Abstract
PTP1B is involved in the oncogenesis of breast cancer. In addition, neoadjuvant therapy has been widely used in breast cancer; thus, a measurement to assess survival improvement could be pathological complete response (pCR). Our objective was to associate PTP1B overexpression with outcomes of breast cancer patients who underwent neoadjuvant chemotherapy. Forty-six specimens were included. Diagnostic biopsies were immunostained using anti-PTP1B antibody. Expression was categorized as negative (<5%) and overexpression (≥5%). Patients’ responses were graded according to the Miller–Payne system. Sixty-three percent of patients overexpressed PTP1B. There was no significant association between PTP1B overexpression and pCR (P = 0.2). However, when associated with intrinsic subtypes, overexpression was higher in human epidermal growth factor receptor 2-positive-enriched specimens (P = 0.02). Ten-year progression-free survival showed no differences. Our preliminary results do not show an association between PTP1B over-expression and pCR; however, given the limited sample and heterogeneous treatment in our cohort, this hypothesis cannot be excluded.
Keywords: PTP1B protein, breast cancer, neoadjuvant therapy
Introduction
Breast cancer is one of the most common cancers in women around the world, with a higher incidence in developing countries.1,2 Breast cancer treatment is multimodal, and although it has been demonstrated that there is no difference in outcomes when comparing adjuvant and neoadjuvant therapies,3–5 the latter is currently the standard treatment for large and locally advanced tumors, allowing conservative surgeries without compromising the results in overall survival (OS). In addition, prognostic and treatment options can be influenced by clinical and pathological characteristics of breast cancer given the heterogeneity of this condition.6,7 Breast cancer molecular subtypes were originally defined by their genetic profile8,9 but can be assessed using immunohistochemistry for estrogen receptor (ER), progesterone receptor, Ki67, and erbB-2 receptor protein-tyrosine kinase (PTK; human epidermal growth factor receptor 2 (HER2)).10,11 These subtypes are known for their different epidemiologic risk factors and response to treatment.10 On the other hand, pathological complete response (pCR) is a surrogate marker for outcomes in breast cancer. According to Cortazar et al,12 a pCR of 18% was achieved in all subtypes, with a higher response in luminal B (HER2+) (30%), triple-negative (34%), and no luminal HER2+ (50%) subtypes, showing a pCR <10% in luminal A subtype.
In 1981, the World Health Organization (WHO) first published tumor response criteria, mainly for use in trials where tumor response was the primary endpoint. The WHO criteria introduced the concept of an overall assessment of tumor burden by summing the products of dimensional lesion measurements and determined response to therapy by evaluation of change from baseline while on treatment.13 However, in the decades that followed their publication, cooperative groups and pharmaceutical companies that used these criteria often made some modifications, which led to confusion in the interpretation of trial results,14 and the application of varying response criteria led to very different conclusions about the efficacy of the same regimen.15 In response to these problems, an International Working Party was formed in the mid-1990s to standardize and simplify response criteria. The Response Evaluation Criteria in Solid Tumors (RECIST) was introduced in 2000 by an International Working Party to standardize and simplify tumor response criteria.16 RECIST has subsequently been widely accepted as a standardized measure of tumor response, particularly in oncology clinical trials in which the primary endpoints are objective response or time to progression.17 pCR after chemotherapy in primary breast cancer tumors has been an important prognostic factor for survival.18 Despite a high specificity using imaging methods, sensitivity remains low, since not all patients with clinical response achieve a pCR, making it necessary to evaluate other measurement methods based on predicting factors.19
Furthermore, when patients do not achieve a pCR to chemotherapy, the response has to be classified according to a grading system. There is no reference definition for pathological response. Miller and Payne grading system can predict OS and disease-free interval in patients with large and locally advanced breast cancers, assessing the histological response to primary chemotherapy. They reported a significant correlation between pathological response using this new grading system and OS (P = 0.02).20 However, a standard validated method for assessing pathological response in specimens after neoadjuvant therapy has not been established.
