial hypothalamic nuclues (E), amygdala (F), and central thalamus (G). No appreciable specific binding was observed in equivalent sections of ORL1 knockout mouse brain.
In native tissues and recombinant cell lines the NOP receptor is functionally coupled to inhibition of adenylate cyclase, activation of MAP kinase, activation of K+ conductance, and inhibition of Ca+2 conductance (for review see Civelli, 2008). Activation of NOP by N/OFQ or small molecule agonists, produces inhibition of neurotransmitter release, including glutamate, gamma-amino butyric acid (GABA), dopamine, acetylcholine, substance P and CGRP. While related structurally to the opioid peptides particularly dynorphin, N/OFQ exhibits no significant cross reactivity to classical opioid receptors mu, delta and kappa. Moreover, the classical opioid ligands (naloxone, enkephalins, endorphin, and dynorphin) do not have high affinity binding to the NOP. Selectivity of N/OFQ for NOP receptors is derived in part from the N-terminal sequence FGGF present in the N/OFQ peptide instead of the YGGF sequence that is conserved among all classical opioid peptides. Indeed, substitution of the phenylalanine in position 1 with tyrosine along with substitutions in positions 7, 10, 14 and 15 produces a nonselective ORL1+kappa receptor agonist (Reinscheid et al., 1998). Similarly, replacement of the second extracellular loop of the kappa receptor with the corresponding sequence from NOP confers binding and functional activation of N/OFQ to this chimeric kappa receptor without impairing responsiveness to dynorphin (Mollereau et al., 1999). Therefore, sequence differences within these specific peptide domains confer the high degree of receptor selectivity exhibited by N/OFQ. Moreover, in some models, N/OFQ exhibits anti-opioid activity in vivo (for example N/OFQ exhibits anti-analgesic properties in some models).
In vivo, N/OFQ modulates several physiological functions and behaviors in nonclinical animal models including depression, stress and anxiety, feeding, locomotor activity, body temperature, substance abuse, memory, and pain (for review see Lambert, 2008). Interestingly, although initially described as producing hyperalgesia and anti-opioid activity, antinociceptive effects of N/OFQ and small molecule agonists have been reported. Therefore, the effects of N/OFQ signalling in pain pathways are complex depending on the site of activity (spinal or supraspinal pain pathways). Unfortunately, to date, little clinical data exist to support validation of N/OFQ agonists or antagonists for the treatment of any of the aforementioned clinical indications.
Obesity
The distribution of N/OFQ and NOP localizes within key nuclei of the hypothalamus involved in the regulation of appetite and metabolism. Indeed, in rodents a high density of NOP receptors are found within the ventromedial nucleus of the hypothalamus, with lower amounts seen in the paraventricular and arcuate nuclei (Florin et al., 2000; Gehlert et al., 2006) (Fig. 1). Not surprisingly, the highly efficacious and potent orexigenic effects of exogenously administered N/OFQ peptide analogues and small molecule agonists are well documented (Economidou et al., 2006; Civelli, 2008). Moreover, N/OFQ is known to decrease core body temperature following i.c.v. administration (Chen et al., 2001; Blakley et al., 2004; Matsushita et al., 2009). These aforementioned effects are similar to those observed following administration of another orexigenic peptide, neuropeptide Y (NPY) (Bouali et al., 1994; Kotz et al., 1998; Hwa et al., 1999).
Numerous studies have demonstrated that activation of NOP receptors produces a robust orexigenic response in rodents. Table 1 summarizes some of these data. Acute hyperphagia has been consistently observed following i.c.v. administration of N/OFQ (Economidou et al., 2006; Pomonis et al., 1996; Rodi et al., 2002). Moreover, sustained increases in food intake and body weight gain were produced following a 12-day i.c.v. infusion of N/OFQ in mice fed either chow or moderately high fat diet (Matsushita et al., 2009). In the same study, when N/OFQ-infused mice were pair fed to vehicle controls, total body mass remained unaltered, while fat mass, plasma leptin, insulin and cholesterol levels increased, suggesting that N/OFQ affects energy metabolism by mechanisms other than increasing calorie intake alone. N/OFQ-induced hyperphagia was produced following 3rd ventricle administration, but not when the peptide was injected into the 4th ventricle (Polidori et al., 2000), suggesting that the hypothalamus may be a key site of N/OFQ orexigenic activity. Lending support to this hypothesis, site specific microinjection of N/OFQ into the hypothalamic ventromedial nucleus, but not in the lateral hypothalamus, stimulated food intake (Stratford et al., 1997). As well, neuroanatomical evidence exists supporting that i.c.v. administration of N/OFQ stimulates c-fos expression in CNS centers associated with the regulation of food intake including the hypothalamic paraventricular nucleus, NTS, central amygdala, lateral septum and habenular nuclei (Olszewski et al., 2000). Interestingly, unlike other opioid peptides, N/OFQ does not increase preferred diets/macronutrients, but rather produces a general increase in food intake (Olszewski et al., 2002, 2010).
Table 1.
Evidence supporting a role for N/OFQ in obestity
| Preparation | Findings | Reference |
|---|---|---|
| N/OFQ into brain of sated mice and rats | Stimulation of feeding | Olszewski and Levine, 2004 |
| N/OFQ into the CNS of fat-prefering rats | Stimulation of feeding | Olszewski et al., 2002 |
| N/OFQ into the CNS of rats | Stimulation of c-fos expression in the NTS, PVN, central amygdala, lateral septum and habenular nuclei | Olszewski et al., 2000 |
| N/OFQ and N/OFQ agoinst Ro 64-6198 | Inhibitition of anorectic effects of CRF N/OFQ | Ciccocioppo et al., 2002; 2004 |
| Chronic central administration of N/OFQ | Increased body weight by inducing hyperphagia and by decreasing energy expenditure | Matsushita et al., 2009 |
| N/OFQ antagonist SB612111 | Inhibition of fasting-induced feeding; effect absent in NOP receptor −/− mice | Figure 2 |
| Fasting in rats | Reduces N/OFQ and ORL1/NOP gene expression within the CNS | Rodi et al., 2002; Przydzial et al., 2009 |
DIO: diet-induced obese
NTS: nucleus of the solitary tract
PVN: paraventricular nucleus
The orexigenic actions of N/OFQ has been proposed to occur via inhibition of anorexigenic signalling rather than from the activation of orexigenic pathways. Indeed, N/OFQ inhibits cfos expression in arcuate nucleus POMC neurons (precursor for α-melanocyte-stimulating hormone) associated with meal termination (Bomberg et al., 2006). Moreover, iontophoretically applied N/OFQ inhibited mEPSC frequency in whole cell patch clamp recordings of arcuate POMC neurons via activation of a G-protein activated, inwardly rectifying K+ channel (GIRK) (Farhang et al., 2010). The inhibitory effects of N/OFQ extend to other hypothalamic nuclei involved in the regulation of food intake and metabolism. Recently, Chee et al. (2011) reported that N/OFQ inhibited anorexigenic leptin receptor containing neurons in whole cell patch clamp recordings of hypothalamic ventromedial nucleus neurons, via activation of a GIRK. Moreover, activation of NOP receptors via N/OFQ or by anorexia elicited by stress or by central injection of corticotrophin-releasing factor (CRF), a major mediator of stress in mammals (Ciccocioppo et al., 2001, 2002, 2004) again supporting the idea that N/OFQ modulates feeding via inhibition of anorexigenic signaling within the CNS. Thus, N/OFQ regulates food intake by inhibition of anorexigenic neurons via activation of a GIRK at least in the arcuate, ventromedial and paraventricular nuclei. However, data suggesting a role for the bed nucleus the stria terminalis in N/OFQ-induced reversal of CRF-mediated anorexia have been also published (Ciccocioppo et al., 2003). Presently, no data exist on the neuroanatomical and cellular mechanism by which N/OFQ inhibits body temperature and energy expenditure; however, inhibition of POMC neurons within the nucleus of the solitary tract that are known to activate thermogenesis in rodents is a likely target (Li et al., 2007; Zhang et al., 2011)
Of note, in a recent study of the effect of N/OFQ in a preclinical model of binge eating (BE), it was discovered that the peptide has very limited efficacy in controlling BE, whereas repeated cycles of food restrictions used to induced the BE markedly increased the sensitivity of rats to the hyperphagic effect of N/OFQ for palatable food. This effect was associated to an increase of NOP receptor transcript in the ventromedial hypothalamus (Micioni Di et al., 2013. Altogether, these data point to the possibility that NOP receptor antagonism may be useful in obesity as well as in the treatment of eating disorders such as binge eating disorder (BED). Some additional data supporting the proposition that NOP receptors are regulators of food intake and body weight are shown in figure 2.
Figure 2.
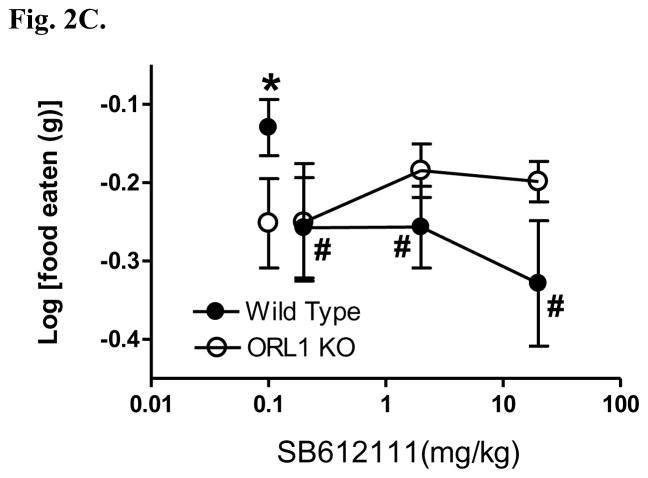
The NOP receptor antagonist SB612111 produces a dose-dependent inhibition of (A) food intake and (B) body weight gain in lean Long-Evans rats (n = 6 rats/group) eating a high fat/high sugar diet (Teklad Research Diets, TD95217). Following initial presention of the TD95217 diet animals become hyperphagic and rapidly gain weight. Animals were treated once daily with SB612111 by the oral route for 3 consecutive days and daily food intake and body weight were measured. * p<0.01; ** p<0.001 vs vehicle treated rats. (C) The NOP receptor antagonist SB612111 in ORL1 knockout mice inhibits fasting-induced 1 hr food intake in a genotype-dependent manner. ORL1 knockout mice and wildtype 129/S6 littermates (n = 6–8 mice/group) were fasted overnight and presented chow 1 hour into the light cycle. The NOP antagonist SB612111 was administered by oral route 1 hr prior to the presentation of food. The amount of food consumed was measured for 1 hr following food presentation, and represents the period during which the most rapid food consumption occurs. * p<0.05; # p<0.05 vs vehicle treated wild type mice.
Stress, Anxiety, and Mood
The available anatomical localization and biological pathway data, together with current neurochemical and behavioral data in the literature are consistent with an hypothesis that antagonism of NOP will improve the symptoms of depression; in contrast, the current literature is not as consistent on a prediction as to whether either agonists or antagonists would attenuate anxiety-related behaviours.
Evaluation of the data from the archival scientific literature suggests a modulatory role for N/OFQ in the control of physiological systems regulating stress and stress reactivity. Figure 3 summarizes this body of data. The findings that lead to this current understanding come from diverse sources that include the impact of stress on NOP receptors and the impact of N/OFQ on biochemical, neurochemical and behavioral effects relevant to stress processing. The figure directs attention toward a dual input/output function that handles stress physiology through the central nervous system as well as the stress system of the hypothalamic/pituitary adrenal (HPA) axis.
Figure 3.
Interplay of NOP receptors with stress pathways, and neuropeptide and non-peptide neurotransmitters. Graphics are drawn from data in the scientific literature cited in the text.
What are the data implicating N/OFQ in stress physiology? One set of data comes from the localization of N/OFQ and its receptors in brain areas that transduce and integrate stressful events and behavioral action (Herman and Culliman, 1997). In situ hybridization studies of the receptor isolated from the cDNA clone exhibited high levels of mRNA in the cerebral cortex, hippocampus, amygdala, hypothalamus, thalamus, and dorsal raphe nuclei (Lachowicz et al., 1995) (Fig. 1). The high localization in limbic brain structures of rodent (Neal et al., 1999a,b), Rhesus monkey (Kimura et al., 2011), and human brain (Lohith et al., 2012) is a clue to the role that this peptide might play in the physiolgical regulation of stress and the pathophysiological states of stress, emotion, mood, and anxiety. Additional information on the localization of relevant CNS structures comes from the findings of elevated expression of the immediate early gene, c-fos, in several brain regions including the paraventricular nucleus, supraoptic nucleus, central amygdaloid nucleus, lateral septum, as well as the brainstem and nucleus tractus solitarius (Olszewski et al., 2000).
Another set of data describes the impact of stress on N/OFQ, NOP receptor function and stress physiology (Table 2). Some of the data summarized from the current experimental literature point to the conclusion that stress mobilizes the N/OFQ system. For example, restraint stress in rats was shown to decrease the levels N/OFQ in the basal forebrain and that these levels were returned to baseline 24 hours later (Devine et al., 2003). Thus, restraint stress is hypothesized to have produced peptide release that further engaged synthetic machinery. Direct measurements of N/OFQ by immunohistochemical localization studies in rat brain have confirmed this hypothesis (Nativio et al., 2012). The released N/OFQ could then act upon its receptors to engender downstream biochemical changes. Since the biochemical effects observed with N/OFQ are comparable to those engendered by stressors (Table 2), the peptide system is likely engaged in the transduction of stress signaling to the HPA axis and central nervous system as discussed further below. In contrast, repeated restraint stress did not induce changes in N/OFQ (Devine et al., 2003) suggesting a tight physiological regulation/adaptation of this system. Glucocorticoids might function as mediators of the stress-induced release of N/OFQ peptides as suggested by studies in adrenalectomized rats supplemented with corticosterone (Nativio et al., 2012). In addition to central nervous system impacts of stress, peripheral tissues are also affected. For example, restraint stress in rats can reduce the density of mucosal mast cells in the gastrointestinal tract. Stress-induced decreases in mucosal mast cell density were mimicked by peripheral infusion of N/OFQ, an effect prevented by co-dosing with the N/OFQ antagonist UFP-101 (Grandi et al., 2011).
Table 2.
