Abstract
Background
Despite significant progress made in recent decades in preventing childhood lead poisoning in the United States through the control or elimination of lead sources in the environment, it continues to be an issue in many communities, primarily in low-income communities with a large percentage of deteriorating housing built before the elimination of lead in residential paint. The purpose of this study is to determine whether state laws aimed at preventing childhood lead poisoning are also effective in preventing recurring lead poisoning among children previously poisoned.
Methods
An evaluation was conducted to determine whether laws in two representative states, Massachusetts and Ohio, have been effective in preventing recurrent lead poisoning among children less than 72 months of age previously poisoned, compared to a representative state (Mississippi) which at the time of the study had yet to develop legislation to prevent childhood lead poisoning.
Results
Compared to no legislation, unadjusted estimates showed children less than 72 months old, living in Massachusetts, previously identified as being lead poisoned, were 73% less likely to develop recurrent lead poisoning. However, this statistically significant association did not remain after controlling for other confounding variables. We did not find such a significant association when analyzing data from Ohio.
Conclusions
While findings from unadjusted estimates indicated that state lead laws such as those in Massachusetts may be effective at preventing recurrent lead poisoning among young children, small numbers may have attenuated the power to obtain statistical significance during multivariate analysis. Our findings did not provide evidence that state lead laws, such as those in Ohio, were effective in preventing recurrent lead poisoning among young children. Further studies may be needed to confirm these findings.
Keywords: Lead, Childhood lead poisoning, Lead law, Blood lead levels
Introduction
Despite the significant progress made in recent decades in preventing childhood lead poisoning in the United States through the elimination of lead in paint and fuel, lead poisoning continues to be a problem in some communities, primarily low-income communities with a large percentage of deteriorating housing built before the elimination of lead in paint. Several community based randomized controlled trials have been conducted to examine the effectiveness of dust control (Lanphear et al., 1996a), soil abatement (Weitzman et al., 1993) and health education (Brown et al., 2006) in reducing blood lead levels (BLLs) among children living in urban neighborhoods with high incidence of childhood lead poisoning. Results suggest that these interventions did not significantly lower BLLs. Results from meta-analyses also seems to suggest that there’s insufficient evidence that these interventions are effective in reducing blood lead levels in children (Yeoh et al., 2014).
In the two studies that examined the effectiveness of a state specific lead paint hazard risk reduction law in either preventing new cases of childhood lead poisoning in housing where previous cases were identified (primary prevention) or in preventing recurring incidences of childhood lead poisoning among previously-poisoned children (secondary prevention), the evidence suggests that laws are effective in controlling or eliminating lead hazards found in housing units where previous hazards were observed (Brown et al., 2001; Korfmacher et al., 2012).
In 2009, 27 (64%) of 42 state health departments with lead poisoning prevention programs funded by the Centers for Disease Control and Prevention (CDC), implemented specific laws aimed at reducing or eliminating childhood lead poisoning. We conducted a study to examine the effectiveness of the lead risk reduction laws in two such states: Massachusetts (MA) and Ohio (OH). Both states have specific laws aimed at preventing or decreasing lead poisoning among children living in housing built prior to 1978 (i.e., when lead-based paint was banned from residential use in the US). Mississippi (MS), which does not have laws requiring lead hazard abatement even for housing where children with lead poisoning have been identified, served as the control state for comparison in this study. These three states were selected based on their willingness to participate in the study and, in the case of the two lead law states, on the strength of their lead laws and the length of time that these laws had been enacted (prior to 2000 for MA, and since 2004 for OH).
The Massachusetts Lead Law, promulgated in 1971 and amended in 1987 and 1993, focuses on primary and secondary prevention and requires disciplinary action at several levels. The owner of a dwelling occupied by children less than six (6) years of age and found to have lead-based paint hazards is responsible for complying with measures to contain or abate all such hazards in and around the residence, regardless of the BLL of the resident children. The owner is held liable for any damages sustained by a child who is lead poisoned due to the owner’s failure to comply with provisions to contain or abate lead paint hazards (LPPCR, 2011).
The Ohio law, enacted in 2004, stipulates that when a child is identified as lead poisoned, the Ohio Department of Health may enter the suspected offending dwelling with the permission of the occupant or owner, or can obtain a court order to enter the property if the occupant or owner does not grant permission, to conduct a risk assessment at the property. If the risk assessment reveals lead hazards, a lead hazard control order may be issued, and until the time at which a clearance examination has been passed, the control order may include a requirement that occupants vacate the unit. The owner or manager can choose a method of controlling each lead hazard, which must be approved by the Ohio Department of Health. Criminal and civil action can be taken if any licensing or work practice requirements are violated in the course of correcting lead hazards (Law Writer, 2005).
While there is evidence suggesting that state laws aimed at primary prevention of lead poisoning among children less than 72 months of age have been effective in achieving this goal (Brown et al., 2001; Kennedy et al., 2014), no evidence was available at the time of this study demonstrating these laws were also effective in preventing recurrent poisoning in children who were previously poisoned. This study therefore sought to determine whether state laws aimed at preventing lead poisoning among young children were also effective in preventing recurring lead poisoning among those poisoned previously.
