Abstract
Sodium is essential for cellular homeostasis and physiological function. Excess dietary sodium has been linked to elevations in blood pressure (BP). Salt-sensitivity of BP varies widely, but certain subgroups tend to be more salt-sensitive. The mechanisms underlying sodium-induced increases in BP are not completely understood, but may involve alterations in renal function, fluid volume, fluid regulatory hormones, the vasculature, cardiac function, and the autonomic nervous system. Recent pre-clinical and clinical data support that even in the absence of an increase in BP, excess dietary sodium can adversely affect target organs, including the blood vessels, heart, kidneys, and brain. In this review, we address these issues and the epidemiological literature relating dietary sodium to BP and cardiovascular health outcomes, addressing recent controversies. We also provide information and strategies for reducing dietary sodium.
Keywords: diet, sodium-restricted, hypertension, kidney, sodium chloride, dietary
INTRODUCTION
Sodium is essential for fluid balance and cellular homeostasis. Claude Bernard was the first to highlight the “milieu intérieur.” Walter Cannon more explicitly defined homeostasis when he referred to the "fluid matrix" of the body and emphasized the role of sodium (1). In the last several decades, there has been a tremendous amount of work exploring dietary sodium and health. The amount of sodium needed to maintain homeostasis in adults is exceedingly low (<500 mg) compared to the average intake of most Americans (>3,200 mg)(2). We review the effects of dietary sodium on blood pressure (BP) and outcomes, emphasizing that excess sodium has direct adverse effects on target organs, beyond the increased risk of hypertension (HTN). We also review strategies for reducing sodium.
PATHOPHYSIOLOGY: SALT SENSITIVITY OF BP
BP responses to alterations in dietary sodium vary widely, leading to the concept of salt-sensitive (SS) BP (3,4). There are no standardized guidelines or firm BP cutoffs for classifying individuals as having SS BP. If BP increases during a period of high dietary sodium or declines during a period of low sodium, the individual is SS. If there is no change in BP with sodium restriction, an individual is salt-resistant (SR). Limited evidence supports the reproducibility of these responses (5,6). While individuals are commonly dichotomized as SS or SR (3), BP responses to sodium manipulation follow a Gaussian distribution (7,8). Table 1 lists groups that tend to be SR or SS (4,9-14). Salt-sensitivity in normotensive adults predicts future HTN (14,15) and SS BP has been associated with increased mortality (16). While there is interest in studying the pathophysiology of SS BP, there is less interest in its routine clinical assessment (17).
Table 1.
Salt Sensitivity in Various Groups*
| Salt-Resistant | Salt-Sensitive |
|---|---|
| Young | Aged |
| Middle-aged | Hypertensive |
| Normotensive | African-American |
| Caucasian | Chronic kidney disease |
| History of pre-eclampsia | |
| Low birth weight |
PATHOPHYSIOLOGY: MECHANISMS OF SODIUM-INDUCED INCREASES IN BP
The physiological mechanisms underlying SS BP are not fully elucidated, but involve alterations in renal function, fluid hormones, the vasculature, the heart, and/or alterations in central sympathetic outflow (Central Illustration). There are also genetic mechanisms related to the SS phenotype (18-20).
Central Illustration. Dietary Salt and Health: Mechanisms Mediating Dietary Salt-Induced Alterations in BP.
High dietary sodium can potentially exert its influence through various mechanisms to cause an increase in BP through alterations in cardiac output and total peripheral resistance. The change (Δ) in BP varies considerably, even within a given population (as depicted in the distribution). AT1 = angiotensin II receptor, type1; BP = blood pressure.
Guyton’s studies demonstrated that sodium loading caused extracellular volume expansion and volume-loaded HTN in the context of induced renal dysfunction in dogs (21), consistent with clinical studies in patients with chronic kidney disease (10). The DOCA-salt model of experimental HTN in rats requires removal of 1 kidney, further supporting the kidney’s role in expression of salt-sensitivity (22).
Impaired hormonal (renin-angiotensin-aldosterone) responsiveness during a sodium manipulation is linked to a SS BP response. Indeed, African Americans have a blunted plasma renin response to a sodium manipulation (23). The molecular signaling pathways involved in sodium-induced increases in BP are not known, but likely involve angiotensin II, type 1 (AT1) receptors (24), found in the renal and nonrenal vasculature, and the central nervous system, which are important for BP and/or fluid regulation. Mice lacking renal AT1 receptors become SS (24).
Smooth muscle in the peripheral vasculature has also been implicated in SS BP responses. Studies suggest that elevated dietary sodium expands the extracellular volume and increases cardiac output, which will increase BP if there is no compensatory decline in peripheral resistance (25). Thus, an unchanged or increased peripheral resistance, coupled with a sodium-induced increase in cardiac output, results in a SS BP response, as observed in African Americans (25).
The autonomic nervous system may play an important role in SS BP. Rodent studies demonstrate modest plasma sodium elevations from high dietary sodium may signal to the brain, causing elevated sympathetic outflow (26). Other studies demonstrate a central interaction between sodium and angiotensin II, which increases sympathetic outflow, which targets splanchnic (27) and renal (28) circulations, and may be an important mechanism in SS BP. Studies in humans support a link between plasma sodium and BP (29,30) and osmolality and sympathetic outflow (31), but these findings are not consistent (32). Extremely low sodium diets (~230 mg) over a short time period (6 days) are associated with increased sympathetic outflow in humans (33).