Neurochemical evidence supporting a negative impact of N/OFQ in stress, anxiety, and mood
| Preparation | Findings | Reference |
|---|---|---|
| Rat dorsal raphe slice | N/OFQ increases in K+-driven rectifying current | Vaughn and Chrisie, 1996 |
| Rat cortical synaptosomes | N/OFQ decrease of 5-HT and NE release | Siniscalchi et al., 1999; Marti et al., 2003; Mela et al., 2004 |
| Rat neocortical slice | N/OFQ decrease of NE release | Okawa et al., 2001; Siniscalchi et al., 2002 |
| Rat locus coeruleus in vivo | N/OFQ decrease of NE release | Okawa et al., 2001 |
| CORT and ACTH levels after N/OFQ (icv) | Increases in plasma levels under mild stress conditions | Devine et al., 2001, 2003; Nicholson et al., 2002; Fernandez et al., 2004; Leggett et al., 2006 |
| Acute restaint stress in rats | Decreases in NOC/OFC that are replenished in 24 hrs | Devine et al., 2003 |
| N/OFQ (icv) | Increased brain experession of CRF and POMC | Leggett et al., 2006 |
| Corticosterone levels after central N/OFQ | Cort levels increased after dosing in lateral ventricle and BNST but not amygdala | Green et al., 2007 |
| N/OFQ into dorsal raphe nucleus of rats | Deccreasd 5-HT release and efflux that is prevented by an antagonist | Tao et al., 2007 |
| Rat brain synaptosomes and slices | N/OFQ down-regulation of DA function | Olianas et al., 2008 |
| Acute and repeat defeat stress | Prepro-N/OFQ and receptor mRNA increased in limbic structures | Green and Devine, 2009 |
| Forced-Swim | decreased 5-HT outflow from DR neurons that is exacerbated by N/OFQ | Nazzaro et al., 2009 |
| Restraint Stress - rat | Increased expression of N/OFQ in hippocampal CA1, CA3 and the dentate gyrus; increased plasam corticosterone | Nativio et al., 2012 |
ACTH: adrenocorticotropic hormone
BNST: bed nucleus of stria terminalis
DA: dopamine
HPA: hypothalamic-pituitary axis
5-HT: 5-hydroxytryptamine or serotonin
Icv: intracerebroventricular
NE: norepinephrine
A causal role of N/OFQ in stress physiology can also be deduced from the results of literature experiments showing that N/OFQ alters the biological substrates of stress and stress reactivity (Table 2). As shown in table 2, central administration of N/OFQ induces increases in activation of the HPA axis; specifically, increases in plasma levels of the stress hormones, corticosterone and adrenocorticotropic hormone (ACTH) are observed. However, if N/OFQ is given after more extreme stress conditions such as restraint, the increases in stress hormones are not observed (Devine et al., 2001). In contrast, increased expression of N/OFQ peptide was observed in hippocampus and dentate gyrus of rats after immobilization stress (Nativio et al., 2011). Thus, these data support a physiological role of N/OFQ in stress reactivity and demonstrate that the regulation by N/OFQ might be dependent upon the basal state of the system. The effects observed after central administration of the peptide are similar to the biological changes observed after acute stress (e.g., Harbuz and Lightman, 1989).
Further evidence for the engagement of the HPA axis by the N/OFQ system comes from the literature findings that central administration of an antagonist of these receptors, UFP-101, was without effect on either plasma markers or central increases in CRF or POMC expression when administered alone (Leggett et al., 2006). However, UFP-101 blocked N/OFQ-induced HPA axis activation, as measured by plasma corticosterone and central CRF/POMC expression (Leggett et al., 2006). While the evidence clearly demonstrates that the N/OFQ system is involved in stress modulation, the lack of suppression by UFP-101 alone suggests that theN/OFQN/OFQ system is not tonically active. Thus, NOP receptors drive the agonist-induced rise in HPA axis activation and UFP-101 blocked these effects of centrally-administered N/OFQ (Leggett et al., 2006).
To the possibility that the basal state of the HPA axis can modulate the effects of stress and the impact of N/OFQ receptor interventions, Leggett and colleagues (2007) conducted studies in the morning (nadir of HPA axis activity) and compared the findings to those obtained in the evening (peak). In this work, it was found that restraint stress in the morning induced increases in plasma corticosterone that were enhanced by the antagonist UFP-101. Restraint stress in the morning also increased expression of CRF in the paraventricular nucleus; UFP-101 prevented this increase. In the evening, there was no enhancement in corticosterone increases by the antagonist. These data point to the dynamic nature of the N/OFQ system in the control of stress-markers. The reduction in reported expression of c-fos in the suprachiasmatic nucleus (SCN) by N/OFQ (Sugino et al., 2006), has been used to suggest the possiblity that N/OFQ is a regulator of circadian cycling of the HPA axis (e.g., Leggett et al., 2007).
In addition to modulation of stress biomarkers, stress and N/OFQ also induce behavioural changes consistent with the diagram of figure 3. Some of these data from the current scientific literature are summarized in table 3.
Table 3.
Behavioral evidence supporting a negative impact of N/OFQ in stress, anxiety, and mood
| Preparation | Findings | Reference |
|---|---|---|
| Defensive test battery and rat confrontation in mice | N/OFQ (icv) decreased some of the defensive behaivors; diazepam blocked all. | Griebel et al., 1999 |
| FST in mice | N/OFQ antagonist, [Nphe1]-nociceptin (1–13)-NH2 had antidepressant-like effects (icv) | Redrobe et al., 2002 |
| FST in mice | N/OFQ antagonist, J-113397, had antidepressant-like effects (ip) | Redrobe et al., 2002 |
| FST in mice | N/OFQ −/− mice had an antidepressant-like phenotype relative to N/OFQ +/+ mice | Gavioli et al., 2003 |
| FST in mice | N/OFQ antagonist, UFP-101 (icv), had antidepressant-like effects that were prevented by N/OFQ | Gavioli et al., 2003 |
| Elevated plus maze, open field, light dark apparatus | N/OFQ (icv) increased anxiety-like behaviors like FG-7142 | Fernandez et al., 2004 |
| Mouse TST | Antidepressant-like effect of antagonist UFP-101 | Gavioli et al., 2004 |
| N/OFQ in mouse TST | Attenuation of antidepressant-like effect of UFP-101 | Gavioli et al., 2004 |
| TST in mice | Antidepressant-like phenotype of N/OFQ −/− mice relative to N/OFQ +/+ mice | Gavioli et al., 2004 |
| Para-chlorophenylalanine in mouse TST | Prevention of antidepressant-like effect of UFP-101(icv) | Gavioli et al., 2004 |
| NOC antagonist UFP-101 in rat FST | Antidepressant-like effect | Gavioli et al., 2004 |
| Hole-board test in mice | N/OFQ (icv) had anxioltyic-like effects; higher doses had anxiogenic-like effects | Kamei et al., 2004 |
| Mouse TST and mouse FST | Antidepressant-like effect of N/OFQ antagonist SB-612111 | Rizzi et al., 2007 |
| FST and TST in mice | Prevention of antidepressant-like effect of SB-612111 by N/OFQ (icv) and in N/OFQ −/− mice | Rizzi et al., 2007 |
| Elevated plus maze and light/dark test in mice | N/OFQ −/− mice had anxiety-related behaviors in these but not in other assays | Gavioli et al., 2007 |
| Open field after N/OFQ into brain areas of rats | Anxiety-like behaviors – dosing in lateral ventricle, BNST, and amygdala | Green et al., 2007 |
| Chronic mild stress in rats | Subchronic antagonist UFP-101 attenuation of FST and sucrose deficits | Vitale et al., 2009 |
| FST and TST in mice | UFP-101 into dorsal hippocampus produces antidpepressant-like effects | Goeldner et al., 2010 |
| Elevated T-maze in rats | Anxiolytic-like effect of antagonist UFP-101 reversed by N/OFQ administration | Duzzioni et al., 2011 |
| FST in rats | Antidepressant-like phenotype of N/OFQ −/− rats | Rizzi et al., 2011 |
ACTH: adrenocorticotropic hormone
BNST: bed nucleus of stria terminalis
Cort: corticosterone
FST: forced-swim test
icv: intracerebroventricular
ip: intraperitoneal
TST: tail-suspsension test
N/OFQ inhibits the release of central monoamines that are critical to stress and to the processing of stress input (anxiety, mood) both in vitro and in vivo. For example, in synaptosomes prepared from rat neocortex, K+-induced release of serotonin and norepinephrine are inhibited by N/OFQ, an effect that is attenuated by N/OFQr[Nphe1]N/OFQ(1-13)NH2 and UFP-101, J-113397 and JTC-801, respectively). On-target activity of the peptide was confirmed by showing that the inhibition of serotonin overflow was lost in N/OFQ −/− mice (Mela et al., 2004). Both in vitro and in vivo data indicate that this effect of the peptide on neurotransmitter release occurs at multiple levels. Thus, cell bodies in the dorsal raphe nucleus regulating serotonin release have been shown to be inhibited by N/OFQ; N/OFQ produced potent increases in inwardly rectifying K+ currents in dorsal raphe neurons in brain slices that were not sensitive to naloxone (Vaughan and Christie, 1996). These findings are consistent with the localization of N/OFQ mRNA in this brain area (Lachowicz et al., 1995). N/OFQ-induced suppression of serotonin neurons in the dorsal raphe was later verified both in vitro and in vivo via electrophysiological methods and likely involves increases in inwardly rectifying K+ currents (Nazzaro et al. 2010). Similarly, N/OFQ has been reported to decrease release of NE from neocortical cells from rat brain in vitro, an effect blocked by an N/OFQ antagonist (Okawa et al., 2001; Siniscalchi et al., 2002).
In addtion to the experimental literature thus far disussed, there are ample data that contradict the hypothesis that N/OFQ produces stress-like biological responses as simplified in figure 3 (some of the data of which is summarized in tables 2 and 3). In these studies, data from both agonists of N/OFQ and from N/OFQ −/− mice are in the opposite direction to the idea that N/OFQ induces stress-related biological responses (table 4). Indeed, much of these data suggest that N/OFQ functions as an anxiolytic similar to diazepam. However, there are exceptions within this data set. For example, whereas the agonist, Ro 64-6198, produced diazepam-like behavioral effects in three assays, it lacked anti-panic-like effects or anticonvulsant properties (Jenck et al., 2000). Furthermore, whether or not N/OFQ −/− mice displayed anxiolytic-like phenotypes was dependent upon the specific behavioral assay studied (Gavioli et al., 2007), which might, as speculated above, be related to the tight physiological regulation of stress reactivity over time.
Table 4.
Evidence contradicting a negative impact of N/OFQ in stress, anxiety, and mood
| Preparation | Findings | Reference |
|---|---|---|
| Light/Dark Preference in mice | N/OFQ (icv) increased time in light area like diazepam | Jenck et al., 1997 |
| Elevated Plus maze in rats | N/OFQ (icv) increased time in open arms like diazepam | Jenck et al., 1997 |
| Punished responding in mice | N/OFQ (icv) increased punished responding like diazepam | Jenck et al., 1997 |
| Urocortin-induced locomotor suppression in mice | N/OFQ (icv) attenuated suppressed locomotion like diazepam | Jenck et al., 1997 |
| Open field in mice | N/OFQ −/− mice were less active and spent less time in center | Köster et al., 1999 |
| Elevated plus maze in mice | N/OFQ −/− mice spent less time in open arms | Köster et al., 1999 |
| Light/dark preference in mice | N/OFQ −/− mice had preference for dark | Köster et al., 1999 |
| Swim stress effects on tail flick test - adaptation to repeat stress in mice | N/OFQ −/− mice were impaired in adaptation | Köster et al., 1999 |
| Basal and stress-induced corticosterone plasma levels | Corticosterone increased in N/OFQ −/− mice | Köster et al., 1999 |
| Elevated plus maze in rats | Agonist Ro 64-6198 increased time in open arms like diazepam | Jenck et al., 2000 |
| Fear-potentiated startle in rats | Agonist Ro 64-6198 decreased startle like diazepam | Jenck et al., 2000 |
| Elevated plus maze | Agonist Ro 64-6198 increased time in open arms after acute and 15 day dosing | Dautzenberg et al., 2001 |
| Punished responding in rats | Agonist Ro 64-6198 increased punished responding like diazepam | Jenck et al., 2000 |
| Elevated plus maze in mice | N/OFQ (icv) increased time in open arms | Gavioli et al., 2002 |
| Various Stress Assays in Individually-house vs group-house mice without the N/OFQ precursor gene | No difference from WT control in individual housing; stress-like reactitivty great than WT under group housing pressure | Ouagazzal et al., 2003 |
| Hole-board test in mice | N/OFQ (icv) had anxioltyic-like effects; higher doses had anxiogenic-like effects | Kamei et al., 2004 |
| Vogel conflict in rats | Agonist Ro 64-6198 increased punished responding like chlordiazepoxide | Varty et al., 2005 |
| Isolation induced vocalizations in rat and guinea pig pups | Agonist Ro 64-6198 reduced vocalizations | Varty et al., 2005 |
| Geller conflict test in mice | Agonist Ro 64-6198 increased punished responding in WT but not in N/OFQ −/− mice | Varty et al., 2005 |
| Conditioned defensive burying in rats | N/OFQ (icv) had anxioltyic-like effects after single and double injections | Vitale et al., 2006 |
| Elevated plus maze in rats | N/OFQ (icv) had anxioltyic-like effects after double but not single injection | Vitale et al., 2006 |
| Novelty-suppressed feeding and elevated T-maze in mice | N/OFQ −/− mice had anxiolytic-like phenotypes in these but not in other assays | Gavioli et al., 2007 |
| Restraint stress in rats | Cort increases were enhanced by the antagonist UFP-101 | Leggett et al., 2007 |
| Elevated plus maze in rats and guinea pigs | Anxioltyic-like effects in increasing open arm time | Varty et al., 2008 |
| Vogel conflict (rat), conditioned lick suppression (rats), fear-potentiated startle (rats), and pup separation-induced vocalization (guinea pigs) | Anxioltyic-like effects | Varty et al., 2008 |
| Vogel conflict in rats | Increases in punished responding attenuated by N/OFQ antagonist J-113397 | Varty et al., 2008 |
| Vogel conflict and fear-potentiated startle in rats | Anxioltyic-like effects | Lu et al., 2011 |
| Guinea pig vocalization | Anxiolytic-like effects | Lu et al., 2011 |
| Punished responding and marble-burying in mice | Anxiolytic-like effects | Lu et al., 2011 |
| Elevated plus maze | Anxiogenic-like effects of N/OFQ −/− rats | Rizzi et al., 2011 |
Cort: corticosterone
FST: forced-swim test
TST: tail-suspension test
WT: wild-type
Findings reported in the literature also indicate that, at the extrahypothalamic level, activation of N/OFQ receptors leads to anti-stress effects and reduces anxiety. In fact, central administration of N/OFQ or systemic injection of brain penetrant selective NOP agonists both leads to marked anxiolytic-like effects in rodents (Gavioli et al., 2006;Varty et al., 2005; Jenck et al., 1997; Goeldner et al., 2012). These effects appear to be particularly robust under stressful conditions; for example alcohol withdrawal following intoxication or after exposure to restraint stress (Economidou et al., 2008). This may depend upon the ability of N/OFQ to act as a functional antagonist for the extrahypothalamic CRF1 receptors. It has been reported that N/OFQ blocks the anxiogenic-like effect of CRF and abolishes the anorectic effect of restraint stress and CRF within the BNST and the CeA, the sites of the interaction between the two systems (Rodi et al., 2008; Ciccocioppo et al., 2003; Uchiyama et al., 2008). Of note, ex vivo electrophysiological recording from brain slices containing CeA revealed that N/OFQ treatment completely prevent the ability of CRF to stimulate GABAergic neurotransmission in this area (Cruz et al., 2012).
To reconcile the apparently opposite effect of N/OFQ on the HPA axis where it mimics a stress-like response and on extrahypothalamic sites where it shows anti-stress and anxiolytic-like effects is possible to hypothesize that this peptidergic system is part of the stress-coping mechanisms associated with physiological responses to stress. After exposure to an acute stimulus the N/OFQ system is recruited to facilitate the adaptation of the organism to the new environmental condition by recruiting the hormonal stress system. However, following protracted exposure to a stressful condition stimulation of N/OFQ activity at extra-hypothalamic sites may serve to attenuate the pathological consequences associated with chronic stress. It can be hypothesized that such a possibility might help to reconcile the discordant data in the experimental literature where both agonism and antagonism of NOP receptors has been suggested to counter or facilitate stress-related disorders such as anxiety and possibly post-traumatic stress.