Methods
Design and data sources
CDC conducted a cross-sectional study to examine the effectiveness of the lead risk reduction laws in preventing recurring lead poisoning among confirmed cases in the two states with laws requiring control of lead paint hazards in housing with a child who meets the state definition of childhood lead poisoning (Massachusetts and Ohio) compared to the state without lead laws (Mississippi). Previously published evidence using this data suggests that lead risk reduction laws were effective in primary prevention of lead poisoning among young children (Kennedy et al., 2014). The methods used in the acquisition of data used in this study have been described elsewhere (Kennedy et al., 2014). Briefly, data for this study were obtained through examination of records from the Childhood Blood Lead Surveillance (CBLS) database. The CBLS is the central repository of blood lead surveillance data, submitted on a quarterly basis by state and local childhood lead Poisoning Prevention Programs (CLPPPs), who are supported through cooperative agreement with the CDC. Data provided to the CBLS include results of blood lead tests performed by public and private clinical laboratories as well as case management and environmental data.
The definition of a lead poisoned case was based on a state-specified threshold that would have triggered an environmental investigation. In MA, BLL ≥ 25 μg/dL would have triggered an environmental investigation, whereas in MS and OH, BLL ≥ 15 μg/dL was the threshold value. Each case file was randomly selected to give each child an equal opportunity of being selected into the study. The specific method used for randomization has been described elsewhere (Kennedy et al., 2014).
Institutional Review Board (IRB) approvals or waivers were sought for and obtained from the Centers for Disease Control and Prevention, the Battelle Memorial Institute, the Massachusetts Department of Public Health, the Mississippi State Department of Health and the Ohio Department of Health. All statistical analyses of study data were conducted using SAS® version 9.3, SAS Institute, Cary N.C.
Variable definitions
The following variable definitions were used during analyses. Lead poisoning: Because this analysis examines whether the rates of recurring lead poisoning among previously-poisoned children are declining as a result of actions taken under existing state lead laws, the term “lead poisoning” has a specific meaning in this report. Here, a child is determined to be “lead poisoned” if his or her blood lead concentration is at or above a specified threshold set by the state in which the child resides, for which an environmental investigation would be deemed necessary. For the three states considered in this study, the threshold levels for determining lead poisoning are as follows:
Massachusetts: ≥25 μg/dL,
Ohio: ≥15 μg/dL, and
Mississippi (the control state): ≥15 μg/dL.
Confirmed case of lead poisoning
Based on the outcome of a specific blood sample analysis, a child is classified as a “confirmed” lead poisoning case if the blood lead measurement is at or above the threshold level of the state in which the child resides. The sample must also satisfy one of the following two criteria: the blood sample was collected using venous technique or the sample was collected using capillary techniques and the measurement associated with a previous capillary blood sample, collected no more than 12 weeks (84 days) earlier, and is at or above the state threshold. If the blood lead measurement was lower for the venous sample, deference was always given to the venous blood lead sample.
Recurrent lead poisoning
Any identified lead poisoning case occurring over 2 or more non-consecutive years.
Cohort year
The year in which a confirmed case of lead poisoning was selected for this study is labeled the case’s “cohort year.” A child could be selected as a case multiple times, corresponding to multiple cohort years. Thus, a “selected case” is uniquely identified by the child and cohort year.
Sample size calculation
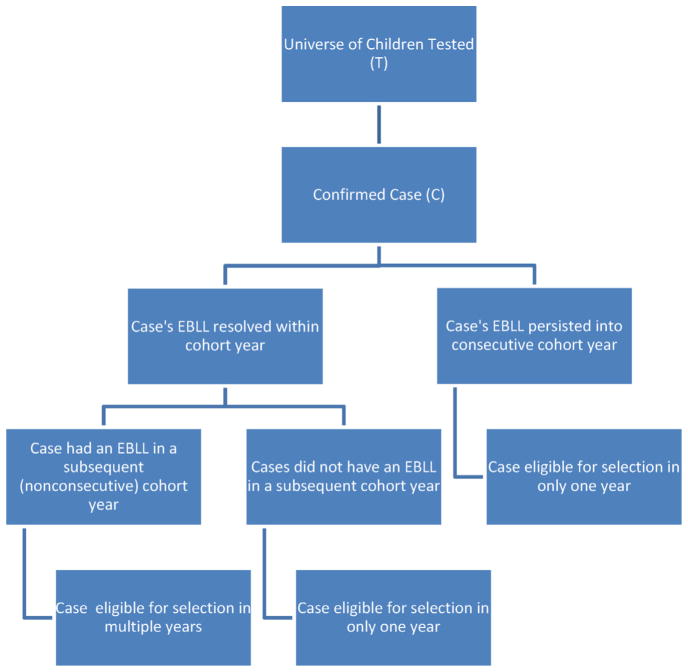
The sample size needed to conduct this study was determined by using the following formula: PF = CF/TF and PM = CM/TM, where PF and PM were the proportion of elevated blood lead levels (EBLLs) for females and males, respectively, CF and CM were the number of confirmed EBLLs for females and males, respectively, and TF and TM were the total number tested, for female and male children, respectively. Since male children compared to female children, are more likely to have EBLLs (Walter et al., 1980), sex specific proportions were determined separately. A specific description of how the formula was operationalized has been described elsewhere (Kennedy et al., 2014). Fig. 1, however, provides a visual illustration of the mechanism of case selection.