The standard laboratory rat is SR, but SS strains have been bred (34). For example, Dahl fed Sprague-Dawley rats high-sodium chow and grouped them according to BP response, developing the Dahl SS and SR lineages (35). While SS BP is heritable, outside of monogenic renal tubular disorders causing sodium retention and HTN, the genetic underpinnings of “routine” salt sensitivity in humans are unknown (19). There has been recent progress in understanding genetic mechanisms, such as the GenSalt studies in China that identified AT1 gene variants predictive of salt-sensitivity (18). Genome-wide association studies and overall BP studies have had limited success—genetic loci associated with a small effect on BP have been identified, (36), which is likely to be the case with SS BP responses.
PATHOPHYSIOLOGY: SODIUM AND TARGET ORGAN EFFECTS
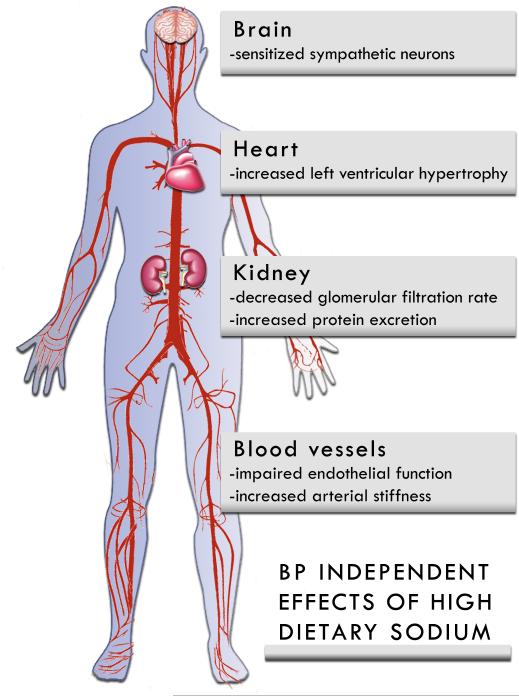
There is evidence that in the absence of increased BP, elevated dietary sodium can adversely affect multiple target organs and tissues (19), including the vasculature, heart, kidneys, and areas of the brain that control autonomic outflow (Figure 1).
Figure 1. BP Independent Effects of High Dietary Sodium.
High dietary sodium can cause target organ damage and may have direct effects on the brain, heart, kidneys, and vasculature. These effects can be independent of changes in BP.
ARTERIES
Rodent studies demonstrated impaired endothelial function during sodium loading, without alterations in BP (37-40). Sodium loading in normotensive men reduced endothelial function (41), and sodium restriction in adults with elevated BP improved endothelial function (42). Additionally, high sodium impairs endothelial function in normotensive SR humans, providing support for a BP-independent effect of sodium on the endothelium (43,44). Sodium’s deleterious effects on endothelial function likely results from reactive oxygen species (38,44), such as superoxide (39,40), resulting in reduced nitric oxide bioavailability. Cell culture studies support that high sodium exposure stiffens endothelial cells and damages the glycocalyx (45).
Animal studies show that elevated dietary sodium can increase arterial stiffness independent of BP (46). In human studies, increased arterial stiffness was observed in groups consuming a higher sodium intake, independent of BP (47,48). This increased stiffness is likely related to the profibrotic effects of transforming growth factor-β (49). Thus, high sodium stiffens the arteries, and reducing dietary sodium lowers arterial stiffness in hypertensive patients (50,51).
HEART AND KIDNEYS
Increased BP is a major risk factor for left ventricular (LV) hypertrophy; high dietary sodium may increase LV wall thickness (52) and mass (53), independent of HTN status. For example, among a cohort of healthy adults with minimal HTN, those with the highest sodium excretion had greater LV mass (53). High aldosterone levels may be important in mediating the effect of dietary salt on LV mass (54). Also, a 12-month sodium restriction intervention in hypertensive patients has been shown to reduce LV hypertrophy (55).
There are a limited number of studies of subjects without kidney disease, but evidence suggests that high sodium is associated with reduced renal function (56). Sodium loading in spontaneously hypertensive rats increased renal vascular resistance, glomerular pressure, serum creatinine, and proteinuria; sodium loading also caused a decline in single-nephron plasma flow. This decline in renal function was observed with only a minimal additional increase in BP (57). Sodium restriction has been shown to reduce protein excretion and BP in black hypertensive patients (58). Similarly, in the LowSalt CKD study (59), low salt reduced proteinuria, albuminuria, and BP.
BRAIN
Sodium may affect brainstem nuclei that control BP (34). Chronically elevated dietary sodium may "sensitize" sympathetic neurons in the rostral ventral lateral medulla of rodents (60-62), causing a greater sympathetic response to a variety of stimuli (63), including skeletal muscle contraction (64). This increased responsiveness has been associated with increased BP variability, even without an elevation in average BP (65); this is relevant due to the association of BP variability with target organ damage (66). Even in the absence of increased BP, chronically increased sympathetic outflow may have deleterious target organ effects.
EPIDEMIOLOGY: DIETARY SODIUM, BP, AND CARDIOVASCULAR OUTCOMES
Studying the effect of salt restriction on clinical outcomes raises significant challenges, including: 1) assessment of sodium intake (best evaluated by multiple measurements of 24-h sodium urine excretion); 2) long-term maintenance on a defined salt intake regimen; and 3) the necessity for large numbers of patients and long-term follow-up to obtain enough outcomes for analysis. Randomized controlled clinical trials (RCTs) between groups with different amounts of sodium in the diet would reduce bias, but have largely been limited to short-term evaluation of the effect of salt restriction on BP, as larger studies with the longer time frames required to evaluate the effects of sodium on cardiovascular (CV) events have not been feasible.