Protein phosphorylation on tyrosine residues is an important event in post-transduction signaling of cells in order to regulate diverse responses. The regulation during these processes is crucial to maintain several biological effects, such as cellular proliferation, differentiation, migration, and apoptosis. PTP1B, a non-transmembrane member of this family, has long been studied as a negative regulator of insulin and leptin signaling, but has recently received renewed attention as a factor in oncogenesis.21,22 Supporting a possible oncogenic role of PTP1B in breast cancer is the finding that overexpression of PTP1B in the mouse mammary gland leads to spontaneous mammary tumor development, suggesting that this tyrosine phosphatase can act as an oncogene on its own.23 In human beings, overexpression of PTP1B in breast epithelial cells distorts the normal acinar morphology and causes uninhibited proliferation and loss of polarity.24 PTP1B has been potentially associated with the HER2 and its modulation of signaling. Wiener et al25 reported that 21 out of 29 human breast cancers stained strongly for PTP1B when compared to normal breast tissue. This strong association between PTP1B and HER2 expression suggests that PTP1B and HER2 may cooperate in the pathogenesis of this subtype of breast cancers. More recently, Soysal et al26 reported that PTP1B expression in breast cancer is associated with significantly improved clinical outcome.
To further explore the role of PTP1B in human breast cancer, we conducted an immunohistochemical study using 46 formalin-fixed breast cancer tissues of patients who underwent neoadjuvant chemotherapy, with clinical information and outcome data. The aim of our study was to investigate the association between PTP1B overexpression and pCR to study the impact of PTP1B on prognosis.
Materials and Methods
Breast cancer patients treated with neoadjuvant therapy, identified in a national health institute in Mexico City, Mexico (Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán), from 2003 to 2015, with available paraffin-embedded initial diagnostic biopsy and surgical specimens (mastectomy/conservative surgery) were included. This protocol was approved by the institutional research and ethics committees under the register HEM-1501.
Data collection
All the information was collected from the hospital archives. Electronic records, including imaging and pathology, were also revised.
Immunohistochemistry
Immunohistochemical study was performed on formalin-fixed, paraffin-embedded breast cancer tissue using a polyclonal anti-PTP1B antibody (H-135: SC-14021, Santa Cruz Biotechnology) in a dilution of 1:200. Spleen was used as positive control tissue. No negative control was used. Tissues were sectioned at 5 μm and floated on distilled water at 45°C. Sections were mounted on chemically charged glass slides followed by drying at room temperature and incubated overnight at 57°C. The sections were deparaffinized according to established guidelines and quenched with 3% hydrogen peroxide for five minutes. They were rinsed in distilled water followed by Tris-buffered saline. Afterward, heat-induced antigen retrieval with citrate buffer at pH 6 was performed for 30 minutes followed by 15-minute cool-down period. Standard Dako EnVision + System-labeled Polymer Anti-rabbit + Liquid DAB + Substrate Chromogen System (DAB) technique was performed. Slides were developed, rinsed with distilled water, and counterstained with hematoxylin solution. Slides were mounted using aqueous solution, and the percentage of cells with a distinctive strong cytoplasmic staining was estimated by a light microscope.
Evaluation of biopsies
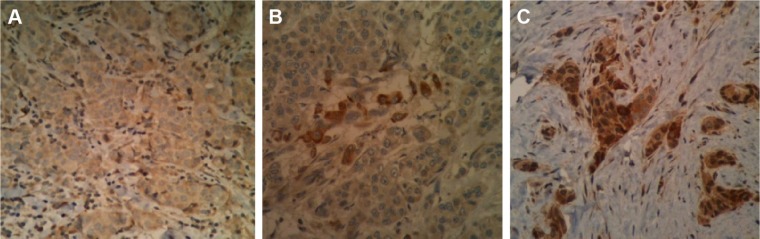
The percentage of cells with a strong PTP1B cytoplasmic staining was estimated by two independent pathologists. All cases with a cytoplasmic PTP1B expression of <5% were considered negative (Fig. 1A), and ≥5% were considered positive (overexpression). Posteriorly, pathologic response to neoadjuvant chemotherapy was evaluated in specimens from the surgical procedure by two independent pathologists, which was graded according to Miller and Payne grading system (Tables 1 and 2). pCR was defined as absence of residual tumor in breast and axillary lymph nodes. Also, breast cancer subtypes were reanalyzed in the available immunostained biopsies.
Figure 1.
Cytoplasmic expression of PTP1B: (A) negative (<5%) and (B) and (C) overexpression (≥5% and >50%, respectively).
Table 1.
Miller and Payne grading system for evaluation of breast tissue.20
| GRADE | DESCRIPTION |
|---|---|
| G5 | No evidence of residual tumor |
| G4 | Microscopic foci of invasive carcinoma |
| G3 | Marked reduction of invasive tumor (30%–90%) |
| G2 | Discrete reduction of invasive tumor (<30%) |
| G1 | Minimal changes in invasive tumor |
Table 2.