A few studies have documented that the anxiolytic-like effects of N/OFQ agonists are not due to opioid/non-N/OFQ-related pharmacology; naltrexone did not attenuate their effects (e.g., Varty et al., 2005, 2008). Furthermore, data exist to show that sedative-like effects of agonists (e.g., locomotor activity) are absent in N/OFQ −/− mice (Lu et al., 2011). Although there are few studies, anxiolytic-like effects of N/OFQ agonists have also been shown to be deleted in N/OFQ −/− mice (Varty et al., 2005). On-target activity of anxiolytic-like effects have also been demonstrated by showing attenuation of agonist-induced anxiolytic-like effects with an antagonist (e.g., Varty et al., 2005).
It is worth noting that the data in Table 3 are behavioral in nature and, in the absence of corroborating findings at other mechanistic levels, one could make the argument that the specificity of the effects is questionable because other data have shown comparable effects of N/OFQ receptor antagonists. For example, UFP-101 had anxiolytic-like effects after acute dosing in rats in an elevated T-maze (Duzzioni et al., 2011). Although anxiolytic-like effects of acute dosing of agonists has been reported (Table 4), there are exceptions that complicate general understanding of the N/OFQ system at this time. Thus, Varty et al. (2005) reported a lack of effect of central N/OFQ administration in two anxiolytic-detecting assays in rats; after subchronic dosing, anxiolytic-like efficacy was achieved. Whether this qualitative change in behavioral effect was due to tolerance to the sedative-like effects of N/OFQ or to changes in receptor density/function, remain to be tested. Thus, although the data in tables 2 and 3 predict that an agonist at N/OFQ receptors would be a stress-mimic, the anxiolytic-like behavioral effects reported for N/OFQ and synthetic agonists might involve rapid agonist-mediated receptor desensitization that is reported to occur in vitro after agonist treatment (Ma et al., 1997; Dautzenberg et al., 2001; Corbani et al. 2004) where some agonists produce receptor internalization while others do not (Spampinato et al., 2007). Further, the processing of receptors in the cycle of agonist-induced endocytosis followed by receptor recycling and reactivation has been shown to bring forth compensatory events that can result in the supersensitivity of specific biochemical signaling pathways in vitro (Spampinato et al., 2007). It is thus hypothesized that for the behavioral output of anxiolytic-detecting behavioral assays, receptor dynamics for agonists in vivo underly the expression of anxiolytic-like effects, in addition to suprahypothalamic mechanisms discussed above. Additional data that build on these relationships along with subchronic agonist dosing experiments in vivo and comparisons of effects to N/OFQ receptor antagonists are needed.
In contrast to the behavioral findings in assays that detect anxiolytic and anti-stress drug effects, uniform findings in the area of the behavioral pharmacology of the antidepressant-like effects of N/OFQ ligands has been reported. Intracerebroventricular administration of N/OFQ did not affect behavior in the forced-swim or tail-suspension tests; however, antagonists of N/OFQ receptors have positive effects in these assays (table 3). The dissociation of findings in antidepressant- and anxiolytic-detecting assays has sometimes even been observed within studies. For example, whereas the N/OFQ antagonist UFP-101 produced antidepressant-like effects (comparable to imipramine) in the mouse forced-swim and tail-suspension tests, neither UFP-101 nor N/OFQ (into the dorsal hippocampus) produced anxiolytic-like effects in the light/dark test (Goeldner et al., 2010). Importantly too, unlike the data reported in the anxiolytic-detecting assays (table 4), Noc −/− mice have been used to substantiate the on-target activity of antagonists in antidepressant-detecting assays (table 3). For example, the antidepressant-like effects of UFP-101 (Gavioli et al., 2003) and of SB-612111 (Rizzi et al., 2007) were eliminated in Noc −/− mice. Further, the antidepressant-like effects of the antagonist UFP-101 were prevented by N/OFQ administration in the mouse forced-swim test (Gavioli et al., 2003). Both mice (Gavioli et al., 2003, 2004) and rats without N/OFQ receptors (Rizzi et al., 2011) display antidepressant-like behavioral phenotypes. In contrast, N/OFQ −/− mice display both anxiolytic-like and anxiogenic like effects in anxiolytic-detecting assays (Gavioli et al., 2007), a phenomenon that corresponds to the bitonic anxiolytic/anxiogenic-like behavioral induction by N/OFQ (i.c.v.) in mice (Kamei et al., 2004).
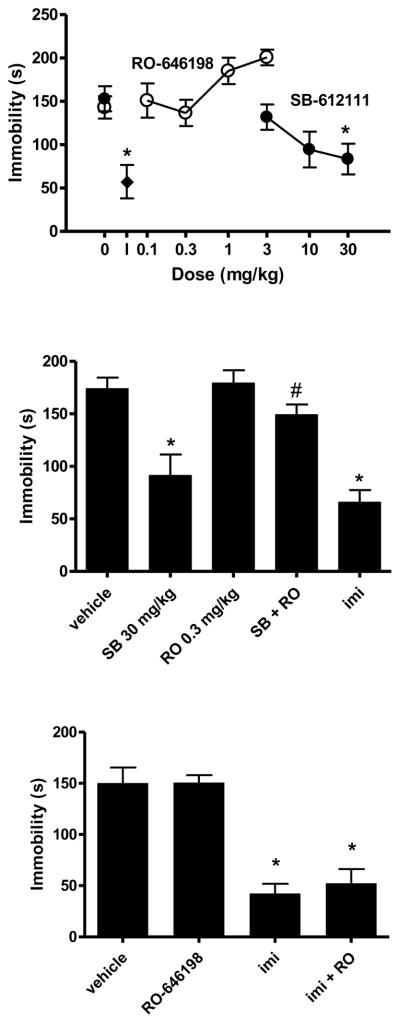
An example of data from NOP agonists and antagonists is shown in figure 4. In contrast to imipramine and to the NOP receptor antagonist SB-612111, the NOP agonist, Ro 64-6198 did not produce an antidepressant-like phenotype in the mouse forced-swim assay (top panel). However, Ro 64-6194 completely prevented the antidepressant-like effects of SB612111 (middle panel). The specificity of the inhibition of SB-612111 effects by Ro 64-6198 is illustrated by the inability of Ro 64-6198 to prevent the antidepressant-like actions of imipramine (bottom panel).
Figure 4.
Anti-immobility effects of the NOP receptor antagonist SB-61211 in the mous forced-swim assay. TOP. Lack of effect of NOP receptor agonist RO-646198. NIH Swiss, male mice, n=4–6/group. *p<0.05 compared to vehicle (Dunnett’s test). I: Effect of 15 mg/kg imipramine. MIDDLE. Prevention of effects of SB-61211(30 mg/kg, p.o.) by RO-646198 (0.3 mg/kg). NIH Swiss male mice, n=8/group. *p<0.05 compared to vehicle (Dunnett’s test); #p<0.05 compared to 2817412 alone (t-test). Imi: imipramine (15mg/kg, i.p., n=4), 30 min prior). LOWER. RO-646198 (0.3 mg/kg, p.o.) was not effective in preventing the anti-immobility effects of imipramine (15 mg/kg, i.p.). NIH Swiss male mice, n=8/group. *p<0.05 compared to vehicle (Dunnett’s test). Behavioral methods are comparable to Li et al. (2005).
Several small open-label and controlled clinical studies have demonstrated that the mixed opiate pharmacology compound buprenorphine exhibits rapid onset antidepressant activity in patients with treatment refractory depression (Emrich et al. 1982; Bodkin et al. 1995; Kosten et al. 1990). The mixed opiate pharmacology of buprenorphine is complicated; consisting of robust partial agonist activity at mu, antagonist activity at both kappa and delta, and low efficacy partial agonist at nociceptin receptors (Hawkinson et al., 2000; Huang et al., 2001; Bloms-Funke et al., 2000; Wnendt et al., 1999). In addition, two active metabolites of buprenorphine produced at relevant exposures following buprenorphine administration also bind at NOP receptors (Brown et al. 2011). The potential role of N/OFQ receptors in the antidepressant efficacy of buprenorphine would constitute convergent data suggesting that blockade of N/OFQ receptors might be antidepressant in humans. The potential role of N/OFQ receptors in the antidepressant efficacy of buprenorphine would constitute convergent data suggesting that blockade of N/OFQ receptors might be antidepressant in humans.
Additional data that support the idea the N/OFQ receptor blockade might have antidepressant effects comes from studies reporting increased nociceptin plasma levels in patients with depression including post-partum depression, major depressive disorder (MDD) and bipolar disorder (Gu et al., 2003; Wang et al., 2009; Zhang et al., 2009). Confirmation of these findings and extension to central measures of N/OFQ (e.g., cerebral spinal fluid and central NOP receptor occupancy) would be important data to link to the preclinical findings that have generated the stress/depression hypothesis. If N/OFQ is indeed increased in the CNS with stress/depression in humans, the ability to monitor N/OFQ levels in combination with various antidepressant therapies would be additional key information to collect.
Mood disorders (major depression, dysthymia and biopolar disorders) and obesity are highly comorbid in the general population (Preiss et al., 2013). Epidemiological studies consistently report mood disorders in obese persons seeking weight loss. Consistent with these findings, studies in patients with mood disorders demonstrated a significant increase in the incidence of being overweight and obesity. Moreover, in patients with binge eating disorder (BED), obesity and mood disorders are common comorbidities. Of particular relevance is that pharmacotherapies prescribed to treat mood disorders (particularly bipolar disorders) are known to cause significant weight gain, resulting in difficulty with treatment compliance and placing the patient at risk for developing obesity related diseases such as Type II diabetes. Therefore, a treatment for patients with mood disorders that is free of weight gain side effects either with or without comorbid obesity and/or BED could be helpful in the pharmacotherapy of MDD and associated mood disorders. In addition, MDD and obese patients also have a high prevalence of alcohol abuse disorder and abuse (c.f., Procopio et al., 2013). Given the potential impact of N/OFQ receptor antagonists in mood disorder and in alcohol abuse disorders (see data and discussion below), such a therapeutic could have additional value in impacting these convergent medical diseases. Finally, since increased serotonin neurtransmission/levels are implicated in the sexual dysfunction observed with some patients taking selective serotonin reuptake inhibitor (SSRI) antidepressants (Dueñas et al., 2011), N/OFQ receptor antagonists, by virtue of their non-serotonin increasing effects, would be predicted to have a lower sexual dysfunction-inducing liability than SSRIs and hence better medication compliance.
Drug Addiction
N/OFQ and NOP receptors are widely distributed in brain areas that regulate motivated behaviors and stress reactivity (fig. 1). In these areas, the peptide and the receptor are largely co-expressed known to interactively control drug-taking and addictive behaviors (Koob, 2013; Sim et al., 2013) suggesting local neurocircuitry modulation. They have been identified in the central nucleus of the amygdala (CeA), the bed nucleus of stria terminalis (BNST), medial prefrontal cortex (mPFC), ventral tegmental area (VTA), lateral hypothalamus (LH), nucleus accumbens (NAcc), and some brain stem areas, including the locus caeruleus and dorsal raphe (Darland, 1998; Neal et al., 1999a,b). Several lines of evidence suggest that the N/OFQ system subserves an important role in the regulation of various aspects of abused drugs such as opioids, psychostimulants, and ethanol.
Opioids
Interestingly, N/OFQ, despite being an opioid-like peptide, has been found to act in the brain with functional anti-opioid mechanisms. It blocks opioid-induced supraspinal analgesia (Mogil, 1996) and, more importantly, morphine reward measured in the conditioned place preference paradigm (Ciccocioppo, 2000; Murphy, 1999).
A key neurochemical correlate to the behavioral finding that N/OFQ abolishes morphine-induced conditioned place preference (CPP) is offered by microdialysis data showing that N/OFQ reduced morphine-induced dopamine (DA) release in the NAcc of conscious rats (Di Giannuario, 2000). Importantly, the same doses of N/OFQ did not modify basal extracellular levels of DA, in keeping with the finding that N/OFQ per se produces neither preference nor aversion in the place conditioning paradigm. In fact, drugs that reduce release in the NAcc (such as kappa opioid receptor agonists) induce place aversion (Herz, 1997).
Further support for this potential mechanism comes from immunohistochemistry experiments indicating that N/OFQ blocks the expression of c-fos, a marker of neuronal activation, induced by morphine in the shell portion of the NAcc (Ciccocioppo, 2000). In fact, there is evidence suggesting that rewarding stimuli, including morphine, potently increase c-fos expression in this area, reflecting activation of dopamine (DA) receptor-containing neurons (Barrot et al., 1999).
Additional studies have investigated the role of N/OFQ in the development of tolerance to the analgesic effect of morphine. Repeated treatments with morphine increased N/OFQ levels in different brain areas, including the amygdala (Yuan et al.,1999). Accordingly, treatment with selective NOP receptor antagonists prevented the development and expression of tolerance following chronic treatment with morphine (Scoto et al., 2010). Moreover, knockout mice for the NOP receptor gene showed a 50% reduction in tolerance to the analgesic effect of morphine (Ueda et al., 1997). These data point to the possibility that N/OFQ may also influence the development of tolerance to other central effects of opiates (i.e., reward). In this respect, the effect of NOP receptor antagonists should also be evaluated for their potential ability to prevent the escalation of opioid self-administration.
Another behavioral outcome associated with drug addiction is locomotor sensitization, a phenomenon in which repeated intermittent administration of drugs of abuse leads to a progressive increase in locomotor activity of rodents (Robinson et al., 1993). According to the incentive sensitization theory of addiction, this phenomenon may reflect the increase in drug “wanting” that occurs following repeated drug experiences (Robinson et al., 1993). The effect of N/OFQ on morphine-induced sensitization was studied with negative results (Ciccocioppo et al., 2000). Another negative finding comes from the only study in which NOP agonism was tested against opioid self-administration. In this study, acute treatment with N/OFQ was unable to prevent the intravenous infusion rate of heroin (Walker et al., 1998).
Although the mixed opiate pharmacology compound buprenorphine is often described as a mu opioid partial agonist and kappa/delta opioid antagonist, it also exhibits prominent N/OFQ activity in recombinant systems and native tissues (Bloms-Funke et al. 2000; Lutfy et al. 2003; Lester and Traynor, 2006). Preclinical studies evaluating the efficacy of buprenorphine against opioid self-administration have been mixed, with some studies indicating suppression of heroin self-administration (Mello et al. 1983; Chen et al. 2006), while others show no effect (Sorge and Stewart 2006). One study demonstrated that buprenorphine suppressed both heroin-induced heroin seeking and heroin-induced DA release in the NAcc (Sorge et al. 2005). It has also been shown that a buprenorphine/naltrexone combination blocks the reinstatement of a morphine-induced CPP (Cordery et al. 2012). Numerous clinical trials have also demonstrated that buprenorphine, alone and in combination with the mu-preferring opioid antagonist naloxone, is an effective maintenance treatment for opioid dependent individuals (see Tetrault and Fiellin, 2012 for a review). Indeed, buprenorphine monotherapy and the buprenorphine/naloxone combination therapy are now available in 73 countries for the treatment of opioid dependence (Farrell et al. 2012). Considering the NOP activity of buprenorphine, it is conceivable that some of its efficacy in reducing heroin and non-prescribed opioid abuse and the promotion of treatment retention is attributable to its modulation of the N/OFQ system, particularly when the mu partial agonist properties are blocked with naloxone or naltrexone.