SALT INTAKE AND BP
Multiple meta-analyses and systematic reviews of RCTs have shown a strong positive association between sodium intake and systolic BP (67-73) and a significant reduction in systolic BP with sodium restriction (74,75). A recent meta-analysis of 103 randomized interventions confirmed these results, showing a linear association between salt restriction and systolic BP. The reduction was larger with older age, among blacks, and among hypertensive patients (76,77). The incidence of HTN also decreased following a sodium intake reduction intervention in the Trials of HTN Prevention II RCT (78).
SALT INTAKE AND CV OUTCOMES
Few randomized trials have sufficient power and long enough follow-up to examine the effects of sodium restriction on CV outcomes (79). In a meta-analysis of 7 randomized sodium-reduction trials with follow-ups of at least 6 months, Taylor et al. did not find any effect of sodium restriction on all-cause mortality, CV mortality, or CV morbidity (80). However, He and colleagues replicated this analysis after excluding 1 trial with methodology issues related to the patient population (patients with heart failure) and showed that a modest reduction in salt intake resulted in a significant 20% decrease in CV and stroke events 73 (80,81).
Most of the studies examining the association between salt intake and CV events are observational cohort studies and, as described in an American Heart Association report (AHA)(82), are subject to multiple methodological issues. As listed in Table 2, errors with the greatest potential to alter the association in either direction are: 1) systematic errors in sodium assessment, most frequently related to measurements of sodium intake through food frequency questionnaires, 24-h recalls, spot or overnight urine collections, or 24-h urine collection without evidence of quality control measures; and 2) reverse causality related to recruiting sick patients who may consume less sodium as part of a therapeutic strategy or reduced overall food consumption, or to not excluding sick participants from general population studies (82). Cobb et al found evidence of systematic error in sodium assessment in 77% of studies that showed a direct association between salt intake and CV events, in 75% of those with an inverse association, in 100% (only 2 studies) of those with a J-shaped association, and in 100% of those that showed a null association. Reverse causality was found in 31% of those that showed a direct association, in 38% of those with an inverse association, in 50% of those with a J-shaped association, and in none of those with a null association (82). Considering these results, and that 3 to 4 methodological issues were identified in each study (82), systematic reviews and meta-analyses of observational studies should be evaluated with circumspection. Since AHA methodology report was published, O’Donnell et al, in an analysis of the Prospective Urban Rural Epidemiology (PURE) data, a prospective observational study of 101,945 adults, described a J-relationship between sodium excretion and CV events, with increased CV events at <3g/day and ≥7g/day (83). This was maintained, even after restricting the population to a low-risk cohort and excluding events occurring within 2 years to limit reverse causality. However, each patient’s sodium excretion was estimated from a single fasting morning urine specimen, overestimating the 24-h urinary excretion and likely introducing a systematic error in sodium assessment according to the AHA Science Advisory’s classification (82). There may also have been reverse causality. A subsequent analysis of the observational follow-up of the Trials of Hypertension Prevention, which, according to the AHA methodology report, has a low potential for reverse causality and for systematic error in sodium assessment, showed a 17% increase in CV events for every 1,000 mg/d increase in sodium (p = 0.054) and no evidence for a J-relationship (84).
Table 2.
Methodological Issues With Cohort Studies That Relate Sodium Intake to Cardiovascular Disease*
| Errors with the greatest potential to alter the direction of association in either direction |
| • Systematic error in sodium assessment |
| • Reverse causality |
| Errors with some potential to alter the direction of association in either direction |
| • Residual confounding |
| • Inadequate follow-up |
| Errors with potential to lead to a false null result |
| • Random error in sodium assessment |
| • Insufficient power |
Reference 82.
In conclusion, a large body of evidence confirms the biological plausibility of the association between high sodium intake and increases in BP and CV events, whereas the evidence for an association between low salt intake and adverse events is unclear and largely conjectural. The association of low sodium intake with mortality has been speculated to result from elevated renin-aldosterone activity, sympathetic activation, and lipid abnormalities (85). However, meta-analyses suggest no significant effect of low sodium on lipids and no effect on catecholamines (74). Also, while dramatic short-term reductions in sodium may increase renin-angiotensin-aldosterone activity (86), modest-to-moderate longer-term reductions, as suggested by the AHA or IOM (87,88), may only produce minimal increases (69). Finally, because of the multiple methodological issues associated with cohort studies and the difficulty of organizing a trial to assess the association between sodium intake and CV events, the AHA recommends sodium on the basis of the large body of evidence linking sodium intake to BP (82).
SODIUM IN THE DIET: LIMITING SODIUM IN THE DIET
Sodium is ubiquitous in the diet of most developed countries. While some sodium intake is necessary, recommendations vary for adequate intake and tolerable upper intake levels (79,89,90). Physiological requirements for sodium are <500 mg/day in most healthy individuals, but the average consumption in the United States is over 3,200 mg/day (2,88,91,92). NHANES data reveal that sodium consumption increased between the early 1970s and the early 1990s (2,91). NHANES data from 2003 to 2008, using 24-h dietary recall, show that 99.4% and 97% of U.S. adults consumed more sodium than recommended by the AHA and the 2010 U.S. Department of Health and Human Services Dietary Guideline for Americans, respectively (79,90). While there is general consensus that current sodium consumption levels are excessive and contribute to CV risk (79,88), the U.S. Food and Drug Administration considers sodium added in food preparation to be “generally regarded as safe,” and there are no standards for its safe use in food (88).
