Abstract
Objectives
Impulse control disorders (ICD) are commonly associated with dopamine replacement therapy (DRT) in patients with Parkinson’s disease (PD). Our aims were to estimate ICD heritability and to predict ICD by a candidate genetic multivariable panel in patients with PD.
Methods
Data from de novo patients with PD, drug-naïve and free of ICD behaviour at baseline, were obtained from the Parkinson’s Progression Markers Initiative cohort. Incident ICD behaviour was defined as positive score on the Questionnaire for Impulsive-Compulsive Disorders in PD. ICD heritability was estimated by restricted maximum likelihood analysis on whole exome sequencing data. 13 candidate variants were selected from the DRD2, DRD3, DAT1, COMT, DDC, GRIN2B, ADRA2C, SERT, TPH2, HTR2A, OPRK1 and OPRM1 genes. ICD prediction was evaluated by the area under the curve (AUC) of receiver operating characteristic (ROC) curves.
Results
Among 276 patients with PD included in the analysis, 86% started DRT, 40% were on dopamine agonists (DA), 19% reported incident ICD behaviour during follow-up. We found heritability of this symptom to be 57%. Adding genotypes from the 13 candidate variants significantly increased ICD predictability (AUC=76%, 95% CI (70% to 83%)) compared to prediction based on clinical variables only (AUC=65%, 95% CI (58% to 73%), p=0.002). The clinical-genetic prediction model reached highest accuracy in patients initiating DA therapy (AUC=87%, 95% CI (80% to 93%)). OPRK1, HTR2A and DDC genotypes were the strongest genetic predictive factors.
Conclusions
Our results show that adding a candidate genetic panel increases ICD predictability, suggesting potential for developing clinical-genetic models to identify patients with PD at increased risk of ICD development and guide DRT management.
INTRODUCTION
Impulse control disorders (ICD) and related behaviours are defined by failure to resist an impulse to perform a self-rewarding act that will cause longer term harm and are referred to as ‘behavioural addictions’.1 In Parkinson’s disease, ICDs are associated with dopamine replacement therapy (DRT). ICDs (either formal diagnosis or symptoms) are estimated to occur in 14–40% of patients with Parkinson’s disease (PD) once DRT, in particular dopamine agonist (DA) treatment, is initiated, greatly exceeding the prevalence in the general population.2–4 Commonly reported presentations in patients with PD include compulsive gambling, eating, buying and sexual behaviours, and multiple comorbid ICDs are common.5,6 These behavioural disorders represent an important public health problem because of their potential socioeconomic and legal impact, leading to reconsideration of the benefit/risk ratio of initiating DA therapy.7,8
However, not all treated patients with PD develop ICDs, suggesting a shared clinical and neurobiological contribution to individual ICD susceptibility. Identification of such predictive factors may allow a tailored therapeutic approach in subpopulations at risk. Clinical features that have been associated with ICD in PD in cross-sectional studies include depression, anxiety, a personal or family history of alcohol abuse or gambling, increased impulsivity, novelty-seeking traits, younger age, early PD onset, unmarried status and past or current smoking.6–12
In the general population, family, adoption and twin studies have provided evidence that genetic factors might contribute up to 60% of the variance in the risk for substance use disorders and pathological gambling.13,14 So far, ICD heritability has not been studied in the PD population. ICDs and substance use disorders might share common neurobiological mechanisms with involvement of monoaminergic, glutamatergic and opioid neurotransmitter systems.15,16 Several genetic studies reported that single genetic variants involved in these pathways are associated with addiction and impulsivity in non-PD cohorts.17,18 Only four genetic association studies have been published on ICD in patients with PD so far.19–22 These studies were of cross-sectional design and included a relatively small number of patients.
We utilised a large, longitudinal cohort of de novo patients with PD with extensive clinical and genetic data. Our main objectives were to first assess ICD heritability in PD, and then to evaluate the contribution of a preselected panel of candidate gene variants in predicting ICD when added to clinical variables.
PATIENTS AND METHODS
Study design
The Parkinson’s Progression Markers Initiative (PPMI) is an ongoing longitudinal multicentre international study designed to identify biomarkers of PD progression in de novo and drug-naïve (at baseline) patients with PD. Data acquisition follows standardised protocols: PD diagnosis is made following established diagnostic criteria and confirmed by reduced striatal dopamine transporter (DAT) binding at enrolment. PPMI is a public–private partnership, sponsored by The Michael J Fox Foundation. Details on study design, study goals and funding are described on the PPMI website (http://www.ppmi-info.org). The PPMI study was conducted in accordance with Good Clinical Practice and any applicable national and local regulations. All patients signed an informed consent form before their participation in the PPMI study.
The PPMI database (http://www.ppmi-info.org/data) provided all clinical and genetic information for our analysis; download was performed on 16 December 2014 for clinical information, 19 March 2015 for genetic data and PD medication data was updated on 26 March 2015.
Genetic data included the NeuroX genotyping array, containing a selection of 292 313 variants, specifically designed for neurological disease studies, as well as whole exome sequencing. The methodology was previously published and its usage in PPMI described on the PPMI website.23–26
Participants and clinical measures
For our study, we included only patients with PD who screened negative for ICD behaviour at baseline and had available genetic data on both NeuroX and exomes. Incident ICD behaviour was defined as a positive score for any symptom (ie, compulsive gambling, sex, buying, eating, hobbyism, simple motor activities or walkabout) of the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP)-short form at any annual postbaseline visit. The QUIP is a validated screening tool to assess ICD behaviours, related compulsive behaviours and compulsive medication use in patients with PD.27
Participants were considered to be on DRT (ie, levodopa, DA, amantadine or monoamine oxidase-B inhibitor) from the first time it was recorded at an annual study visit. Participants lacking either QUIP or DRT data were excluded from analysis.