Miller and Payne grading system for evaluation of lymph nodes.20
| GRADE | DESCRIPTION |
|---|---|
| A | Negative lymph nodes without changes due to chemotherapy |
| B | Positive lymph nodes without changes due to chemotherapy |
| C | Positive lymph nodes with evidence of partial response |
| D | Negative lymph nodes due to chemotherapy |
Statistical analysis
Patients’ clinical and histological characteristics between PTP1B-positive and PTP1B-negative tumors were compared using chi-square test, Wilcoxon rank-sum test, or two-sample t-test, as appropriate. The association between PTP1B expression and other factors was also described using Spearman’s correlation (ρ). OS was defined as the time from treatment initiation to death due to any cause. Survivors were censored at the date of last contact. Progression-free survival (PFS) was defined as the time from treatment to relapse. Survival curves by PTP1B status were estimated using the Kaplan–Meier method and compared by log-rank test. All analyses were two sided, and significance was set at a P value of 0.05. Statistical analyses were performed using SPSS v.21.
Results
The final analysis included 46 breast cancer patients treated with neoadjuvant therapy based on the sequence of paclitaxel and doxorubicin plus cyclophosphamide.
The mean age at diagnosis was 55.4 years (range 32–80 years). Fifty-two percent of patients were diagnosed and treated after 2011. The mean tumor size at diagnosis was 3.5 cm (range 0.5–20 cm). Twenty-one patients (46%) presented positive lymph nodes demonstrated by imaging or physical examination, and 50% of patients had tumors of stage IIA. The most common breast cancer histological subtype was invasive ductal carcinoma (85%), followed by invasive lobular carcinoma (9%) and ductal carcinoma in situ (4%; Table 3).
Table 3.
Patients’ demographic data.
| DEMOGRAPHIC DATA | |
|---|---|
| Clinic or pathological parameter | |
| Mean age at diagnosis (years) | 55.4 (32–80) |
| Diagnosis after 2011 | 24 (52%) |
| Mean of tumor size at diagnosis (cm) | 3.5 (0.5–20) |
| n (%) | |
| Clinical stage | |
| IA | 2 (4) |
| IIA | 23 (50) |
| IIB | 8 (18) |
| IIIA | 5 (11) |
| IIIB | 6 (13) |
| Unknown | 2 (4) |
| Tumor grade | |
| 1 | 10 (22) |
| 2 | 18 (39) |
| 3 | 17 (37) |
| Unknown | 1 (2) |
| Pathologic stage (tumor) | |
| pTis | 1 (2) |
| pTmic | 2 (4) |
| pT1a | 2 (4) |
| pT1b | 7 (15) |
| pT1c | 10 (22) |
| pT2 | 10 (22) |
| pT3 | 2 (4) |
| pCR | 12 (26) |
| Histological subtype | |
| DCIS | 0 |
| LCIS | 2 (4) |
| IDC | 39 (85) |
| ILC | 4 (9) |
| Other | 1 (2) |
| Lymph nodes at diagnosis | |
| + | 21 (46) |
| − | 20 (43) |
| Unknown | 5 (11) |
| Molecular subtype | |
| Luminal A | 16 (35) |
| Luminal B | 11 (24) |
| HER2+ | 10 (22) |
| Triple negative | 8 (17) |
| Unknown | 1 (2) |
Consistent with the known cytoplasmic localization of PTP1B, breast cancers expressing this phosphatase showed strong PTP1B staining (≥5% and >50%, as shown in Fig. 1B and C). Twenty-nine patients (63%) overexpressed PTP1B (Table 4). PTP1B expression was not associated with tumor size, lymph node status, tumor grade, and pathological or clinical stages (Table 5). However, statistical significance was found when comparing age in PTP1B overexpression and PTP1B negative groups (59 vs 50 years, respectively, P = 0.015). It is important to mention that overexpression of PTP1B was not associated with ER status (57% ER+ vs 39% ER−, P = 0.62, Spearman’s P = 0.075) or HER2 status (36% HER2+ vs 61% HER2−, P = 0.9, Spearman’s P = 0.19; Table 5).
Table 4.
PTP1B cytoplasmic expression.
| PTP1B EXPRESSION | n (%) |
|---|---|
| Negative (<5%) | 17 (37) |
| Overexpression (≥5%) | 29 (63) |
Table 5.