Most people like some level of salt in food. Conceptually, there is a “bliss point” where the effect of sodium on flavor is optimum (93). However, this “bliss point” is malleable, and most people will adapt (94) to a reduction in dietary sodium (88). Sudden sodium changes are harder to accept, but if the United States gradually moves to a diet with less sodium, many people will likely make the transition with little difficulty (94-96).
Approximately 70% of sodium in the diet is in processed foods (97-99) and used to prepare foods as ubiquitous as bread (100). Sodium added in food preparation and at the table contributes less (88,98). Restaurants are also more likely to have saltier foods and more people are eating out in recent decades.
Market forces are a factor in the large amount of sodium in the diet, so without a societal approach, pressure on individual stakeholders is likely to be resisted. Food processors have marketed low-sodium alternatives without much success (101). A partnership of food processors and restaurant associations with groups like the AHA is more likely to successfully change diets (79). Efforts in Finland and the United Kingdom have successfully reduced sodium (102-104).
A number of approaches can decrease the dietary sodium: 1) decrease the sodium content of foods; 2) a switch by the consumer from high-sodium to low-sodium foods by avoiding processed foods and reading labels; 3) switch to substitute salts (105-107); 4) reduce sodium while increasing other flavors (96,100); and 5) use engineering approaches to provide salty taste with less sodium or food processing with less sodium (88,108). Importantly, flavor must be maintained because taste is the driving force behind salty foods (88,109). A decrease in sodium from current levels will represent a major change in our food supply and may be most successful as a series of small steps over several years. Coordination between food processors, restaurants, and advocacy groups is crucial and currently lacking (88). Importantly, increased dietary potassium intake may decrease salt sensitivity and favorably effect BP (110).
Altered dietary sodium intake targets should be considered for individuals engaging in high physical activity or exposed to heat stress (111). Although there may be some sweat loss accommodation in response to sodium restriction (112), sodium recommendations should be assessed to ensure that sodium intake matches sweat loss. While there is general consensus that the current sodium intake in the United States (3,200 mg per day) should be lowered, the Institute of Medicine cautioned against sodium diets <2,300 mg per day for selected groups (113).
CONCLUSIONS
BP correlates with sodium intake, with multiple mechanisms underlying this relation. Preclinical and clinical studies demonstrate that sodium adversely affects multiple target organs independent of BP. Clinical trials have shown decreased BP with decreased sodium intake, but the studies relating sodium consumption to CV events have significant limitations related to difficulty in assessment of sodium intake and confounding (79,82,114). Lack of power has been a barrier to demonstrating an effect of reduced sodium on hard outcomes in normotensive people. The difficulties of adhering to a sodium restriction diet over years may be an insurmountable hurdle for an RCT with enough power to detect a difference in CV events that could be generalizable to the entire population. Because of the weight of evidence in favor of salt reduction and the difficulties in organizing a clinical trial, the AHA recommends a population-wide reduction in sodium intake (87). Reducing sodium will take a coordinated effort involving organizations like the AHA, food producers and processors, restaurants, and public policy aimed at education.
Acknowledgements
NIH grants R01 HL104106, 5P20RR016472, U54-GM104941, and 8P20 GM103446 supported the authors’ work. All authors contributed to the writing of this review article.
ABBREVIATIONS
- AHA
American Heart Association
- BP
blood pressure
- CV
cardiovascular
- HTN
hypertension
- LV
left ventricle
- RCTs
randomized controlled clinical trials
- SR
salt-resistant
- SS
salt-sensitive
Footnotes
Disclosures: The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9:399–431. [Google Scholar]
- 2.Bernstein AM, Willett WC. Trends in 24-h urinary sodium excretion in the United States, 1957-2003: a systematic review. Am J Clin Nutr. 2010;92:1172–80. doi: 10.3945/ajcn.2010.29367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawasaki T, Delea CS, Bartter FC, et al. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med. 1978;64:193–8. doi: 10.1016/0002-9343(78)90045-1. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger MH, Miller JZ, Luft FC, et al. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127–34. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 5.Weinberger MH. Is salt-sensitivity of blood pressure a reproducible phenomenon-commentary. J Hypertens. 1996;14:1461–2. doi: 10.1097/00004872-199612000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Gu D, Zhao Q, Chen J, et al. Reproducibility of blood pressure responses to dietary sodium and potassium interventions: the GenSalt study. Hypertension. 2013;62:499–505. doi: 10.1161/HYPERTENSIONAHA.113.01034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, Gu D, Chen J, et al. GenSalt Collaborative Research Group. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens. 2009;27:48–54. doi: 10.1097/hjh.0b013e328316bb87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Leeuw PW, Kroon AA. Salt and sensitivity. Hypertension. 2013;62:461–2. doi: 10.1161/HYPERTENSIONAHA.113.01831. [DOI] [PubMed] [Google Scholar]