Candidate gene and SNP selection
We identified all frequent (MAF >0.2) single nucleotide polymorphisms (SNPs) on exome sequencing and NeuroX genotyping data, which we then extracted using plink software (plink, pngu.mgh.harvard.edu/~purcell/plink/).28 We selected 15 autosomal candidate genes for their involvement in monoaminergic systems or based on published ICD literature: SLC6A3, DRD2, DRD3, DRD4, DDC, TPH2, HTR1A, HTR2A, HTR2C, SLC6A4, COMT, OPRK1, OPRM1, GRIN2B and ADRA2C. For each candidate gene, all variants present on the NeuroX or exome data within its genomic coordinates were determined. We then chose a maximum of two SNPs per gene to prevent overfitting in the multiple regression model. SNPs were selected following predefined selection criteria: (1) we looked for variants that have been associated with ICD or addictive behaviour in the literature;17,18 (2) if none were present on either NeuroX or exome sequencing data, we sought variants in linkage disequilibrium with these SNPs (D’>0.8); (3) if not available, we chose among the frequent SNPs (MAF>0.2) present on the gene region; (4) for each step we filtered the variants based on their functional category, with preferential inclusion as follows: missense>coding synonymous>UTR’3/UTR’5>Intron>the most frequent SNP of the preferred category.
Statistical analysis
Heritability of ICD behaviour was estimated performing restricted maximum likelihood (REML) analysis, using Genome-wide Complex Trait Analysis (GCTA) software (http://www.complextraitgenomics.com/software/gcta). We determined variance of incident ICD behaviour explained by autosome-wide SNPs, while taking into account predefined clinical covariates known to be associated with ICD.5 Clinical variables selected were age, sex, PD treatment (no treatment, DA treatment and other DRT) and duration of follow-up in the study.
Incident ICD behaviour predictability was estimated with receiver operating characteristic (ROC) curves. To assess whether inclusion of genotype information would improve predictability of ICD behaviour incidence, ROC curves were plotted with the preselected clinical variables only, and then with candidate gene information added. Area under the curves (AUCs) were compared using DeLong’s test for two correlated ROC curves.29 Hosmer and Lemeshow goodness-of-fit test was performed to validate our logistic regression model.
Adjusted single factors associated with ICD incidence were assessed using logistic regression models, with variable selection carried out in a backwards stepwise fashion based on the Akaike information criterion (AIC). Since DA treatment is the DRT most strongly associated with ICD, a similar secondary enriched analysis was performed on the subgroup of patients on DA treatment versus no DRT at all. No correction for multiple comparisons was performed due to the exploratory nature of the study.
RESULTS
Patient characteristics
Baseline clinical and genetic data were available for 276 patients with PD, 183 men (66.3%), 96.4% Caucasian, with a mean age of 65.04 (SD 9.6) years, and a mean formal education of 15.58 (SD 3.0) years (table 1). At baseline, mean disease duration was 6.31 (SD 6.3) months, and the mean Unified Parkinson’s Disease Rating Scale part III score was 21.46 (SD 9.0). Of this cohort, follow-up visits occurred for 98% (n=270) at year 1, 84% (n=232) at year 2 and 38% (n=106) at year 3. Across the entire PPMI study, the retention rate at the time of analysis was 92%, so nearly all participants without year 2 or 3 data remain active study participants, but have not yet reached these later time points. In our cohort, 238 patients (86%) started DRT during follow-up, including 111 (40%) patients on a DA. Fifty-two patients (19%) reported incident ICD behaviour during follow-up.
Table 1.
Baseline characteristics
| N | 276 |
|---|---|
| Sex, % male (male: female) | 66.3% (183: 93) |
| Race, % white (n) | 96.4% (266) |
| Age, years | 65.04 [9.6] |
| Formal education, years | 15.58 [3.0] |
| Montreal Cognitive Assessment score | 27·24 [2.3] |
| Duration of PD, months | 6·31 [6.3] |
| MDS-UPDRS part III score | 21.46 [9.0] |
| Duration of follow-up, years | 2.20 [0.8] |
Values are means (SD), n=number.
PD, Parkinson’s disease; MDS-UPDRS, Movement Disorders Society Unified Parkinson’s Disease Rating Scale.
Candidate gene selection
We identified at least one frequent polymorphism in 12 of the 15 candidate genes; for the DRD4 and HTR1A gene, no frequent variant was found, so these genes were not included in the analysis. For the DDC gene we included a second variant, as no single SNP has been implicated in ICD or addictive behaviours so far, no frequent SNP in the coding region was found and the gene is large in size (107 020 bp). Thus, our final set of candidate variants consisted of 13 SNPs in 12 candidate genes. Allele and genotype distributions of all 13 variants respected the Hardy-Weinberg equilibrium (table 2).
Table 2.
