Abstract
As an exceedingly recalcitrant and highly aggressive tumor type without Food and Drug Administration-approved treatment or a known cure, the prognosis of recurrent extensive stage platinum-resistant/refractory small cell lung cancer (SCLC) is worse than other types of lung cancer, and many other tumor types, given a response rate of less than 10% and an overall survival of less than six months. It was broadly classified into three groups based on the initial response to cisplatin/etoposide therapy, platinum-refractory, platinum-resistant, and platinum-sensitive, extensive stage SCLC inevitably relapses, at which point the only standard options are to rechallenge with the first-line chemotherapeutic regimen in the case of sensitive disease or to start the topoisomerase I inhibitor, topotecan. Sensitive disease is defined by a response to the first-line therapy and a treatment-free interval of at least 90 days, while the definitions of refractory and resistant disease, respectively, are nonresponse to the first-line treatment or relapse within 90 days. As an important predictor of response to the second-line treatment, the clinical cutoff of three months (or two months in some cases) for resistant and sensitive disease, which along with performance status prognostically separates patients into high- and low-risk categories, dictates subsequent management. This case report presents a resistant SCLC patient enrolled on a Phase II clinical trial called QUADRUPLE THREAT (formerly TRIPLE THREAT; NCT02489903) who responded to reintroduced platinum doublets after sequential priming with the resistance-reversing epi-immunotherapeutic agent, RRx-001. In the QUADRUPLE THREAT clinical trial, both during priming with RRx-001 and during sequential treatment with platinum doublets, the patient maintained a good quality of life and performance status.
Keywords: resistant SCLC, RRx-001, resistance reversal, resensitization, platinum doublets, epigenetic
Introduction
As a major cause of treatment failure, therapeutic tumor resistance, whether innate or acquired, is mediated by multifactorial compensatory and adaptive events,1 and following the known principle of “fighting fire with fire”, multifactorial inactivation or inhibition is likely required to bypass or reverse it. These multiple events,2 too numerous to list in full here, which include the upregulation of antioxidant response pathways, drug efflux, poor blood flow, hypoxia, acidity, genetic mutations, epigenetic modifications, enhanced DNA repair, quiescence, and inactivation of apoptotic proteins, lead to an insidious drug-resistant state,3 not only to one particular therapeutic regimen per se but also to related ones in a phenomenon known as cross- or pan-resistance,4 resulting in an ever-increasing uphill battle for the treating oncologist. Such pleiotropy is an argument in favor of the use of combination therapy; however, additive or synergistic therapeutic effects may be accompanied by additive or synergistic toxicities, rendering the combination infeasible due to safety concerns and/or poor compliance. An alternative to combination therapy is a multitargeted single agent,5 such as RRx-001.
RRx-001 is a systemically nontoxic reactive oxygen species-mediated epi-immunotherapeutic agent, acting on DNA methyltransferases and histone deacetlyases6 with vascular normalizing properties7 that multifactorially reverse the tumor cell resistance8 and chemo–immuno–radiosensitize the cancer cells.9–11 A lack of toxicity is a major advantage in this context, because approved epigenetic inhibitors, such as the hypomethylating agents, azacitidine and decitabine, which have been administered adjunctively to reverse broad-spectrum chemoresistance, are associated with prolonged dose-limiting myelosuppression and gastrointestinal toxicity even at lower doses.12
In contrast, the nonmyelosuppressive properties of RRx-001,6 which generally lead to the recovery of normal blood counts during treatment, are the basis for a sequential schedule of RRx-001 administered until radiologic progression followed by introduction or reintroduction of the first-line chemotherapy (Fig. 1).
Figure 1.
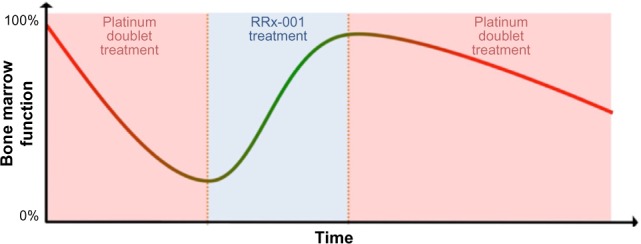
Bone marrow function recovery period with RRx-001 treatment prior to reintroduction of platinum doublets.