- 9.de Boer MP, Ijzerman RG, de Jongh RT, et al. Birth weight relates to salt sensitivity of blood pressure in healthy adults. Hypertension. 2008;51:928–32. doi: 10.1161/HYPERTENSIONAHA.107.101881. [DOI] [PubMed] [Google Scholar]
- 10.Koomans HA, Roos JC, Boer P, et al. Salt sensitivity of blood pressure in chronic renal failure. Evidence for renal control of body fluid distribution in man. Hypertension. 1982;4:190–7. doi: 10.1161/01.hyp.4.2.190. [DOI] [PubMed] [Google Scholar]
- 11.Martillotti G, Ditisheim A, Burnier M, et al. Increased salt sensitivity of ambulatory blood pressure in women with a history of severe preeclampsia. Hypertension. 2013;62:802–8. doi: 10.1161/HYPERTENSIONAHA.113.01916. [DOI] [PubMed] [Google Scholar]
- 12.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–90. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 13.Weinberger MH. Is salt sensitivity of blood pressure linked to the cardiometabolic syndrome? J Cardiometab Syndr 2006 Summer. 1:217–9. doi: 10.1111/j.1559-4564.2006.05613.x. [DOI] [PubMed] [Google Scholar]
- 14.Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension. 1991;18:67–71. doi: 10.1161/01.hyp.18.1.67. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan JM. Salt sensitivity. Definition, conception, methodology, and long-term issues. Hypertension. 1991;17:I61–8. doi: 10.1161/01.hyp.17.1_suppl.i61. [DOI] [PubMed] [Google Scholar]
- 16.Weinberger MH. Salt sensitivity is associated with an increased mortality in both normal and hypertensive humans. J Clin Hypertens (Greenwich) 2002;4:274–6. doi: 10.1111/j.1524-6175.2002.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu D, Kelly TN, Hixson JE, et al. Genetic variants in the renin-angiotensin-aldosterone system and salt sensitivity of blood pressure. J Hypertens. 2010;28:1210–20. [PMC free article] [PubMed] [Google Scholar]
- 19.Kotchen TA, Cowley AW, Jr., Frohlich ED. Salt in health and disease--a delicate balance. New Engl J Med. 2013;368:1229–37. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- 20.Meneton P, Jeunemaitre X, de Wardener HE, et al. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 21.Langston JB, Guytom AC, Douglas BH, et al. Effect of Changes in Salt Intake on Arterial Pressure and Renal Function in Partially Nephrectomized Dogs. Circ Res. 1963;12:508–13. [Google Scholar]
- 22.Jacob F, Clark LA, Guzman PA, et al. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol. 2005;289:H1519–29. doi: 10.1152/ajpheart.00206.2005. [DOI] [PubMed] [Google Scholar]
- 23.He FJ, Markandu ND, Sagnella GA, et al. Importance of the renin system in determining blood pressure fall with salt restriction in black and white hypertensives. Hypertension. 1998;32:820–4. doi: 10.1161/01.hyp.32.5.820. [DOI] [PubMed] [Google Scholar]
- 24.Crowley SD, Gurley SB, Oliverio MI, et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–9. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidlin O, Sebastian AF, Morris RC., Jr. What initiates the pressor effect of salt in salt-sensitive humans? Observations in normotensive blacks. Hypertension. 2007;49:1032–9. doi: 10.1161/HYPERTENSIONAHA.106.084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks VL, Haywood JR, Johnson AK. Translation of salt retention to central activation of the sympathetic nervous system in hypertension. Clin Exp Pharmacol Physiol. 2005;32:426–32. doi: 10.1111/j.1440-1681.2005.04206.x. [DOI] [PubMed] [Google Scholar]
- 27.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–56. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- 28.Guild SJ, McBryde FD, Malpas SC, et al. High dietary salt and angiotensin II chronically increase renal sympathetic nerve activity: a direct telemetric study. Hypertension. 2012;59:614–20. doi: 10.1161/HYPERTENSIONAHA.111.180885. [DOI] [PubMed] [Google Scholar]
- 29.He FJ, Markandu ND, Sagnella GA, et al. Plasma sodium: ignored and underestimated. Hypertension. 2005;45:98–102. doi: 10.1161/01.HYP.0000149431.79450.a2. [DOI] [PubMed] [Google Scholar]
- 30.Schmidlin O, Forman A, Sebastian A, et al. Sodium-selective salt sensitivity: its occurrence in blacks. Hypertension. 2007;50:1085–92. doi: 10.1161/HYPERTENSIONAHA.107.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farquhar WB, Wenner MM, Delaney EP, et al. Sympathetic neural responses to increased osmolality in humans. Am J Physiol Heart Circ Physiol. 2006;291:H2181–6. doi: 10.1152/ajpheart.00191.2006. [DOI] [PubMed] [Google Scholar]
- 32.Brown MD, Hogikyan RV, Dengel DR, et al. Sodium-sensitive hypertension is not associated with higher sympathetic nervous system activity in older hypertensive humans. Am J Hypertens. 2000;13:873–83. doi: 10.1016/s0895-7061(00)00256-9. [DOI] [PubMed] [Google Scholar]