Fig. 1. Case selection diagram.
Case selection was conducted separately for male and female children <72 months of age within specific cohort years.
Data abstraction
The types of abstracted data included case management, environmental, and dwelling specific data. Case management data were used to identify cases for this study as well as obtain information on the child’s demographic characteristics. Environmental assessment data, which was obtained from case management records included information on dust–lead loadings (from floors and window sills) at the child’s primary dwelling at the time of lead poisoning. Information on the child’s primary dwelling, obtained from both case-management and tax assessor data, included information of year dwelling built and ownership status.
Case management data and the case selection process
Using information from the case management data set, eligible cases were identified in each year from 2000 to 2007 based on his or her maximum blood lead measurement in that year, giving deference to the venous measurement. For children classified as an eligible case, we determined whether the child had multiple tests within and across subsequent years. This was important as a child could be eligible for selection into this study during multiple cohort years but not in consecutive years. The child was not counted as a newly identified case in a consecutive cohort year to account for the time it may take BLLs to decrease (Manton et al., 2000; Roberts et al., 2001), as well as the time it may take any remediation activities in the home to be implemented (Aschengrau et al., 1997; Lanphear et al., 1996b). Therefore, for any given cohort year, a child was only counted once as a case. If the child had blood lead measurements in subsequent years in which he or she was recorded as being lead poisoned, the child was selected as a case in multiple, non-consecutive cohort years.
Random numbers were assigned to each child eligible for selection as a case within gender and year stratum. An auto-generated list of randomly selected cases was then presented to the grantees for file selection. The grantee started at the top of each year/gender grouping and selected files as available until the targeted number of cases (for that year/gender combination, which was provided to the grantee) was achieved. If a file was available and pulled for a child in a given year, that child became a selected case in that year. To ensure that sufficient numbers of cases would be selected, the targeted number of cases in each year/gender combination was increased by three prior to providing them to the grantees. Selected children were labeled according to the cohort year identified and by a unique four digit questionnaire ID.
Once the sample cases were selected, we identified those who had recurrent lead poisoning in later years and who were identified as a case in multiple non-consecutive cohort years. Counts of these recurrent cases represented the numerator when calculating the rate of lead poisoning in at least one year following their cohort year.
Socio-demographic/environmental/housing data
Case-management records were used to obtain information on each participant’s demographic characteristics, which included information on age, race/ethnicity, gender, provider type requesting the blood lead test and health insurance status. Information on the environmental investigation was also obtained from case management records. At the time a child is identified as being lead poisoned, an environmental investigation is conducted at the child’s primary dwelling. The environmental investigation included measuring the amount of lead dust found on dwelling floors and window sills as well as determining whether there was any evidence of peeling or chipping paint on interior or exterior walls.
Case management and tax assessor data were used to obtain information of dwelling ownership status as well as year the structure was built. Situations where tax assessor information was not readily available online resulted in manual extraction of the data through telephone calls.
Analytic strategy
To examine the demographic characteristics of the selected cases and their dwellings in states with and without lead laws, sample means and frequencies were calculated for selected socio-demographic and housing variables. For categorical variables, Fisher’s exact tests or chi-square tests were performed to determine whether the distribution of values among categories differed significantly between states, and in particular, between intervention and control states. For continuous variables (i.e., dust–lead loadings), Kruskal–Wallis non-parametric tests were used to determine whether differences in ranked mean responses differed significantly between states. In each test, when significant differences were observed among states at the 0.05 level, pairwise tests were performed to compare each of MA and OH to the control state (MS) to determine which of the intervention states differed significantly from the control state. Each pairwise test was performed at a 0.05/2 = 0.025 significance level to correct for multiple comparison, and to ensure that the Type I error rate (i.e., incorrectly indicating significant difference from MS in at least one test) was no higher than 0.025 between the two tests.
The main effects inquiry examined the effectiveness of state specific lead-based paint hazard risk reduction laws in secondary prevention of lead poisoning in children less than 72 months old. Fisher’s exact test was conducted to determine whether there were statistically significant differences between the lead law states and the control state in the percentage of children who remained cases in later cohort years, following their initial identification as a case. Multiple logistic regression analyses were conducted to determine whether differences in the probability of being counted as a case in at least one subsequent cohort year following the initial cohort year remained statistically significant between states with lead laws compared to the state without lead laws, even after controlling for demographic and environmental covariates. The Hosmer–Lemeshow chi-square goodness-of-fit test was conducted to assess the adequacy of the model to fit the data. The logistic regression model was fitted using the LOGISTIC procedure in SAS.
Results
Demographic information
Case level demographic information
Data for a total of 637 selected cases were included in the analyses. These data represent 633 distinct children, with four of these children each representing a selected case in two different cohort years: three children in MS and one in OH. Of these cases, 255 resided in MA, 145 in OH and 237 in MS. The cohort years for these selected cases ranged from 2000 to 2007 for MA and MS and from 2004 to 2007 for OH (Table 1).