In the context of treatment of small cell lung cancer (SCLC), non-SCLC (NSCLC), or high-grade neuroendocrine tumor (HGNET) patients, the first-line therapy is typically a platinum doublet with platinum (cisplatin or carboplatin) matched with etoposide (EP), nab-paclitaxel, or paclitaxel. Food and Drug Administration-approved salvage therapies that would be available to this patient population include docetaxel for NSCLC, topotecan for SCLC, and none for HGNET.
In addition, serial tumor biopsies have demonstrated that RRx-001 initiates an immunologically driven inflammatory and edematous response9,13 and not uncommonly misinterpreted as tumor progression on 6- or 8-week scans,14 which is potentially a further justification for sequential dosing of RRx-001 followed by chemotherapy, since it may take up to 12 weeks to resolve the inflammation and edema and to shrink or stabilize the tumor.
In the four-arm sequentially designed Phase II clinical trial called QUADRUPLE THREAT (formerly TRIPLE THREAT; NCT02489903), patients with SCLC, NSCLC, HGNET, and resistant/refractory ovarian tumors (hence, the QUADRUPLE in the title) are reintroduced to the first-line platinum doublets following disease progression on RRx-001 (Fig. 2).
Figure 2.
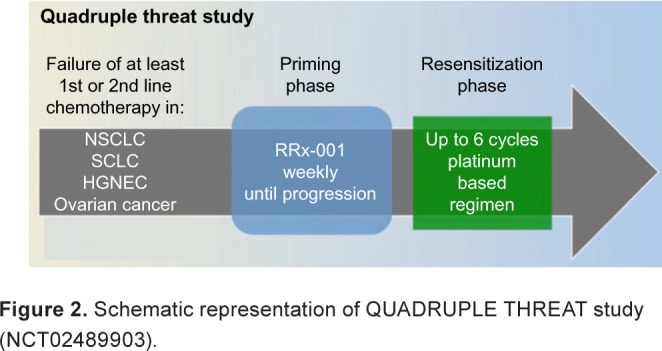
Schematic representation of QUADRUPLE THREAT study (NCT02489903).
Of the tumors studied in this trial, recurrent resistant SCLC (rSCLC) arguably has the most aggressive, drug-resistant phenotype with a response rate (RR) of less than 10%15,16 and an overall survival of less than six months.17 Therefore, resensitization to platinum doublets in this patient population is a virtual impossibility.
Herein, we present an 82-year-old rSCLC patient who achieved a partial response on platinum doublets after 12 weeks of priming with RRx-001 in the QUADRUPLE THREAT clinical trial. After over three decades of no new treatments in SCLC,18 any favorable response is a potentially publishable case, especially in the recurrent-resistant setting,19 where
second-line treatments are not approved (although topotecan is commonly used)20,21;
the five-year survival rate has remained stagnant at 5%;
topotecan yields RRs that range dismally from 6.4% to 8.6%, and the median overall survival has never exceeded 5.7 months22;
rapid clinical deterioration is the rule.
Rather than this expected downward spiral of functional decline, the patient has maintained a good performance status over the course of his participation in the QUADRUPLE THREAT clinical trial.
Case
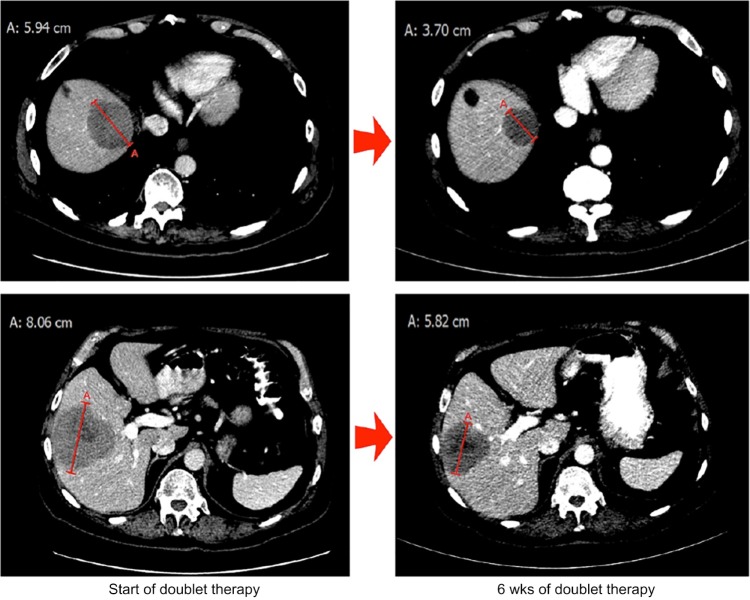
The patient is a 82-year-old white male diagnosed in June 2015 with extrapulmonary SCLC, manifesting HPV-positive small cell carcinoma at the left base of the tongue metastatic to the liver (clinical stage T3, N0, and M1). The patient initially received six cycles of carboplatin–EP on July 7, 2015, but progressed one month after its completion, meeting the definition of resistant disease. The platinum doublets were followed on December 12th, 2015, by palliative radiotherapy (XRT) to the neck. On January 14, 2016, having histologically or cytologically confirmed SCLC with a metastatic liver lesion as the primary lesion, the patient enrolled on NCT02489903 and began weekly intravenous treatment with 4 mg RRx-001 coin-fused with autologous blood. At the time of enrollment, his performance status was ECOG 1 with fatigue and decreased appetite as his chief complaints. Within one week of starting RRx-001, the patient reported normalization of energy and appetite, resulting in an improvement of general demeanor and level of alertness. No systemic side effects related to treatment were observed. The first six-week restaging computerized tomography (CT) scan demonstrated stable disease, which resulted in the continuation of therapy. However, the 12-week CT scan demonstrated progressive disease per RECIST v.1.1 criteria. At this point, per protocol, RRx-001 was discontinued, and carboplatin–EP was reintroduced with the plan to repeat CT scans after every two cycles (six weeks). To date, the EP regimen has been very well tolerated with no myelosuppression. The CT scan at week 6 was classified as a partial response with a 32% reduction in target liver lesions (Fig. 3).