Psychomotor Stimulant Addiction
Little is known about the anti-addictive potential of N/OFQ modulation on psychostimulant drug taking and addiction related neurochemistry. Promising preliminary evidence comes from CPP and microdialysis experiments. For instance, it has been shown that N/OFQ prevents the expression of CPP engendered by either cocaine or methamphetamine (Zhao et al., 2003;Kotlinska et al., 2002), and microdialysis experiments revealed that intracranial N/OFQ injection prevented cocaine from stimulating mesoaccumbal DA efflux (Lutfy et al., 2001). Indirect evidence supporting the ability of N/OFQ to attenuate the rewarding effect of psychostimulants comes also from studies on NOP receptor knockout (KO) mice where these mice have been shown to be more sensitive to the place conditioning effects of cocaine (Sakoori et al., 2009), replicating the previous findings with both morphine and nicotine (Rutten et al., 2011; Marquez et al., 2008). Additional studies with psychostimulants investigated the ability of NOP agonism to prevent the development or the expression of sensitization to cocaine and methamphetamine. Results revealed that N/OFQ blocked cocaine- and amphetamine-induced psychomotor sensitization (Bebawy et al., 2010; Lutfy et al., 2002; Kotlinska et al., 2003), an effect that was absent in NOP receptor KO mice (Bebawy et al., 2010).
Prior to drawing definitive conclusions on the potential of NOP agonists in psychostimulant addiction, their ability to prevent operant drug self-administration and reinstatement should be explored. At present, at least to our knowledge, only one study evaluated the effect of N/OFQ on stress-induced reinstatement of cocaine seeking behaviors. The results were negative (Martin-Fardon et al., 2000).
Preclinical studies have documented that buprenorphine attenuates cocaine-engendered CPP, self-administration, and sensitization (Kosten et al. 1991; Carroll and Lac, 1992; Placenza et al. 2008). However, other studies have reported that buprenorphine potentiated cocaine- and amphetamine-associated behavioral and neurochemical effects, consistent with its mu partial agonist properties and reported abuse liability (Brown et al. 1991; Kimmel and Holtzman, 1997; Sorge et al. 2005). Thus, the preclinical data regarding the potential efficacy of buprenorphine in the treatment of psychostimulant addiction are difficult to interpret, perhaps simply due to the complex pharmacology of buprenorphine and its active metabolites. Alternatively, the discrepant findings may be related to increased glutamate transmission in the NAcc that is stimulated by buprenorphine, which leads to increased sensitivity of AMPA receptors that contributes to sensitization and other physiological changes (Placenza et al. 2008). Importantly, when buprenorphine has been tested in combination with low doses of naltrexone to block the mu partial agonist effects, the combination blocked cocaine self-administration and cocaine-primed reinstatement, indicating that other pharmacological targets of buprenorphine contributed to the efficacy (Mello et al. 1993; Cordery et al. 2012; Wee et al. 2012). Clinically, buprenorphine has produced mixed results on cocaine dependent individuals, with the greatest efficacy being demonstrated at higher doses (above 16 mg/day) and in individuals either co-abusing or co-dependent on opiates (Kosten et al. 1992; Schottenfeld et al. 1993; Montoya et al., 2004). As discussed previously, if at low/intermediate doses buprenorphine specifically binds to the classical mu, delta and kappa opioid receptors at higher doses it may also occupy NOP receptors. Extrapolating from human PET imaging studies with [11C]-carfentanil it is reasonable to assert that at 16 mg/day buprenorphine reach a full occupation of mu opioid receptors (Greenwald et al., 2003). But considering that the affinity of buprenorphine for kappa and delta receptors is approximately equal to that of mu it is likely that at this dose a full occupation of all the classical opioid receptors occurs (Huang et al., 2001). Hence, the increased efficacy of buprenorphine on cocaine addicted individuals that is observable at doses usually higher than 16 mg/day is unlikely attributable to buprenorphine ability to bind to mu delta and kappa receptors. An intriguing possibility is, that at high doses buprenorphine activates NOP receptors which may contribute to the pharmacotherapeutic properties of this agent.
Alcohol (Ethanol) Addiction
Results of several studies from the current scientific literature have been reported that have demonstrated that activation of the N/OFQ system blunts the reinforcing and motivating effects of ethanol across a range of behavioral measures, including ethanol intake (Ciccocioppo et al., 1999), conditioned place preference (Kuzmin et al., 2003; Ciccocioppo et al., 1999), and reinstatement elicited by conditioned stimuli (Ciccocioppo et al., 2004) or stress (Martin-Fardon et al., 2000). The effects of N/OFQ on ethanol-related behaviors have been largely explored in genetically selected alcohol-preferring Marchigian Sardinian Alcohol-Preferring (msP) rats. This rat line is particularly sensitive to suppression of ethanol drinking and relapse by N/OFQ and N/OFQ analogues (Ciccocioppo et al., 1999; Ciccocioppo et al., 2004; Economidou et al., 2008). msP rats exhibit high sensitivity to stress, a high anxiety phenotype, and depression-like symptoms that are ameliorated by ethanol consumption (Ciccocioppo et al., 2006). These findings led to the hypothesis that these animals, at least in part, drink ethanol to “self-medicate” from their innate negative affective state (Ciccocioppo et al., 2006). Considering the possibility (discussed in section titled Stress, Anxiety, and Mood above) that activation of NOP receptors mediates a potent anxiolytic and anti-stress effect, it is possible that part of the N/OFQ effect in msP rats may be due to the peptide’s ability to alleviate their genetically-determined negative affective state.
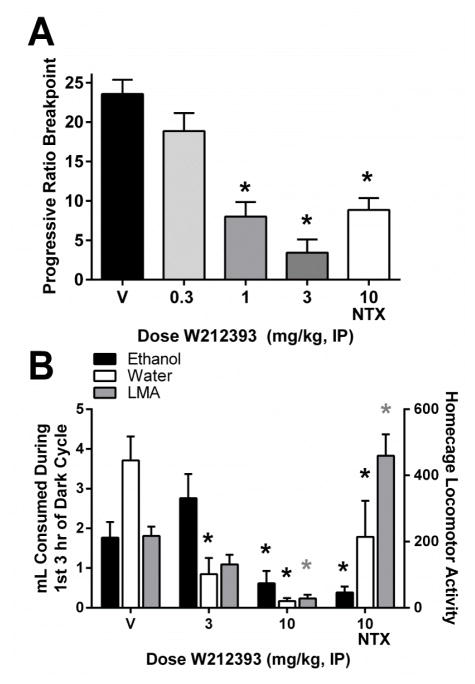
Some of the work in the msP rat line has been replicated in another line of selectively-bred high-ethanol drinking rats, the Alcohol-Preferring (P) rats. In these rats, activation of NOP receptors via the small molecule agonist, W212393, reduced responding under a progressive ratio schedule maintained by ethanol (15% v/v; fig 5A). In another study, W212393 also reduced ethanol drinking in P rats with continuous home cage access to ethanol and water (fig. 5B). In that study, however, the dose that attenuated ethanol self-administration also significantly decreased food intake and locomotor activity, suggesting that behavioral effects of W212393 were not specific to ethanol-related motivational processes. These data replicate the attenuation of ethanol-self administration by NOP agonists previously reported in the msP rats.
Figure 5.
Effects of the NOP receptor agonist W212393 on ethanol self-administration in Alcohol-Preferring (P) rats. Panel A: data from P rats maintained under a progressive ratio operant schedule of reinforcement (0.1 ml 15% ethanol v/v in water/reinforcer). Panel B: data in P rats with continuous homecage access to ethanol (15% v/v in water) and water. Methods used for homecage ethanol drinking in P rats were similar to those reported in Rorick-Kehn et al. (Neuropharmacology, in press), except that W212393 was dissolved in 20% Captisol in phosphate buffer and dosed IP immediately before onset of the dark cycle, using a within-subjects design (exception: W212393 was dosed 30 min prior to progressive ratio operant experiments). Homecage locomotor activity is shown on the right y-axis (gray bars). *p<0.05 compared to vehicle (V). NTX = naltrexone, LMA = locomotor activity.
Notably, msP rats exhibit elevated expression of N/OFQ and NOP receptor mRNA, especially in the BNST and CeA (Economidou et al., 2008), and increased NOP receptor binding in the CeA, BNST, VTA, and several cortical structures, indicating an endogenous up-regulation of the N/OFQ-NOP system in this rat line (Economidou et al., 2008). However, despite elevated NOP receptor expression and binding, msP rats show a distinct pattern of N/OFQ functional abnormalities in the CeA, where N/OFQ-stimulated [35S]-GTPγS binding was significantly lower than in Wistar rats (Economidou et al., 2008). Thus, uncoupling of the NOP receptor from G-protein-mediated signal transduction in the CeA may lead to regionally selective hypofunction of the N/OFQ system, which could facilitate ethanol drinking and possibly anxiety in msP rats. This hypothesis is corroborated by data showing that alcohol self-administration in msP rats is reduced by site-specific injection of N/OFQ into the CeA (Economidou et al., 2008).
The efficacy of N/OFQ in preventing the expression of somatic and affective alcohol withdrawal signs in ethanol-dependent Wistar rats was also investigated. Intracranial peptide administration completely abolished somatic signs associated with acute withdrawal, while the expression of anxiety measured one week after termination of ethanol exposure (protracted withdrawal) was markedly reduced (Economidou et al., 2011). Paradoxically, in another study in Sprague-Dawley rats, acute ethanol intoxication (1 day binge exposure), ethanol dependence (5 day binge exposure), and ethanol withdrawal (1 day after 5 day binge exposure) were associated with significant increases in pronociceptin mRNA levels in the amygdala (D’Addario et al. 2013). Perhaps the increase in pronociceptin transcript reflects an allostatic process, or the body’s attempt to adapt to and counter the high levels of ethanol administered in that study. Overall, the data suggest that activation of NOP receptors might have beneficial effects not only on ethanol drinking and relapse but also in treating withdrawal.
However, it has also been shown that complex adaptive changes in the N/OFQ system may occur following protracted exposure to intoxicating doses of ethanol which may potentially limit the therapeutic efficacy of NOP receptor agonists in alcoholism. In fact, Wistar rats made dependent on ethanol and then tested for ethanol self-administration 1 week following withdrawal, were sensitive to the suppressant effects and to the anxiolytic-like action of N/OFQ whereas nondependent controls rats remained less sensitive (Economidou et al., 2011; Martin-Fardon et al., 2010; Aujla et al., 2013). However, after 3 weeks post-intoxication, N/OFQ engendered anxiogenic-like actions in ethanol-dependent rats but continued to exert anxiolytic-like actions in non-dependent controls. Unfortunately, no data are available on the effects of N/OFQ on ethanol self-administration at time points longer than 3 weeks post-withdrawal.
Surprisingly, relatively few studies have been conducted to assess the efficacy of buprenorphine in reducing ethanol-motivated behaviors. Studies in outbred rats and Rhesus monkeys demonstrated that buprenorphine attenuated ethanol self-administration (Martin et al. 1983; Carroll et al. 1992; June et al. 1998). The most convincing evidence for the N/OFQ contribution to the efficacy of buprenorphine comes from a study in msP rats demonstrating that buprenorphine at low intermediate doses increased alcohol intake while at higher doses it suppressed it. Pretreatment with naltrexone specifically blocked the increase in drinking at low buprenorphine doses whereas pretreatment with the NOP antagonist UFP-101, which had no effect when administered alone, selectively prevented buprenorphine-induced inhibition of intake (Ciccocioppo et al. 2007). Consistent with the anti-opioid effects of NOP agonism and the NOP modulation by buprenorphine, it has been reported that the rewarding and motor stimulant properties of buprenorphine were enhanced in mice lacking NOP receptors, despite a lack of altered responsiveness to morphine (Marquez et al. 2008). In a small open-label clinical trial, buprenorphine at high doses was shown to significantly reduce alcohol drinking in heroin addicts with comorbid alcohol abuse (Nava et al. 2008).
Possible mechanisms of action for the effects of NOP agonism on addiction
As discussed above, the current experimental literature findings indicate that NOP receptor agonism attenuates several physiological effects of drugs of abuse, including reward, sensitization, tolerance, withdrawal, craving and relapse. Moreover, these effects have been observed across different classes of drugs of abuse (i.e., alcohol, opioids, and psychostimulants). Based upon this evidence, the literature proposes a few key hypotheses that can be tested based on N/OFQ physiopharmacology: the dopamine modulation hypothesis and the anxiolytic CRF hypothesis.
As noted above, the experimental literature supports the idea that NOP receptor activation results in a reduction of drug-induced activation of mesolimbic DA neurotransmission. Considering that stimulation of the mesocorticolimbic DA system has been shown to reflect, at least in part, drug reward and reinforcement (Luscher et al., 2006; Di Chiara et al., 2007; Wise et al., 1989), it is hypothesized that the peptide’s ability to reduce drug seeking and self-administration may be directly linked to the ability of N/OFQ to blunt this core process involving drugs of abuse: DA release in mesolimbic reward pathways. Particularly intriguing is also the mechanism through which NOP receptors might attenuate ethanol reward and reinforcement. In fact, the effects of ethanol on the mesolimbic DA system is likely dependent upon its ability to indirectly activate the endogenous opioid system (Herz et al., 1997; Johnson et al., 1992).
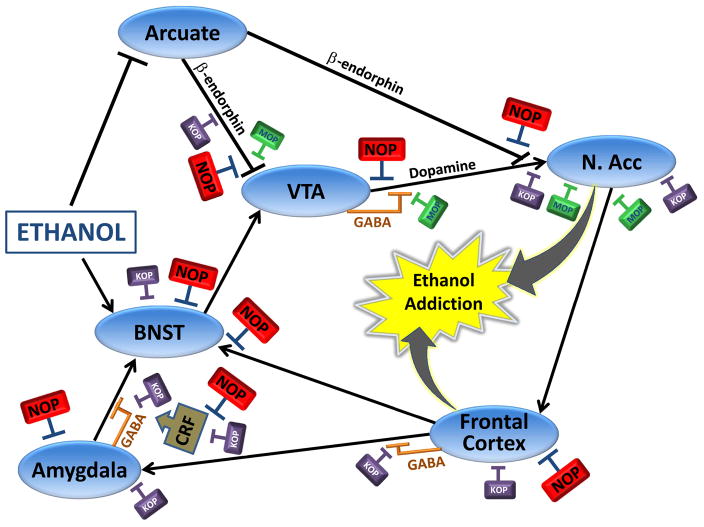
It has been suggested that activation of β-endorphinergic neurons of the arcuate nucleus may represent an important mechanism by which ethanol stimulates reward (Herz et al., 1997) (fig. 6). These β-endorphin neurons project, in part, to the NAcc where they may stimulate DA neurotransmission by activating mu opioid receptors located on DA nerve terminals. Other β-endorphin neurons project from the arcuate to the VTA, thus stimulating mu receptors located on GABAergic interneurons of the VTA, which tonically inhibit DA neurons (Johnson et al., 1992). In support of these mechanisms, electrophysiological evidence indicates that N/OFQ inhibits the activity of β-endorphinergic neurons of the arcuate (Wagner et al., 1998). Alternatively, it is possible that N. stria terminalis N/OFQ may directly inhibit DA neurotransmission in the NAcc by activating NOP receptors localized on DA neurons in the VTA (Murphy et al., 1999). Indeed, the VTA expresses relatively high levels of intermediate-size nociceptin-containing neurons, and a high density of NOP receptors (Ikeda et al., 1998). Consistent with this hypothesis, high levels of tyrosine hydroxylase-containing neurons in the VTA and substantia nigra co-express NOP mRNA, but not N/OFQ mRNA (Norton et al., 2002) indicating that N/OFQ could be in an optimal position to regulate DA neurotransmission. Interestingly, N/OFQ mRNA tends to be expressed in GABAergic neurons within these brain areas (Norton et al. 2002), suggesting a local inhibitory feedback loop.
