Abstract
Sex differences in brain and behavior were investigated across the lifespan. Parameters include neurobehavioral measures linkable to neuroanatomic and neurophysiologic indicators of brain structure and function. Sexual differentiation of behavior has been related to organizational factors during sensitive periods of development, with adolescence and puberty gaining increased attention. Adolescence is a critical developmental period where transition to adulthood is impacted by multiple factors that can enhance vulnerability to brain dysfunction.
Here we highlight sex differences in neurobehavioral measures in adolescence that are linked to brain function. We summarize neuroimaging studies examining brain structure, connectivity and perfusion, underscoring the relationship to sex differences in behavioral measures and commenting on hormonal findings. We focus on relevant data from the Philadelphia Neurodevelopmental Cohort (PNC), a community-based sample of nearly 10,000 clinically and neurocognitively phenotyped youths age 8–21 of whom 1600 have received multimodal neuroimaging. These data indicate early and pervasive sexual differentiation in neurocognitive measures that is linkable to brain parameters. We conclude by describing possible clinical implications.
Keywords: Neurocognition, Sexual differentiation, Brain structure, Brain function, Neurodevelopment
Introduction
An extensive literature on brain and behavior has documented sex differences in cognitive, affective and brain imaging parameters. Such measures have been informative in evaluating aberrations in neurodevelopmental disorders where sex differences are prominent, including attention deficit, learning disabilities and autism spectrum disorder. Sexual differentiation of behavior has been related to organizational factors during sensitive periods of development, with the prenatal period most investigated across species. There is growing evidence that puberty is another organizational period with long lasting effects on brain and behavior. Adolescence presents an especially informative and dynamic period as brain maturation is accelerated, hormonal changes associated with puberty emerge and social factors increase their impact. The transition to adulthood is influenced by complex interactions where the effects of this critical period may differ for males and females with implications for healthy functioning and psychopathology.
We will begin this review by highlighting sex differences in neurobehavioral measures in adolescence that are linked to brain function. We will then summarize neuroimaging studies examining brain structure, connectivity and perfusion. We will conclude by summarizing literature on the role of hormonal measures and discuss clinical implications.
Behavior linked to brain function
The developmental course of specific behavioral domains has been well documented. Executive-control (e.g., Conklin, et al., 2007; Goldberg, Maurer, & Lewis, 2001; Pickering, 2001), language and reasoning (e.g., Friederici & Wartenburger, 2010; Kuhl, 2010) and, more recently, social cognition (e.g., Burnett, et al., 2011; Shaw et al., 2012) show improved performance from childhood to young adulthood, especially pronounced during adolescence for executive domains of attention and working memory (Ang & Lee, 2010). Neural substrates for such age-related differences are being examined extensively with structural and functional neuroimaging, initially in cross-sectional studies and more recently expanding to longitudinal investigations. Results highlight childhood and adolescence as periods during which important age-related differences are observed in parameters of neural structure and function (Casey, Duhoux & Malter, 2010; Giedd, et al., 1999; Matsuzawa et al., 2001; Shaw et al., 2008). Integrating neuroimaging with behavioral findings, Jung and Haier (2007) identified a central role for frontal and parietal regions in the neurodevelopment of cognition, and this hypothesis has received support in large-scale studies (Deary, Penke & Johnson, 2010).
Sex differences have been extensively documented in behavioral measures (e.g., Halpern, et al., 2007; Hines, 2010). Males perform better than females on spatial (Linn & Petersen, 1985; Voyer, Voyer & Bryden, 1995) and motor tasks (e.g., Moreno-Briseño, et al., 2010; Thomas & French, 1985), while females perform better than males on some verbal and memory tasks (e.g., Hedges & Nowell, 1995; Hyde & Linn, 1988; Saykin et al., 1995) as well as measures of social cognition (Erwin et al., 1992, Gur et al., 2010, 2012; Moore et al., 2015; Williams et al., 2008). Some sex differences have been related to structural neuroimaging (e.g., De Bellis et al., 2001; Goldstein et al., 2001; Gur et al., 1999; Lenroot, et al., 2007) and functional imaging measures (e.g., Gur et al., 1982; 1995; 2000; Lenroot & Giedd, 2010), including volumetric differences in executive and memory related areas, supporting neural substrates for sex differences in cognition. However, the developmental course of sex differences in brain-behavior relationship, especially in adolescence and across neurobehavioral domains, remains to be elucidated, particularly with longitudinal studies.
Shortcomings of most cognitive measures currently used limit their applicability in establishing further links between brain function and behavioral domains. Most are broadly defined and load heavily on the “g factor” (Salthouse, 2004) without separating accuracy from speed. This feature precludes rigorous testing of hypotheses on the effects of brain connectivity on performance, which is expected to differentially influence processing speed. Additionally, the paper-and pencil administration format of many tests precludes their use in large-scale neuroimaging genomic studies. More narrowly defined behavioral tasks, used in functional neuroimaging, have been adapted as computerized tests to obtain rapid and efficient quantification of individual differences (Gur, Erwin & Gur, 1992, Gur et al., 2010). The literature is especially limited in the application of an identical neurocognitive test battery across a population ranging from childhood through puberty and young adulthood.
The Philadelphia Neurodevelopmental Cohort (PNC) includes a large well-characterized community sample of youths, age 8–21 years. The PNC received a computerized neurocognitive battery (CNB; Gur et al., 2010, 2012, Roalf et al., 2014a) that is based on functional neuroimaging studies (Roalf et al., 2014b), has established validity (Moore et al., 2015) and heritability (Calkins et al., 2010; Greenwood et al., 2007; Gur et al., 2007). The age range from childhood to young adulthood enables to examine the pattern of performance, both accuracy and speed, during adolescence. The cross sectional sample was obtained between 2009–2011 and a subsample of about 500 is followed longitudinally with clinical, neurocognitive and neuroimaging measures.
Performance scores on each neurocognitive domain at baseline were standardized to the average of the entire sample (n= 9,122: 4405 males, 4,717 females). The z-scores were entered into a repeated-measures ANOVA in SAS (SAS Institute Inc., Cary, NC, operating on Linux LIN 64 platform), using PROC GLM separately on the 12 Accuracy measures and the 14 Speed measures with Age group (7 levels, 2-year spans from 8–21) and Sex as grouping factors and Test as a repeated measures (within–group) factor. The Age group, Sex, and Test effects and their interactions for each test are presented in Table 1.
Table 1.
Summary of overall ANOVA results for neurocognitive domains by test for main effects of age group (A), sex (S), Test (T) and their interactions (upper Table) and followup ANOVAs by neurocognitive domain and Test (Lower Table).