Characteristics of the selected genetic variants
| Gene | Variant | Alleles | Location in gene | MAF |
|---|---|---|---|---|
| SLC6A3 (DAT1) | rs27072 | C/T | UTR’3 | T=0.205 |
| DRD2 | rs1800497 | G/A | Exon, missense | A=0.326 |
| DRD3 | rs6280 | C/T | Exon, missense | C=0.486 |
| GRIN2B | rs7301328 | C/G | Exon, synonymous | C=0.442 |
| HTR2A | rs6313 | G/A | Exon, synonymous | A=0.441 |
| TPH2 | rs7305115 | A/G | Exon, synonymous | A=0.458 |
| SLC6A4 (SERT) | rs7224199 | G/T | UTR’3 | G=0.419 |
| ADRA2C | rs76337672 | C/G | UTR’3 | C=0.422 |
| DDC | rs3837091 (DIV) | −/CTCT | UTR’5 | −=0.293 |
| DDC | rs1451375 | A/C | Intron | A=0.346 |
| COMT | rs4680 | A/G | Exon, missense | A=0.369 |
| OPRM1 | rs1799971 | A/G | Exon, missense | G=0.223 |
| OPRK1 | rs702764 | C/T | Exon, synonymous | C=0.245 |
DIV, deletion/insertion variation; MAF, minor allele frequency as reported in the dbSNP database; UTR′3, three prime untranslated region; UTR′5, five prime untranslated region.
ICD heritability
Variance of ICD incidence explained by all frequent autosomal SNPs from whole exome sequencing (MAF >0.2, 44 504 SNPs) was estimated to be 57% (SE ±39.8%).
ICD prediction based on clinical and genetic variables
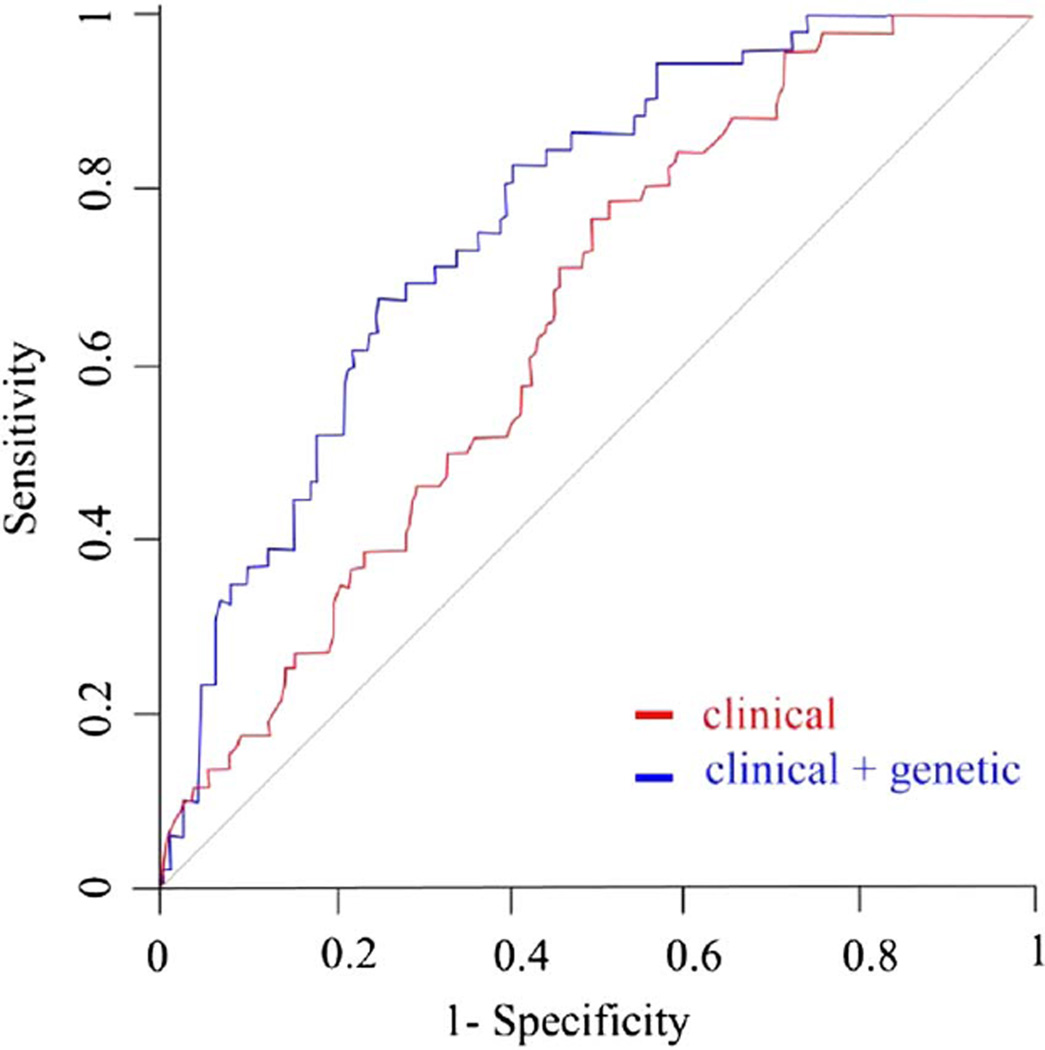
ROC curves plotted with clinical variables (sex, age, PD treatment and length of follow-up) with or without genetic variables are displayed in figure 1. The AUC was 0.65 (95% CI 0.58 to 0.73) when only clinical variables were used in the model, and increased by 11% to 0.76 (95% CI 0.70 to 0.83) (p=0.002, DeLong’s test) when adding genotype data for the 13 SNPs.
Figure 1.