Association of PTP1B overexpression and clinical/pathological parameters.
| PARAMETER | PTP1B OVEREXPRESSION | PTP1B NEGATIVE | SPEARMAN’S P | P |
|---|---|---|---|---|
| Mean of tumor size at diagnosis (cm) | 2.9 | 4.4 | −0.195 | 0.22 |
| Mean age at diagnosis | 59 | 50 | 0.35 | 0.015 |
| n (%) | n (%) | SPEARMAN’S P | P | |
| Clinical stage | 0.165 | 0.29 | ||
| I–IIA | 13 (46) | 12 (67) | ||
| IIB–IIIA/B | 13 (46) | 6 (33) | ||
| Tumor grade | −0.159 | 0.28 | ||
| 1 | 6 (21) | 4 (22) | ||
| 2 | 14 (50) | 4 (22) | ||
| 3 | 8 (29) | 9 (50) | ||
| Perineural invasion | 0.12 | 0.48 | ||
| IPN+ | 3 (11) | 1 (6) | ||
| IPN− | 18 (64) | 14 (78) | ||
| Lymphovascular invasion | 0.16 | 0.32 | ||
| ILV+ | 8 (29) | 3 (17) | ||
| ILV− | 16 (57) | 13 (72) | ||
| ERs | 0.075 | 0.62 | ||
| ER+ | 16 (57) | 12 (67) | ||
| ER− | 11 (39) | 6 (33) | ||
| HER2 | −0.019 | 0.9 | ||
| HER2+ | 10 (36) | 7 (39) | ||
| HER2− | 17 (61) | 11 (61) |
Moreover, PTP1B overexpression was associated with the subtypes of breast cancer, according to those defined at St. Gallen. PTP1B overexpression was higher in HER2-enriched subtypes (90%, P = 0.026; Table 6).
Table 6.
Association of PTP1B overexpression and molecular subtypes of breast cancer.
| MOLECULAR SUBTYPE | PTP1B OVEREXPRESSION n (%) | PTP1B NEGATIVE n (%) | P |
|---|---|---|---|
| Luminal A HER2+ | 0 | 3 (100) | 0.026 |
| Luminal A HER2− | 10 (77) | 3 (23) | |
| Luminal B HER2+ | 3 (75) | 1 (25) | |
| Luminal B HER2− | 4 (57) | 3 (43) | |
| Triple negative | 3 (37) | 5 (63) | |
| HER2+ enriched | 9 (90) | 1 (10) |
Breast pathological response was as follows: G1 9% (n = 4), G2 24% (n = 11), G3 26% (n = 12), G4 13% (n = 6), and G5 28% (n = 13). Axillary pathological response was as follows: A 11% (n = 5), B 7% (n = 3), C 17% (n = 8), and D 24% (n = 11). Ten patients (22%) achieved pCR (Miller and Payne G5 + A or D; Table 7). There was no significant association between PTP1B overexpression and grades of pathological response in breast and axillary lymph nodes (P = 0.58 and P = 0.87, respectively; Table 8).
Table 7.
Pathological response (Miller and Payne grading system).
| MILLER AND PAYNE GRADING SYSTEM | |||
|---|---|---|---|
| BREAST | AXILLARY LYMPH NODES | ||
| PATHOLOGICAL RESPONSE GRADE | n (%) | PATHOLOGICAL RESPONSE GRADE | n (%) |
| G1 | 4 (9) | 0 | 19 (41) |
| G2 | 11 (24) | A | 5 (11) |
| G3 | 12 (26) | B | 3 (7) |
| G4 | 6 (13) | C | 8 (17) |
| G5 | 13 (28) | D | 11 (24) |
Table 8.
Association between PTP1B expression and pathological response in breast and axillary lymph nodes.
| PTP1B EXPRESSION | PATHOLOGICAL RESPONSE (BREAST) | ||||
|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | |
| Negative | 2 (4) | 3 (6.5) | 4 (9) | 2 (4) | 6 (13) |
| Overexpression | 2 (4) | 8 (17.5) | 8 (18) | 4 (9) | 7 (15) |
| PATHOLOGICAL RESPONSE (AXILLARY LYMPH NODES) | |||||
| A | B | C | D | 0 | |
| Negative | 2 (4.3) | 1 (2) | 2 (4.3) | 5 (11) | 7 (15.3) |
| Overexpression | 3 (6.3) | 2 (4.3) | 6 (13) | 6 (13) | 12 (26.5) |
PTP1B overexpression (≥5%) and pCR (G5 + A or D) were associated, showing that 50% of patients who overexpressed PTP1B had no pCR compared to 28% of the PTP1B negative group (P = 0.3; Table 9).
Table 9.
Association of overexpression of PTP1B and pCR (P = 0.3).
| PTP1B OVEREXPRESSION | pCR | |
|---|---|---|
| YES n (%) | NO n (%) | |
| Yes n (%) | 6 (13) | 22 (48) |
| No n (%) | 6 (13) | 12 (26) |
The median follow-up was 41 months (range 5–116 months). Neither nine-year OS curve (92 vs 91%, respectively, P = 0.9; Fig. 2) nor nine-year PFS (55 vs 77%, respectively, P = 0.9; not shown) was statistically significant when comparing patients with PTP1B overexpression vs PTP1B negative.