| ANOVA | ACCURACY | SPEED | |||||
|---|---|---|---|---|---|---|---|
| DF | F | P | DF | F | P | ||
| A | 6;8516 | 588.76 | <.0001 | 6;8354 | 374.80 | <.0001 | |
| S | 1;8516 | <1 | NS | 1;8354 | <1 | NS | |
| T | 11;93676 | 3.36 | 0.0001 | 13;108602 | 7.81 | <.0001 | |
| A*S | 6;8516 | 1.87 | 0.0826 | 6;8354 | 2.56 | 0.0177 | |
| A*T | 66;93676 | 34.89 | <.0001 | 78;108602 | 99.06 | <.0001 | |
| S*T | 11;93676 | 60.47 | <.0001 | 13;108602 | 67.43 | <.0001 | |
| A*S*T | 66;93676 | 1.47 | 0.0074 | 78;108602 | 2.83 | <.0001 | |
| DOMAIN | TEST | Effect | DF | Accuracy F | P | Speed F | P |
| EXECUTIVE | ABF | A | 6;8354 | 82.85 | <.0001 | 22.99 | <.0001 |
| S | 1;8354 | 23.16 | <.0001 | 0.02 | 0.8916 | ||
| A*S | 6;8354 | 0.46 | 0.8386 | 2.63 | 0.0152 | ||
| ATT | A | 6;8354 | 297.91 | <.0001 | 902.90 | <.0001 | |
| S | 1;8354 | 35.50 | <.0001 | 80.03 | <.0001 | ||
| A*S | 6;8354 | 1.60 | 0.1433 | 6.26 | <.0001 | ||
| WM | A | 6;8354 | 231.70 | <.0001 | 247.23 | <.0001 | |
| S | 1;8354 | 0.03 | 0.865 | 71.66 | <.0001 | ||
| A*S | 6;8354 | 0.79 | 0.5798 | 2.61 | 0.0156 | ||
| MEMORY | VMEM | A | 6;8354 | 31.95 | <.0001 | 373.99 | <.0001 |
| S | 1;8354 | 29.63 | <.0001 | 14.15 | 0.0002 | ||
| A*S | 6;8354 | 0.25 | 0.9605 | 0.98 | 0.4342 | ||
| FMEM | A | 6;8354 | 144.12 | <.0001 | 93.06 | <.0001 | |
| S | 1;8354 | 21.21 | <.0001 | 0.61 | 0.4343 | ||
| A*S | 6;8354 | 0.69 | 0.6576 | 0.51 | 0.7977 | ||
| SMEM | A | 6;8354 | 11.87 | <.0001 | 63.66 | <.0001 | |
| S | 1;8354 | 13.41 | 0.0003 | 3.58 | 0.0584 | ||
| A*S | 6;8354 | 2.64 | 0.0148 | 0.90 | 0.4957 | ||
| COMPLEX | LAN | A | 6;8354 | 486.77 | <.0001 | 220.88 | <.0001 |
| COGNIITION | S | 1;8354 | 7.29 | 0.007 | 41.30 | <.0001 | |
| A*S | 6;8354 | 2.65 | 0.0144 | 0.23 | 0.9684 | ||
| NVR | A | 6;8354 | 169.29 | <.0001 | 36.15 | <.0001 | |
| S | 1;8354 | 35.10 | <.0001 | 64.46 | <.0001 | ||
| A*S | 6;8354 | 3.57 | 0.0015 | 3.25 | 0.0034 | ||
| SPA | A | 6;8354 | 229.96 | <.0001 | 17.93 | <.0001 | |
| S | 1;8354 | 221.85 | <.0001 | 28.68 | <.0001 | ||
| A*S | 6;8354 | 1.08 | 0.373 | 3.92 | 0.0006 | ||
| SOCIAL | EID | A | 6;8354 | 149.50 | <.0001 | 201.12 | <.0001 |
| COGNITION | S | 1;8354 | 36.75 | <.0001 | 161.12 | <.0001 | |
| A*S | 6;8354 | 1.40 | 0.2093 | 3.22 | 0.0037 | ||
| EDI | A | 6;8354 | 272.77 | <.0001 | 30.46 | <.0001 | |
| S | 1;8354 | 36.48 | <.0001 | 11.36 | 0.0008 | ||
| A*S | 6;8354 | 1.66 | 0.1273 | 2.69 | 0.0132 | ||
| ADI | A | 6;8354 | 306.22 | <.0001 | 13.28 | <.0001 | |
| S | 1;8354 | 58.32 | <.0001 | 16.75 | <.0001 | ||
| A*S | 6;8354 | 2.40 | 0.0254 | 3.10 | 0.005 | ||
| SENSORIMOTOR | MOT | A | 6;8354 | 738.10 | <.0001 | ||
| SPEED | S | 1;8354 | 213.44 | <.0001 | |||
| A*S | 6;8354 | 10.13 | <.0001 | ||||
| SM | A | 6;8354 | 121.92 | <.0001 | |||
| S | 1;8354 | 26.35 | <.0001 | ||||
| A*S | 6;8354 | 0.75 | 0.6057 |
Abbreviations: ABF-Abstraction and Mental flexibility; ATT-Attention; WM-Working Memory; VMEM-Verbal Memory; FMEM-Facial Memory; SMEM-Sptial Memory; LAN-Language; NVR-Nonverbal Reasoning; SPA-Spatial Processing; EID-Emotion Identification; EDI-Emotion Differentiation; ADI-Age Differentiation; MOT-Motor Speed; SM-Sensorimotor Speed.
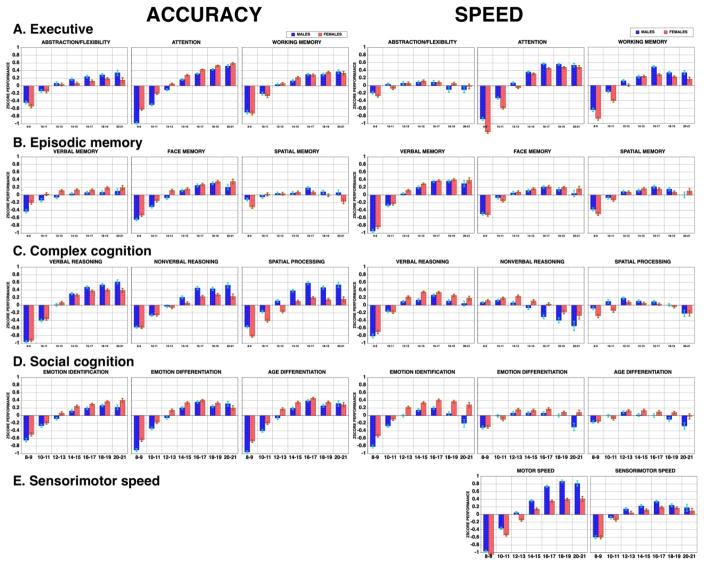
Figure 1 shows performance scores on each domain. As can be seen, there is overwhelming age associated improvement in performance across multiple neurobehavioral domains. Against that background, there is some variability among domains and between accuracy and speed measures and, most importantly, sex differences modulate these effects in a manner related to adolescence.
Figure 1.
Performance effect sizes in standard deviation units (+/-SEM) of males (blue) and females (red) in the Philadelphia Neurodevelopmental Cohort on the Computerized Neurocognitive Battery domains for Accuracy (left) and Speed (right).
Several effects are notable in Figure 1. Among the Executive domain measures (A), abstraction and mental flexibility shows the least age related improvement in accuracy and speed shows a trend toward decline post pubescence. Attention shows the greatest improvement in both accuracy and speed while working memory has intermediate age-related effect sizes. Sex differences are not prominent in executive functions except for higher accuracy in females for attention and greater working memory speed for males. Both effects emerge after age 11. For Episodic Memory tests (B), effect sizes are considerably smaller than for attention; memory is apparently a major strength of the developing brain already in childhood. Age-related improvement is most pronounced for verbal memory speed and for face memory, two domains in which females outperform males across the age range. As with sex differences in the Executive domain, the magnitude of the sex difference increases in post pubescence age bands. For the Complex Cognition domain (C), age-related improvement is seen primarily in accuracy where the effect sizes are large as well as in verbal reasoning speed. Sex differences appear again after age 11, where males begin to show improved accuracy while females begin to show better speed. For Social Cognition (D), females outperform males from childhood onward in both accuracy and speed across all three measures. Nonetheless, this difference seems accentuated in post pubescent years, especially for speed. The opposite sex difference is observed for motor speed, where males outperform females across the age range. Here too, however, these differences become greater in the post pubescent age groups. Thus, while most age-related trajectories flatten after age 18, both the rate of age-related differences and the magnitude of the sex differences increase after age 11.