ROC curves for prediction of ICD incidence in the whole population. The red ROC curve was plotted with clinical variables only (age, gender, DRT and duration of follow-up period). The blue ROC curve was plotted with clinical and genetic variables combined. The genetic variables consisted of genotype data on 13 preselected SNPs. p Value refers to AUC comparison of the two curves. AUC, area under the curve, DRT, dopamine replacement therapy; ICD, Impulse control disorders; ROC, receiver operating characteristic SNPs, single nucleotide polymorphisms.
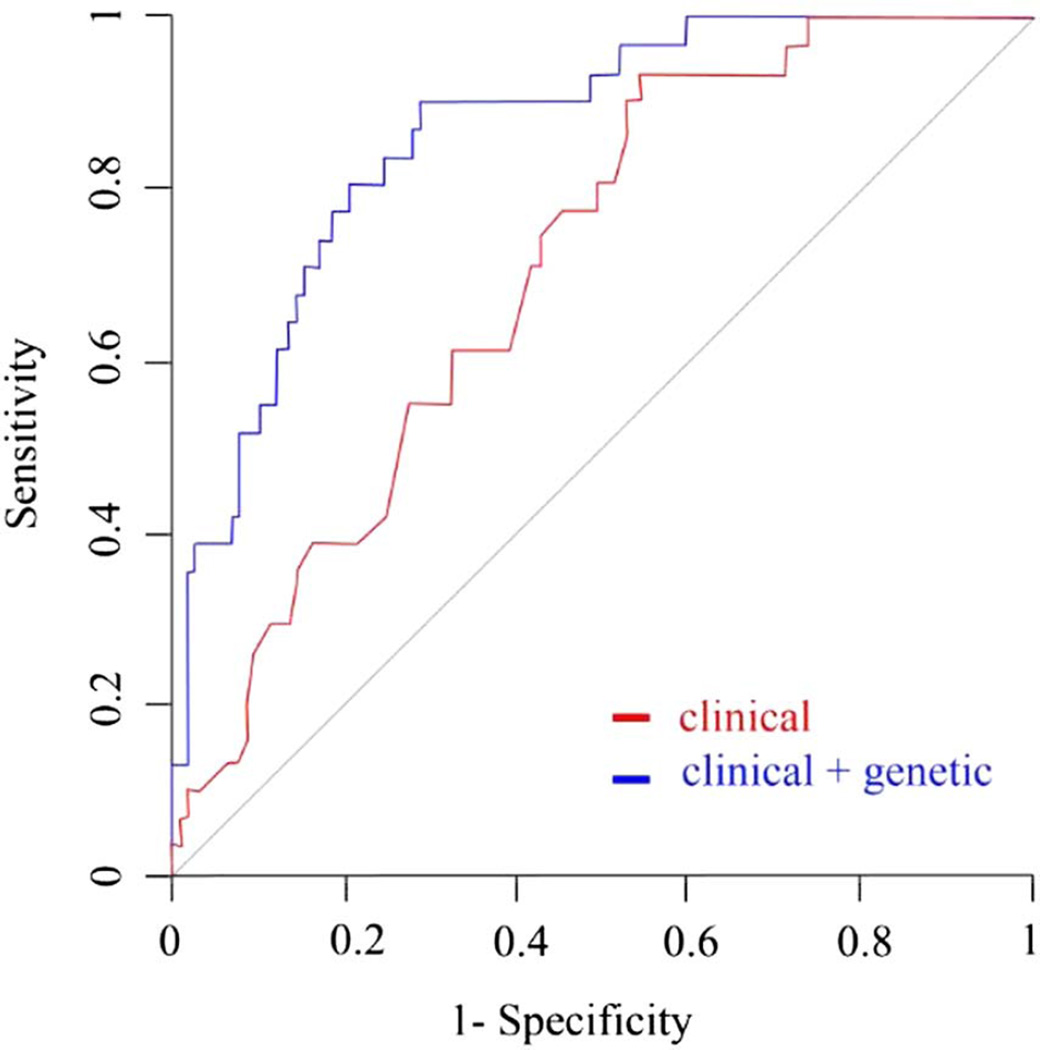
The subgroup analysis of the 149 patients either initiating DA treatment or not on DRT during follow-up revealed even greater ICD predictability with genetic data added to the model compared to clinical variables alone. When genotype data were added, AUC increased significantly by 16% (from 0.71 (95% CI 0.62 to 0.8) to 0.87 (95% CI 0.80 to 0.93), p<0.001; figure 2).
Figure 2.
ROC curves for prediction of ICD incidence in patients using dopamine agonists. The red ROC curve was plotted with clinical variables only (age, gender, DRT and duration of follow-up period). The blue ROC curve was plotted with clinical and genetic variables combined. The genetic variables consisted of genotype data on 13 preselected SNPs. p Value refers to AUC comparison of the two curves. AUC, area under the curve, DRT, dopamine replacement therapy; ICD, impulse control disorders; ROC, receiver operating characteristic SNPs, single nucleotide polymorphisms.
Stepwise regression
In order to identify individual factors that contribute to ICD predictability, we performed a backward stepwise regression. DRT, age and the length of follow-up were significant clinical predictors. The significant genetic predictors were TPH2 and OPRK1 genotypes.
The same analysis was performed in the subset of patients either initiating DA or on no DRT during follow-up, revealing age, male sex, DRT and ADRA2C, DRD2, DDC, HTR2A and OPRK1 genotypes as significant predictors of incident ICD behaviour.