Figure 6.
Circuitry underlying ethanol addiction as controlled by NOP receptors. The diagram represents neuroanatomical, neurochemical, and electrophysiological evidence describing modulation of mesocortical and mesolimbic dopamine circuits by NOP receptors, and their relationship to KOP and MOP receptors. Blue ovals = nuclei; ↑ = excitatory connection; T = inhibitory connection. Abbreviations: NOP, nociceptin opioid receptor; MOP, mu opioid receptor; KOP, kappa opioid receptor; VTA, ventral tegmental area, NAcc, nucleus accumbens; BNST, bed nucleus of the stria terminalis; GABA, g-aminobutyric acid; CRF = corticotropin releasing factor.
In addition to the dopamine modulation hypothesis presented above, it has been suggested based on the data generated in genetically-selected rats that NOP receptor agonism may attenuate drug-seeking and self-administration through the anxiolytic and anti-stress actions produced by the peptide and small molecule agonists (Gavioli et al., 2006; Varty et al., 2005). This may depend upon the ability of N/OFQ to act as a functional antagonist of the extrahypothalamic CRF1 receptor system. It has been shown, for example, that N/OFQ blocks the anxiogenic-like effect of CRF and abolishes the anorectic effect of restraint stress and CRF, with the BNST being the critical site of the interaction between the two systems (Rodi et al., 2008; Ciccocioppo et al., 2003). In addition, in a recent electrophysiological study, it was shown that N/OFQ opposes the ability of CRF to facilitate GABAergic transmission in the CeA (Cruz et al., 2012). Interestingly this inhibitory effect was more pronounced in animals withdrawn from intoxicating doses of alcohol which are known to have an overactive CRF neurotransmission (Cruz et al., 2012). Noteworthy, the CeA appears to be the brain site of action of N/OFQ on alcohol drinking (Gilpin and Roberto, 2012). Taken as a whole, these data provide converging evidence supporting the possibility that the anti-addictive properties of N/OFQ may be related to its anxiolytic and anti-stress effects, especially when the CRF system is recruited. This view is confirmed by gene expression data showing that exposure to stressful conditions such as alcohol withdrawal or intracranial CRF administration lead to over-expression of NOP receptors in the BNST which may explain, at least in part, the higher efficacy of the peptide under these conditions (Rodi et al., 2008; Martin-Fardon et al., 2010).
Clearly, the anti-opioid and anti-CRF nature of N/OFQ, that has been described in the experimental literature and discussed above, offers a strong rationale to help account for the effect of NOP receptor agonism in addiction summarized above. Based on current knowledge, it is tempting to hypothesize that both properties N/OFQ may contribute to the attenuation of drug intake: on the one hand, by attenuating drug-induced positive reinforcement (opioid antagonism) and, on the other hand, by blocking negative reinforcement resulting from adaptive changes associated with protracted drug use (CRF antagonism). With respect to reinstatement behavior, it is reasonable to believe, based upon current literature data, that cue- and stress-induced relapse are predominantly controlled by the anti-opioid and the anti-CRF effects of N/OFQ, respectively.
N/OFQ and Addiction Future Directions
As reviewed and analyzed in the previous paragraphs, a wealth of data from the current state of data from the experimental literature points to the possibility that NOP receptor agonists may represent a novel approach to treat addiction. Particularly robust are data supporting the potential of targeting the N/OFQ system for alcohol dependence. Scattered findings also suggest the possibility that pharmacological activation of NOP receptors might also be useful for the treatment of psychostimulant and opioid dependence. However, this body of data is not without inconsistency. For instance, it has been shown that behavioral sensitization to methamphetamine is attenuated in NOP receptor KO mice and is prevented by the antagonist UFP-101 in wild type mice (Sakoori et al. 2008). NOP receptor KO mice also drink less ethanol compared to their wild type counterparts, and in the place conditioning paradigm, the sensitization produced by chronic drug treatment is absent in NOP receptor KO mice (Sakoori et al., 2008). Although these apparent literature disparities are currently unexplained, clues to reconcilliation might be found in the comparable divided data sets in the area of anxiety (discussed at length above).
In addition, it needs to be borne in mind that addictive behaviors may be controlled through a variety of different or even opposite mechanisms. For instance, motivation underlying drug taking or to relapse during abstinence can be attenuated by substituting the drug of abuse but also by blocking rewarding effects. A prototypical example is the opioid system where both agonists like methadone or buprenorphine, and antagonists like naltrexone or nalmefene, have been successfully developed for addiction treatment (Stotts et al., 2009).
Clinical Development
Clinical validation for any of the aforementioned indications is lacking. However, important clinical tools have been successfully developed enabling hypothesis testing for disease modification as well as advanced study of NOP receptors in human subjects. Recently, a small molecule antagonist [11C]-labeled PET ligand for NOP receptors has been developed (Pike et al., 2011; Kimura et al., 2011; Lohith et al., 2012). Studies with this ligand revealed rapid uptake of the radioligand and a high density of binding sites throughout the CNS of healthy human volunteers. A [3H]-labeled version of the antagonist tracer also exists and exhibited a high degree of receptor selectivity in sections of mouse brain (fig. 1). Therefore, the [3H]-radioligand should offer great support for nonclinical ex vivo or in situ based studies. Moreover, several studies have employed measurement of N/OFQ in plasma, CSF and synovial fluids from patients presenting affective disorders, angina, osteoarthritis, sepsis, cholitis, liver cancer and migraine (Brooks et al., 1998; Raffaeli et al., 2006; Chiou et al., 2007; Wang et al., 2009; Krepuska et al., 2011; Spadaro et al., 2006; Ertsey et al., 2005; Anderberg et al., 1998) Thus, these tools will enable clinical testing of potential therapeutic indications suggested by the biological data on N/OFQ and its receptor NOP as discussed above. To date, several molecules have been reported in clinical development (Table 5).
Table 5.
NOP receptor ligands in clinical development
| Molecule | Molecular Features | Development Agent | Latest Known Status |
|---|---|---|---|
| GRT-6005 | Agonist | Grunenthal/Forest | Phase II for diabetic neuropathy, Neuropathic pain |
| MT-7716 | Agonist | Mitsubishi Tanabe | Phase 1 for pain |
| SER-100 | Agonist | Serodus/Zealand Pharma | Phase 1 for pain |
| LY2940094 | Antagonist | Eli Lilly and Company | Phase II for major depressive disorder |
| JTC-801 | Antagonist | Japan Tobacco | Discontinued for pain |
| SB-612111 | Antagonist | Galaxo Smith-Kline | No current development |
Conclusions
Since its de-orphinization, a growing body of scientific findings have been reported that have helped begin to define the biological functions of N/OFQ and its receptors in normal and pathophsyiological conditions. Although the present review and commentary focuses on the disease areas of obesity, depression, stress reactivity, anxiety, and drug dependencies, other biological systems are impacted by this N/OFQ. The preclinical literature has utilized the research tools of selective NOP agonists and antagonists and receptor deficient mice and rats to aid in this interrogation of biological function. Convergent evidence suggests that N/OFQ is involved in feeding, weight gain, and obesity, where antagonism at NOP receptors has been suggested to have therapeutic potential in these disease areas. Likewise, multiple pieces of data suggest that N/OFQ is involved in stress, mood, and anxiety. For antidepressant-related activities the preclinical data indicates the possibility that NOP receptor blockade might be a viable therapeutic strategy. In contrast, the predominance of current scientific evidence points to NOP receptor agonism as potentially beneficial for the treatment of anxiety and addictions. Although multiple agonist and antagonist small molecules have been put into clinical play, no clinical findings are currently available to confirm or refute hypotheses based upon preclinical evidence. The discovery and implementation of PET ligands for NOP receptors combined with pharmacological tools, and a rich biological spectrum to explore bodes well for a rapid advancement of clinical understanding.
Abbreviations
- ACTH
adrenocorticotropic hormone
- BED
binge eating disorder
- BNST
bed nucleus of stria terminalis
- CeA
central nucleus of the amygdala
- CGRP
Calcitonin gene related peptide
- CPP
conditioned place preference
- CRF
corticotrophin-releasing factor
- CTA
conditioned taste aversion
- DA
dopamine
- EPSC
excitatory post-synaptic current
- FST
forced-swim test
- GIRK
G-protein activated, inwardly rectifying K+ channel
- GPCR
G-protein-coupled receptor
- HPA
hypothalamic-pituitary axis
- 5-HT
5-hydroxytryptamine or serotonin
- J-113397
(±)trans-1-[1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one, a NOP receptor antagonist
- JTC-801
N-(4-amino-2-methylquinolin-6-yl)-2-(4-ethylphenoxymethyl)benzamide hydrochloride, a NOP receptor antagonist
- KO
knockout
- MDD
major depressive disorder
- mPFC
medial prefrontal cortex
- msP
Marchigian Sardinian Alcohol-Preferring
- NAcc
nucleus accumbens
- NE
norepinephrine
- N/OFQ
Nociceptin/Orphinan FQ (F:phenylalanine;Q:glutamine; the amino acids that begin and end the peptide sequence)
- NOP
Nociceptin opioid peptide or Nociceptin opioid peptide receptor
- NPY
neuropeptide Y
- ORL
opioid-receptor-like
- P rats
Alcohol-prefering rats
- POMC
Pro-opiomelanocortin
- Ro 64-6198
(1S,3aS)-8- (2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza- spiro[4.5]decan-4-one, a NOP receptor agonist
- SCH 221510
8-[bis(2-methylphenyl)-methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol
- SCH 655842
endo-8-[bis(2-chlorophenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octane-3-carboxamide, a NOP receptor agonist
- SB-612111
[(−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol, a NOP receptor antagonist
- TST
tail-suspension test
- UFP-101
[Nphe1,Arg14,Lys15]N/OFQ-NH2, a NOP receptor antagonist
- VTA
ventral tegmental area
- W212393
2-{3-[1-((1R)-acenaphthen-1-yl)piperidin-4-yl]-2,3-dihydro-2-oxo-benzimidazol-1-yl}-N-methylacetamide, a NOP receptor agonist
Footnotes
This paper is dedicated to the memory of Dr. Toni S. Shippenberg. Although she left life at a young age, her contributions to the neuroscience of drug dependence, to hedonic valuation, and to specific opiate and non-opiate neural pathways are tremendous. Her dedication to the field and to her friends and colleagues will always be remembered.
Contributor Information
Jeffrey M. Witkin, Lilly Research Labs, Eli Lilly and Company, Indianapolis, IN, USA
Michael A. Statnick, Lilly Research Labs, Eli Lilly and Company, Indianapolis, IN, USA
Linda M. Rorick-Kehn, Lilly Research Labs, Eli Lilly and Company, Indianapolis, IN, USA
John E. Pintar, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, Piscataway, NJ, USA
Michael Ansonoff, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, Piscataway, NJ, USA.
Yanyun Chen, Lilly Research Labs, Eli Lilly and Company, Indianapolis, IN, USA.
R. Craig Tucker, Lilly Research Labs, Eli Lilly and Company, Indianapolis, IN, USA.
Roberto Ciccocioppo, School of Pharmacy, University of Camerino, Camerino, Italy.