- 33.Anderson EA, Sinkey CA, Lawton WJ, et al. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–83. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 34.Stocker SD, Monahan KD, Browning KN. Neurogenic and sympathoexcitatory actions of NaCl in hypertension. Curr Hypertens Rep. 2013;15:538–46. doi: 10.1007/s11906-013-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahl LK, Heine M, Tassinari L. Effects of chronic excess salt ingestion. Evidence that genetic factors play an important role in susceptibility to experimental hypertension. J Exp Med. 1962;115:1173–90. doi: 10.1084/jem.115.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrap SB. Blood pressure genetics: time to focus. J Am Soc Hypertens. 2009;3:231–7. doi: 10.1016/j.jash.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Lenda DM, Boegehold MA. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol. 2002;282:H395–402. doi: 10.1152/ajpheart.0354.2001. [DOI] [PubMed] [Google Scholar]
- 38.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol. 2000;279:H7–H14. doi: 10.1152/ajpheart.2000.279.1.H7. [DOI] [PubMed] [Google Scholar]
- 39.Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1550–6. doi: 10.1152/ajpregu.00703.2006. [DOI] [PubMed] [Google Scholar]
- 40.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res. 2007;44:382–90. doi: 10.1159/000102955. [DOI] [PubMed] [Google Scholar]
- 41.Tzemos N, Lim PO, Wong S, Struthers AD, et al. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension. 2008;51:1525–30. doi: 10.1161/HYPERTENSIONAHA.108.109868. [DOI] [PubMed] [Google Scholar]
- 42.Jablonski KL, Racine ML, Geolfos CJ, et al. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61:335–43. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DuPont JJ, Greaney JL, Wenner MM, et al. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens. 2013;31:530–6. doi: 10.1097/HJH.0b013e32835c6ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greaney JL, DuPont JJ, Lennon-Edwards SL, et al. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol. 2012;590:5519–28. doi: 10.1113/jphysiol.2012.236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oberleithner H, Peters W, Kusche-Vihrog K, et al. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Archiv. 2011;462:519–28. doi: 10.1007/s00424-011-0999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safar ME, Thuilliez C, Richard V, et al. Pressure-independent contribution of sodium to large artery structure and function in hypertension. Cardiovasc Res. 2000;46:269–76. doi: 10.1016/s0008-6363(99)00426-5. [DOI] [PubMed] [Google Scholar]
- 47.Avolio AP, Clyde KM, Beard TC, et al. Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis. 1986;6:166–9. doi: 10.1161/01.atv.6.2.166. [DOI] [PubMed] [Google Scholar]
- 48.Avolio AP, Deng FQ, Li WQ, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71:202–10. doi: 10.1161/01.cir.71.2.202. [DOI] [PubMed] [Google Scholar]
- 49.Sanders PW. Vascular consequences of dietary salt intake. Am J Physiol Renal Physiol. 2009;297:F237–43. doi: 10.1152/ajprenal.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gates PE, Tanaka H, Hiatt WR, et al. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35–41. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]
- 51.Todd AS, Macginley RJ, Schollum JB, et al. Dietary salt loading impairs arterial vascular reactivity. Am J Clin Nutr. 2010;91:557–64. doi: 10.3945/ajcn.2009.28645. [DOI] [PubMed] [Google Scholar]
- 52.Jin Y, Kuznetsova T, Maillard M, et al. Independent relations of left ventricular structure with the 24-hour urinary excretion of sodium and aldosterone. Hypertension. 2009;54:489–95. doi: 10.1161/HYPERTENSIONAHA.109.130492. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez CJ, Bibbins-Domingo K, Jin Z, et al. Association of sodium and potassium intake with left ventricular mass: coronary artery risk development in young adults. Hypertension. 2011;58:410–6. doi: 10.1161/HYPERTENSIONAHA.110.168054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.du Cailar G, Fesler P, Ribstein J, et al. Dietary sodium, aldosterone, and left ventricular mass changes during long-term inhibition of the renin-angiotensin system. Hypertension. 2010;56:865–70. doi: 10.1161/HYPERTENSIONAHA.110.159277. [DOI] [PubMed] [Google Scholar]
- 55.Jula AM, Karanko HM. Effects on left ventricular hypertrophy of long-term nonpharmacological treatment with sodium restriction in mild-to-moderate essential hypertension. Circulation. 1994;89:1023–31. doi: 10.1161/01.cir.89.3.1023. [DOI] [PubMed] [Google Scholar]
- 56.Smyth A, O'Donnell MJ, Yusuf S, et al. Sodium intake and renal outcomes: a systematic review. Am J Hypertens. 2014;27:1277–84. doi: 10.1093/ajh/hpt294. [DOI] [PubMed] [Google Scholar]
- 57.Matavelli LC, Zhou X, Varagic J, et al. Salt loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H814–9. doi: 10.1152/ajpheart.00671.2006. [DOI] [PubMed] [Google Scholar]
- 58.Swift PA, Markandu ND, Sagnella GA, et al. Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: a randomized control trial. Hypertension. 2005;46:308–12. doi: 10.1161/01.HYP.0000172662.12480.7f. [DOI] [PubMed] [Google Scholar]
- 59.McMahon EJ, Bauer JD, Hawley CM, et al. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol. 2013;24:2096–103. doi: 10.1681/ASN.2013030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adams JM, Bardgett ME, Stocker SD. Ventral lamina terminalis mediates enhanced cardiovascular responses of rostral ventrolateral medulla neurons during increased dietary salt. Hypertension. 2009;54:308–14. doi: 10.1161/HYPERTENSIONAHA.108.127803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ito S, Gordon FJ. Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol. 1999;276:R1600–7. doi: 10.1152/ajpregu.1999.276.6.R1600. [DOI] [PubMed] [Google Scholar]