Independent factor associations
The significant variables from the stepwise regression were used in multivariate logistic regression, which showed significant associations of single genetic variants with ICD behaviour incidence (table 3). The heterozygous genotype of OPRK1 was significantly associated with incident ICD behaviour (p=0.03), suggesting a dominant effect of the minor allele C. There was a suggestion of an association with the minor homozygous genotype of the TPH2 polymorphism (p=0.07) and for the duration of follow-up (p=0.07). In addition, ICD behaviours occurred at increased frequency in patients on DA, compared with untreated patients (p=0.04), whereas no significant difference was found for patients using DRT other than DA, compared with untreated patients (p=0.13).
Table 3.
Single factor associations with ICD incidence in the multivariable non-linear model, whole population
| Factor | Multivariable p Value |
|---|---|
| (Intercept) | 0.217 |
| OPRK1: rs702764: TC genotype | 0.033 |
| OPRK1: rs702764: CC genotype | 0.989 |
| TPH2: rs7305115: GA genotype | 0.819 |
| TPH2: rs7305115: AA genotype | 0.077 |
| Dopamine agonist use | 0.036 |
| Other DRT | 0.125 |
| Age | 0.125 |
| Duration of follow-up | 0.072 |
Sets of variables were determined using stepwise regression. p Values for each variable were adjusted for the other covariates.
DRT, dopamine replacement therapy; ICD, impulse control disorders.
Multivariate logistic regression in the subcohort of patients either treated with a DA or on no DRT during follow-up revealed that patients who started DA treatment were more likely to develop ICD behaviours, compared with untreated patients (p=0.001; table 4). Male sex was significantly associated with ICD behaviour incidence (p=0.01). Heterozygous genotype of the OPRK1 and the HTR2A variants was significantly associated with incident ICD behaviours (p=0.04, and p=0.008). Significant associations were also found for the heterozygous and minor homozygous genotype of the rs3837091 DDC polymorphism (p=0.01 and p=0.04), as well as the minor homozygous genotype of the rs1451375 DDC SNP (p=0.04). No significant association was found for the ADRA2C and DRD2 variants.
Table 4.
Single factor associations with ICD incidence in the multivariable non-linear model, patients taking DA only
| Factor | Multivariable p Value |
|---|---|
| (Intercept) | 0.578 |
| OPRK1: rs702764: TC genotype | 0.038 |
| OPRK1: rs702764: CC genotype | 0.997 |
| HTR2A: rs6313: GA genotype | 0.008 |
| HTR2A: rs6313: AA genotype | 0.456 |
| DDC: rs383709: −/AGAG genotype | 0.01 |
| DDC: rs3837091: −/− genotype | 0.043 |
| DDC: rs1451375: CA genotype | 0.122 |
| DDC: rs1451375: AA genotype | 0.037 |
| ADRA2C: rs76337672: GC genotype | 0.705 |
| ADRA2C: rs76337672: CC genotype | 0.995 |
| DRD2: rs1800497: GA genotype | 0.655 |
| DRD2: rs1800497: AA genotype | 0.993 |
| Age | 0.071 |
| Sex | 0.014 |
| Dopamine agonist use | 0.001 |
Sets of variables were determined using stepwise regression. p Values for each variable were adjusted for the other covariates. Subgroup analysis was performed on 149 patients who either were on DA treatment or did not receive DRT during follow-up.
DA, dopamine agonist; DRT, dopamine replacement therapy; ICD, impulse control disorders.
DISCUSSION
This is the first study to evaluate ICD heritability in a prospective cohort of de novo patients with PD. Incident ICD behaviour was common in this cohort, with a cumulative frequency of 19%, and initiation of DA treatment significantly increased risk, compared with other DRT classes. Although the majority of the literature addresses ICD prevalence rather than incidence, the rates in our study are within the range reported in cross-sectional studies.2–4
Based on whole exome data, we found common genetic variants to account for more than half (57%) of the variance of ICD incidence in patients with PD. This finding is comparable to previous estimations on heritability for substance addiction and pathological gambling in the general population.13,14
A broad genetic screening technique such as whole exome sequencing may be important in stratifying patients with PD’s’ ICD risk, since the contribution of single genetic variants to ICD susceptibility may be small, multiple gene interactions may play a role and several neurotransmitter systems may contribute to ICD pathogenesis.30 By selecting a multipolymorphism profile comprised of genes implicated in monoaminergic, glutamatergic and opioid signalling pathways, we found a substantial 11–16% increase in ICD behaviour predictability compared to examining clinical variables alone. Incident ICD behaviour predictability was particularly strengthened in patients initiating DA treatment versus those patients with Parkinson’s disease remaining untreated during follow-up, with an AUC approaching 90%. The latter group controlled for the suboptimal specificity of the QUIP (ie, the relative high potential for a false-positive incident QUIP in a patient who has not yet initiated DRT) and therefore supports clinical relevance of this finding. This is the first proof-of-concept study demonstrating that clinical-genetic modelling could provide clinically meaningful risk stratification and lead to personalised therapy for patients with PD once validated in independent prospective studies.