Figure 2.
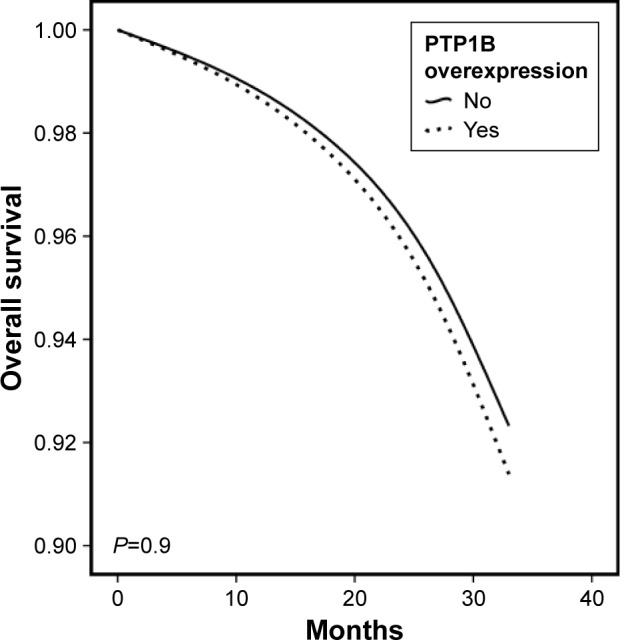
Nine-year OS depending on PTP1B overexpression (dotted line: PTP1B overexpression and solid line: negative PTP1B expression; P = 0.9).
Discussion
PTP1B is a widely expressed non-receptor PTP that is localized on intracellular membranes via a hydrophobic C-terminal targeting sequence. A role for PTP1B in the regulation of many cellular functions has been suggested.27 Clinically, PTP1B is a critical node of insulin signaling due to its ability to dephosphorylate and inactivate the insulin receptor, thereby switching off this pathway.28,29 In fact, PTP1B-deficient mice are a unique model of insulin hypersensitivity due to enhanced insulin action.30–32 PTP1B is also involved in the control of immune cell signaling.33,34
PTP1B expression and its activity have been associated with the oncogenesis of diverse types of cancers such as esophagus, ovarian, colon, and breast cancers.25,35–38 In 1994, an association of the overexpression of PTP1B was first reported in a small cohort of patients with breast cancer.25 However, the exact biological mechanism by which PTP1B facilitates breast cancer progression remains unknown. Previous studies in murine models have shown that the absence of PTP1B in mammary tumors (NDL2/PTP1B−/−) confers a delay in tumor development and a decreased frequency of lung metastases.22 In breast cancer cells, a poor clinical outcome is associated with the amplification or expression of some PTK factors. It has been suggested that PTP1B is necessary for implantation and progression of mammary tumors.39 PTP1B overexpression has also been associated with HER2+ and with a more aggressive breast cancer phenotype.40 In human beings, there is no evidence of PTP1B as a prognostic factor. The existent data report the amplification of chromosome 20q13, where PTP1B gene is located, and it is demonstrated that the amplification of this region is associated with a poor prognosis in breast cancer.41–43 More recently, Soysal et al26 published that 49% of patients with breast cancer express PTP1B and this was associated with a significant improvement in OS; however, we could not confirm this finding in our cohort (P = 0.9).
Our study shows that PTP1B is overexpressed in 63% of breast cancer, which is consistent with previous reports (49–72.4%).25,26 Furthermore, our results did not show an association between PTP1B overexpression and tumor size at diagnosis, tumor grade, clinical stage, lymphovascular or perineural invasion, HER2, or ER. Interestingly, the difference between age at diagnosis was statistically significant when comparing patients overexpressing PTP1B and negative patients (59 vs 50 years, respectively, P = 0.015). In our cohort, breast cancer subtypes were analyzed separately, showing PTP1B overexpression in a significant fraction, more evident in the HER2+-enhanced group (90%, P = 0.02).