Table 1.
Frequencies and percent of demographic and dwelling information among randomly selected lead poisoning cases in MA and MS (2000–2007) and OH (2004–2007)*.
| Demographic characteristics | Massachusetts (MA) | Ohio (OH) | Mississippi (MS) | All states | p-Value1 |
|---|---|---|---|---|---|
| Cohort year | |||||
| 2000 | 45 (17.6%) | – | 44 (18.6%) | 89 (14.0%) | 0.946 (all states, 2004–07) 0.750 (MS vs. OH, 2004–07) 0.015 (MS vs. MA, 2000–07) |
| 2001 | 42 (16.5%) | – | 52 (21.9%) | 94 (14.8%) | |
| 2002 | 35 (13.7%) | – | 50 (21.1%) | 85 (13.3%) | |
| 2003 | 26 (10.2%) | – | 30 (12.7%) | 56 (8.8%) | |
| 2004 | 31 (12.2%) | 44 (30.3%) | 23 (9.7%) | 98 (15.4%) | |
| 2005 | 27 (10.6%) | 36 (24.8%) | 14 (5.9%) | 77 (12.1%) | |
| 2006 | 26 (10.2%) | 35 (24.1%) | 14 (5.9%) | 75 (11.8%) | |
| 2007 | 23 (9.0%) | 30 (20.7%) | 10 (4.2%) | 63 (9.9%) | |
| Total | 255 (100.0%) | 145 (100.0%) | 237 (100.0%) | 637 (100.0%) | |
| Gender | |||||
| Male | 137 (53.7%) | 77 (53.5%) | 124 (53.0%) | 338 (53.4%) | 0.995 (all states) 1.000 (MS vs. OH) 0.928 (MS vs. MA) |
| Female | 118 (46.3%) | 67 (46.5%) | 110 (47.0%) | 295 (46.6%) | |
| Total | 255 (100.0%) | 144 (100.0%) | 234 (100.0%) | 633 (100.0%) | |
| Race/ethnicity | |||||
| Asian or Pacific Islander | 9 (3.5%) | 2 (1.4%) | 1 (0.4%) | 12 (1.9%) | <0.0001 (for all states and each pair of states) |
| Black | 34 (13.3%) | 32 (22.2%) | 164 (70.1%) | 230 (36.3%) | |
| Hispanic | 42 (16.5%) | 5 (3.5%) | 2 (0.9%) | 49 (7.7%) | |
| Other/multiracial | 16 (6.3%) | 11 (7.6%) | 3 (1.3%) | 30 (4.7%) | |
| White | 69 (27.1%) | 83 (57.6%) | 22 (9.4%) | 174 (27.5%) | |
| Unknown/not specified | 85 (33.3%) | 11 (7.6%) | 42 (17.9%) | 138 (21.8%) | |
| Total | 255 (100.0%) | 144 (100.0%) | 234 (100.0%) | 633 (100.0%) | |
| Age at confirmation | |||||
| <12 months | 16 (6.3%) | 12 (8.3%) | 7 (3.0%) | 35 (5.5%) | <0.0001 (all states) <0.0001 (MS vs. OH)0.0003 (MS vs. MA) |
| 12–23 months | 90 (35.3%) | 68 (46.9%) | 61 (25.7%) | 219 (34.4%) | |
| 24–35 months | 80 (31.4%) | 36 (24.8%) | 61 (25.7%) | 177 (27.8%) | |
| 36–71 months | 69 (27.1%) | 29 (20.0%) | 105 (44.3%) | 203 (31.9%) | |
| Unknown/not specified | 0 (0.0%) | 0 (0.0%) | 3 (1.3%) | 3 (0.5%) | |
| Total | 255 (100.0%) | 145 (100.0%) | 237 (100.0%) | 637 (100.0%) | |
| Building ownership | |||||
| Private, owner-occupied | 109 (42.7%) | 34 (23.6%) | 76 (32.5%) | 219 (34.6%) | <0.0001 (all states) 0.0002 (MS vs. OH) <0.0001 (MS vs. MA) |
| Rental, privately-owned | 91 (35.7%) | 85 (59.0%) | 97 (41.5%) | 273 (43.1%) | |
| Rental, publicly-owned2 | 8 (3.1%) | 6 (4.2%) | 10 (4.3%) | 24 (3.8%) | |
| Mix of owner-occupied and rental | 3 (1.2%) | 0 (0.0%) | 25 (10.7%) | 28 (4.4%) | |
| Mix of rental types | 0 (0.0%) | 1 (0.7%) | 2 (0.9%) | 3 (0.5%) | |
| Unknown/not specified | 44 (17.3%) | 18 (12.5%) | 24 (10.3%) | 86 (13.6%) | |
| Total | 255 (100.0%) | 144 (100.0%) | 234 (100.0%) | 633 (100.0%) | |
| Type of provider ordering test or screening site | |||||
| Dept. of Health fixed-site specific to lead | 23 (9.0%) | 1 (0.7%) | 192 (81.0%) | 216 (33.9%) | <0.0001 (for all states and each pair of states) |
| Other fixed-site screening provider (WIC) | 92 (36.1%) | 4 (2.8%) | 0 (0.0%) | 96 (15.1%) | |
| Private health care provider | 55 (21.6%) | 132 (91.0%) | 11 (4.6%) | 198 (31.1%) | |
| Other | 2 (0.8%) | 1 (0.7%) | 2 (0.8%) | 5 (0.8%) | |
| Unknown/Not specified | 83 (32.5%) | 7 (4.8%) | 32 (13.5%) | 122 (19.2%) | |
| Total | 255 (100.0%) | 145 (100.0%) | 237 (100.0%) | 637 (100.0%) | |
| Funding source for test | |||||
| Medicaid | 0 (0.0%) | 15 (10.3%) | 127 (53.6%) | 142 (22.3%) | <0.0001 (MS vs. OH) |
| Private insurance | 0 (0.0%) | 0 (0.0%) | 27 (11.4%) | 27 (4.2%) | |
| Unknown/not specified | 255 (100.0%) | 130 (89.7%) | 83 (35.0%) | 468 (73.5%) | |
| Total | 255 (100.0%) | 145 (100.0%) | 237 (100.0%) | 637 (100.0%) | |
The data in this table represent information obtained for 637 distinct children among 633 selected cases for which the cohort year was 2000 or later for MA and MS, and 2004 or later for OH. Four children represent a case selected in two different cohort years, 3 from MS and 1 from OH.