Our model suggests that genetic variants in several neurotransmitter systems that have been previously associated with behavioural addictions may contribute to ICD risk in PD. Dopamine, serotonin and norepinephrine genes have been shown to contribute to ICD predisposition in the general population.16,30 In addition, considerable evidence supports the importance of glutamatergic and opioid transmitter systems for behaviour and impulsivity regulation.15,16 To date, evaluation of ICD susceptibility in PD has focused on independent associations of single variants, including polymorphisms of the DRD1-5, SLC6A4 (SERT1), HTR2A, GRIN2B, COMT and SLC6A3 (DAT1) genes. We extended the spectrum of monoaminergic ICD candidate genes by adding the DDC gene (which encodes for AADC (aromatic l-amino acid decarboxylase), an enzyme involved in dopamine and serotonin biosynthesis) and the TPH2 gene (which encodes for the rate-limiting enzyme of serotonin synthesis). To the best of our knowledge, this was the first study to also evaluate genes involved in noradrenergic (ADRA2C) and opioid (OPRM1 and ORPK1) signalling in PD ICD. α-2 adrenergic receptors are expressed in the caudate and accumbens nuclei as well as in the hippocampus and cerebral cortex, and are implicated in the regulation of behavioural responses.31 The endogenous opioid system mediates effect of, and motivation as well as reactivity to stress and reward by modulating the mesolimbic dopamine pathway. Mesolimbic neurons may either be excited by μ-opioid receptor activation or inhibited by either μ-opioid or κ-opioid receptors depending on their target projections.15 Both, particularly the μ-opioid receptor, have been extensively studied for their implication in substance addiction with some contradictory results.15,32,33
In the entire cohort, our multivariate model revealed that the OPRK1 polymorphism rs702764 significantly predicted incident ICD behaviour. This variant has previously been reported to be part of a risk haplotype accounting for higher alcohol use and withdrawal symptoms in patients on methadone maintenance.34 However, in another study, no association was found with heroin and alcohol addiction.35 Interestingly, a recent randomised, placebo-controlled clinical trial showed that the opioid receptor antagonist naltrexone decreases ICD symptom severity in patients with PD with ICD.36
In the subcohort of patients initiating DA therapy, multivariable analysis showed independent associations of the OPRK1, DDC and HTR2A variants with new-onset ICD behaviours. The serotonin 2A receptor has been implicated in the modulation of drug addiction, and specifically the functional HTR2A rs6313 variant has been reported to predispose for impulsivity and addiction in the general population as well as ICD in patients with PD.20
This is the first study reporting an association of DDC variants with ICD in PD. DDC polymorphisms/haplotypes have been associated with impulsivity and addiction in the general population.37–39 The DDC rs3837091 promoter deletion polymorphism has recently been reported to influence the motor response to levodopa.40 The discrepancy in genetic predictors between the whole cohort and the DA-treated subgroup may be due to medication-specific drug-gene interactions that mediate the interplay between these neurotransmitter systems and dopamine neurotransmission.
To date, polymorphisms of the DRD1, DRD2, DRD3, HTR2A and GRIN2B genes have been associated with ICD in patients with PD.19–22 None of these genes sustained stepwise regression in our cohort, suggesting that their independent contribution may be too low to be detected. Discrepancy between our findings and previous reports may also be due to differences in study design (eg, cross-sectional vs prospective, method of ICD behaviour assessment and cohort characteristics).
Since our cohort consisted of early patients with PD, disease duration was relatively short, and the length and dose of DRT exposure would be expected to be low overall, which might have contributed to differences in genetic susceptibility to ICD. Indeed, 5HTR2A polymorphism was found to have increased ICD risk only in patients receiving low LEDD.20 Certain genetic variants might enhance ICD risk in the absence of other clinical risk factors (eg, DA use, higher LEDD), while other variants might increase ICD risk only under specific treatments (eg, DA treatment). Ethnic background also needs to be considered, and our results must be confirmed across more diverse ethnicities. The only previous study in patients with PD with European ancestry showed no significant association with the DRD2 and COMT genes, similar to our findings.21
In terms of study limitations, the candidate selection of SNPs analysed in our study may have been too restrictive and the global genetic effect of DRT on ICD behaviour in patients with PD may be further elucidated by future genome-wide gene-drug interaction studies in larger cohorts. Another limitation was assessment of ICD behaviours by the QUIP, which might have led to a falsely high incidence rate due to the high sensitivity and lower specificity of the QUIP. Formal ICD diagnostic criteria should be applied in future studies. In addition, we were not able to assess the impact of DRT doses. Different dopamine agonists might have differential risks for ICD development, as recently shown for subcutaneous forms.41 Since the sample size was limited, dopamine agonists were considered as a class in our analysis. A larger cohort of patients will be needed to investigate potential drug-specific genetic risk factors for ICD behaviours. Although PPMI is the largest prospective cohort of de novo patients with PD to date, our proposed genetic algorithm must be replicated and refined in similar longitudinal cohorts before it can be translated into clinical practice. Then, a ‘personalised medicine’ approach may be developed for the management of patients with PD based on their genetic profile. For instance, patients at significantly increased risk for ICD development based on their demographic, clinical and genetic profile, might not be initiated on a dopamine agonist, instead being treated with other antiparkinsonian medications.
In summary, we found incident ICD behaviours to have a substantial hereditability in early PD. A 13-SNP genetic panel significantly increased ICD predictability, leading to a predictive model reaching clinically relevant accuracy. We provide supportive evidence that the HTR2A variant rs6313 is an independent risk factor for ICD development, and for the first time report that OPRK1 and DDC polymorphisms are associated with incident ICD behaviours in PD. Our results suggest that there may be premorbid genetically determined neurobiological risk factors for ICD in PD, although it is unlikely that a single genetic variant or genetic variation in a single neurotransmitter system will be sufficient to predict this complex condition. Additional studies are needed to replicate these findings before applying them to clinical practice, to further disentangle the relationships among clinical, pharmacologic and genetic risk factors for ICD development in PD, and to determine the functional relevance of genetic risk factors.
