Abstract
There is significant clinical demand for functional tendon grafts in human and veterinary medicine. Tissue engineering techniques combining cells, scaffolds and environmental stimuli may circumvent the shortcomings of traditional transplantation processes. In this study, the influence of cyclic mechanical stimulation on graft maturation and cellular phenotype was assessed in an equine model. Decellularized tendon scaffolds from four equine sources were seeded with syngeneic bone marrow-derived mesenchymal stem cells and subjected to 0%, 3% or 5% strain at 0.33Hz for up to one hour daily for 11 days. Cells cultured at 3% strain integrated deep into their scaffolds, altered extracellular matrix composition, adopted tendon-like gene expression profiles, and increased construct elastic modulus and ultimate tensile strength to native levels. This bioreactor protocol is therefore suitable for cultivating replacement tendon material or as an in vitro model for studying differentiation of stem cells toward tendon.
Indexing Keywords: tendon, tissue engineering, bioreactor, extracellular matrix, mesenchymal stem cell
Introduction
Tendon dysfunction occurs with high morbidity in both humans and animals, compromising freedom of movement and quality of life. Tendons are predominantly composed of hierarchically organized, aligned collagen fibrils1, and the specialized structure of tendon extracellular matrix (ECM) provides tensile strength while transferring mechanical stimuli to resident cells2–3. There is a reciprocal relationship between ECM properties and cellular behavior, and success of in vitro cultivation of tendon is dependent on recapitulating the natural environment of the tissue.
The horse is a model organism for studies of human tendon pathophysiology4–5. Injury of the flexor digitorum superficialis tendon (FDST) is particularly common6, and significant research has been dedicated to addressing the poor intrinsic regenerative capacity of this tissue7. Mesenchymal stem cell (MSC) implantation has been safely used in the treatment of tendon degeneration, and there is some evidence that the multipotency and immunomodulatory properties of MSCs may improve healing8. Seeding cells on scaffolds influences cellular behavior9 and supports endogenous repair10, but the utility of current commercial tendon augmentation products remains limited11. An equine decellularized tendon scaffold (DTS) has been previously developed in our laboratory as a step toward graft material production and as an in vitro model for tendon injury and repair12.
According to a recent review, inadequate knowledge of the tendon progenitor cell niche is a primary barrier inhibiting the development of effective cell-based therapies13. Proteins isolated from tendon extracellular matrix alone enhance proliferation and tenogenesis in three-dimensional culture14, yet intact, naturally-derived scaffolds have the benefit of 1) biochemical composition, 2) three-dimensional topography and 3) tissue-relevant mechanical properties. DTS is suitable for MSC culture under static conditions, and it was hypothesized that subjecting these constructs to mechanical stimulation would induce differentiation toward tendon and produce viable regenerative graft materials. From previous bioreactor studies on tendon it is evident that, while loading is required for maintenance of a differentiated tenocytic phenotype15 and tissue biomechanical properties16, mechanically stimulated tendon constructs exhibit sensitivity to the characteristics of mechanical stimuli. This cellular response is likely tissue- and model-dependent, requiring optimization based on construct properties and environmental conditions.
The aim of this experiment was to compare three deformation protocols on MSC-seeded DTS by examining the influence of strain on MSC phenotype. Two dynamic strain regimens of varying amplitude (3% and 5%) were selected based on their physiological relevance and compared to static (0%) controls. The approximate biomechanical transition between the toe region and the linear elastic region of deformation of the tendon stress-strain curve is 3% strain17, while 5% is a standard linear amplitude of normal usage conditions18. Despite the seemingly minor differences between these two groups, it was hypothesized that the distinctive biomaterial behaviors delineating the two deformation regions19 would differentially translate mechanical stimuli to resident cells. We hypothesized that both 3%- and 5%-strained constructs would exhibit stronger evidence of tendon differentiation than static culture. Furthermore, we anticipated the 3% strain protocol would effectively induce tendon differentiation in adult MSCs and promote an anabolic response. Effects of the three strain protocols were evaluated via the expression of tendon marker genes, biomechanical properties and production of ECM following 11 days of bioreactor culture.
Materials and Methods
Experimental design
MSC-seeded DTS was divided into three groups by strain amplitude: referenced in the text as the 0%, 3% and 5% experimental groups. Microscopy, composition and biomechanics data references either initial DTS (iDTS), control DTS (cDTS), or both as negative controls. iDTS is the freshly prepared scaffold material, subject to no further manipulation. cDTS was not seeded with cells, but underwent identical incubation conditions to the 0% experimental group. Adult tendon (FDST) was used as a control to compare bioreactor gene expression data with mature whole tissue (n=4).