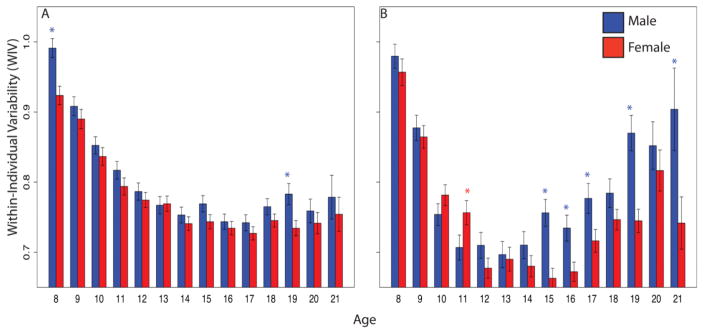
In addition to the uneven rate at which cognitive systems mature, abilities develop unevenly within individuals (e.g., Luna, et al., 2004). The study of within-individual variability (WIV) in performance can shed light on the developmental course of variability in performance on different domains, across tasks, within a single individual. While most studies of development examine age effects on average performance, a parameter that reflects the degree of variability in performance within an individual would be sensitive to deviations of specific abilities from the global level of performance of that individual. There are likely individual differences in WIV, with low values indicating “cognitive generalists” and high values identifying “cognitive specialists” and changes in WIV could reflect differential improvement or deterioration in specific performance domains. Thus, assessing variability could provide important information about typical development (e.g., Van Geert & Van Dijk, 2002) and help identify individuals at risk for brain disorders affecting neurocognition. Notably, WIV is higher in developmental disorders such as attention deficit–hyperactivity disorder (Leth-Steensen, Elbaz, & Douglas, 2000) and schizophrenia (Roalf, et al., 2013). The PNC enables to study WIV across domains through development and examine sex differences in a sample that received the same measures across the age range.
WIV showed a non-linear, U-shaped, relationship with age (Roalf et al., 2014a) both for accuracy (Figure 2A) and for speed (Figure 2B). WIV decreased with age from childhood to adolescence, indicating the expected leveling of performance with maturation. Unexpectedly, however, WIV increased after age 17 for accuracy and after age 13 for speed into early adulthood, especially in males. Notably, WIV is consistently higher in males but this sex difference becomes accentuated after age 13 and into adulthood for both accuracy and speed. These results suggest that after maturation reaches a level of evenness among cognitive abilities, further maturation of behavioral performance is characterized by increased variability, most likely related to specialization. That specialization is more strongly reflected in speed variability than in accuracy.
Figure 2.
Within-individual variability (WIV) in performance of males (blue) and females (red) in the Philadelphia Neurodevelopmental Cohort on the Computerized Neurocognitive Battery domains for Accuracy (a) and Speed (b). (From Roalf DR, Gur RE, Ruparel K, Calkins ME, Satterthwaite TD, Bilker WB, Hakonarson H, Harris LJ, Gur RC. Within-individual variability in neurocognitive performance: age- and sex-related differences in children and youths from ages 8 to 21. Neuropsychology. 2014;28:506–518).
Structural Neuroimaging
Volumetric MRI
An extensive literature shows that adolescence is associated with changes in brain structure, including reduced gray matter (GM) volume and increased white matter (WM) volume, which have been related to sex differences (Blakemore, Burnett & Dahl, 2010; Giedd et al., 1999; Lenroot and Giedd, 2006; Paus, 2005; Sowell et al., 2003). Such maturational processes have been linked to cognitive and affective development during adolescence. Sex differences have been reported in overall brain volume as well as in regional volumes. For example, a steeper age-related increase in WM was reported in males compared with females (Giedd et al. 1999; De Bellis et al., 2001). Lenroot et al. (2007) compared the trajectories of WM development in a large sample and reported the same pattern of sex differences across the brain, and after covarying for total brain volume differences in WM trajectories between males and females in the frontal lobe persisted.
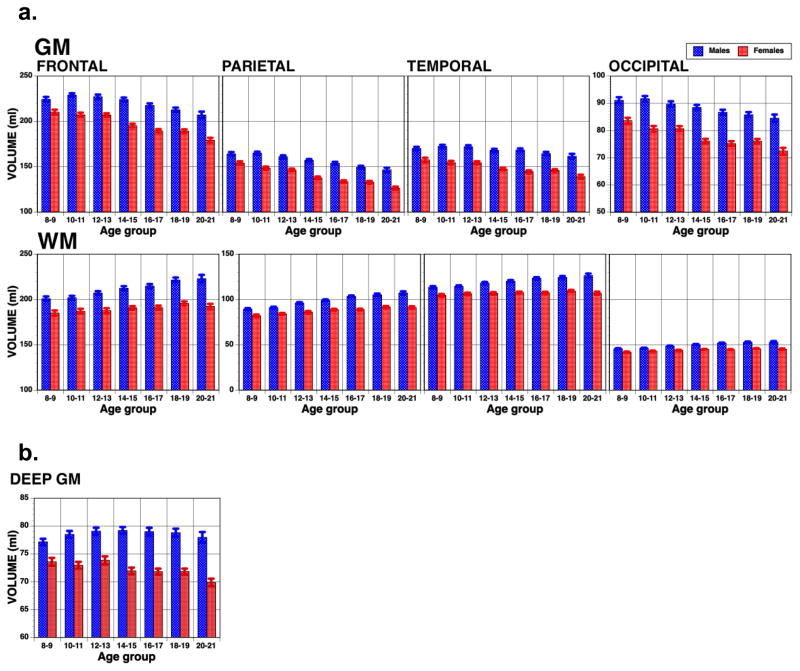
In the PNC sample, as previously detailed (Satterthwaite et al., 2014b), imaging data was acquired at a single site, on a single 3T Siemens TIM Trio whole-body scanner. Signal excitation and reception were obtained using a quadrature body coil for transmit and a 32-channel head coil for receive. Gradient performance was 45mT/m, with a maximum slew rate of 200 T/m/s. Volumetric analysis was performed using deformable registration via attribute matching and mutual-saliency weighting (DRAMMS; Ou et al., 2011, 2014). We examined age group and sex differences applying ANOVA to the volume measures obtained from the T1 weighted images in a sample of 1571 participants (745 males, 826 females). The ANOVA tested main effects for Age group, which divided the sample into seven groups (2-year spans from 8–21), and Sex as grouping factors, and Region (Frontal, Temporal, Parietal, Occipital) as repeated measures (within-group) factor, separately for GM and WM. Age group and Sex effects on deep GM were examined in a separate ANOVA. The overall ANOVAs for GM and WM and for individual regions are presented in Table 2 and the regional volumes are shown in Figure 3.
Table 2.
Summary of ANOVA results for volumetric analysis for main effects of age (A), sex (S), region (R) and their interactions overall and univariate by region.
| OVERALL | GM | WM | ||||
|---|---|---|---|---|---|---|
| Effect | DF | F | P | F | P | |
| A | 6;1557 | 28.33 | <.0001 | 18.32 | <.0001 | |
| S | 1;1557 | 395.27 | <.0001 | 328.15 | <.0001 | |
| R | 3;4671 | 51659.50 | <.0001 | 68268.20 | <.0001 | |
| A*S | 6;1557 | 2.19 | 0.0416 | 3.44 | 0.0022 | |
| A*R | 18;4671 | 23.96 | <.0001 | 8.06 | <.0001 | |
| S*R | 3;4671 | 144.18 | <.0001 | 182.30 | <.0001 | |
| A*S*R | 18;4671 | 1.85 | 0.0153 | 1.39 | 0.1248 | |
| REGION | ||||||
| FRONTAL | A | 6;1557 | 28.53 | <.0001 | 11.66 | <.0001 |
| S | 1;1557 | 334.77 | <.0001 | 264.91 | <.0001 | |
| A*S | 6;1557 | 2.31 | 0.0321 | 2.19 | 0.0417 | |
| TEMPORAL | A | 6;1557 | 13.42 | <.0001 | 10.21 | <.0001 |
| S | 1;1557 | 376.61 | <.0001 | 344.91 | <.0001 | |
| A*S | 6;1557 | 2.17 | 0.0437 | 4.06 | 0.0005 | |
| PARIETAL | A | 6;1557 | 46.90 | <.0001 | 40.07 | <.0001 |
| S | 1;1557 | 359.56 | <.0001 | 342.52 | <.0001 | |
| A*S | 6;1557 | 2.17 | 0.0437 | 4.51 | 0.0002 | |
| OCCIPITAL | A | 6;1557 | 17.89 | <.0001 | 21.77 | <.0001 |
| S | 1;1557 | 364.78 | <.0001 | 264.48 | <.0001 | |
| A*S | 6;1557 | 1.42 | 0.2051 | 3.75 | 0.001 | |
| DEEP GRAY | A | 6;1557 | 2.05 | 0.0561 | ||
| S | 1;1557 | 304.22 | <.0001 | |||
| A*S | 6;1557 | 2.36 | 0.0283 |
A=Age group; S=Sex; R=Region
Figure 3.