References
- Aujla H, Cannarsa R, Romualdi P, Ciccocioppo R, Martin-Fardon R, Weiss F. Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict Biol. 2010;18:467–79. doi: 10.1111/j.1369-1600.2012.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderberg UM, Liu Z, Berglund L, Nyberg F. Plasma levels on nociceptin in female fibromyalgia syndrome patients. Z Rheumatol. 1998;57(Suppl 2):77–80. doi: 10.1007/s003930050241. [DOI] [PubMed] [Google Scholar]
- Armstead WM. Nociceptin/orphanin phenylalanine glutamine (FQ) receptor and cardiovascular disease. Cardiovasc Ther. 2011;29(1):23–28. doi: 10.1111/j.1755-5922.2010.00241. [DOI] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. Functional heterogeneity in dopamine release and in the expression of Fos-like proteins within the rat striatal complex. Eur J Neurosci. 1999;11:1155–66. doi: 10.1046/j.1460-9568.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- Bebawy D, Marquez P, Samboul S, Parikh D, Hamid A, Lutfy K. Orphanin FQ/nociceptin not only blocks but also reverses behavioral adaptive changes induced by repeated cocaine in mice. Biol Psychiatry. 2011;68:223–30. doi: 10.1016/j.biopsych.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakley GG, Pohorecky LA, Benjamin D. Behavioral and endocrine changes following antisense oligonucleotide-induced reduction in the rat NOP receptor. Psychopharmacology (Berl) 2004;171(4):421–428. doi: 10.1007/s00213-003-1597-5. [DOI] [PubMed] [Google Scholar]
- Bloms-Funke P, Gillen C, Schuettler AJ, Wnendt S. Agonistic effects of the opioid buprenorphine on the nociceptin/OFQ receptor. Peptides. 2000;21(7):1141–1146. doi: 10.1016/s0196-9781(00)00252-7. [DOI] [PubMed] [Google Scholar]
- Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15(1):49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Bomberg EM, Grace MK, Levine AS, Olszewski PK. Functional interaction between nociceptin/orphanin FQ and alpha-melanocyte-stimulating hormone in the regulation of feeding. Peptides. 2006;27(7):1827–1834. doi: 10.1016/j.peptides.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Bouali SM, Fournier A, St-Pierre S, Jolicoeur FB. In vivo central actions of NPY(1-30), an N-terminal fragment of neuropeptide Y. Peptides. 1994;15(5):799–802. doi: 10.1016/0196-9781(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Brooks H, Elton CD, Smart D, Rowbotham DJ, McKnight AT, Lambert DG. Identification of nociceptin in human cerebrospinal fluid: comparison of levels in pain and non-pain states. Pain. 1998;78(1):71–73. doi: 10.1016/S0304-3959(98)00130-4. [DOI] [PubMed] [Google Scholar]
- Brown EE, Finlay JM, Wong JT, Damsma G, Fibiger HC. Behavioral and neurochemical interactions between cocaine and buprenorphine: implications for the pharmacotherapy of cocaine abuse. J Pharmacol Exp Ther. 1991;256(1):119–126. [PubMed] [Google Scholar]
- Brown SM, Holtzman M, Kim T, Kharasch ED. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251–1260. doi: 10.1097/ALN.0b013e318238fea0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Carmona GN, May SA, Buzalsky S, Larson C. Buprenorphine’s effects on self-administration of smoked cocaine base and orally delivered phencyclidine, ethanol and saccharin in rhesus monkeys. J Pharmacol Exp Ther. 1992;261(1):26–37. [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Effects of buprenorphine on self-administration of cocaine and a nondrug reinforcer in rats. Psychopharmacology (Berl) 1992;106(4):439–446. doi: 10.1007/BF02244812. [DOI] [PubMed] [Google Scholar]
- Chee MJ, Price CJ, Statnick MA, Colmers WF. Nociceptin/orphanin FQ suppresses the excitability of neurons in the ventromedial nucleus of the hypothalamus. J Physiol. 2011;589(Pt 13):3103–3114. doi: 10.1113/jphysiol.2011.208819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31(12):2692–2707. doi: 10.1038/sj.npp.1301008. d. [DOI] [PubMed] [Google Scholar]
- Chen X, McClatchy DB, Geller EB, Liu-Chen L, Tallarida RJ, Adler MW. Possible mechanism of hypothermia induced by intracerebroventricular injection of orphanin FQ/nociceptin. Brain Res. 2001;904(2):252–258. doi: 10.1016/s0006-8993(01)02467-2. [DOI] [PubMed] [Google Scholar]
- Chiou LC, Lee HJ, Ho YC, Chen SP, Liao YY, Ma CH, … Wang SJ. Orexins/hypocretins: pain regulation and cellular actions. Curr Pharm Des. 2010;16(28):3089–3100. doi: 10.2174/138161210793292483. [DOI] [PubMed] [Google Scholar]
- Chiou LC, Liao YY, Fan PC, Kuo PH, Wang CH, Riemer C, Prinssen EP. Nociceptin/orphanin FQ peptide receptors: pharmacology and clinical implications. Curr Drug Targets. 2007;8(1):117–135. doi: 10.2174/138945007779315605. [DOI] [PubMed] [Google Scholar]
- Chung S, Pohl S, Zeng J, Civelli O, Reinscheid RK. Endogenous orphanin FQ/nociceptin is involved in the development of morphine tolerance. J Pharmacol Exp Ther. 2006;318(1):262–267. doi: 10.1124/jpet.106.103960. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 1999;141:220–4. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol. 2000;404:153–9. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F, Massi M. Nociceptin/orphanin FQ inhibits stress- and CRF-induced anorexia in rats. Neuroreport. 2001 May 8;12(6):1145–9. doi: 10.1097/00001756-200105080-00019. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R. It has been reported that N/OFQ blocks the anxiogenic-like effect of CRF and abolishes the anorectic effect of restraint stress and CRF within the BNST and the CeA 2003 [Google Scholar]
- Ciccocioppo R, Biondini M, Antonelli L, Wichmann J, Jenck F, Massi M. Reversal of stress- and CRF-induced anorexia in rats by the synthetic nociceptin/orphanin FQ receptor agonist, Ro 64-6198. Psychopharmacology (Berl) 2002;161(2):113–119. doi: 10.1007/s00213-002-1020-7. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Cippitelli A, Economidou D, Fedeli A, Massi M. Nociceptin/orphanin FQ acts as a functional antagonist of corticotropin-releasing factor to inhibit its anorectic effect. Physiol Behav. 2004;82(1):63–68. doi: 10.1016/j.physbeh.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Hyytia P. The genetics of alcoholism: learning from 50 years of research. Addict Biol. 2006;11:193. doi: 10.1111/j.1369-1600.2006.00028.x. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Rimondini R, Sommer W, Massi M, Heilig M. Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol Psychiatry. 2007;61(1):4–12. doi: 10.1016/j.biopsych.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O. The orphanin FQ/nociceptin (OFQ/N) system. Results Probl Cell Differ. 2008;46:1–25. doi: 10.1007/400_2007_057. [DOI] [PubMed] [Google Scholar]
- Corbani M, Gonindard C, Meunier JC. Ligand-regulated internalization of the opioid receptor-like 1: a confocal study. Endocrinology. 2004;145(6):2876–2885. doi: 10.1210/en.2004-0062. [DOI] [PubMed] [Google Scholar]
- Cordery SF, Taverner A, Ridzwan IE, Guy RH, Delgado-Charro MB, Husbands SM, Bailey CP. A non-rewarding, non-aversive buprenorphine/naltrexone combination attenuates drug-primed reinstatement to cocaine and morphine in rats in a conditioned place preference paradigm. Addict Biol. 2012 doi: 10.1111/adb.12020. [DOI] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, Roberto M. Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biol Psychiatry. 2012;71:666–76. doi: 10.1016/j.biopsych.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Addario C, Caputi FF, Rimondini R, Gandolfi O, Del Borrello E, Candeletti S, Romualdi P. Different alcohol exposures induce selective alterations on the expression of dynorphin and nociceptin systems related genes in rat brain. Addict Biol. 2013;18(3):425–433. doi: 10.1111/j.1369-1600.2011.00326.x. [DOI] [PubMed] [Google Scholar]
- Darland T, Heinricher MM, Grandy DK. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–21. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Wichmann J, Higelin J, Py-Lang G, Kratzeisen C, Malherbe P, … Jenck F. Pharmacological characterization of the novel nonpeptide orphanin FQ/nociceptin receptor agonist Ro 64-6198: rapid and reversible desensitization of the ORL1 receptor in vitro and lack of tolerance in vivo. J Pharmacol Exp Ther. 2001;298(2):812–819. [PubMed] [Google Scholar]
- Devine DP, Hoversten MT, Ueda Y, Akil H. Nociceptin/orphanin FQ content is decreased in forebrain neurones during acute stress. J Neuroendocrinol. 2003;15(1):69–74. doi: 10.1046/j.1365-2826.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- Devine DP, Watson SJ, Akil H. Nociceptin/orphanin FQ regulates neuroendocrine function of the limbic-hypothalamic-pituitary-adrenal axis. Neuroscience. 2001;102(3):541–553. doi: 10.1016/s0306-4522(00)00517-0. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S. Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides. 2000;21:1125–30. doi: 10.1016/s0196-9781(00)00250-3. [DOI] [PubMed] [Google Scholar]
- Dueñas H, Brnabic AJ, Lee A, Montejo AL, Prakash S, Casimiro-Querubin ML, … Raskin J. Treatment-emergent sexual dysfunction with SSRIs and duloxetine: effectiveness and functional outcomes over a 6-month observational period. Int J Psychiatry Clin Pract. 2011;15(4):242–254. doi: 10.3109/13651501.2011.590209. [DOI] [PubMed] [Google Scholar]
- Duzzioni M, Duarte FS, Leme LR, Gavioli EC, De Lima TC. Anxiolytic-like effect of central administration of NOP receptor antagonist UFP-101 in rats submitted to the elevated T-maze. Behav Brain Res. 2011;222(1):206–211. doi: 10.1016/j.bbr.2011.03.056. [DOI] [PubMed] [Google Scholar]
- Economidou D, Fedeli A, Fardon RM, Weiss F, Massi M, Ciccocioppo R. Effect of novel nociceptin/orphanin FQ-NOP receptor ligands on ethanol drinking in alcohol-preferring msP rats. Peptides. 2006;27(12):3299–3306. doi: 10.1016/j.peptides.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, et al. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry. 2008;64:211–8. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Policani F, Angellotti T, Massi M, Terada T, Ciccocioppo R. Effect of novel NOP receptor ligands on food intake in rats. Peptides. 2006;27(4):775–783. doi: 10.1016/j.peptides.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, et al. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin Exp Res. 2011;35:747–55. doi: 10.1111/j.1530-0277.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emrich HM, Vogt P, Herz A, Kissling W. Antidepressant effects of buprenorphine. Lancet. 1982;2(8300):709. doi: 10.1016/s0140-6736(82)90727-9. [DOI] [PubMed] [Google Scholar]
- Ertsey C, Hantos M, Bozsik G, Tekes K. Plasma nociceptin levels are reduced in migraine without aura. Cephalalgia. 2005;25(4):261–266. doi: 10.1111/j.1468-2982.2004.00849. [DOI] [PubMed] [Google Scholar]
- Farhang B, Pietruszewski L, Lutfy K, Wagner EJ. The role of the NOP receptor in regulating food intake, meal pattern, and the excitability of proopiomelanocortin neurons. Neuropharmacology. 2010;59(3):190–200. doi: 10.1016/j.neuropharm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M, Wodak A, Gowing L. Maintenance drugs to treat opioid dependence. BMJ. 2012;344:e2823. doi: 10.1136/bmj.e2823. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Misilmeri MA, Felger JC, Devine DP. Nociceptin/orphanin FQ increases anxiety-related behavior and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacology. 2004;29(1):59–71. doi: 10.1038/sj.npp.1300308. [DOI] [PubMed] [Google Scholar]
- Fioravanti B, Vanderah TW. The ORL-1 receptor system: are there opportunities for antagonists in pain therapy? Curr Top Med Chem. 2008;8(16):1442–1451. doi: 10.2174/156802608786264227. [DOI] [PubMed] [Google Scholar]
- Florin S, Meunier J, Costentin J. Autoradiographic localization of [3H]nociceptin binding sites in the rat brain. Brain Res. 2000;880(1–2):11–16. doi: 10.1016/s0006-8993(00)02669-x. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Calo G. Antidepressant- and anxiolytic-like effects of nociceptin/orphanin FQ receptor ligands. Naunyn Schmiedebergs Arch Pharmacol. 2006;372(5):319–330. doi: 10.1007/s00210-006-0035-8. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Marzola G, Guerrini R, Bertorelli R, Zucchini S, De Lima TC, … Calo G. Blockade of nociceptin/orphanin FQ-NOP receptor signalling produces antidepressant-like effects: pharmacological and genetic evidences from the mouse forced swimming test. Eur J Neurosci. 2003;17(9):1987–1990. doi: 10.1046/j.1460-9568.2003.02603.x. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Rae GA, Calo G, Guerrini R, De Lima TC. Central injections of nocistatin or its C-terminal hexapeptide exert anxiogenic-like effect on behaviour of mice in the plus-maze test. Br J Pharmacol. 2002;136(5):764–772. doi: 10.1038/sj.bjp.0704739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavioli EC, Vaughan CW, Marzola G, Guerrini R, Mitchell VA, Zucchini S, De Lima TC, Rae GA, Salvadori S, Regoli D, Calo’ G. Antidepressant-like effects of the nociceptin/orphanin FQ receptor antagonist UFP-101: new evidence from rats and mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:547–553. doi: 10.1007/s00210-004-0939-0. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Rizzi A, Marzola G, Zucchini S, Regoli D, Calo G. Altered anxiety-related behavior in nociceptin/orphanin FQ receptor gene knockout mice. Peptides. 2007;28(6):1229–1239. doi: 10.1016/j.peptides.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Romao PR. NOP Receptor Ligands as Potential Agents for Inflammatory and Autoimmune Diseases. J Amino Acids. 2011;2011:836569. doi: 10.4061/2011/836569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Gackenheimer SL, Shaw JL. Distribution of nociceptin and Ro64-6198 activated [35S]-GTPgammaS binding in the rat brain. Neuropeptides. 2006;40(2):95–105. doi: 10.1016/j.npep.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Roberto M. Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci Biobehav Rev. 2012 Feb;36(2):873–88. doi: 10.1016/j.neubiorev.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeldner C, Reiss D, Kieffer BL, Ouagazzal AM. Endogenous nociceptin/orphanin-FQ in the dorsal hippocampus facilitates despair-related behavior. Hippocampus. 2010;20(8):911–916. doi: 10.1002/hipo.20760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeldner C, Spooren W, Wichmann J, Prinssen EP. Further characterization of the prototypical nociceptin/orphanin FQ peptide receptor agonist Ro 64-6198 in rodent models of conflict anxiety and despair. Psychopharmacology (Berl) 2012 Jul;222(2):203–14. doi: 10.1007/s00213-012-2636-x. [DOI] [PubMed] [Google Scholar]
- Grandi D, Massi M, Guerrini R, Calo G, Morini G. Role of nociceptin/orphanin FQ receptors in the decrease of mucosal mast cells caused by acute stress in the rat colon. Life Sci. 2011;89(19–20):735–740. doi: 10.1016/j.lfs.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Green MK, Barbieri EV, Brown BD, Chen KW, Devine DP. Roles of the bed nucleus of stria terminalis and of the amygdala in N/OFQ-mediated anxiety and HPA axis activation. Neuropeptides. 2007;41(6):399–410. doi: 10.1016/j.npep.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Green MK, Devine DP. Nociceptin/orphanin FQ and NOP receptor gene regulation after acute or repeated social defeat stress. Neuropeptides. 2009;43(6):507–514. doi: 10.1016/j.npep.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Johanson CE, Moody DE, Woods JH, Kilbourn MR, Koeppe RA, Schuster CR, Zubieta JK. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003 Nov;28(11):2000–9. doi: 10.1038/sj.npp.1300251. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Sanger DJ. Orphanin FQ, a novel neuropeptide with anti-stress-like activity. Brain Res. 1999;836(1–2):221–224. doi: 10.1016/s0006-8993(99)01684-4. [DOI] [PubMed] [Google Scholar]
- Gu H, Hu D, Hong XR, Mao J, Cui Y, Hui N, Sha JY. Changes and significance of orphanin and serotonin in patients with postpartum depression. Zhonghua Fu Chan Ke Za Zhi. 2003;38(12):727–728. [PubMed] [Google Scholar]