Chi-square or Fisher’s exact test used to examine differences in proportion at the p = 0.05 level between MA, OH and MS.
Includes Section 8 or subsidized housing.
Approximately 53% of the selected cases in each state were male. White and Black/African-American race accounted for the largest distribution of selected cases, where 27.1%, 57.6% and 9.4% of selected cases in MA, OH, and MS, respectively, were White, and 13.3%, 22.2% and 70.1% of selected cases in MA, OH and MS, respectively, were Black/African-American. Hispanic ethnicity accounted for 16.5%, 3.5% and 0.9% of selected cases in MA, OH and MS, respectively. Less than 6% of selected cases were <12 months old. Children 12–23 months of age accounted for the largest proportion, corresponding to 35.3%, 46.9% and 25.7% of all selected cases in MA, OH and MS, respectively. Across all states, most of the selected cases resided in dwellings that were privately owned rental property, corresponding to 35.7%, 59.0% and 41.5% of cases sampled in MA, OH and MS, respectively (Table 1).
Among selected cases in OH, 91.0% had lead tests performed which were ordered by a private health care provider, while in MS, 81.0% of tests were ordered by the Department of Public Health, Childhood Lead Poisoning Prevention Program. In MA, the distribution of lead tests ordered by public screening providers (e.g., Supplemental Program for Women Infant and Children, [WIC]) and by private health care providers was similar: 36.1% and 21.6%, respectively. Medicaid was the most cited source of payment for lead tests in MS (53.6%), while no payment information was available in MA, and little if any information was available for OH (Table 1).
Secondary prevention of child lead poisoning
Of the 633 children represented among the selected cases, 425 had blood lead measurements available in multiple years following their cohort year, thereby allowing them to be included in this analysis. This included 179 of the 255 cases in MA, 93 of the 144 selected cases in OH, and 153 of the 234 selected cases in MS. MA had the lowest percentage of cases that remained a lead poisoning case in subsequent years following their cohort year (17.9%) while MS had the highest percentage (44.4%). In OH, 35.5% of cases had recurrent lead poisoning in subsequent years following their cohort year (Table 2).
Table 2.
Proportion of 425 distinct cases* classified as a lead poisoning case in at least one year following their cohort year.
| Proportion of selected cases (n) | ||||
|---|---|---|---|---|
|
| ||||
| Massachusetts (MA) | Ohio (OH) | Mississippi (MS) | p-Value1,2 | |
| Proportion of participants who were recurrent cases in later years (number of cases) | 17.9% (32) | 35.5% (33) | 44.4% (68) | <0.0001 (MA vs. MS) 0.183 (OH vs. MS) |
| Proportion of participants who were not recurrent cases in later years (number of cases) | 82.1% (147) | 64.5% (60) | 55.6% (85) | |
| Total | 179/255 | 93/144 | 153/234 | |
Results reflect 425 cases who had sufficient blood lead measurements in multiple years following their cohort year. 425 individual cases represent 179 distinct children in MA, 93 distinct children in Ohio and 153 distinct children in Mississippi.
Fisher’s exact test used to examine differences in percentages of Yes versus No. (cases classified as “not specified” were excluded from this analysis).
p-Value significance level adjusted to account for multiple comparisons. p < 0.05/2 = 0.025 Statistically significant.
The proportion of selected cases who remained a lead poisoning case in subsequent years following their cohort year was significantly different between MA and MS, p < 0.0001; however, no significant difference was noted between OH and MS, p = 0.183 (Table 2).