Figure 3.
CT scan performed on August 24, 2011, demonstrating liver metastases with the maximum diameters of 5.94 and 8.06 cm (left) that showed significant shrinkage to 3.70 and 5.82 cm (right).
The management plan for this patient is to continue treatment with carboplatin–EP for up to six cycles, with repeat CT every six weeks until disease progression or intolerable toxicity.
Discussion
Progress in SCLC, a relentlessly aggressive, rapidly metastasizing, and highly fatal neoplasm,23 has remained lamentably stagnant for decades, and an active, nontoxic chemotherapeutic regimen, especially in the resistant/recurrent setting, is notably lacking for this disease. The rarity of desirable responses in rSCLC coupled with a lack of effective, nontoxic therapies is the motivation for this case report, which describes clinical benefit in the form of a >30% reduction in tumor sizes after only two cycles (six weeks) of reintroduced platinum therapy following a priming period with RRx-001 and maintenance/improvement of ECOG performance status. While it is manifestly too early to generalize and extrapolate the efficacy of RRx-001 in SCLC from this patient, partial responses have also been reported for two other rSCLC patients in the QUADRUPLE THREAT trial,9 which suggests a developing trend.
Two of the key RRx-001 antiresistance mechanisms are epigenetic inhibition24 and immune stimulation9,13; however, a hallmark of any successful sensitizer is target promiscuity, and RRx-001, which is no exception,25 also possesses prooxidant, apoptotic,24,26 antiangiogenic,27 and P-gp inhibitory properties. Recently published case reports of partial responses in NSCLC13,28 in the QUADRUPLE THREAT trial raise the possibility that RRx-001 may broadly alter the multidrug resistance phenotype, sensitizing or resensitizing heavily pretreated patients with platinum-based resistance in other non-thoracic tumor types where doublet regimens are approved or considered the mainstay of care, such as gastroesophageal, transitional cell and bladder cancer, cervical, testicular, and head and neck cancers. Additional studies are needed in support of this hypothesis and early observations.
Conclusion
This case report describes a new rechallenge strategy with RRx-001 in the management of a patient with recurrent SCLC/rSCLC, a tumor type known for its poor clinical response to chemotherapy. Additional cases are needed to determine whether RRx-001 is beneficial in SCLC.
Statement of Ethics
The patient described in this case report has given his written, informed consent to participate in the TRIPLE THREAT clinical study (NCT02489903), and for publication of details and images of his case in this report. This study protocol has been approved by the Walter Reed National Military Medical Center Institutional Review Board. The research was conducted in accordance with the principles of the Declaration of Helsinki.
Footnotes
ACADEMIC EDITOR: William Chi-shing Cho, Editor in Chief
PEER REVIEW: Seven peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1264 words, excluding any confidential comments to the academic editor.
FUNDING: The clinical trial in which this case occurred was funded by EpicentRx, the makers of RRx-001. EpicentRx received funding for the trial from InterWest Partners. BO, SC, JS and CL are employees of EpicentRx. AO is an investor with InterWest Partners and board member of EpicentRx.