Magnetic Resonance Imaging volumetric measures (Means+/-SEM), for gray matter (GM) and white matter (WM), males (blue) and females (red), in the Philadelphia Neurodevelopmental Cohort. Age groups are in 2-year intervals, and results are shown for the four lobes (3a) and deep gray matter (3b).
Thus, we observed reduced cortical GM volume and increased WM volume in all lobes associated with age, which appeared more pronounced in post-pubescent groups compared to pre-pubescent children. In contrast, deep GM volume showed minimal age related changes. Sex differences in WM replicate earlier findings that indicate steeper increase in males from pre - to post–pubescence, especially in the frontal lobe (Lenroot et al., 2007).
Sex differences were also observed in the deep GM of the medial temporal lobe. Giedd et al. (2006) reported post-pubescence increase in hippocampal volume in females, but not in males. This effect may relate to better memory performance in females and the post-pubescence enhancement of this sex differences in memory described above (Gur et al., 2012). The PNC sample afforded the opportunity to test this link directly by examining pre-pubescent and post-pubescent hippocampal and amygdala volume in relation to memory performance (Satterthwaite et al., 2014a). We found that whereas pre-pubertal males and females had similar hippocampal volumes, post-pubertal females had larger hippocampi bilaterally. This effect was absent in the amygdala. Notably, post-pubertal sex differences were most prominent in the lateral aspect of the hippocampi corresponding to the CA1 subfield. The sex differences in hippocampal volume correlated with performance on memory tests.
There is need to establish, rather than assume, a link between sex differences and puberty or age. Disentangling the effects of age from pubescence is difficult in cross sectional studies although some inroads can be made by examining same age groups at different stages of pubescence (Satterthwaite et al., 2014a). Elucidating effects of pubescence and separating age effects from hormonal effects requires longitudinal studies in which hormonal measures are obtained and pubescence stage is rigorously established. While the literature on longitudinal effects is growing thus far efforts to separate age from hormonal effects are limited. For example, Dennison et al. (2013) examined volumetric changes in subcortical structures of 60 adolescents (28 females, 32 males) at ages 12.5 and 16.5 years. Brain regions showed a heterogeneous pattern of maturation indicating that hemispheric specialization and volumetric sex differences play a role in maturation. Vijayakumar et al. (2016), in a mixed longitudinal design, examined maturation of cortical thickness, surface area and volume in 90 participants (ages 11–20 years). Surface area, across most of the cortex, showed non-linear increases, whereas thickness and volume were characterized by non-linear decreasing and increasing trajectories. Sex differences in volume and surface area were observed across time, but there were no differences in thickness. The authors consider their findings to suggest that thickness and surface area may be driven by different underlying mechanisms of brain development. However, neither study examined hormonal status and therefore age and pubescence effects could not be disentangled.
Diffusion Tensor Imaging Connectivity
Diffusion tensor imaging (DTI) has been increasingly applied to examine sex differences in WM microstructure and fiber tracts that connect among regions. For example, Schmithorst, Holland and Dardzinski (2008) reported that females had higher fractional anisotropy (FA) in the splenium of the corpus callosum than males. Males, however, had a higher FA in several regions including the frontal lobes. Furthermore, in females age and FA had a positive correlation across regions, while males showed no such correlation between FA and age. For mean diffusivity (MD), males had higher values, compared with females, in the corticospinal tract and in the frontal lobe, while females had higher MD values in several other regions. Thus, structural properties of WM are not uniform throughout the brain or across males and females.
Perrin et al. (2009) examined sex differences in the maturation of WM during adolescence, measuring lobular volumes throughout the brain to estimate a myelination index using magnetization-transfer ratio. They reported in male adolescents age-related increases in WM lobular volumes with decreases in the lobular values of WM magnetization-transfer ratio. Furthermore, WM density in the cortico-spinal tract decreased with age. This pattern was not evident in female adolescents. The investigators suggest that sex-specific mechanisms may underlie WM growth during adolescence, involving age-related increases in axonal caliber in males and increased myelination in females. The literature on sex differences in DTI-based measures such as FA, as highlighted above, indicates increased FA values in major WM regions and tracts in males (Clayden et al., 2012; Hertig et al., 2011; Hsu et al., 2008;), and in the corpus callosum in females (Kannan et al. 2012). Indeed, our volumetric finding of greater cortical WM volume in males and greater callosal prominence in females led us to hypothesize that male brains are optimized to communicate within a hemisphere, whereas female brains are optimized for inter hemispheric communication (Gur et al., 1999).
More recently, DTI has been used to study the communication architecture of brain networks (Bava et al., 2011; Clayden et al., 2012; Herting et al., 2011; Hsu et al., 2008; Ingalhalikar et al., 2014). The structural connectome examines brain connectivity locally and globally. The identification of network properties such as communities or the communication backbone can advance the understanding of how complex behavior emerges from the integration of segregated neuronal clusters (Schwarz, Gozzi &Bifone, 2008). We have evaluated the structural connectome to elucidate sex differences in the PNC and found stronger intra-hemispheric connectivity bilaterally in males and stronger inter-hemispheric connectivity in females (Ingalhalikar et al., 2014).
To examine the developmental course of sex differences requires large datasets spanning age ranges that include adolescence. The PNC dataset (Satterthwaite et al., 2014b), which includes structural, functional and behavioral parameters, provides a unique opportunity to identify age-related differences in the subnetworks of the structural connectome and elucidate how these differences relate to sex differences in behavior. We evaluated subnetworks in order to establish a reliable link between brain structure and behavior (Tunc et al., 2016). Our results suggest that sex differences in functional and behavioral dimensions are associated with related differences in the network properties of the structural connectome. We observed increased structural connectivity related to the motor, sensory and executive function subnetworks in males. In females, subnetworks associated with social cognition, attention and memory tasks had higher connectivity. Another measure of network structure is modularity, which indicates the prominence of division of networks into modules (sometimes referred to as “communities”). Highly modular networks are characterized by dense connections among nodes within a module relative to sparse connections among nodes that belong to different modules. We found that males showed higher modularity compared to females, with females having higher inter-modular connectivity. Thus, an increased separation between males and females emerges in the course of development, in behavioral patterns and in associated brain parameters. However, it is still unclear how specifically a greater within hemispheric modularity in males and higher inter-modular and inter-hemispheric connectivity in females contribute to sex differences in particular behavioral domains. We can speculate that tasks that require depth of processing within a single domain, verbal or spatial, would be more easily processed by males, whereas tasks that require integration of domains, such as fusion of verbal and spatial aspects of stimuli, would be better facilitated in a brain with female connectome features.
Longitudinal DTI studies provide information on developmental trajectories indicating WM growth during adolescence. Most studies include small samples and two time points (Bava et al., 2010; Giorgio et al., 2009; Lebel and Beaulieu, 2011; Wang et al., 2012). Simmonds et al., (2014) conducted repeated annual examinations over a course of five years in a large sample of 128 youths (ages 8–28). The age range enabled evaluation of patterns of growth from childhood to young adulthood. During adolescence, WM microstructure in fronto-cortical, fronto-subcortical and cerebellar connections reached adult levels, whereas cortico-limbic connectivity matured in adulthood. Sex differences were observed with females showing growth especially in mid-adolescence whereas males showing continuous WM growth across the age range. Notably, maturation was related to cognitive performance.
Functional Neuroimaging
Perfusion
Cerebral blood flow (CBF), critical for healthy brain function, is coupled to regional metabolism, responds to activation with cognitive tasks and shows a marked decline throughout childhood and adolescence (Chiron et al., 1992; Takahashi et al., 1999). Compared to the extensive literature on cognitive and structural brain parameters in relation to development, the literature on CBF has been limited. Early technologies to measure CBF, including the Kety–Schmidt nitrous oxide method, 133Xe clearance and 15O labeled water with PET, were limited in their application to developmental samples due to invasiveness and use of ionizing radiation. Yet, consistently across studies CBF was found to be elevated during childhood then declining throughout adolescence. Small sample sizes did not allow examining sex differences in youth. Studies of adults have demonstrated increased perfusion in females (e.g., Gur et al., 1982, Ragland et al., 2000).