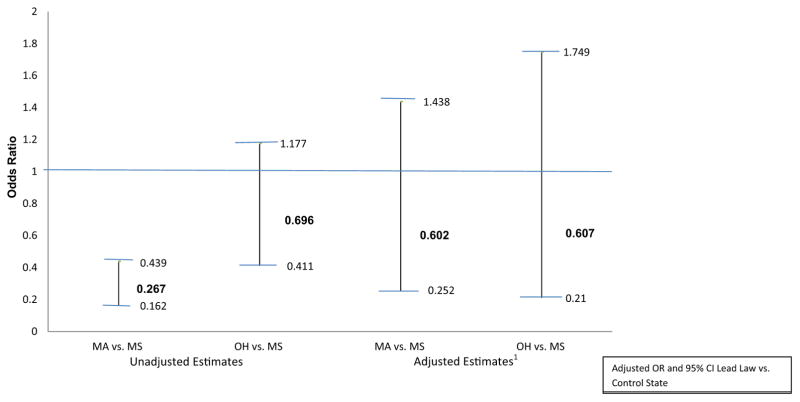
Unadjusted estimates found, compared to cases in MS, those in MA were 73% less likely (OR = 0.27, p = 0.0001) to be counted as a case in subsequent cohort years (Fig. 2). However, this association was no longer statistically significant after controlling for other factors such as gender, race, age at confirmation, building ownership, provider type, funding source of test, presence of deteriorated paint, visible paint chips, mean floor dust–lead loading and mean window sill dust–lead loading (Table 3). Non-significant findings may be partly explained by the reduced number of cases having data for all covariates, where the sample size went to less than 100 cases in each of the three states, as this reduced sample limits the statistical power of the test (i.e., the probability of rejecting the null hypothesis when it was in fact false).
Fig. 2.
Unadjusted and adjusted odds ratio and 95% confidence interval of remaining a lead poisoning case in subsequent years following their cohort years, with comparisons made between MA vs. MS and OH vs. MS. 1 Adjusted for demographic characteristics, building characteristics and environmental factors.
Table 3.
Logistic regression model showing unadjusted and adjusted estimates of recurrent lead poisoning case in subsequent years following the cohort year, with comparisons made between Massachusetts and Mississippi and Ohio and Mississippi.
| Covariate | No. of cases1 | Slope parameters | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| MA vs. MS | OH vs. MS | Odds ratio of lead law states vs. control state2 | ||||||
|
|
|
|||||||
| Estimate | Std. error | p-Value | Estimate | Std. error | p-Value | |||
| Case in subsequent year (unadjusted) | 429 | −1.3188 | 0.2529 | <0.0001 | −0.3621 | 0.2683 | 0.1771 | OR (MA vs. MS): 0.267 OR (OH vs. MS): 0.696 |
| Adjusted estimates | ||||||||
| Case in subsequent year (adjusted full model)3 | 147 | −0.5070 | 0.4441 | 0.2535 | −0.4997 | 0.5404 | 0.3552 | OR (MA vs. MS): 0.602 OR (OH vs. MS): 0.607 |
| Gender (female vs. male) | 429 | −1.3294 | 0.2536 | <0.0001 | −0.3768 | 0.2692 | 0.1615 | |
| Race (others vs. African-American) | 346 | −1.0210 | 0.3583 | 0.0044 | −0.1346 | 0.3398 | 0.6920 | |
| Age at confirmation (others vs. <12 months) | 427 | −1.3279 | 0.2540 | <0.0001 | −0.3489 | 0.2706 | 0.1973 | |
| Building ownership (others vs. private owner-occupied) | 372 | −1.4694 | 0.2857 | <0.0001 | −0.3356 | 0.2846 | 0.2383 | |
| Type of provider ordering Test (others vs. private health care) | 353 | −1.4145 | 0.3226 | <0.0001 | −0.2564 | 0.4710 | 0.5862 | |
| Presence of deteriorated paint (absence vs. presence) | 286 | −1.2736 | 0.3084 | <0.0001 | −0.4157 | 0.3364 | 0.2166 | |
| Presence of visible paint chips (absence vs. presence) | 225 | −0.7585 | 0.4105 | 0.0646 | −0.2987 | 0.3419 | 0.3822 | |
| Mean floor dust–lead loading | 389 | −1.3086 | 0.2695 | <0.0001 | −0.3650 | 0.2745 | 0.1837 | |
| Mean sill dust–lead loading | 356 | −1.4824 | 0.2862 | <0.0001 | −0.5449 | 0.2843 | 0.0553 | |
No. of cases represent 425 distinct children counted as a recurrent lead poisoning—four children were selected as a new case in multiple years, and thus, may have different values for the adjustment factors.
Odds ratios are calculated as the exponential of the parameter estimates in this table.
The Hosmer–Lemeshow test chi-square value for the goodness of fit test was 7.7417, p = 0.4591. Model is a good fit to the data, given p > 0.05.
The Hosmer–Lemeshow chi-square goodness of fit test provided evidence that the model was an adequate fit to the data, given a chi-square value of 7.7417, p = 0.4591. Since the p-value was >0.05, there is evidence that the model was a good fit to the data.