The association between PTP1B expression in breast cancer and treatment based on two taxanes, paclitaxel and docetaxel, has been studied in vitro. The expression of this phosphatase correlated with the opposite sensitivity to these drug agents.44 However, the molecular mechanism of this effect is not yet known. Both taxanes act by disrupting the function of microtubules.45 Microtubule instability is necessary for cellular functions and is regulated by microtubule-associated proteins (MAPs), which in their dephosphorylating state bind to microtubules stabilizing them45–48 and act as a coupling site for some kinases and phosphatases.48,49 MAPs/microtubule affinity-regulating kinases (MARKs), which regulate phosphorylation state of MAPs, are regulated by two phosphatases: phosphatase 2A and PTP1B.46 It is possible that an increase in PTP1B levels conditions a major dynamic instability due to MARK dephosphorylation and, at the same time, a chemoresistance to paclitaxel.45,50
Our study could not associate PTP1B overexpression and pCR in breast cancer patients treated with neoadjuvant chemotherapy (P = 0.3). An important factor is that half of our cohort was diagnosed and treated before 2011, prior to the introduction of a government health insurance program, which provided breast cancer treatment including trastuzumab. The non-statistically significant results in our cohort could be due to 48% of our patients not receiving the optimal treatment due to high costs and also due to our small sample size.
Finally, recent in vitro studies are investigating the role of PTP1B and its interaction with other proteins51–53 involved in breast cancer oncogenesis,54,55 which eventually could help establish correlations between non-clinical and clinical settings.
Importantly, we emphasize that, at present, evaluation of pathological response is not yet standardized, making it important to continue evaluating prognostic and predictive markers in breast cancer, especially because pCR associates with better OS in some subtypes treated with neoadjuvant chemotherapy.56,57
Conclusion
Given our results and the results of other two previously reported studies,25,26 PTP1B is overexpressed in >50% of breast cancer patients; hence, it seems that this phosphatase plays an important role in breast cancer. It is necessary to have more studies, preferably in a large cohort, evaluating its role in different aspects of breast cancer, including chemosensitization.
Acknowledgments
We thank the Oncology Department for acquiring the PTP1B Antibody essential for conducting our research.
Footnotes
ACADEMIC EDITOR: Goberdahn P. Dimri, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 997 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: BMB. Contributed to the biostatistical analysis: AAA. Analyzed the data: MMRF. Wrote the first draft of the manuscript: MMRF. Contributed to the writing of the manuscript: ELR. Agreed with manuscript results and conclusions: MMRF, ELR, BMB, LGVR, MdlLSG, AAA. Jointly developed the structure and arguments for the article: MMRF. Made critical revisions and approved the final version: ELR. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378(9801):1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 3.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 5.Mieog JSD, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. 2007;18(2):CD005002. doi: 10.1002/14651858.CD005002.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson JF, Gray R, Dressler LG, et al. Prognostic value of histologic grade and proliferative activity in axillary node-positive breast cancer: results from the Eastern Cooperative Oncology Group Companion Study, EST 4189. J Clin Oncol. 2000;18(10):2059–2069. doi: 10.1200/JCO.2000.18.10.2059. [DOI] [PubMed] [Google Scholar]
- 7.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11(16):5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 8.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 9.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5(1):5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes: dealing with the diversity of breast cancer—highlights of the St. Gallen International Expert Consensus on the primary therapy of early breast cancer. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blows FM, Driver KE, Schmidt MK, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7(5):e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 13.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Tonkin K, Tritchler D, Tannock I. Criteria of tumor response used in clinical trials of chemotherapy. J Clin Oncol. 1985;3(6):870–875. doi: 10.1200/JCO.1985.3.6.870. [DOI] [PubMed] [Google Scholar]