Production of decellularized tendon scaffolds (DTS)
DTS was produced using sterile technique in accordance with methods developed in our laboratory12. FDSTs were surgically excised from the forelimbs of four adult sport horses aged 4.5±1.7 years, euthanized as a result of unrelated conditions in accordance with the Institutional Animal Care and Use Committee of Virginia Tech. Tendons from these horses were longitudinally sectioned using an electric dermatome (Integra Lifesciences, USA) into ribbons 400μm in thickness, then divided into samples 10×45mm in surface area. Briefly, these samples were decellularized by four freeze-thaw cycles, a 48-hour detergent infusion with 2% SDS (Sigma, USA) in 1M Tris-HCl, pH 7.8 (Fisher Scientific, USA) at 4°C, incubations in 0.05% trypsin-EDTA (Gibco, USA), 10μg/mL DNase-I (STEMCELL Technologies, Canada) and 95% ethanol (Sigma, USA), followed by repeated washings in H2O in a gyratory shaker (Brunswick Scientific, USA). The resulting scaffolds were frozen at −20°C prior to use. Reference FDST samples for RNA analysis were flash frozen using liquid nitrogen and stored at −80°C prior to processing in the same manner as the DTS samples (described below).
Derivation of primary mesenchymal stem cell (MSC) lines
MSCs were collected and assessed via routine processing techniques20 using bone marrow aspirate collected from the sternum of the same four donor horses as the DTS material. Cells were cultured at 37°C, 5% CO2 and 90% humidity in standard MSC media: low-glucose GlutaMAX DMEM with 110μg/mL sodium pyruvate (Gibco, USA) plus 10% MSC FBS (Gibco, USA) and 100U/mL sodium penicillin, 100μg/mL streptomycin sulfate (Sigma, USA). Cells were expanded in monolayer culture to 80% confluence and passaged twice. Flow cytometry was conducted on these four separate cell lines with a BD FACSCalibur using monoclonal antibodies previously validated in our laboratory (data not shown). The results demonstrated that approximately 90% of cells were positive for CD-90 and CD-44, common MSC surface antigens, as well as Oct-4, which is indicative of a naïve stemness found in both embryonic21 and adult22 stem cell populations.
Construct seeding and bioreactor culture
Following MSC expansion, DTS was thawed and saturated in tendon cell culture media: standard MSC media as previously described with the addition of 35.7μg/mL L-ascorbic acid (Sigma, USA). This medium was used for the remainder of the study. DTS samples were clamped into the bioreactor vessels along their natural axis of alignment (Figure 1), obscuring 0.5cm on each end. MSC suspensions were deposited via micropipette over syngeneic DTS at a density of 20,000 cells/cm2, which equates to the approximate surface density of a 40% confluent monolayer and had previously been validated12. Seeded DTS was subsequently placed in an incubator for 72 hours to allow cells to adhere, with the vessels filled to their maximum media volume of 6mL after the first 24 hours. Following this seeding period, bioreactor culture was initiated, and half of the media was changed every 2–3 days.
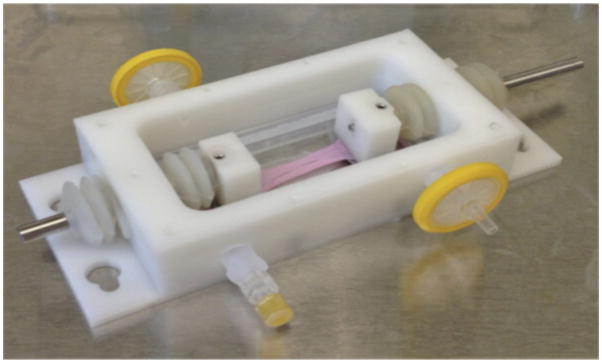
Figure 1.
The bioreactor consists of interchangeable, enclosed modular vessels. This picture portrays an MSC-laden DTS construct with 10×35mm of exposed surface area immediately following seeding.
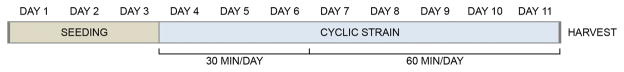
A custom bioreactor at the Tissue Engineering Resource Center was used in this study. The device, described previously23, incorporates self-contained tissue culture vessels that allow samples to be individually clamped and mechanically manipulated. A LabVIEW program (National Instruments, USA) operates four stepper motors in parallel stages. MSC-seeded DTS constructs were cultured in this bioreactor for a total of 11 days: three days without stimulation, three days subject to displacement for 30 minutes per day, then five days at 60 minutes per day16 (Figure 2). Samples underwent linear deformation at 0.33Hz according to their experimental group. These parameters were selected due to their physiological relevance as well as reports that as few as 5–7 days of bioreactor culture at 0.0167–0.5Hz are sufficient to observe improvements in material properties in fibroblast-laden tendon/ligament constructs24–25. All groups were repeated in triplicate for a total of 36 vessels: four horses, three experimental groups, and three replicates. After the final day, constructs were removed from their vessels, divided for assays and either flash frozen in liquid nitrogen or immersed in a fixative for preservation prior to analysis.