Discussion
The decline in the prevalence of childhood lead poisoning among young children in the U.S. is considered one of public health’s great victories. Owing to stronger regulations banning the use of lead in household paint in 1978 and the U.S. Environmental Protection Agency’s (EPA) gradual phase out and eventual ban in the use of lead in gasoline from 1973 to 1986, a significant decrease of exposure to lead in children’s environments has occurred (EPA, 1996).
The National Health and Nutrition Examination Surveys (NHANES) conducted between 1976–1980 and 1999–2004 show a decline from 77.8% to 1.4% in the percentage of children aged 1–5 years with BLLs ≥ 10 μg/dL, corresponding to a 98% decline (Jones et al., 2009). In addition, the geometric mean blood lead level (GMBLL) in children has also declined substantially, from 2.7 μg/dL in the 1991–1994 survey, to 1.9 μg/dL in the 1999–2004 survey (Jones et al., 2009). However, in many metropolitan cities with homes built prior to 1978, there continues to be an unacceptably high prevalence of children living in residences containing lead-based paint. These children are exposed to high concentrations of lead and suffer adverse health conditions as a result. Thus, these children tend to historically have had, on average, higher mean blood lead levels compared to the remainder of the population (CDC, 2012).
For example, recent evidence from the CDC found that African-American children and children living below the poverty level have significantly higher GMBLLs compared to White, non-Hispanic children and children who live at or above the poverty level (CDC, 2013). While the GMBLL among children aged 1–5 years old as estimated by the 2007–2010 NHANES survey (1.3 μg/dL) is lower than that of even a decade ago (1.9 μg/dL based on the 1999–2004 NHANES survey), lead exposure continues to be a pervasive public health issue that requires a concerted effort across multiple agencies at the local, state, and federal level for its elimination (CDC, 2013). Efforts to mitigate lead exposures are a critical component to preventing EBLLs in young children.
While the study suggests, during unadjusted analyses, that previously lead poisoned children in Massachusetts are less likely to experience recurrent poisoning when compared to children living in the control state this association did not remain statistically significant after control of socio-demographic and environmental factors. This may have been due in part to sparse numbers, where of the original 429 cases used during unadjusted analyses, only 147 cases identified in all three states with complete covariate information, were used during multivariate analyses. Sparse numbers may have limited the precision to detect actual associations. Additionally, differences in BLL thresholds may have resulted in an “artificial” lower probability of detecting a recurrent case in MA compared to OH and MS.
Our findings did not provide evidence that previously lead poisoned children in OH were less likely to experience recurrent poisoning when compared to children living in the control state. While one may argue that sparse numbers may be a reasonable explanation for non-significant findings, one may also argue that the laws in OH, which were enacted in 2004, five years before the start of data collection, may not have had enough time to have an effect on preventing recurrent lead poisoning among young children. Additionally, the stringency of the laws may also partly explain non-significant findings in OH. For example, according to OH lead laws, while civil or criminal actions can be taken if any licensing or work practice requirements are violated during the course of correcting lead hazards (Law Writer, 2005), there is no indication, from the way in which the law is written, that the owner also face similar actions. The urgency to correct residential lead hazards may not be as strong in instances where punitive actions are not taken against the owner compared to when the owner may face civil or criminal actions.
While no safe level of lead exposure has been found, a concerted effort to prevent primary exposure to lead as well as re-exposure among those previously poisoned appears to be a viable option in successful elimination of childhood lead poisoning. In recognition of no safe lead exposure levels, the CDC in 2012, under advisement from the Advisory Committee on Childhood Lead Poisoning Prevention (ACCLPP) accepted the recommendation to redefine the EBLL classification from ≥10 μg/dL to ≥ the 97.5th percentile of the distribution of blood lead levels in a nationally representative, probabilistic sample of children less than 72 months of age. Currently, if a child has a blood lead level ≥5 μg/dL, this would warrant further investigation into possible lead exposures. The recommendation also called for the elimination of the term “level of concern,” as any detectable blood lead level in a child is of potential concern. The findings from this study suggest that additional studies, with more complete information on covariates and larger sample sizes, may be needed to clearly delineate the association between lead laws and recurrent lead poisoning among young children.
Acknowledgments
We thank Dr. Beryl Polk, Program Director, Childhood Lead Poisoning Prevention Program, Mississippi Department of Public Health, Mr. Paul Hunter, Program Director, Childhood Lead Poisoning Prevention Program, Massachusetts Department of Public Health and Mr. John Belt, Program Administrator, Childhood Lead Poisoning Prevention Program, Ohio Department of Public Health for their insightful review of the study protocol and final manuscript. Without their support, this study, as conducted, would not have been possible. We thank Mr. Peter Ashley, Department of Housing and Urban Development, for providing the opportunity to conduct this study.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- CLPPP
Childhood Lead Poisoning and Prevention Program
- CBLS
Childhood Blood Lead Surveillance
- EBLL
elevated blood lead level
- HHLPPP
Healthy Homes and Lead Poisoning Prevention Program
- MA
Massachusetts
- MS
Mississippi
- OH
Ohio
Footnotes
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention and the Agency for Toxic Substances and Disease Registry.