- 62.Pawloski-Dahm CM. Gordon FJ. Increased dietary salt sensitizes vasomotor neurons of the rostral ventrolateral medulla. Hypertension. 1993;22:929–33. doi: 10.1161/01.hyp.22.6.929. [DOI] [PubMed] [Google Scholar]
- 63.Stocker SD, Madden CJ. Sved AF. Excess dietary salt intake alters the excitability of central sympathetic networks. Physiol Behav. 2010;100:519–24. doi: 10.1016/j.physbeh.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamauchi K, Tsuchimochi H, Stone AJ, et al. Increased dietary salt intake enhances the exercise pressor reflex. Am J Physiol Heart Circ Physiol. 2014;306:H450–4. doi: 10.1152/ajpheart.00813.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simmonds SS, Lay J. Stocker SD. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension. 2014;64:583–9. doi: 10.1161/HYPERTENSIONAHA.114.03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parati G, Ochoa JE, Lombardi C, et al. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–55. doi: 10.1038/nrcardio.2013.1. [DOI] [PubMed] [Google Scholar]
- 67.Dickinson HO, Mason JM, Nicolson DJ, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24:215–33. doi: 10.1097/01.hjh.0000199800.72563.26. [DOI] [PubMed] [Google Scholar]
- 68.He FJ. MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2004:CD004937. doi: 10.1002/14651858.CD004937. [DOI] [PubMed] [Google Scholar]
- 69.He FJ, Marciniak M, Visagie E, et al. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension. 2009;54:482–8. doi: 10.1161/HYPERTENSIONAHA.109.133223. [DOI] [PubMed] [Google Scholar]
- 70.Hooper L, Griffiths E, Abrahams B, et al. UK Heart Health and Thoracic Dietitians Specialist Group of the British Dietetic Association. Dietetic guidelines: diet in secondary prevention of cardiovascular disease (first update, June 2003) J Hum Nutr Diet. 2004;17:337–49. doi: 10.1111/j.1365-277X.2004.00533.x. [DOI] [PubMed] [Google Scholar]
- 71.Jurgens G, Graudal NA. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride. Cochrane Database Syst Rev. 2004:CD004022. doi: 10.1002/14651858.CD004022.pub2. [DOI] [PubMed] [Google Scholar]
- 72.Matyas E, Jeitler K, Horvath K, et al. Benefit assessment of salt reduction in patients with hypertension: systematic overview. J Hypertens. 2011;29:821–8. doi: 10.1097/HJH.0b013e3283442840. [DOI] [PubMed] [Google Scholar]
- 73.Meland E, Aamland A. Salt restriction among hypertensive patients: modest blood pressure effect and no adverse effects. Scand J Prim Health Care. 2009;27:97–103. doi: 10.1080/02813430802661795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aburto NJ, Ziolkovska A, Hooper L, et al. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346:f1326. doi: 10.1136/bmj.f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- 76.Mente A, O'Donnell MJ, Rangarajan S, et al. PURE Investigators. Association of urinary sodium and potassium excretion with blood pressure. New Engl J Med. 2014;371:601–11. doi: 10.1056/NEJMoa1311989. [DOI] [PubMed] [Google Scholar]
- 77.Mozaffarian D, Fahimi S, Singh GM, et al. Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Global sodium consumption and death from cardiovascular causes. New Engl J Med. 2014;371:624–34. doi: 10.1056/NEJMoa1304127. [DOI] [PubMed] [Google Scholar]
- 78.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157:657–67. [PubMed] [Google Scholar]
- 79.Whelton PK, Appel LJ, Sacco RL, et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126:2880–9. doi: 10.1161/CIR.0b013e318279acbf. [DOI] [PubMed] [Google Scholar]
- 80.Taylor RS, Ashton KE, Moxham T, et al. Reduced dietary salt for the prevention of cardiovascular disease: a meta-analysis of randomized controlled trials (Cochrane review) Am J Hypertens. 2011;24:843–53. doi: 10.1038/ajh.2011.115. [DOI] [PubMed] [Google Scholar]
- 81.He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet. 2011;378:380–2. doi: 10.1016/S0140-6736(11)61174-4. [DOI] [PubMed] [Google Scholar]
- 82.Cobb LK, Anderson CA, Elliott P, et al. American Heart Association Council on Lifestyle and Metabolic Health. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129:1173–86. doi: 10.1161/CIR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 83.O'Donnell M, Mente A, Rangarajan S, et al. PURE Investigators. Urinary sodium and potassium excretion, mortality, and cardiovascular events. New Engl J Med. 2014;371:612–23. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 84.Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129:981–9. doi: 10.1161/CIRCULATIONAHA.113.006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Graudal N, Jurgens G, Baslund B, et al. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens. 2014;27:1129–37. doi: 10.1093/ajh/hpu028. [DOI] [PubMed] [Google Scholar]
- 86.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review). Am J Hypertens. 2012;25:1–15. doi: 10.1038/ajh.2011.210. [DOI] [PubMed] [Google Scholar]
- 87.Appel LJ, Frohlich ED, Hall JE, et al. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation. 2011;123:1138–43. doi: 10.1161/CIR.0b013e31820d0793. [DOI] [PubMed] [Google Scholar]
- 88.Henney JE, Taylor CL, Boon CS, editors. Strategies to Reduce Sodium Intake in the United States. National Academies Press; Washington, D.C.: 2010. Institute of Medicine (US) Committee on Strategies to Reduce Sodium Intake. [PubMed] [Google Scholar]
- 89.Panel on Dietary Reference Intakes for Electrolytes and Water; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Food and Nutrition Board; Institute of Medicine of the National Academies . Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. The National Academies Press; Washington, D.C.: 2005. [Google Scholar]