- Hallberg M, Nyberg F. Neuropeptide conversion to bioactive fragments--an important pathway in neuromodulation. Curr Protein Pept Sci. 2003;4(1):31–44. doi: 10.2174/1389203033380313. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Lightman SL. Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J Endocrinol. 1989;122(3):705–711. doi: 10.1677/joe.0.1220705. [DOI] [PubMed] [Google Scholar]
- Hawkinson JE, Acosta-Burruel M, Espitia SA. Opioid activity profiles indicate similarities between the nociceptin/orphanin FQ and opioid receptors. Eur J Pharmacol. 2000;389(2–3):107–114. doi: 10.1016/s0014-2999(99)00904-8. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology. 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Mul JD, de Wit E, Cuppen E. Complete knockout of the nociceptin/orphanin FQ receptor in the rat does not induce compensatory changes in mu, delta and kappa opioid receptors. Neuroscience. 2009;163(1):308–315. doi: 10.1016/j.neuroscience.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther. 2001;297(2):688–695. [PubMed] [Google Scholar]
- Hwa JJ, Witten MB, Williams P, Ghibaudi L, Gao J, Salisbury BG, … Parker EM. Activation of the NPY Y5 receptor regulates both feeding and energy expenditure. Am J Physiol. 1999;277(5 Pt 2):R1428–1434. doi: 10.1152/ajpregu.1999.277.5.R1428. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Watanabe M, Ichikawa T, Kobayashi T, Yano R, Kumanishi T. Distribution of pre-pro-nociceptin/orphanin FQ mRNA and its receptor mRNA in developing and adult mouse central nervous systems. J Comp Neurol. 1998;399:139–51. doi: 10.1002/(sici)1096-9861(19980914)399:1<139::aid-cne11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Jr, … Civelli O. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci U S A. 1997;94(26):14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, … Kilpatrick G. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci U S A. 2000;97(9):4938–4943. doi: 10.1073/pnas.090514397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–8. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Cason CR, Chen SH, Lewis MJ. Buprenorphine alters ethanol self-administration in rats: dose-response and time-dependent effects. Psychopharmacology (Berl) 1998;140(1):29–37. doi: 10.1007/s002130050735. [DOI] [PubMed] [Google Scholar]
- Kamei J, Matsunawa Y, Miyata S, Tanaka S, Saitoh A. Effects of nociceptin on the exploratory behavior of mice in the hole-board test. Eur J Pharmacol. 2004;489(1–2):77–87. doi: 10.1016/j.ejphar.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Holtzman SG. Mu opioid agonists potentiate amphetamine- and cocaine-induced rotational behavior in the rat. J Pharmacol Exp Ther. 1997;282(2):734–746. [PubMed] [Google Scholar]
- Kimura Y, Fujita M, Hong J, Lohith TG, Gladding RL, Zoghbi SS, … Innis RB. Brain and whole-body imaging in rhesus monkeys of 11C-NOP-1A, a promising PET radioligand for nociceptin/orphanin FQ peptide receptors. J Nucl Med. 2011;52(10):1638–1645. doi: 10.2967/jnumed.111.091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Marby DW, Nestler EJ. Cocaine conditioned place preference is attenuated by chronic buprenorphine treatment. Life Sci. 1991;49(24):PL201–206. doi: 10.1016/0024-3205(91)90490-3. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Morgan C, Kosten TA. Depressive symptoms during buprenorphine treatment of opioid abusers. J Subst Abuse Treat. 1990;7(1):51–54. doi: 10.1016/0740-5472(90)90035-o. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rosen MI, Schottenfeld R, Ziedonis D. Buprenorphine for cocaine and opiate dependence. Psychopharmacol Bull. 1992;28(1):15–19. [PubMed] [Google Scholar]
- Köster A, Montkowski A, Schulz S, Stube EM, Knaudt K, Jenck F, … Reinscheid RK. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc Natl Acad Sci U S A. 1999;96(18):10444–10449. doi: 10.1073/pnas.96.18.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlinska J, Wichmann J, Legowska A, Rolka K, Silberring J. Orphanin FQ/nociceptin but not Ro 65-6570 inhibits the expression of cocaine-induced conditioned place preference. Behav Pharmacol. 2002;13:229–35. doi: 10.1097/00008877-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Rafalski P, Biala G, Dylag T, Rolka K, Silberring J. Nociceptin inhibits acquisition of amphetamine-induced place preference and sensitization to stereotypy in rats. Eur J Pharmacol. 2003;474:233–9. doi: 10.1016/s0014-2999(03)02081-8. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Briggs JE, Grace MK, Levine AS, Billington CJ. Divergence of the feeding and thermogenic pathways influenced by NPY in the hypothalamic PVN of the rat. Am J Physiol. 1998;275(2 Pt 2):R471–477. doi: 10.1152/ajpregu.1998.275.2.R471. [DOI] [PubMed] [Google Scholar]
- Krepuska M, Sotonyi P, Csobay-Novak C, Szeberin Z, Hartyanszky I, Zima E, … Tekes K. Plasma nociceptin/orphanin FQ levels are lower in patients with chronic ischemic cardiovascular diseases--A pilot study. Regul Pept. 2011;169(1–3):1–5. doi: 10.1016/j.regpep.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther. 2003;304:310–8. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Madjid N, Johansson B, Terenius L, Ogren SO. The nociceptin system and hippocampal cognition in mice: a pharmacological and genetic analysis. Brain Res. 2009;1305(Suppl):S7–19. doi: 10.1016/j.brainres.2009.09.075. [DOI] [PubMed] [Google Scholar]
- Lachowicz JE, Shen Y, Monsma FJ, Jr, Sibley DR. Molecular cloning of a novel G protein-coupled receptor related to the opiate receptor family. J Neurochem. 1995;64(1):34–40. doi: 10.1046/j.1471-4159.1995.64010034.x. [DOI] [PubMed] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov. 2008;7(8):694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- Largent-Milnes TM, Vanderah TW. Recently patented and promising ORL-1 ligands: where have we been and where are we going? Expert Opin Ther Pat. 2010;20(3):291–305. doi: 10.1517/13543771003602004. [DOI] [PubMed] [Google Scholar]
- Leggett JD, Dawe KL, Jessop DS, Fulford AJ. Endogenous nociceptin/orphanin FQ system involvement in hypothalamic-pituitary-adrenal axis responses: relevance to models of inflammation. J Neuroendocrinol. 2009;21(11):888–897. doi: 10.1111/j.1365-2826.2009.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett JD, Harbuz MS, Jessop DS, Fulford AJ. The nociceptin receptor antagonist [Nphe1,Arg14,Lys15]nociceptin/orphanin FQ-NH2 blocks the stimulatory effects of nociceptin/orphanin FQ on the HPA axis in rats. Neuroscience. 2006;141(4):2051–2057. doi: 10.1016/j.neuroscience.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Leggett JD, Jessop DS, Fulford AJ. The nociceptin/orphanin FQ antagonist UFP-101 differentially modulates the glucocorticoid response to restraint stress in rats during the peak and nadir phases of the hypothalamo-pituitary-adrenal axis circadian rhythm. Neuroscience. 2007;147(3):757–764. doi: 10.1016/j.neuroscience.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Lester PA, Traynor JR. Comparison of the in vitro efficacy of mu, delta, kappa and ORL1 receptor agonists and non-selective opioid agonists in dog brain membranes. Brain Res. 2006;1073–1074:290–296. doi: 10.1016/j.brainres.2005.12.066. [DOI] [PubMed] [Google Scholar]
- Li G, Zhang Y, Rodrigues E, Zheng D, Matheny M, Cheng KY, Scarpace PJ. Melanocortin activation of nucleus of the solitary tract avoids anorectic tachyphylaxis and induces prolonged weight loss. Am J Physiol Endocrinol Metab. 2007;293(1):E252–258. doi: 10.1152/ajpendo.00451.2006. [DOI] [PubMed] [Google Scholar]
- Li X, Need AB, Baez M, Witkin JM. mGlu5 Receptor Antagonism is Associated with Antidepressant-Like effects in Mice. J Pharmacol Exp Ther. 2006;319:254–259. doi: 10.1124/jpet.106.103143. [DOI] [PubMed] [Google Scholar]
- Lohith TG, Zoghbi SS, Morse CL, Araneta MF, Barth VN, Goebl NA, … Fujita M. Brain and whole-body imaging of nociceptin/orphanin FQ peptide receptor in humans using the PET ligand 11C-NOP-1A. J Nucl Med. 2012;53(3):385–392. doi: 10.2967/jnumed.111.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SX, Higgins GA, Hodgson RA, Hyde LA, Del Vecchio RA, Guthrie DH, … Varty GB. The anxiolytic-like profile of the nociceptin receptor agonist, endo-8-[bis(2-chlorophenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octane-3-carboxami de (SCH 655842): comparison of efficacy and side effects across rodent species. Eur J Pharmacol. 2011;661(1–3):63–71. doi: 10.1016/j.ejphar.2011.04.034. [DOI] [PubMed] [Google Scholar]
- Luscher C, Ungless MA. The mechanistic classification of addictive drugs. PLoS Med. 2006;3:e437. doi: 10.1371/journal.pmed.0030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Do T, Maidment NT. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl) 2001;154:1–7. doi: 10.1007/s002130000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Khaliq I, Carroll FI, Maidment NT. Orphanin FQ/nociceptin blocks cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 2002;164:168–76. doi: 10.1007/s00213-002-1192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, … Evans CJ. Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci. 2003;23(32):10331–10337. doi: 10.1523/JNEUROSCI.23-32-10331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Cheng ZJ, Fan GH, Cai YC, Jiang LZ, Pei G. Functional expression, activation and desensitization of opioid receptor-like receptor ORL1 in neuroblastoma x glioma NG108-15 hybrid cells. FEBS Lett. 1997;403(1):91–94. doi: 10.1016/s0014-5793(97)00031-8. [DOI] [PubMed] [Google Scholar]
- Marquez P, Borse J, Nguyen AT, Hamid A, Lutfy K. The role of the opioid receptor-like (ORL1) receptor in motor stimulatory and rewarding actions of buprenorphine and morphine. Neuroscience. 2008;155(3):597–602. doi: 10.1016/j.neuroscience.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Stocchi S, Paganini F, Mela F, De Risi C, Calo G, … Morari M. Pharmacological profiles of presynaptic nociceptin/orphanin FQ receptors modulating 5-hydroxytryptamine and noradrenaline release in the rat neocortex. Br J Pharmacol. 2003;138(1):91–98. doi: 10.1038/sj.bjp.0705005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Pilotto R, Singer G, Oei TP. The suppression of ethanol self injection by buprenorphine. Pharmacol Biochem Behav. 1983;19(6):985–986. doi: 10.1016/0091-3057(83)90403-3. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–43. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res. 2010;1314:145–161. doi: 10.1016/j.brainres.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Borse J, Nguyen AT, Hamid A, Lutfy K. The role of the opioid receptor-like (ORL1) receptor in motor stimulatory and rewarding actions of buprenorphine and morphine. Neuroscience. 2008;155:597–602. doi: 10.1016/j.neuroscience.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita H, Ishihara A, Mashiko S, Tanaka T, Kanno T, Iwaasa H, … Kanatani A. Chronic intracerebroventricular infusion of nociceptin/orphanin FQ produces body weight gain by affecting both feeding and energy metabolism in mice. Endocrinology. 2009;150(6):2668–2673. doi: 10.1210/en.2008-1515. [DOI] [PubMed] [Google Scholar]
- Mela F, Marti M, Ulazzi L, Vaccari E, Zucchini S, Trapella C, … Morari M. Pharmacological profile of nociceptin/orphanin FQ receptors regulating 5-hydroxytryptamine release in the mouse neocortex. Eur J Neurosci. 2004;19(5):1317–1324. doi: 10.1111/j.1460-9568.2004.03220.x. [DOI] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Mendelson JH. Comparison of buprenorphine and methadone effects on opiate self-administration in primates. J Pharmacol Exp Ther. 1983;225(2):378–386. [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Mendelson JH, Drieze J. Naltrexone-buprenorphine interactions: effects on cocaine self-administration. Neuropsychopharmacology. 1993;9(3):211–224. doi: 10.1038/npp.1993.57. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, … Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377(6549):532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Micioni Di Bonaventura MV, Ubaldi M, Liberati S, Ciccocioppo R, Massi M, Cifani C. Caloric restriction increases the sensitivity to the hyperphagic effect of nociceptin/orphanin FQ limiting its ability to reduce binge eating in female rats. 2013 doi: 10.1007/s00213-013-3013-0. [DOI] [PubMed] [Google Scholar]
- Mika J, Obara I, Przewlocka B. The role of nociceptin and dynorphin in chronic pain: implications of neuro-glial interaction. Neuropeptides. 2011;45(4):247–261. doi: 10.1016/j.npep.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Mouledous L, Lapalu S, Cambois G, Moisand C, Butour JL, Meunier JC. Distinct mechanisms for activation of the opioid receptor-like 1 and kappa-opioid receptors by nociceptin a Psychopharmacology (Berl). 2013 Jul;228(1):53–63. nd dynorphin A. Mol Pharmacol. 1999;55(2):324–331. doi: 10.1124/mol.55.2.324. [DOI] [PubMed] [Google Scholar]
- Montoya ID, Gorelick DA, Preston KL, Schroeder JR, Umbricht A, Cheskin LJ, Lange WR, Contoreggi C, Johnson RE, Fudala PJ. Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmacol Ther. 2004 Jan;75(1):34–48. doi: 10.1016/j.clpt.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NP, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–70. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Noda Y, Mamiya T. The role of nociceptin in cognition. Brain Res. 1999;848(1–2):167–173. doi: 10.1016/s0006-8993(99)01906-x. [DOI] [PubMed] [Google Scholar]
- Nativio P, Pascale E, Maffei A, Scaccianoce S, Passarelli F. Effect of stress on hippocampal nociceptin expression in rat. Stress. 2012 Jul;15(4):378–84. doi: 10.3109/10253890.2011.627071. [DOI] [PubMed] [Google Scholar]
- Nava F, Manzato E, Leonardi C, Lucchini A. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867–1872. doi: 10.1016/j.pnpbp.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Nazzaro C, Barbieri M, Varani K, Beani L, Valentino RJ, Siniscalchi A. Swim stress enhances nociceptin/orphanin FQ-induced inhibition of rat dorsal raphe nucleus activity in vivo and in vitro: role of corticotropin releasing factor. Neuropharmacology. 2010 Feb;58(2):457–64. doi: 10.1016/j.neuropharm.2009.09.004. (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzaro C, Barbieri M, Varani K, Beani L, Valentino RJ, Siniscalchi A. Swim stress enhances nociceptin/orphanin FQ-induced inhibition of rat dorsal raphe nucleus activity in vivo and in vitro: role of corticotropin releasing factor. Neuropharmacology. 2010;58(2):457–464. doi: 10.1016/j.neuropharm.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid RK, Nothacker H-P, Civelli O, Akil H, Watson SJ., Jr Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol. 1999a;412:563–605. [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid RK, Nothacker H-P, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999b;406:503–547. [PubMed] [Google Scholar]
- Nicholson JR, Akil H, Watson SJ. Orphanin FQ-induced hyperphagia is mediated by corticosterone and central glucocorticoid receptors. Neuroscience. 2002;115:637–643. doi: 10.1016/s0306-4522(02)00290-7. [DOI] [PubMed] [Google Scholar]
- Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, … Sugimoto T. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. EMBO J. 1997;16(8):1858–1864. doi: 10.1093/emboj/16.8.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton CS, Neal CR, Kumar S, Akil H, Watson SJ. Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J Comp Neurol. 2002 Mar 18;444(4):358–68. doi: 10.1002/cne.10154. [DOI] [PubMed] [Google Scholar]
- Okawa H, Kudo M, Kudo T, Guerrini R, Lambert DG, Kushikata T, Yoshida H, Matsuki A. Effects of nociceptinNH2 and [Nphe1]nociceptin(1-13)NH2 on rat brain noradrenaline release in vivo and in vitro. Neurosci Lett. 2001;303:173–176. doi: 10.1016/s0304-3940(01)01721-9. [DOI] [PubMed] [Google Scholar]