The application of arterial spin labeling (ASL; Aguirre & Detre, 2012; Detre et al., 1992) with MRI provides a quantitative noninvasive measure of CBF that has been validated with PET (Ye et al., 2000) and applied to pediatric samples. Taki et al. (2011) replicated prior findings of declining perfusion in adolescence and also reported that females had higher perfusion in the posterior cingulate cortex, with a steeper decline rate of CBF in males. The effects of puberty on such sex differences have not been evaluated. Using ASL data from the PNC, we examined developmental patterns of cerebral perfusion in males and females in relation to puberty (Satterthwaite et al., 2014c). We demonstrated differential patterns of developmental CBF in males and females with divergent nonlinear trajectories in multiple brain regions, including hubs of the executive and default mode networks. The decline in CBF was similar between males and females in early puberty but diverged in mid-puberty, with CBF increasing in females and continuing to decrease in males. Thus, higher CBF previously noted in adult females emerges in mid-puberty already and the contribution of hormonal levels needs to be established.
Resting State Functional Connectivity
Resting-state functional connectivity MRI (rsfc-MRI; Biswal, VanKylen & Hyde, 1997; Fox & Raichle 2007) offers a potentially effective tool for examining functional brain networks (Power et al., 2011; Yeo et al., 2011). Several studies reported sex differences in functional connectivity (Biswal et al., 2010; Tian et al., 2011; Wang et al., 2012; Wu et al., 2013; Zuo et al., 2010), but most of this work has been done in adults. Developmental studies confronted a methodological obstacle since motion affects rsfc-MRI and confounds interpretation because of its association with age (Power et al., 2012; Satterthwaite et al., 2012; van Dijk, Sabuncu & Buckner, 2011).
We examined sex differences in functional connectivity in relation to cognition in the PNC (Satterthwaite et al., 2015). We found that sex differences in cognitive performance were related to multivariate patterns of rsfc-MRI. Males outperformed females on motor and spatial cognitive tasks and displayed more between-module connectivity, whereas females were faster in tasks of emotion identification and nonverbal reasoning, showing more within-module connectivity. Multivariate pattern analysis with support vector machines classified subject’s sex on the basis of their cognitive profile with 63% accuracy, but was more accurate using functional connectivity data (71% accuracy). Notably, “masculinity” of a participant’s cognitive profile was related to that of their pattern of brain connectivity. These findings demonstrate that sex differences in cognition are associated with divergent patterns of brain functional connectivity. Whereas analysis of the structural connectome indicates greater modularity in males, functional connectome shows greater modularity in females. The relationship between sex differences in structural connectivity described in the section on DTI and functional connectivity based on resting state measures of regional co-activation is a topic of current interest that is yet to be elucidated. Such investigations require multimodal convergent analyses with datasets geared to test hypotheses in developmental samples. For example, it is not clear whether structural connectivity constrains the development of functional connectivity or, conversely the establishment of a functional connectome triggers the layering of myelinated fibers that create the structural connectome. Longitudinal studies in which the relative timing of the establishment of regional connectivity can be determined are prerequisite for answering such questions.
Activation with functional MRI
Cognitive and affective processes have been probed in fMRI paradigms in adolescents, commonly in separate studies (e.g. Forbes, et al., 2011; Luna et al., 2001, Moore et al., 2012). The elucidation of the interaction between cognitive and emotion processing measures has been more limited in adolescence (e.g., Somerville et al., 2011). Sex differences have been noted in fMRI studies of adolescents probing inhibitory control (e.g., Rubia et al., 2013), attention (e.g., Rubia et al., 2010), working memory (e.g., Alarcon et al., 2014), and emotion processing (e.g., Schneider et al., 2011). It is difficult to generalize from a growing number of studies that use divergent tasks and approaches to data analysis and often with relatively small samples. Nonetheless, these studies generally indicate that sex difference are evident already in adolescence and parameters of regional brain activation show changes related to improved performance. Of note, hormonal effects have been occasionally evaluated with fMRI (e.g. Alcaron et al., 2014; Goddings et al., 2012; Cservenka et al., 2015), as described below.
Hormonal Modulation
The extent to which human behavioral sex differences are influenced by sex hormones that change during sensitive periods of development has been an important area of investigation. Hormonal effects on behavior in early development have been well documented in animal studies establishing the prenatal and early neonatal stage as a sensitive period. More recently, based on rodent research, puberty has been considered as another sensitive period along the continuum where brain organization is influenced by sex hormones (Schultz, Molenda-Figueira & Sisk, 2009; Sisk & Zehr, 2005). This line of research is germane to advancing the understanding of how puberty during adolescence impacts brain organization.
In a comprehensive review, Berenbaum and Beltz (2011) suggest that sex differences in human behavior relate to hormonal exposure at multiple developmental periods, including puberty. While most literature evaluated the effects of androgens on male-typical behavior, there is a growing body of research supporting the role of ovarian hormones in female-typical behavior. Viewing sensitive periods as a continuum implies that variations in timing of puberty impact brain organization and behavior and may contribute to understanding sex differences during adolescence. Lacking is data from large-scale longitudinal studies in adolescence where cognitive performance, combined with parameters of brain structure and function, are obtained during specific time points. As noted in our summary of the neurocognitive findings, some sex differences in behavior remain constant throughout development, such as better performance of females on social cognition measures, while others emerge with pubescence.
Similar effects are reported for several parameters of brain structure and function (Raznahan et al., 2010). MRI studies of brain structure have linked increased testosterone to WM development in males (Perrin et al., 2009; Paus, 2010) and a negative correlation was reported between estradiol level and age-corrected GM volume in adolescent females (Pepper et al., 2008). Similarly, Satterthwaite et al., (2014c, 2015) using the PNC data, linked both structural and functional parameters to puberty and age-related differences in neurocognitive performance. Again, longitudinal studies are needed with concurrent measurements of performance, brain parameters and hormonal status. Such studies could focus on the period of transition from pre-pubescence to pubescence.
There is variability between sexes and within each sex in onset of puberty. Physical changes are evident earlier in females, about age 10, than males, about age 11.5 (Marshall and Tanner, 1969). There is also a notable concomitant increase in sex hormones - estradiol in females and testosterone in males (Nottelmann et al., 1987). Longitudinal studies enabling repeated measures of maturation can provide important information on onset and progression of puberty in relation to brain parameters. Several studies have undertaken such paradigms with structural MRI measures. For example, Raznahan and colleagues (2010) examined the relation between variation in signaling efficacy of the androgen receptor and neuroanatomic brain maturation (ages 9 to 22 years). Findings suggest sex specific and brain region specific effects: greater androgen receptor signaling attenuated age-related decreases in superior parietal and parts of temporal lobe in males, while accelerating age-related decreases in the left inferior frontal gyrus in females. Nguyen and colleagues (2013a; 2013b) evaluated sex differences in the association between androgen levels and cortical thickness in pre-pubertal and post-pubertal males and females (ages 4 to 22 years). They reported that higher dehydroepiandrosterone (DHEA) in males and females and testosterone in females predicted increases in cortical thickness in the pre-pubertal participants, while higher testosterone predicted decreases in cortical thickness in post-pubertal males and females. Tanner stages in a sample of males and females (ages 7 to 22 years) were noted to predict changes in subcortical volume of several structures including the hippocampus, amygdala, and caudate (Goddings et al. 2013). In a sample of 126 adolescents (ages 10 to 14 years) Hertig et al. (2014) employed growth curve modeling to examine how testosterone and estradiol relate to changes in subcortical brain volumes obtained in a longitudinal design 2-years apart. Hormonal levels and Tanner Stage predicted WM and right amygdala growth for males and females across adolescence independent of age. Such studies illustrate the power of integrating measures of puberty and developmental epoch in the study of brain and behavior. Efforts to examine gene environment contributions to structural brain measures were enhanced in the twin paradigm (Brouwer et al., 2015). Non-shared environmental factors contributed in females, ages 9–12 years, to the association between follicle stimulating hormone and regional GM density. Shared environmental factors contributed to the association of higher estradiol levels with lower regional GM density.