- 15.Baar J, Tannock I. Analyzing the same data in two ways: a demonstration model to illustrate the reporting and misreporting of clinical trials. J Clin Oncol. 1989;7(7):969–978. doi: 10.1200/JCO.1989.7.7.969. [DOI] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Kuerer HM, Newman LA, Buzdar AU, et al. Pathologic tumor response in the breast following neoadjuvant chemotherapy predicts axillary lymph node status. Cancer J Sci Am. 1998;4(4):230–236. [PubMed] [Google Scholar]
- 19.Mukherjee P, Sharma S, Sheikh ZA, Vijaykumar DK. Correlation of clinicopathologic and radiologic parameters of response to neoadjuvant chemotherapy in breast cancer. Indian J Cancer. 2014;51(1):25–29. doi: 10.4103/0019-509X.134610. [DOI] [PubMed] [Google Scholar]
- 20.Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12(5):320–327. doi: 10.1016/s0960-9776(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 21.Woodford-Thomas TA, Rhodes JD, Dixon JE. Expression of a protein tyrosine phosphatase in normal and v-src-transformed mouse 3T3 fibroblasts. J Cell Biol. 1992;117(2):401–414. doi: 10.1083/jcb.117.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bentires-Alj M, Neel BG. Protein-tyrosine phosphatase 1B is required for HER2/Neu-induced breast cancer. Cancer Res. 2007;67(6):2420–2424. doi: 10.1158/0008-5472.CAN-06-4610. [DOI] [PubMed] [Google Scholar]
- 23.Julien SG, Dubé N, Read M, et al. Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis. Nat Genet. 2007;39(3):338–346. doi: 10.1038/ng1963. [DOI] [PubMed] [Google Scholar]
- 24.Arias-Romero LE, Saha S, Villamar-Cruz O, et al. Activation of Src by protein tyrosine phosphatase 1B Is required for ErbB2 transformation of human breast epithelial cells. Cancer Res. 2009;69(11):4582–4588. doi: 10.1158/0008-5472.CAN-08-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiener JR, Kerns BJ, Harvey EL, et al. Overexpression of the protein tyrosine phosphatase PTP1B in human breast cancer: association with p185c-erbB-2 protein expression. J Natl Cancer Inst. 1994;86(5):372–378. doi: 10.1093/jnci/86.5.372. [DOI] [PubMed] [Google Scholar]
- 26.Soysal S, Obermann EC, Gao F, et al. PTP1B expression is an independent positive prognostic factor in human breast cancer. Breast Cancer Res Treat. 2013;137(2):637–644. doi: 10.1007/s10549-012-2373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haj FG, Markova B, Klaman LD, Bohmer FD, Neel BG. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J Biol Chem. 2003;278(2):739–744. doi: 10.1074/jbc.M210194200. [DOI] [PubMed] [Google Scholar]
- 28.Salmeen A, Andersen JN, Myers MP, Tonks NK, Barford D. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol Cell. 2000;6(6):1401–1412. doi: 10.1016/s1097-2765(00)00137-4. [DOI] [PubMed] [Google Scholar]
- 29.Seely BL, Staubs PA, Reichart DR, et al. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes. 1996;45(10):1379–1385. doi: 10.2337/diab.45.10.1379. [DOI] [PubMed] [Google Scholar]
- 30.Elchebly M, Payette P, Michaliszyn E, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283(5407):1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 31.Haj FG, Zabolotny JM, Kim YB, Kahn BB, Neel BG. Liver-specific protein-tyrosine phosphatase 1B (PTP1B) re-expression alters glucose homeostasis of PTP1B−/−mice. J Biol Chem. 2005;280(15):15038–15046. doi: 10.1074/jbc.M413240200. [DOI] [PubMed] [Google Scholar]
- 32.Klaman LD, Boss O, Peroni OD, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20(15):5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adachi M, Sekiya M, Arimura Y, et al. Protein-tyrosine phosphatase expression in pre-B cell NALM-6. Cancer Res. 1992;52(3):737–740. [PubMed] [Google Scholar]
- 34.Yi T, Cleveland JL, Ihle JN. Identification of novel protein tyrosine phosphatases of hematopoietic cells by polymerase chain reaction amplification. Blood. 1991;78(9):2222–2228. [PubMed] [Google Scholar]
- 35.Warabi M, Nemoto T, Ohashi K, Kitagawa M, Hirokawa K. Expression of protein tyrosine phosphatases and its significance in esophageal cancer. Exp Mol Pathol. 2000;68(3):187–195. doi: 10.1006/exmp.2000.2303. [DOI] [PubMed] [Google Scholar]
- 36.Zhai YF, Beittenmiller H, Wang B, et al. Increased expression of specific protein tyrosine phosphatases in human breast epithelial cells neoplastically transformed by the neu oncogene. Cancer Res. 1993;53(10 suppl):2272–2278. [PubMed] [Google Scholar]
- 37.Wiener JR, Hurteau JA, Kerns BJ, et al. Overexpression of the tyrosine phosphatase PTP1B is associated with human ovarian carcinomas. Am J Obstet Gynecol. 1994;170(4):1177–1183. doi: 10.1016/s0002-9378(94)70118-0. [DOI] [PubMed] [Google Scholar]