- Okuda-Ashitaka E, Minami T, Tachibana S, Yoshihara Y, Nishiuchi Y, Kimura T, Ito S. Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature. 1998;392(6673):286–289. doi: 10.1038/32660. [DOI] [PubMed] [Google Scholar]
- Olianas MC, Dedoni S, Boi M, Onali P. Activation of nociceptin/orphanin FQ-NOP receptor system inhibits tyrosine hydroxylase phosphorylation, dopamine synthesis, and dopamine D(1) receptor signaling in rat nucleus accumbens and dorsal striatum. J Neurochem. 2008 Oct;107(2):544–56. doi: 10.1111/j.1471-4159.2008.05629.x. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Billington CJ, Levine AS. Fos expression in feeding-related brain areas following intracerebroventricular administration of orphanin FQ in rats. Brain Res. 2000;855(1):171–175. doi: 10.1016/s0006-8993(99)02239-8. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Grace MK, Fard SS, Le Greves M, Klockars A, Massi M, … Levine AS. Central nociceptin/orphanin FQ system elevates food consumption by both increasing energy intake and reducing aversive responsiveness. Am J Physiol Regul Integr Comp Physiol. 2010;299(2):R655–663. doi: 10.1152/ajpregu.00556.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski PK, Grace MK, Sanders JB, Billington CJ, Levine AS. Effect of nociceptin/orphanin FQ on food intake in rats that differ in diet preference. Pharmacol Biochem Behav. 2002;73(3):529–535. doi: 10.1016/s0091-3057(02)00821-3. [DOI] [PubMed] [Google Scholar]
- Ouagazzal AM, Moreau JL, Pauly-Evers M, Jenck F. Impact of environmental housing conditions on the emotional responses of mice deficient for nociceptin/orphanin FQ peptide precursor gene. Behav Brain Res. 2003 Sep 15;144(1–2):111–7. doi: 10.1016/s0166-4328(03)00066-4. [DOI] [PubMed] [Google Scholar]
- Pike VW, Rash KS, Chen Z, Pedregal C, Statnick MA, Kimura Y, … Innis RB. Synthesis and evaluation of radioligands for imaging brain nociceptin/orphanin FQ peptide (NOP) receptors with positron emission tomography. J Med Chem. 2011;54(8):2687–2700. doi: 10.1021/jm101487v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placenza FM, Rajabi H, Stewart J. Effects of chronic buprenorphine treatment on levels of nucleus accumbens glutamate and on the expression of cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 2008;200(3):347–355. doi: 10.1007/s00213-008-1210-z. [DOI] [PubMed] [Google Scholar]
- Polidori C, Calo G, Ciccocioppo R, Guerrini R, Regoli D, Massi M. Pharmacological characterization of the nociceptin receptor mediating hyperphagia: identification of a selective antagonist. Psychopharmacology (Berl) 2000;148(4):430–437. doi: 10.1007/s002130050073. [DOI] [PubMed] [Google Scholar]
- Pomonis JD, Billington CJ, Levine AS. Orphanin FQ, agonist of orphan opioid receptor ORL1, stimulates feeding in rats. Neuroreport. 1996;8(1):369–371. doi: 10.1097/00001756-199612200-00072. [DOI] [PubMed] [Google Scholar]
- Preiss K, Brennan L, Clarke D. A systematic review of variables associated with the relationship between obesity and depression. Obes Rev. 2013 Jun 30; doi: 10.1111/obr.12052. [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Procopio DO, Saba LM, Walter H, Lesch O, Skala K, Schlaff G, … Tabakoff B. Genetic markers of comorbid depression and alcoholism in women. Alcohol Clin Exp Res. 2013;37(6):896–904. doi: 10.1111/acer.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przydzial MJ, Heisler LK. Nociceptin/orphanin FQ peptide receptor as a therapeutic target for obesity. Mini Rev Med Chem. 2008;8(8):796–811. doi: 10.2174/138955708784912139. [DOI] [PubMed] [Google Scholar]
- Raffaeli W, Samolsky Dekel BG, Landuzzi D, Caminiti A, Righetti D, Balestri M, … Candeletti S. Nociceptin levels in the cerebrospinal fluid of chronic pain patients with or without intrathecal administration of morphine. J Pain Symptom Manage. 2006;32(4):372–377. doi: 10.1016/j.jpainsymman.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Caló G, Regoli D, Quirion R. Nociceptin receptor antagonists display antidepressant-like properties in the mouse forced swimming test. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:164–167. doi: 10.1007/s00210-001-0511-0. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Civelli O. The orphanin FQ/nociceptin knockout mouse: a behavioral model for stress responses. Neuropeptides. 2002;36(2–3):72–76. doi: 10.1054/npep.2002.0901. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Higelin J, Henningsen RA, Monsma FJ, Jr, Civelli O. Structures that delineate orphanin FQ and dynorphin A pharmacological selectivities. J Biol Chem. 1998;273(3):1490–1495. doi: 10.1074/jbc.273.3.1490. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, … Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270(5237):792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Gavioli EC, Marzola G, Spagnolo B, Zucchini S, Ciccocioppo R, Trapella C, Regoli D, Calò G. Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol]: in vivo studies. J Pharmacol Exp Ther. 2007 Jun;321(3):968–74. doi: 10.1124/jpet.106.116780. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Molinari S, Marti M, Marzola G, Calo G. Nociceptin/orphanin FQ receptor knockout rats: in vitro and in vivo studies. Neuropharmacology. 2011;60(4):572–579. doi: 10.1016/j.neuropharm.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, Polidori C. Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology (Berl) 2008;196:523–31. doi: 10.1007/s00213-007-0985-7. [DOI] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD, … McKinzie DL. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Röver, Geo Adam S, Cesura Andrea M, Galley Guido, Jenck François, Monsma Frederick J, Jr, Wichmann Jürgen, Dautzenberg Frank M. High-Affinity, Non-Peptide Agonists for the ORL1 (Orphanin FQ/Nociceptin) Receptor. J Med Chem. 2000;43:1329–1338. doi: 10.1021/jm991129q. [DOI] [PubMed] [Google Scholar]
- Rutten K, De Vry J, Bruckmann W, Tzschentke TM. Pharmacological blockade or genetic knockout of the NOP receptor potentiates the rewarding effect of morphine in rats. Drug Alcohol Depend. 2011;114:253–6. doi: 10.1016/j.drugalcdep.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Enhanced nicotine sensitivity in nociceptin/orphanin FQ receptor knockout mice. Neuropharmacology. 2009;56:896–904. doi: 10.1016/j.neuropharm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Endogenous nociceptin (orphanin FQ) suppresses basal hedonic state and acute reward responses to methamphetamine and ethanol, but facilitates chronic responses. Neuropsychopharmacology. 2008;33:877–91. doi: 10.1038/sj.npp.1301459. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes J, Ziedonis D, Kosten TR. Buprenorphine: dose-related effects on cocaine and opioid use in cocaine-abusing opioid-dependent humans. Biol Psychiatry. 1993;34(1–2):66–74. doi: 10.1016/0006-3223(93)90258-f. [DOI] [PubMed] [Google Scholar]
- Scoto GM, Arico G, Iemolo A, Ronsisvalle G, Parenti C. Selective inhibition of the NOP receptor in the ventrolateral periaqueductal gray attenuates the development and the expression of tolerance to morphine-induced antinociception in rats. Peptides. 2010;31:696–700. doi: 10.1016/j.peptides.2009.12.028. [DOI] [PubMed] [Google Scholar]
- Serrano-Gomez A, Thompson JP, Lambert DG. Nociceptin/orphanin FQ in inflammation and sepsis. Br J Anaesth. 2011;106(1):6–12. doi: 10.1093/bja/aeq337. [DOI] [PubMed] [Google Scholar]
- Sim HR, Choi TY, Lee HJ, Kang EY, Yoon S, Han PL, … Baik JH. Role of dopamine D2 receptors in plasticity of stress-induced addictive behaviours. Nat Commun. 2013;4:1579. doi: 10.1038/ncomms2598. [DOI] [PubMed] [Google Scholar]
- Siniscalchi A, Rodi D, Beani L, Bianchi C. Inhibitory effect of nociceptin on [3H]-5HT release from rat cerebral cortex slices. Br J Pharmacol. 1999;128:119–123. doi: 10.1038/sj.bjp.0702793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniscalchi A, Rodi D, Morari M, Marti M, Cavallini S, Marino S, Beani L, Bianchi C. Direct and indirect inhibition by nociceptin/orphanin FQ on noradrenaline release from rodent cerebral cortex in vitro. Br J Pharmacol. 2002;136:1178–1184. doi: 10.1038/sj.bjp.0704841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Rajabi H, Stewart J. Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology. 2005;30(9):1681–1692. doi: 10.1038/sj.npp.1300712. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. The effects of chronic buprenorphine on intake of heroin and cocaine in rats and its effects on nucleus accumbens dopamine levels during self-administration. Psychopharmacology (Berl) 2006;188(1):28–41. doi: 10.1007/s00213-006-0485-1. [DOI] [PubMed] [Google Scholar]
- Spadaro A, Ajello A, Luigiano C, Morace C, Resta ML, Berlinghieri G, … Freni MA. Low utility of plasma Nociceptin/orphanin FQ in the diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2006;12(29):4716–4720. doi: 10.3748/wjg.v12.i29.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato S, Baiula M, Calienni M. Agonist-regulated internalization and desensitization of the human nociceptin receptor expressed in CHO cells. Curr Drug Targets. 2007 Jan;8(1):137–46. doi: 10.2174/138945007779315641. [DOI] [PubMed] [Google Scholar]
- Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10:1727–40. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Holahan MR, Kelley AE. Injections of nociceptin into nucleus accumbens shell or ventromedial hypothalamic nucleus increase food intake. Neuroreport. 1997;8(2):423–426. doi: 10.1097/00001756-199701200-00009. [DOI] [PubMed] [Google Scholar]
- Sugino T, Shimazoe T, Ikeda NS. Watanabe Role of nociceptin and opioid receptor-like-1 on entrainment function in the rat suprachiasmatic nucleus. Neuroscience. 2006;137:537–544. doi: 10.1016/j.neuroscience.2005.08.085. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Thakkar MM, McCarley RW, Auerbach SB. Nociceptin/orphanin FQ decreases serotonin efflux in the rat brain but in contrast to a kappa-opioid has no antagonistic effect on mu-opioid-induced increases in serotonin efflux. Neuroscience. 2007 Jun 15;147(1):106–16. doi: 10.1016/j.neuroscience.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Terenius L, Sandin J, Sakurada T. Nociceptin/orphanin FQ metabolism and bioactive metabolites. Peptides. 2000;21(7):919–922. doi: 10.1016/s0196-9781(00)00228-x. [DOI] [PubMed] [Google Scholar]
- Tetrault JM, Fiellin DA. Current and potential pharmacological treatment options for maintenance therapy in opioid-dependent individuals. Drugs. 2012;72(2):217–228. doi: 10.2165/11597520-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama H, Toda A, Hiranita T, Watanabe S, Eyanagi R. Role of amygdaloid nuclei in the anxiolytic-like effect of nociceptin/orphanin FQ in rats. Neurosci Lett. 2008 Jan 24;431(1):66–70. doi: 10.1016/j.neulet.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Ueda H, Yamaguchi T, Tokuyama S, Inoue M, Nishi M, Takeshima H. Partial loss of tolerance liability to morphine analgesia in mice lacking the nociceptin receptor gene. Neurosci Lett. 1997;237:136–8. doi: 10.1016/s0304-3940(97)00832-x. [DOI] [PubMed] [Google Scholar]
- Varty GB, Hyde LA, Hodgson RA, Lu SX, McCool MF, Kazdoba TM, Del Vecchio RA, Guthrie DH, Pond AJ, Grzelak ME, Xu X, Korfmacher WA, Tulshian D, Parker EM, Higgins GA. Characterization of the nociceptin receptor (ORL-1) agonist, Ro64-6198, in tests of anxiety across multiple species. Psychopharmacology (Berl) 2005;182:132. doi: 10.1007/s00213-005-0041-4. [DOI] [PubMed] [Google Scholar]
- Varty GB, Lu SX, Morgan CA, Cohen-Williams ME, Hodgson RA, Smith-Torhan A, Zhang H, Fawzi AB, Graziano MP, Ho GD, Matasi J, Tulshian D, Coffin VL, Carey GJ. The anxiolytic-like effects of the novel, orally active nociceptin opioid receptor agonist 8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol (SCH 221510) J Pharmacol Exp Ther. 2008;326:672–682. doi: 10.1124/jpet.108.136937. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Increase by the ORL1 (opioid receptor-like 1) ligand, nociceptin, of inwardly rectifying K conductance in dorsal raphe nucleus neurones. Br J Pharmacol. 1996;117:1609–1611. doi: 10.1111/j.1476-5381.1996.tb15329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G, Arletti R, Ruggieri V, Cifani C, Massi M. Anxiolytic-like effects of nociceptin/orphanin FQ in the elevated plus maze and in the conditioned defensive burying test in rats. Peptides. 2006 Sep;27(9):2193–200. doi: 10.1016/j.peptides.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Vitale G, Ruggieri V, Filaferro M, Frigeri C, Alboni S, Tascedda F, Brunello N, Guerrini R, Cifani C, Massi M. Chronic treatment with the selective NOP receptor antagonist [Nphe 1, Arg 14, Lys 15]N/OFQ-NH 2 (UFP-101) reverses the behavioural and biochemical effects of unpredictable chronic mild stress in rats. Psychopharmacology (Berl) 2009 Dec;207(2):173–89. doi: 10.1007/s00213-009-1646-9. [DOI] [PubMed] [Google Scholar]
- Volta M, Viaro R, Trapella C, Marti M, Morari M. Dopamine-nociceptin/orphanin FQ interactions in the substantia nigra reticulata of hemiparkinsonian rats: involvement of D2/D3 receptors and impact on nigro-thalamic neurons and motor activity. Exp Neurol. 2011;228(1):126–137. doi: 10.1016/j.expneurol.2010.12.024. [DOI] [PubMed] [Google Scholar]
- Walker JR, Spina M, Terenius L, Koob GF. Nociceptin fails to affect heroin self-administration in the rat. Neuroreport. 1998;9:2243–7. doi: 10.1097/00001756-199807130-00017. [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Ronnekleiv OK, Grandy DK, Kelly MJ. The peptide orphanin FQ inhibits beta-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology. 1998;67:73–82. doi: 10.1159/000054301. [DOI] [PubMed] [Google Scholar]
- Wang LN, Liu LF, Zhang JX, Zhao GF. Plasma levels of nociceptin/orphanin FQ in patients with bipolar disorders and health adults. Zhonghua Yi Xue Za Zhi. 2009;89(13):916–918. [PubMed] [Google Scholar]
- Wee S, Vendruscolo LF, Misra KK, Schlosburg JE, Koob GF. A combination of buprenorphine and naltrexone blocks compulsive cocaine intake in rodents without producing dependence. Sci Transl Med. 2012;4(146):146ra110. doi: 10.1126/scitranslmed.3003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann J, Adam Geo, Röver Stephan, Hennig Michael, Scalone Michelangelo, Cesura Andrea M, Dautzenberg Frank M. François Jenck Synthesis of (1S, 3aS)-8-(2, 3, 3a, 4, 5, 6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1, 3, 8-triaza-spiro[4.5]decan-4-one, a potent selective orphanin FQ (OFQ) receptor agonist with anxiolytic-like properties. Eur J Med Chem. 2000;35:839–851. doi: 10.1016/s0223-5234(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Wnendt S, Krüger T, Janocha E, Hildebrandt D, Englberger W. Agonistic effect of buprenorphine in a nociceptin/OFQ receptor-triggered reporter gene assay. Mol Pharmacol. 1999 Aug;56(2):334–8. doi: 10.1124/mol.56.2.334. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Yuan L, Han Z, Chang JK, Han JS. Accelerated release and production of orphanin FQ in brain of chronic morphine tolerant rats. Brain Res. 1999;826:330–4. doi: 10.1016/s0006-8993(99)01337-2. [DOI] [PubMed] [Google Scholar]
- Zaveri NT. The nociceptin/orphanin FQ receptor (NOP) as a target for drug abuse medications. Curr Top Med Chem. 2011;11(9):1151–1156. doi: 10.2174/156802611795371341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rodrigues E, Li G, Gao Y, King M, Carter CS, … Scarpace PJ. Simultaneous POMC gene transfer to hypothalamus and brainstem increases physical activity, lipolysis and reduces adult-onset obesity. Eur J Neurosci. 2011;33(8):1541–1550. doi: 10.1111/j.1460-9568.2011.07633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang nocicpetin levels in depressed patients 2009 [Google Scholar]
- Zhao RJ, Woo RS, Jeong MS, Shin BS, Kim DG, Kim KW. Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport. 2003;14:2383–5. doi: 10.1097/00001756-200312190-00019. [DOI] [PubMed] [Google Scholar]