In addition to hormonal relations to structural brain parameters, highlighted above, some studies with fMRI likewise made efforts to evaluate pubertal status in relation to brain function (e. g., Forbes, et al., 2011; Klapwijk et al., 2013; Moore et al., 2012), including hormonal levels (e.g., Alcaron et al., 2014; Goddings et al., 2012; Cservenka et al., 2015). The convergence of brain measures with evaluation of hormonal studies will contribute to elucidating the mechanisms underlying sex differences. However, as indicated above, disentangling hormonal effects from age effects requires longitudinal studies with rigorous measurements of all related parameters.
Clinical implications
Here we focused on normative sex differences in brain and behavior. These differences should be considered when interpreting effects of diverse brain disorders with manifested psychopathology. Developmental disorders may emerge early and are more prevalent in males. Anxiety and mood disorder commonly emerge later in development and are more frequent in females. Some of the normative sex differences may explain or modulate effect of disorders such as schizophrenia (e.g., Ragland et al., 1999; Gur et al., 2004; Calkins et al., 2013). Therefore, comparative samples of males and females are needed when examining disorders. Such samples should represent the lifespan because hormonal factors mediate these sex differences and likely interact with the disorder in generating the symptoms. Future treatments can be informed by these relationships in tailoring interventions to males and females at different stages of development.
How could such future clinical application look like? For example, there is increased awareness that sport-related mild traumatic brain injuries can have cumulative adverse effects. With the increased participation of females in sports, normative growth charts for neurocognitive development and, eventually, neuroimaging based growth charts for brain development, it will be possible to identify and monitor such effects. Importantly, such a developmental database would also enable disentangling these effects from those of other insults to the brain that may relate to other adverse conditions characteristic of adolescence such as substance use, car crashes and brain disorders. Of note, many of these events affect frontal executive as well as limbic and striatal brain systems that would increase risk-taking behavior and potentiate sex characteristic psychiatric manifestations with depression in females and externalizing behaviors in males. Knowledge about normative sex differences is necessary to interpret and intervene.
Summary and future directions
Sex differences in brain organization that are evident in adults become accentuated during adolescence, implicating hormonal effects of pubescence. Improved executive function and complex and social cognition is associated with increase in the magnitude of sex differences in these domains and such changes in cognition are paralleled by age-related differences in brain parameters.
Results of the PNC, where the sample size permits detection of relatively small effects, show a striking ubiquity of significant sex differences on nearly all behavioral and brain parameters. We replicated effects from the literature, but also found sex differences that have not been observed before both in individual parameters and in age-related differences across the developmental epoch we examined. The prevalence of such differences indicates that the human species demonstrates complementarity between the sexes in behavior and underlying brain structure and function. While some of these differences are small their effects across humanity can be substantial and with clinical and societal implications. On the other hand, none of the results are of the kind that would justify considering the human brain “sexually dimorphic”, certainly not in the same sense as other sexually dimorphic organs in the human body. Furthermore, environmental effects certainly modulate sex differences and their interaction with the developing brain needs further study. Finally, biological sex can interact with gender identity in ways that can be illuminating and merit investigation. Thus, sex differences in brain and behavior should be taken in perspective. They are interesting and informative, but male brains and correspondingly behavior is more alike that of females than it is different.
What we have learned from the PNC data is that the period between childhood through adolescence and into young adulthood is characterized by pronounced improvement in accuracy and speed of performance especially in executive and reasoning tasks, combined with reduced within individual variability from childhood through adolescence followed by increased variability especially in speed. Sex differences were evident on most tasks already at childhood but their magnitude increased with development. These age-related effects were paralleled by differences in brain parameters of anatomy and its connectivity and of physiology and its connectivity. Novel approaches for data analysis are needed and have been applied to examine development of these parameters and they generally indicate pruning of gray matter accompanied by myelination and complementary age-related effects on inter-regional anatomic connectivity. Sex differences in anatomic connectivity indicate greater modularity and within hemispheric connectivity in males and greater inter-module and interhemispheric connectivity in females. This pattern of sex differences emerges during adolescence and becomes more pronounced during young adulthood. It is still unclear and a topic of further investigation how the anatomic connectivity relates to physiologic connectivity and how both relate to performance.
These general relationships, however, are yet to be examined extensively in a longitudinal context and only such studies can be sensitive to detect effects of changes and document trajectories. Longitudinal studies are also needed to elucidate effects of sex hormones related to puberty. Multimodal longitudinal studies are needed for advancing the understanding of sex differences in both healthy development and the effect of aberrant conditions that lead to illness. The multitude of variables involved in such deep phenotyping and the need to consider multiple social and environmental factors mandates large-scale studies. In this context, community studies have an advantage over investigation of help-seeking patients and convenience samples of control individuals because the former provide information on the distribution of continuous dimensions and may afford a better appraisal of the need for intervention regardless of specific conditions affecting the likelihood of help seeking.
The prospect of large-scale multimodal studies in which deeply phenotyped populations are evaluated longitudinally is daunting, such an effort is however necessary to have the information needed for mechanistic accounts of sex differences in behavior and neuropsychiatric disorders. With such information we will be in a position to detect early signs of impending psychopathology that may differ in boys and girls, and we will be equipped with evidence-based models for gender optimized prevention and intervention.
Highlights.
Notable sex differences
Adolescence is a critical period
Multimodal data merging needed
Acknowledgments
We thank the many colleagues and dedicated staff who made the PNC possible and the many research participants who offered their time and efforts. Supported by NIMH Grants RC2 MH089983, P50MH096891, and R01 MH107235.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, Detre JA. The development and future of perfusion fMRI for dynamic imaging of human brain activity. Neuroimage. 2012;62:1279–1285. doi: 10.1016/j.neuroimage.2012.04.039. [DOI] [PubMed] [Google Scholar]
- Alarcon G, Cservenka A, Fair DA, Nagel BJ. Sex differences in the neural substrates of spatial working memory during adolescence are not mediated by endogenous testosterone. Brain Research. 2014;1593:40–54. doi: 10.1016/j.brainres.2014.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang SY, Lee K. Exploring developmental differences in visual short-term memory and working memory. Developmental Psychology. 2010;46:279–285. doi: 10.1037/a0017554. [DOI] [PubMed] [Google Scholar]