- 38.Zhu S, Bjorge JD, Fujita DJ. PTP1B contributes to the oncogenic properties of colon cancer cells through Src activation. Cancer Res. 2007;67(21):10129–10137. doi: 10.1158/0008-5472.CAN-06-4338. [DOI] [PubMed] [Google Scholar]
- 39.Balavenkatraman KK, Aceto N, Britschgi A, et al. Epithelial protein-tyrosine phosphatase 1B contributes to the induction of mammary tumors by HER2/Neu but is not essential for tumor maintenance. Mol Cancer Res. 2011;9(10):1377–1384. doi: 10.1158/1541-7786.MCR-11-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernández-Flores O, Esparza-López J, Escobar-Arriaga E, León-Rodríguez E, Ibarra-Sánchez MJ. Sobreexpresión de PTP1B induce mayor proliferación celular en cultivos primarios de cáncer de mama. GAMO. 2013;12(1):4–9. [Google Scholar]
- 41.Dubé N, Bourdeau A, Heinonen KM, Cheng A, Loy AL, Tremblay ML. Genetic ablation of protein tyrosine phosphatase 1B accelerates lymphomagenesis of p-53-null mice through the regulation of B-cell development. Cancer Res. 2005;65(21):10088–10095. doi: 10.1158/0008-5472.CAN-05-1353. [DOI] [PubMed] [Google Scholar]
- 42.Tanner MM, Tirkkonen M, Kallioniemi A, et al. Independent amplification and frequent co-amplification of three nonsyntenic regions on the long arm of chromosome 20 in human breast cancer. Cancer Res. 1996;56(15):3441–3445. [PubMed] [Google Scholar]
- 43.Courjal F, Cuny M, Rodriguez C, et al. DNA amplifications at 20q13 and MDM2 define distinct subsets of evolved breast and ovarian tumours. Br J Cancer. 1996;74(12):1984–1989. doi: 10.1038/bjc.1996.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garibay-Díaz JC, López-Zelada KA, Escobar-Arriaga E, León-Rodríguez E, Esparza-López J, Ibarra-Sánchez MJ. PTP1B como factor predictivo de respuesta a paclitaxel y docetaxel en cultivos primarios de cáncer de mama. GAMO. 2014;13(2):91–96. [Google Scholar]
- 45.Le XF, Bast RC., Jr Src family kinases and paclitaxel sensitivity. Cancer Biol Ther. 2011;12(4):260–269. doi: 10.4161/cbt.12.4.16430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89(2):297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 47.Feijoo C, Campbell DG, Jakes R, Goedert M, Cuenda A. Evidence that phosphorylation of the microtubule-associated protein Tau by SAPK4/p38delta at Thr50 promotes microtubule assembly. J Cell Sci. 2005;118(pt 2):397–408. doi: 10.1242/jcs.01655. [DOI] [PubMed] [Google Scholar]
- 48.Illenberger S, Drewes G, Trinczek B, et al. Phosphorylation of microtubule-associated proteins MAP2 and MAP4 by the protein kinase p110mark. Phosphorylation sites and regulation of microtubule dynamics. J Biol Chem. 1996;271(18):10834–10843. doi: 10.1074/jbc.271.18.10834. [DOI] [PubMed] [Google Scholar]
- 49.Mandelkow E, Mandelkow EM. Microtubules and microtubule-associated proteins. Curr Opin Cell Biol. 1995;7(1):72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 50.Izbicka E, Campos D, Carrizales G, Patnaik A. Biomarkers of anticancer activity of R115777 (Tipifarnib, Zarnestra) in human breast cancer models in vitro. Anticancer Res. 2005;25(5):3215–3223. [PubMed] [Google Scholar]
- 51.Jové M, Planavila A, Laguna JC, Vázquez-Carrera M. Palmitate-induced interleukin 6 production is mediated by protein kinase C and nuclear-factor kappa B activation leads to glucose transporter 4 down-regulation in skeletal muscle cells. Endocrinology. 2005;146(7):3087–3095. doi: 10.1210/en.2004-1560. [DOI] [PubMed] [Google Scholar]
- 52.Weigert C, Brodbeck K, Staiger H, et al. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J Biol Chem. 2004;279(23):23942–23952. doi: 10.1074/jbc.M312692200. [DOI] [PubMed] [Google Scholar]
- 53.Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem. 2008;283(21):14230–14241. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Charafe-Jauffret E, Ginestier C, Birnbaum D. Breast cancer stem cells: tools and models to rely on. BMC Cancer. 2009;9:202. doi: 10.1186/1471-2407-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ponti D, Costa A, Zaffaroni N, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65(13):5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 56.Brown-Shimer S, Johnson KA, Hill DE, Bruskin AM. Effect of protein tyrosine phosphatase 1B expression on transformation by the human neu oncogene. Cancer Res. 1992;52(2):478–482. [PubMed] [Google Scholar]
- 57.Arena S, Benvenuti S, Bardelli A. Genetic analysis of the kinome and phosphatome in cancer. Cell Mol Life Sci. 2005;62(18):2092–2099. doi: 10.1007/s00018-005-5205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]