Conflict of interest statement
The authors have no relevant financial or non-financial competing interest in this article.
Authors’ contribution
CK: Served as the Principal Investigator of this study and was instrumental in the conception and design of the study protocol as well as draft of the manuscript. RL: Served as the senior statistician on this study and was instrumental in the analysis of the data as well as help with the draft of the manuscript. MSS: Served as the technical advisor and study coordinator and was instrumental in training the field data collection staff as well as reviewing and editing the manuscript. RB: Served as the senior data collector and monitor. She also assisted with data analysis. MJB: Served as the senior advisor on the manuscript and provided critical revisions to the content. All authors read and approved the final manuscript.
Competing interests
This study was supported by funding from the National Center for Environmental Health in agreement with the Department of Housing and Urban Development.
References
- Aschengrau A, Beiser A, Bellinger D. Residential lead-based-paint hazard remediation and soil lead abatement: their impact among children with mildly elevated blood lead levels. Am J Public Health. 1997;87:1698–1702. doi: 10.2105/ajph.87.10.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MJ, McLaine P, Dixon S. A randomized, community-based trial of home visiting to reduce blood lead levels in children. Pediatrics. 2006;117:2328–2329. doi: 10.1542/peds.2004-2880. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Gardner J, Sargent JD. The effectiveness of housing policies in reducing children’s lead exposure. Am J Public Health. 2001;91:621–624. doi: 10.2105/ajph.91.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Blood lead levels in children aged 1–5 years—United States, 1999–2010. MMWR. 2013;62(April):245–248. [PMC free article] [PubMed] [Google Scholar]
- CDC. Advisory Committee on Childhood Lead Poisoning Prevention. US Department of Health and Human Services, CDC; Atlanta, GA: 2012. Low level lead exposure harms children: a renewed call for primary prevention. Available at 〈 http://www.cdc.gov/nceh/lead/acclpp/finaldocument030712.pdf〉. [Google Scholar]
- EPA. EPA Takes Final Step in Phaseout of Leaded Gasoline. United States Environmental Protection Agency press release. 1996 Jan; Available at 〈 http://www2.epa.gov/aboutepa/epa-takes-final-step-phaseout-leaded-gasoline〉.
- Jones RL, Homa DM, Meyer PA, Brody DJ, Caldwell KL, Pirkle JL, Brown MJ. Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988–2004. Pediatrics. 2009;123:e376–e385. doi: 10.1542/peds.2007-3608. [DOI] [PubMed] [Google Scholar]
- Kennedy CM, Lordo R, Sucosky MS, Boehm R, Brown MJ. Primary prevention of lead poisoning in children: evaluation of state specific lead-based paint hazard risk reduction laws in preventing lead poisoning in children. Environ Health. 2014;13:93. doi: 10.1186/1476-069X-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfmacher KS, Ayoob M, Morley R. Rochester’s lead law: evaluation of a local environmental health policy innovation. Environ Health Persp. 2012;120:309–315. doi: 10.1289/ehp.1103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Winter NL, Apetz L. A randomized trial of the effect of dust control on children’s blood lead levels. Pediatrics. 1996a;98:35–40. [PubMed] [Google Scholar]
- Lanphear BP, Weitzman M, Winter NL. Lead-contaminated house dust and urban children’s blood lead levels. Am J Public Health. 1996b;86:1416–1421. doi: 10.2105/ajph.86.10.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law Writer. Ohio laws and rules, Ohio revised code title [37] XXXVII health-safety-morals. Lead Abatement, 3742.37 Lead Hazard Control Order. 2005 Available at 〈 http://codes.ohio.gov/orc/3742.37〉 (Chapter 3742)
- Lead Poisoning Prevention and Control Regulation. 105 CMF 460.000. Executive Office of Health and Human Service; Massachusetts (Mass.gov): 2011. Available at 〈 http://www.mass.gov/eohhs/docs/dph/regs/105cmr460.pdf〉. [Google Scholar]
- Manton WI, Angle CR, Stanek KL. Acquisition and retention of lead by young children. Environ Res. 2000;82:60–80. doi: 10.1006/enrs.1999.4003. [DOI] [PubMed] [Google Scholar]
- Roberts JR, Reigart JR, Ebeling M. Time required for blood lead levels to decline in nonchelated children. J Toxicol Clin Toxicol. 2001;39:153–160. doi: 10.1081/clt-100103831. [DOI] [PubMed] [Google Scholar]
- Walter SD, Yankel AJ, von Lindern IH. Age-specific risk factors for lead absorption in children. Arch Environ Health. 1980;1:53–58. doi: 10.1080/00039896.1980.10667462. [DOI] [PubMed] [Google Scholar]
- Weitzman M, Aschengrau A, Bellinger D. Lead-contaminated soil abatement and urban children’s blood lead levels. JAMA. 1993;269:1647–1654. [PubMed] [Google Scholar]
- Yeoh B, Woolfenden S, Ridley GF. Household interventions for preventing domestic lead exposure in children. Cochrane Database Syst Rev. 2014;15:CD006047. doi: 10.1002/14651858.CD006047.pub4. [DOI] [PubMed] [Google Scholar]