- Bava S, Boucquey V, Goldenberg D, Thayer RE, Ward M, Jacobus J, Tapert SF. Sex differences in adolescent white matter architecture. Brain Res. 2011;1375:41–48. doi: 10.1016/j.brainres.2010.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM. Sexual differentiation of human behavior: effects of prenatal and pubertal organizational hormones. Front Neuroendocrinol. 2011;32:183–200. doi: 10.1016/j.yfrne.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Biswal BB, VanKylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR in Biomedicine. 1997;10:165–17. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer RM, Koenis MM, Schnack HG, van Baal GC, van Soelen IL, Boomsma DI, Hulshoff Pol HE. Longitudinal development of hormone levels and grey matter density in 9 and 12-year-old twins. Behav Genet. 2015;45:313–323. doi: 10.1007/s10519-015-9708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Cohen Kadosh K, Blakemore SJ. The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience & Biobehavioral Reviews. 2011;35:1654–1664. doi: 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Tepper P, Gur RC, Ragland JD, Klei L, Wiener HW, Richard J, Savage RM, Allen TB, O’Jile J, Devlin B, Kwentus J, Aliyu MH, Bradford LD, Edwards N, Lyons PD, Nimgaonkar VL, Santos AB, Go RC, Gur RE. Project among African-Americans to explore risks for schizophrenia (PAARTNERS): evidence for impairment and heritability of neurocognitive functioning in families of schizophrenia patients. Am J Psychiatry. 2010;167:459–472. doi: 10.1176/appi.ajp.2009.08091351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Ray A, Gur RC, Freedman R, Green MF, Greenwood TA, Light GA, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Sugar C, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL, Lazzeroni LC, Gur RE. Sex differences in familiality effects on neurocognitive performance in schizophrenia. Biological Psychiatry. 2013;73:976–984. doi: 10.1016/j.biopsych.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Duhoux S, Malter, Cohen M. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67:749–760. doi: 10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron C, Raynaud C, Mazière B, Zilbovicius M, Laflamme L, Masure MC, Dulac O, Bourguignon M, Syrota AI. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med. 1992;33:696–703. [PubMed] [Google Scholar]
- Clayden JD, Jentschke S, Muñoz M, Cooper JM, Chadwick MJ, Banks T, Clark CA, Vargha-Khadem F. Normative development of white matter tracts: similarities and differences in relation to age, gender, and intelligence. Cereb Cortex. 2012;22:1738–1747. doi: 10.1093/cercor/bhr243. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: behavioral evidence of protracted frontal lobe development. Developmental Neuropsychology. 2007;31:103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Stroup ML, Etkin A, Nagel BJ. The effects of age, sex, and hormones on emotional conflict-related brain response during adolescence. Brain and Cognition. 2015;99:135–150. doi: 10.1016/j.bandc.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nature Reviews Neuroscience. 2010;11:201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, et al. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Dennison M, Whittle S, Yucel M, Vijayakumar N, Kline A, Simmons J, Allen NB. Mapping subcortical brain maturation during adolescence: evidence of hemisphere- and sex-specific longitudinal changes. Developmental Science. 2013;16:772–791. doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Gur RC, Gur RE, Skolnick B, Mawhinney-Hee M, Smailis J. Facial emotion discrimination: I. Task construction and behavioral findings in normal subjects. Psychiatry Research. 1992;42:231–240. doi: 10.1016/0165-1781(92)90115-j. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: Role of pubertal maturation and relation to measures of negative affect. Developmental Neuropsychology. 2011;36:429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Wartenburger I. Language and brain. Wiley Interdisciplinary Reviews: Cognitive Science. 2010;1:150–159. doi: 10.1002/wcs.9. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H, James AC. Longitudinal changes in grey and white matter during adolescence. NeuroImage. 2009;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Goddings AL, Burnett Heyes S, Bird G, Viner RM, Blakemore SJ. The relationship between puberty and social emotion processing. Developmental Science. 2012;15:801–811. doi: 10.1111/j.1467-7687.2012.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MC, Maurer D, Lewis TL. Developmental changes in attention: the effects of endogenous cueing and of distractors. Developmental Science. 2001;4:209–219. [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;1:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch Gen Psychiatry. 2007;64:1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE. Neurobehavioral probes for physiologic neuroimaging studies. Archives of General Psychiatry. 1992;49:409–414. doi: 10.1001/archpsyc.1992.01820050073013. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Younkin D, Rosen AD, Skolnick BE, Reivich M. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982;217:659–661. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187:254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Mozley LH, Mozley PD, Resnick SM, Karp JS, Alavi A, Gur RE. Sex differences in regional cerebral glucose metabolism during a resting state. Science. 1995;267:528–531. doi: 10.1126/science.7824953. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26:251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Alsop D, Glahn D, Petty R, Swanson CL, Maldjian JA, Turetsky BI, Detre JA, Gee J, Gur RE. An fMRI study of sex differences in regional activation to a verbal and a spatial task. Brain Lang. 2000;74:157–170. doi: 10.1006/brln.2000.2325. [DOI] [PubMed] [Google Scholar]
- Gur RE, Kohler CG, Turetsky BI, Siegel SJ, Kanes SJ, Bilker WB, Brennan AR, Gur RC. A sexually dimorphic ratio of orbitofrontal to amygdala volume is altered in schizophrenia. Biological Psychiatry. 2004;55:512–517. doi: 10.1016/j.biopsych.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, Kanes S, Blangero J, Gur RC. Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, Kanes S, Blangero J, Gur RC. Am J Psychiatry. 2007;164:813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- Halpern DF, Benbow CP, Geary DC, Gur RC, Hyde JS, Gernsbacher MA. The science of sex differences in science and mathematics. Psychological Science in the Public Interest. 2007;8:1–51. doi: 10.1111/j.1529-1006.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV, Nowell A. Sex differences in mental test scores, variability, and numbers of high-scoring individuals. Science. 1995;269:41–45. doi: 10.1126/science.7604277. [DOI] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 2012;22:1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Gautama P, Spielberg JM, Kana E, Dahl RE, Sowell ER. The role of testosterone and estradiol in brain volume changes across adolescence: a longitudinal structural MRI study. Hum Brain Mapp. 2014;35:5633–5645. doi: 10.1002/hbm.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. Sex-related variation in human behavior and the brain. Trends in Cognitive Science. 2010;14:448–456. doi: 10.1016/j.tics.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J-L, Leemans A, Bai C-H, Lee C-H, Tsai Y-F, Chiu H-C, Chen W-H. Gender differences and agerelated white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage. 2008;39:566–577. doi: 10.1016/j.neuroimage.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Linn MC. Gender differences in verbal ability: A meta-analysis. Psychological Bulletin. 1988;104:53–69. [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci USA. 2014;111:823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Kanaan RA, Allin M, Picchioni M, Barker GJ, Daly E, Shergill SS, Woolley J, McGuire PK. Gender differences in white matter microstructure. PLoS ONE. 2012;7:e38272. doi: 10.1371/journal.pone.0038272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapwijk ET, Goddings AL, Burnett Heyes S, Bird G, Viner RM, Blakemore SJ. Increased functional connectivity with puberty in the mentalising network involved in social emotion processing. Hormones and Behavior. 2013;64:314–322. doi: 10.1016/j.yhbeh.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Brain mechanisms in early language acquisition. Neuron. 2010;67:713–727. doi: 10.1016/j.neuron.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72:46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Linn MC, Petersen AC. Emergence and characterization of sex differences in spatial ability: a meta analysis. Child Development. 1985;56:1479–1498. [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa J, Matsui M, Konishi T, Noguchi N, Gur RC, Bilker W, Miyawaki T. Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cerebral Cortex. 2001;11:335–342. doi: 10.1093/cercor/11.4.335. [DOI] [PubMed] [Google Scholar]
- Moore WE, 3rd, Pfeifer JH, Masten CL, Mazziotta JC, Iacoboni M, Dapretto M. Facing puberty: Associations between pubertal development and neural responses to affective facial displays. Social Cognitive and Affective Neuroscience. 2012;7:35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC. Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology. 2015;29:235–246. doi: 10.1037/neu0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Briseno P, Diaz R, Campos-Romo A, Fernandez-Ruiz J. Sex-related differences in motor learning and performance. Behavioral and brain functions. 2010;6:74. doi: 10.1186/1744-9081-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama S. Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex. 2013a;23:1424–1432. doi: 10.1093/cercor/bhs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, McCracken JT, Ducharme S, Cropp BF, Botteron KN, Evans AC, Karama S. Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. The Journal of neuroscience. 2013b;33:10840–10848. doi: 10.1523/JNEUROSCI.5747-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottelmann ED, Susman EJ, Dorn LD, Inoff-Germain G, Loriaux DL, Cutler GB, Jr, Chrousos GP. Developmental processes in early adolescence. Relations among chronologic age, pubertal stage, height, weight, and serum levels of gonadotropins, sex steroids, and adrenal androgens. Journal Adolesc Health Care. 1987;8:246–260. doi: 10.1016/0197-0070(87)90428-1. [DOI] [PubMed] [Google Scholar]