Acknowledgments
The authors thank all PPMI participants and their families. Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (http://www.ppmi-info.org/data). For up-to-date information on the study, visit http://www.ppmi-info.org.
Funding PPMI—a public–private partnership—is funded by the Michael J Fox Foundation for Parkinson’s Research and funding partners, including AbbVie, Avid Radiopharmaceuticals, Biogen, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramal, Roche, Servier and UCB. J-CC, IM and VG were supported by a funding from the programme ‘Investissements d’Avenir’ ANR-10-IAIHU-06; and KS by Medtronic Inc.
KS has received an unrestricted educational grant from Medtronic Inc. DW has received research funding or support from Michael J Fox Foundation for Parkinson’s Research, National Institutes of Health (NINDS), Novartis Pharmaceuticals, Department of Veterans Affairs, Avid Radiopharmaceuticals, Alzheimer’s Disease Cooperative Study, and the International Parkinson and Movement Disorder Society; honoraria from AbbVie, Acadia, Biotie, Clintrex LLC, Otsuka, Teva Pharmaceuticals, UCB and the CHDI Foundation; license fee payments from the University of Pennsylvania for the QUIP and QUIP-RS; royalties from Wolters Kluweland; and fees for legal consultation for a lawsuit related to antipsychotic prescribing in a patient with Parkinson’s disease. VG reports grants from ‘Investissements d’Avenir’ ANR-10-IAIHU-06 during the conduct of the study. IM reports grants from ‘Investissements d’Avenir’ ANR-10-IAIHU-06 during the conduct of the study. J-CC reports grants from the programme ‘Investissements d’Avenir’ ANR-10-IAIHU-06 during the conduct of the study. J-CC reports research grants from the French Ministry of Health, the Michael J Fox Foundation, Ipsen, Sanofi-Aventis, travel grants from Teva, Lundbeck, UCB and Novartis, and honoraria from AbbVie, Pfizer and Zambon, outside the submitted work.
Footnotes
Contributors JK, KS, DW, VG, FC, IM, ABS and J-CC participated in the acquisition of data, the analysis and drafting of the manuscript. MAN and ABS participated in the acquisition of the data. DW and J-CC participated in the conception and the design of the work. All the authors participated in the interpretation of data, revised the work critically for important intellectual content and gave their final approval of the version published.
Competing interests MAN reports no conflict of interest. FC reports no conflict of interest.
Patient consent Obtained.
Ethics approval Ethics committees according to local regulations.
REFERENCES
- 1.Grant JE, Atmaca M, Fineberg NA, et al. Impulse control disorders and “behavioural addictions” in the ICD-11. World Psychiatry. 2014;13:125–127. doi: 10.1002/wps.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weintraub D, Papay K, Siderowf A Parkinson’s Progression Markers I. Screening for impulse control symptoms in patients with de novo Parkinson disease: a case-control study. Neurology. 2013;80:176–180. doi: 10.1212/WNL.0b013e31827b915c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weintraub D, David AS, Evans AH, et al. Clinical spectrum of impulse control disorders in Parkinson’s disease. Mov Disord. 2015;30:121–127. doi: 10.1002/mds.26016. [DOI] [PubMed] [Google Scholar]
- 4.Antonini A, Siri C, Santangelo G, et al. Impulsivity and compulsivity in drug-naive patients with Parkinson’s disease. Mov Disord. 2011;26:464–468. doi: 10.1002/mds.23501. [DOI] [PubMed] [Google Scholar]
- 5.Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 6.Bastiaens J, Dorfman BJ, Christos PJ, et al. Prospective cohort study of impulse control disorders in Parkinson’s disease. Mov Disord. 2013;28:327–333. doi: 10.1002/mds.25291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voon V, Sohr M, Lang AE, et al. Impulse control disorders in Parkinson disease: a multicenter case—control study. Ann Neurol. 2011;69:986–996. doi: 10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- 8.Phu AL, Xu Z, Brakoulias V, et al. Effect of impulse control disorders on disability and quality of life in Parkinson’s disease patients. J Clin Neurosci. 2014;21:63–66. doi: 10.1016/j.jocn.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Joutsa J, Martikainen K, Vahlberg T, et al. Effects of dopamine agonist dose and gender on the prognosis of impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:1079–1083. doi: 10.1016/j.parkreldis.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Hurt CS, Alkufri F, Brown RG, et al. Motor phenotypes, medication and mood: further associations with impulsive behaviours in Parkinson’s disease. J Parkinsons Dis. 2014;4:245–254. doi: 10.3233/JPD-130314. [DOI] [PubMed] [Google Scholar]
- 11.Weintraub D. Impulse control disorders in Parkinson’s disease: prevalence and possible risk factors. Parkinsonism Relat Disord. 2009;15(Suppl 3):S110–S113. doi: 10.1016/S1353-8020(09)70794-1. [DOI] [PubMed] [Google Scholar]
- 12.Valença GT, Glass PG, Negreiros NN, et al. Past smoking and current dopamine agonist use show an independent and dose-dependent association with impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:698–700. doi: 10.1016/j.parkreldis.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Eisen SA, Lin N, Lyons MJ, et al. Familial influences on gambling behavior: an analysis of 3359 twin pairs. Addiction. 1998;93:1375–1384. doi: 10.1046/j.1360-0443.1998.93913758.x. [DOI] [PubMed] [Google Scholar]