COMPETING INTERESTS: BO, SC, JS and CL are employees of EpicentRx. AO is an investor with InterWest Partners and a board member of Applied Genetic Technologies Corporation (AGTC), Centrexion, Drais Pharmaceuticals, Dynavax Technologies (DVAX), EpicentRx, Integrated Diagnostics, PMV Pharma, Potenza Theraputics, Sera Prognostics, TESARO (TSRO) and Tizona Therapeutics. Other authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: BO, SC, CC. Analyzed the data: BO, SC, KZ, MQ, CB, JS, PC, TRR, JBT, NDA, CL, AO, HEL, RMD, CAC. Wrote the first draft of the manuscript: BO. Contributed to the writing of the manuscript: BO, SC, JS, CC. Agree with manuscript results and conclusions: BO, SC, KZ, MQ, CB, JS, PC, TRR, JBT, NDA, CL, AO, HEL, RMD, CAC. Jointly developed the structure and arguments for the paper: BO, SC, KZ, MQ, CB, JS, PC, TRR, JBT, NDA, CL, AO, HEL, RMD, CAC. Made critical revisions and approved final version: BO, SC. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Hazlehurst LA, Foley NE, Gleason-Guzman MC, et al. Multiple mechanisms confer drug resistance to mitoxantrone in the human 8226 myeloma cell line. Cancer Res. 1999;59(5):1021–8. [PubMed] [Google Scholar]
- 2.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 3.Bailey FP, Andreev VI, Eyers PA. The resistance tetrad: amino acid hotspots for kinome-wide exploitation of drug-resistant protein kinase alleles. Methods Enzymol. 2014;548:117–46. doi: 10.1016/B978-0-12-397918-6.00005-7. [DOI] [PubMed] [Google Scholar]
- 4.Brouse C, Ortiz D, Su Y, Oronsky B, Scicinski J, Cabrales P. Impact of hemoglobin nitrite to nitric oxide reductase on blood transfusion for resuscitation from hemorrhagic shock. Asian J Transfus Sci. 2015;9(1):55–60. doi: 10.4103/0973-6247.150952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mencher SK, Wang LG. Promiscuous drugs compared to selective drugs (promiscuity can be a virtue) BMC Clin Pharmacol. 2005;5:3. doi: 10.1186/1472-6904-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid T, Oronsky B, Scicinski J, et al. Safety and activity of RRx-001 in patients with advanced cancer: a first-in-human, open-label, dose-escalation phase 1study. Lancet Oncol. 2015;16(9):1133–42. doi: 10.1016/S1470-2045(15)00089-3. [DOI] [PubMed] [Google Scholar]
- 7.Carter CA, Oronsky BT, Caroen SZ, et al. Partial response to platinum doublets in refractory EGFR-positive non-small cell lung cancer patients after RRx-001: evidence of episensitization. Case Rep Oncol. 2016;9(1):62–7. doi: 10.1159/000443725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das DS, Ray A, Das A, et al. A novel hypoxia-selective epigenetic agent RRx-001 triggers apoptosis and overcomes drug resistance in multiple myeloma cells. Leukemia. 2016 May 24; doi: 10.1038/leu.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter CA, Schmitz B, Peterson PG, et al. Immune reactivity and pseudoprogression or tumor flare in a serially biopsied neuroendocrine patient treated with the epigenetic agent RRx-001. Case Rep Oncol. 2016;9(1):164–70. doi: 10.1159/000444633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MM, Parmar H, Cao Y, et al. Whole brain radiotherapy and RRx-001: two partial responses in radioresistant melanoma brain metastases from a phase I/II clinical trial: a TITE-CRM phase I/II clinical trial. Transl Oncol. 2016;9(2):108–13. doi: 10.1016/j.tranon.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MM, Parmar H, Cao Y, et al. Concurrent whole brain radiotherapy and RRx-001 for melanoma brain metastases. Neuro Oncol. 2016;18(3):455–6. doi: 10.1093/neuonc/nov317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karahoca M, Momparler RL. Pharmacokinetic and pharmacodynamic analysis of 5-aza-2′-deoxycytidine (decitabine) in the design of its dose-schedule for cancer therapy. Clin Epigenetics. 