- 90.U.S. Department of Agriculture, U.S. Department of Health and Human Services . Dietary Guidelines for Americans, 2010. 7th Government Printing Office; Washington, D.C.: 2010. [Google Scholar]
- 91.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–31. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- 92.Cogswell ME, Zhang Z, Carriquiry AL, et al. Sodium and potassium intakes among US adults: NHANES 2003-2008. Am J Clin Nutr. 2012;96:647–57. doi: 10.3945/ajcn.112.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McBride RL. The bliss point as a measure of pleasure. In: Warburton DM, editor. Pleasure, the Politics and the Reality. John Wiley & Sons; New York, New York: 1994. pp. 5–14. [Google Scholar]
- 94.Bertino M, Beauchamp GK, Engelman K. Long-term reduction in dietary sodium alters the taste of salt. Am J Clin Nutr. 1982;36:1134–44. doi: 10.1093/ajcn/36.6.1134. [DOI] [PubMed] [Google Scholar]
- 95.Girgis S, Neal B, Prescott J, et al. A one-quarter reduction in the salt content of bread can be made without detection. Eur J Clin Nutr. 2003;57:616–20. doi: 10.1038/sj.ejcn.1601583. [DOI] [PubMed] [Google Scholar]
- 96.McGuire S, Institute of Medicine . Adv Nutr. Vol. 1. The National Academies Press; Washington, DC: 2010. 2010. Strategies to Reduce Sodium Intake in the United States; pp. 49–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anderson CA, Appel LJ, Okuda N, et al. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: the INTERMAP study. J Am Diet Assoc. 2010;110:736–45. doi: 10.1016/j.jada.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mattes RD, Donnelly D. Relative contributions of dietary sodium sources. J Am Coll Nutr. 1991;10:383–93. doi: 10.1080/07315724.1991.10718167. [DOI] [PubMed] [Google Scholar]
- 99.Centers for Disease Control and Prevention (CDC) Vital Signs: Food Categories Contributing the Most to Sodium Consumption — United States, 2007–2008. MMWR Morb Mortal Wkly Rep. 2012:92–8. [PubMed] [Google Scholar]
- 100.Kilcast D, Angus F, editors. Reducing Salt in Foods: Practical Strategies. Woodhead Publishing/CRC Press; Boca Raton, Florida: 2007. [Google Scholar]
- 101.Arumugam N. Campbell Soup Increases Sodium As New Studies Vindicate Salt. Forbes. 2011 Jul 18; Available at: http://www.forbes.com/sites/nadiaarumugam/2011/07/18/campbell-soup-increases-sodium-as-new-studies-vindicate-salt/. Accessed January 4, 2015.
- 102.Laatikainen T, Pietinen P, Valsta L, et al. Sodium in the Finnish diet: 20-year trends in urinary sodium excretion among the adult population. Eur J Clin Nutr. 2006;60:965–70. doi: 10.1038/sj.ejcn.1602406. [DOI] [PubMed] [Google Scholar]
- 103.Reinivuo H, Valsta LM, Laatikainen T, et al. Sodium in the Finnish diet: II trends in dietary sodium intake and comparison between intake and 24-h excretion of sodium. Eur J Clin Nutr. 2006;60:1160–7. doi: 10.1038/sj.ejcn.1602431. [DOI] [PubMed] [Google Scholar]
- 104.Sadler K, Nicholson S, Steer T, et al. National Diet and Nutrition Survey— Assessment of Dietary Sodium in Adults (Aged 19–64) in London, England, 2011. Department of Health of England. 2012 [Google Scholar]
- 105.Cauvain SP. Reducing salt in bread and other baked products. In: Kilcast DA, Angus F, editors. Reducing Salt in Foods: Practical Strategies. Woodhead Publishing/CRC Press; Boca Raton, Florida: 2007. pp. 283–295. [Google Scholar]
- 106.Desmond E. Reducing salt in meat and dairy products. In: Kilcast DA, Angus F, editors. Reducing Salt in Foods: Practical Strategies. Woodhead Publishing/CRC Press; Boca Raton, Florida: 2007. pp. 233–55. [Google Scholar]
- 107.Guinee TP, O’Kennedy BT. Reducing salt in cheese and dairy spreads. In: Kilcast DA, Angus F, editors. Reducing Salt in Foods: Practical Strategies. Woodhead Publishing/CRC Press; Boca Raton, Florida: 2007. pp. 316–357. [Google Scholar]
- 108.Case Studies. Tate & Lyle; 2013. Making Innovation Second Nature. Available at: http://www.tateandlyle.com/AboutUs/Casestudies/Pages/Makinginnovationsecondnature.aspx. Accessed January 4, 2015. [Google Scholar]
- 109.Gillette M. Flavour effects of sodium chloride. Food Tech. 1985;39:47–52. [Google Scholar]
- 110.Luft FC, Miller JZ, Grim CE, et al. Salt sensitivity and resistance of blood pressure. Age and race as factors in physiological responses. Hypertension. 1991;17:I102–8. doi: 10.1161/01.hyp.17.1_suppl.i102. [DOI] [PubMed] [Google Scholar]
- 111.American College of Sports Medicine. Sawka MN, Burke LM, et al. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007;39:377–90. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 112.Allsopp AJ, Sutherland R, Wood P, et al. The effect of sodium balance on sweat sodium secretion and plasma aldosterone concentration. Eur J Appl Physiol Occup Physiol. 1998;78:516–21. doi: 10.1007/s004210050454. [DOI] [PubMed] [Google Scholar]
- 113.Committee on the Consequences of Sodium Reduction in Populations; Food and Nutrition Board; Board on Population Health and Public Health Practice; Institute of Medicine . In: Sodium Intake in Populations: Assessment of Evidence. Strom BL, Yaktine AL, Oria M, editors. National Academies Press; Washington, D.C.: 2013. [PubMed] [Google Scholar]
- 114.Willett W. Nutritional Epidemiology. Second Oxford University Press; New York, New York: 1998. [Google Scholar]