- 14.Slutske WS, Zhu G, Meier MH, et al. Genetic and environmental influences on disordered gambling in men and women. Arch Gen Psychiatry. 2010;67:624–630. doi: 10.1001/archgenpsychiatry.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol. 2008;75:63–75. doi: 10.1016/j.bcp.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Napier TC, Corvol JC, Grace AA, et al. Linking neuroscience with modern concepts of impulse control disorders in Parkinson’s disease. Mov Disord. 2015;30:141–149. doi: 10.1002/mds.26068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cormier F, Muellner J, Corvol JC. Genetics of impulse control disorders in Parkinson’s disease. J Neural Transm (Vienna) 2013;120:665–671. doi: 10.1007/s00702-012-0934-4. [DOI] [PubMed] [Google Scholar]
- 18.Kim YE, Jeon BS. Genetic susceptibility of impulse control and related behavior in Parkinson’s disease. J Parkinsons Dis. 2014;4:261–272. doi: 10.3233/JPD-130292. [DOI] [PubMed] [Google Scholar]
- 19.Lee JY, Lee EK, Park SS, et al. Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson’s disease. Mov Disord. 2009;24:1803–1810. doi: 10.1002/mds.22678. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Jeon BS, Kim HJ, et al. Genetic variant of HTR2A associates with risk of impulse control and repetitive behaviors in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:76–78. doi: 10.1016/j.parkreldis.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Vallelunga A, Flaibani R, Formento-Dojot P, et al. Role of genetic polymorphisms of the dopaminergic system in Parkinson’s disease patients with impulse control disorders. Parkinsonism Relat Disord. 2012;18:397–399. doi: 10.1016/j.parkreldis.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Zainal Abidin S, Tan EL, Chan SC, et al. DRD and GRIN2B polymorphisms and their association with the development of impulse control behaviour among Malaysian Parkinson’s disease patients. BMC Neurol. 2015;15:59. doi: 10.1186/s12883-015-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nalls MA, Bras J, Hernandez DG, et al. NeuroX, a fast and efficient genotyping platform for investigation of neurodegenerative diseases. Neurobiol Aging. 2015;36:1605.e7–1605.e12. doi: 10.1016/j.neurobiolaging.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DePristo MA, Banks E, Poplin R, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Auwera GA, Carneiro MO, Hartl C, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinform. 2013;11:11 10 1–11 10 33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weintraub D, Hoops S, Shea JA, et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease. Mov Disord. 2009;24:1461–1467. doi: 10.1002/mds.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang CC, Chow CC, Tellier LC, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 30.Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- 31.Philipp M, Brede M, Hein L. Physiological significance of alpha(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol. 2002;283:R287–R295. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- 32.Crist RC, Berrettini WH. Pharmacogenetics of OPRM1. Pharmacol Biochem Behav. 2014;123:25–33. doi: 10.1016/j.pbb.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tejeda HA, Shippenberg TS, Henriksson R. The dynorphin/kappa-opioid receptor system and its role in psychiatric disorders. Cell Mol Life Sci. 2012;69:857–896. doi: 10.1007/s00018-011-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang SC, Tsou HH, Chung RH, et al. The association of genetic polymorphisms in the kappa-opioid receptor 1 gene with body weight, alcohol use, and withdrawal symptoms in patients with methadone maintenance. J Clin Psychopharmacol. 2014;34:205–211. doi: 10.1097/JCP.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 35.Kumar D, Chakraborty J, Das S. Epistatic effects between variants of kappa-opioid receptor gene and A118G of mu-opioid receptor gene increase susceptibility to addiction in Indian population. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:225–230. doi: 10.1016/j.pnpbp.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Galduroz JCF, Ramos AC, Santos-Galduroz RF, et al. Naltrexone for impulse control disorders in Parkinson disease: a placebo-controlled study. Neurology. 2015;84:1386–1387. doi: 10.1212/WNL.0000000000001458. [DOI] [PubMed] [Google Scholar]
- 37.Gray JC, MacKillop J. Genetic basis of delay discounting in frequent gamblers: examination of a priori candidates and exploration of a panel of dopamine-related loci. Brain Behav. 2014;4:812–821. doi: 10.1002/brb3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agrawal A, Verweij KJ, Gillespie NA, et al. The genetics of addiction-a translational perspective. Transl Psychiatry. 2012;2:e140. doi: 10.1038/tp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma JZ, Beuten J, Payne TJ, et al. Haplotype analysis indicates an association between the DOPA decarboxylase (DDC) gene and nicotine dependence. Hum Mol Genet. 2005;14:1691–1698. doi: 10.1093/hmg/ddi177. [DOI] [PubMed] [Google Scholar]
- 40.Devos D, Lejeune S, Cormier-Dequaire F, et al. Dopa-decarboxylase gene polymorphisms affect the motor response to L-dopa in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:170–175. doi: 10.1016/j.parkreldis.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Ruiz PJ, Martinez Castrillo JC, Alonso-Canovas A, et al. Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: a multicentre study. J Neurol Neurosurg Psychiatr. 2014;85:840–844. doi: 10.1136/jnnp-2013-306787. [DOI] [PubMed] [Google Scholar]