2013;5(1):3. doi: 10.1186/1868-7083-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brzezniak C, Schmitz BA, Peterson PG, et al. RRx-001-induced tumor necrosis and immune cell infiltration in an EGFR mutation-positive NSCLC with resistance to EGFR tyrosine kinase inhibitors: a case report. Case Rep Oncol. 2016;9(1):45–50. doi: 10.1159/000443605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oronsky B, Carter CA, Reid TR, et al. Confirmatory trials in the evaluation of anticancer medicinal products in man – pfs2: a measure of therapeutic action-at-a-distance. Neoplasia. 2015;17(9):716–22. doi: 10.1016/j.neo.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glisson BS. Recurrent small cell lung cancer: update. Semin Oncol. 2003;30(1):72–8. doi: 10.1053/sonc.2003.50014. [DOI] [PubMed] [Google Scholar]
- 16.Ardizzoni A. Topotecan in the treatment of recurrent small cell lung cancer: an update. Oncologist. 2004;9(Suppl 6):4–13. doi: 10.1634/theoncologist.9-90006-4. [DOI] [PubMed] [Google Scholar]
- 17.Hagmann R, Hess V, Zippelius A, Rothschild SI. Second-line therapy of small-cell lung cancer: topotecan compared to a combination treatment with adriamycin, cyclophosphamide and vincristine (ACO) – a single center experience. J Cancer. 2015;6(11):1148–54. doi: 10.7150/jca.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scientific Framework for Small Cell Lung Cancer (SCLC) 2014. [Accessed May 5, 2016]. Available at: http://deainfo.nci.nih.gov/advisory/ctac/workgroup/SCLC/SCLCCongressionalResponse.pdf.
- 19.Ardizzoni A, Tiseo M, Boni L. Validation of standard definition of sensitive versus refractory relapsed small cell lung cancer: a pooled analysis of topotecan second-line trials. Eur J Cancer. 2014;50(13):2211–8. doi: 10.1016/j.ejca.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Tiseo M, Ardizzoni A. Current status of second-line treatment and novel therapies for small cell lung cancer. J Thorac Oncol. 2007;2(8):764–72. doi: 10.1097/JTO.0b013e3180986262. [DOI] [PubMed] [Google Scholar]
- 21.Inoue A, Sugawara S, Yamazaki K, et al. Randomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402. J Clin Oncol. 2008;26(33):5401–6. doi: 10.1200/JCO.2008.18.1974. [DOI] [PubMed] [Google Scholar]
- 22.Huber RM, Reck M, Gosse H, et al. Efficacy of a toxicity-adjusted topotecan therapy in recurrent small cell lung cancer. Eur Respir J. 2006;27(6):1183–9. doi: 10.1183/09031936.06.00015605. [DOI] [PubMed] [Google Scholar]
- 23.Maruno K, Absood A, Said SI. Vasoactive intestinal peptide inhibits human small-cell lung cancer proliferation in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95(24):14373–8. doi: 10.1073/pnas.95.24.14373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H, Ning S, Scicinski J, Oronsky B, Knox SJ, Peehl DM. Epigenetic effects of RRx-001: a possible unifying mechanism of anticancer activity. Oncotarget. 2015;6(41):43172–81. doi: 10.18632/oncotarget.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oronsky BT, Carter CA, Oronsky AL, Salacz ME, Reid T. “No patient left behind”: an alternative to “the War on Cancer” metaphor. Med Oncol. 2016;33(6):55. doi: 10.1007/s12032-016-0769-1. [DOI] [PubMed] [Google Scholar]
- 26.Ning S, Bednarski M, Oronsky B, Scicinski J, Saul G, Knox SJ. Dinitroazetidines are a novel class of anticancer agents and hypoxia-activated radiation sensitizers developed from highly energetic materials. Cancer Res. 2012;72(10):2600–8. doi: 10.1158/0008-5472.CAN-11-2303. [DOI] [PubMed] [Google Scholar]
- 27.Das D, Ray A, Song Y, et al. A novel hypoxia-selective epigenetic agent RRx-001 triggers apoptosis and overcomes drug resistance in multiple myeloma cells. Leukemia. 2016 doi: 10.1038/leu.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrouk N, Reid TR, Fisher GA, et al. Statistical analysis of episensitization using transition probability functions and PFS2 for “ROCKET”, a two stage phase II study of RRx-001, a multi-epigenetic agent, investigating resensitization to irinotecan in colorectal cancer. J Clin Oncol. 2016;34(Suppl 4S) abstr TS775. [Google Scholar]