- Ou Y, Sotiras A, Paragios N, Davatzikos C. DRAMMS: deformable registration via attribute matching and mutual-saliency weighting. Medical Image Analysis. 2011;15:622–639. doi: 10.1016/j.media.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Akbari H, Bilello M, Da X, Davatzikos C. Comparative evaluation of registration algorithms in different brain databases with varying difficulty: Results and insights. IEEE Trans On Medical Imaging. 2014;33:2039–2065. doi: 10.1109/TMI.2014.2330355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: Myelin or axon? Brain and Cognition. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Pepper JS, Brouwer RM, Schnack HG, van Baal GCM, Van Leeuwen M, Van den Berg SM, Delemarre-Van de Waal HA, Janke AL, Collins DL, Evans AC, Boomsma DI, Kahn RS, Hulshoff Pol HE. Cerebral white matter in early puberty is associated with luteinizing hormone concentrations. Psychoneuroendocrinology. 2008;33:909–915. doi: 10.1016/j.psyneuen.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, et al. Sex differences in the growth of white matter during adolescence. Neuroimage. 2009;45:1055–1066. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Pickering SJ. The development of visuo-spatial working memory. Memory. 2001;9:423–432. doi: 10.1080/09658210143000182. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Coleman AR, Gur RC, Glahn DC, Gur RE. Sex differences in brain-behavior relationships between verbal episodic memory and resting regional cerebral blood flow. Neuropsychologia. 2000;38:451–461. doi: 10.1016/s0028-3932(99)00086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RE, Klimas BC, McGrady N, Gur RC. Neuropsychological laterality indices of schizophrenia: Interactions with gender. Schizophrenia Bulletin. 1999;25:79–89. doi: 10.1093/oxfordjournals.schbul.a033369. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, et al. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci USA. 2010;107:16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Ruparel K, Verma R, Elliott MA, Gur RE, Gur RC. White matter organization and neurocognitive performance variability in schizophrenia. Schizophr Res. 2013;143:172–178. doi: 10.1016/j.schres.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Gur RE, Ruparel K, Calkins ME, Satterthwaite TD, Bilker WB, Hakonarson H, Harris LJ, Gur RC. Within-individual variability in neurocognitive performance: age- and sex-related differences in children and youths from ages 8 to 21. Neuropsychology. 2014a;28:506–518. doi: 10.1037/neu0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Ruparel K, Gur RE, Bilker W, Gerraty R, Elliott MA, Gallagher RS, Almasy L, Pogue-Geile MF, Prasad K, Wood J, Nimgaonkar VL, Gur RC. Neuroimaging predictors of cognitive performance across a standardized neurocognitive battery. Neuropsychology. 2014b;28:161–176. doi: 10.1037/neu0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Hyde Z, Halari R, Giampietro V, Smith A. Effects of age and sex on developmental neural networks of visual-spatial attention allocation. Neuroimage. 2010;51:817–827. doi: 10.1016/j.neuroimage.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Rubia K, Lim L, Ecker C, Halari R, Giampietro V, Simmons A, et al. Effects of age and gender on neural networks of motor response inhibition: From adolescence to mid-adulthood. Neuroimage. 2013;83:690–703. doi: 10.1016/j.neuroimage.2013.06.078. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Localizing age-related individual differences in a hierarchical structure. Intelligence. 2004;32:541–561. doi: 10.1016/j.intell.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, Hopson R, Jackson C, Keefe J, Riley M, et al. Neuroimaging of the Philadelphia Neurodevelopmental Cohort. Neuroimage. 2014b;86:544–553. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Shinohara RT, Wolf DH, Hopson RD, Elliott MA, Vandekar SN, Ruparel K, Calkins ME, Roalf DR, Gennatas ED, Jackson C, Erus G, Prabhakaran K, Davatzikos C, Detre JA, Hakonarson H, Gur RC, Gur RE. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc Natl Acad Sci U S A. 2014c;111:8643–8648. doi: 10.1073/pnas.1400178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Vandekar S, Wolf DH, Ruparel K, Roalf DR, Jackson C, Elliott MA, Bilker WB, Calkins ME, Prabhakaran K, Davatzikos C, Hakonarson H, Gur RE, Gur RC. Sex differences in the effect of puberty on hippocampal morphology. J Am Acad Child Adolesc Psychiatry. 2014d;53:341–350. doi: 10.1016/j.jaac.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, Gennatas ED, Elliott MA, Smith A, Hakonarson H, Verma R, Davatzikos C, Gur RE, Gur RC. Linked sex differences in cognition and functional connectivity in youth. Cereb Cortex. 2015;25:2383–2894. doi: 10.1093/cercor/bhu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Shtasel DL, Flannery KA, Mozley LH, Malamut BL, Watson B, Mozley PD. Normative neuropsychological test performance: effects of age, education, gender and ethnicity. Applied Neuropsychology. 1995;2:79–88. doi: 10.1207/s15324826an0202_5. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Menz MM, Miedl SF, et al. Boys do it the right way: Sex-dependent amygdala lateralization during face processing in adolescents. Neuroimage. 2011;56:1847–1853. doi: 10.1016/j.neuroimage.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: the organizational–activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz AJ, Gozzi A, Bifone A. Community structure and modularity in networks of correlated brain activity. Magn Reson Imaging. 2008;26:914–920. doi: 10.1016/j.mri.2008.01.048. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DJ, Grosbras M-H, Leonard G, Pike GB, Paus T. Development of the action observation network during early adolescence: a longitudinal study. Social Cognitive and Affective Neuroscience. 2012;7:64–80. doi: 10.1093/scan/nsq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds D, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2014;92:356–368. doi: 10.1016/j.neuroimage.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Shirane R, Sato S, Yoshimoto T. Developmental changes of cerebral blood flow and oxygen metabolism in children. AJNR Am J Neuroradiol. 1999;20:917–922. [PMC free article] [PubMed] [Google Scholar]
- Taki Y, Hashizume H, Sassa Y, Takeuchi H, Wu K, Asano M, Asano K, Fukuda H, Kawashima R. Correlation between gray matter density-adjusted brain perfusion and age using brain MR images of 202 healthy children. Hum Brain Mapp. 2011;32:1973–1985. doi: 10.1002/hbm.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Wang J, Yan C, He Y. Hemisphere- and gender-related differences in small-world brain networks: a resting-state functional MRI study. Neuroimage. 2011;54:191–202. doi: 10.1016/j.neuroimage.2010.07.066. [DOI] [PubMed] [Google Scholar]
- Thomas JR, French KE. Gender differences across age in motor performance: A meta-analysis. Psychological Bulletin. 1985;98:260–282. [PubMed] [Google Scholar]
- Tunç B, Solmaz B, Parker D, Satterthwaite TD, Elliott MA, Calkins ME, Ruparel K, Gur RE, Gur RC, Verma R. Establishing a link between sex-related differences in the structural connectome and behaviour. Philos Trans R Soc Lond B Biol Sci. 2016:371. doi: 10.1098/rstb.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2011;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Geert P, Van Dijk M. Focus on variability: New tools to study intra-individual variability in developmental data. Infant Behavior and Development. 2002;25:340–374. [Google Scholar]
- Vijayakumar N, Allen NB, Youssef G, Dennison M, Yücel M, Simmons JG, Whittle S. Brain development during adolescence: A mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum Brain Mapp. 2016;37:2027–2038. doi: 10.1002/hbm.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychological Bulletin. 1995;117:250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Wang L, Shen H, Tang F, Zang Y, Hu D. Combined structural and resting-state functional MRI analysis of sexual dimorphism in the young adult human brain: an MVPA approach. Neuroimage. 2012;61:931–940. doi: 10.1016/j.neuroimage.2012.03.080. [DOI] [PubMed] [Google Scholar]
- Williams LM, Mathersul D, Palmer DM, Gur RC, Gur RE, Gordon E. Explicit identification and implicit recognition of facial emotions: I. Age effects in males and females across 10 decades. Journal of Clinical and Experimental Neuropsychology. 2008;19:1–21. doi: 10.1080/13803390802255635. [DOI] [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Hashizume H, Sassa Y, Takeuchi H, Thyreau B, He Y, Evans AC, Li X, et al. Topological organization of functional brain networks in healthy children: differences in relation to age, sex, and intelligence. PLoS One. 2013;8:e55347. doi: 10.1371/journal.pone.0055347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye FQ, Berman KF, Ellmore T, Esposito G, van Horn JD, Yang Y, Duyn J, Smith AM, Frank JA, Weinberger DR, McLaughlin AC. H(2)(15)O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magn Reson Med. 2000;44:450–456. doi: 10.1002/1522-2594(200009)44:3<450::aid-mrm16>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30:15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]