Figure 2.
The duration each construct spent in the bioreactor per day gradually increased from 0 to 30 to 60 minutes over the cultivation period.
RNA isolation and gene expression analysis
Half of each sample was used for gene expression analysis, and all samples within each treatment group were pooled. The experiment was repeated in its entirety to produce a replicates. Bioreactor constructs were transferred from storage at −80°C directly into a cryomill (SPEX SamplePrep, USA) and pulverized in liquid nitrogen. RNA was isolated from tissue homogenates using an acid guanidinium thiocyanate extraction protocol in phenol-chloroform, followed by precipitation in isopropanol. The resulting pellets were resuspended and the solutions concentrated in RNeasy spin columns (Qiagen, USA), quantitated with RiboGreen RNA reagent (Life Technologies, USA) and reverse-transcribed with a high-capacity cDNA kit (Life Technologies, USA). cDNA was pre-amplified using a validated commercial TaqMan kit (Life Technologies, USA) prior to reaction in a 7500 Real-Time PCR System (Applied Biosystems, USA) using custom TaqMan probes (Life Technologies, USA) in duplicate. A list of primers, probes and abbreviations used are included in Table 1. Reactions were quantified with the 2-ΔΔCt method using GAPDH as a reference gene and are reported by fold-change with respect FDST.
Table 1.
Custom-designed equine qPCR primers and probes were designed to target: glyceraldehyde 3-phosphate dehydrogenase (GAPDH), scleraxis (SCX), type-I collagen (COL-I), type-III collagen (COL-III), decorin (DCN) and biglycan (BGN).
| GENE | FORWARD | REVERSE | PROBE |
|---|---|---|---|
| GAPDH | CAAGTTCCATGGCACAGTCAAG | GGCCTTTCCGTTGATGACAA | CCGAGCACGGGAAG |
| SCX | CGCCCAGCCCAAACAG | TTGCTCAACTTTCTCTGGTTGCT | TCTGCACCTTCTGCC |
| COL-I | GCCAAGAAGAAGGCCAAGAA | TGAGGCCGTCCTGTATGC | ACATCCCAGCAGTCACCT |
| COL-III | CTGCTTCATCCCACTCTTAT | ATCCGCATAGGACTGACCA | AACAGGAAGTTGCTGAAGG |
| DCN | AAGTTGATGCAGCTAGCCTG | GGCCAGAGAGCCATTGTCA | ATTTGGCTAAATTGGGACTG |
| BGN | TGGACCTGCAGAACAATGAGAT | AGAGATGCTGGAGGCCTTTG | TCTGAGCTCCGAAAGG |
Mechanical testing
A representative sample was collected from one replicate of each FDST and experimental group to undergo failure testing (average dimensions 12.4±1.1mm × 1.7±0.1mm). Following measurement with a digital caliper, samples were elongated at 0.5% per second until failure using a custom materials testing device controlled by National Instruments components, generating stress-strain curves. Elastic modulus was computed as the slope of the total linear region of this relationship. Ultimate tensile strength was calculated as the maximum force per unit area endured prior to failure.
Spectrophotometric biochemistry assays
Sulfated glycosaminoglycan (GAG) content was assayed using the 1,9-dimethylmethylene blue (Sigma, USA) technique, referencing chondroitin sulfate A (Sigma, USA). This procedure was conducted in aliquots obtained during media changes, as well as in each bioreactor construct on day 11. Solid samples were solubilized by enzymatic digestion in papain (Sigma, USA). cDTS was also included in this analysis, in addition to the typical FDST and iDTS controls, to isolate the influence of cells on GAG maintenance over time under experimental conditions and in tendon cell culture media. DNA content was quantified in the same digest solutions using a Quant-iT PicoGreen (Molecular Probes, USA) assay to determine relative cell number. Solubilized collagen was measured using a Sircol kit (Bicolor Ltd., UK) in acid/salt-washed pepsin (Sigma, USA) digests of solid samples following the conclusion of the experiment. Values are reported with respect to dry weight, obtained by dehydration in an oven.
Histology
A portion of each experimental sample, as well as of each FDST and iDTS control, was fixed in a freshly prepared solution of 4% paraformaldehyde (Sigma, USA) and submitted for commercial histological preparation (Histoserv, Inc., USA). Samples were embedded in paraffin, longitudinally sectioned into 5μm slices, and stained with hematoxylin and eosin. Images were acquired using an Olympus IM inverted microscope and a Moticam 10 CMOS camera.
Statistical analysis
Data are reported as mean ± standard error in all figures. One-way multivariate analyses of variance (MANOVA) with a repeated measures designs followed by standard F-tests were used to determine statistical significance of all data points except qPCR data (p≤0.05). A standard one-way MANOVA was used for qPCR results. Results are annotated in figures alphabetically. One-way Student’s t-tests were also used in biochemical and biomechanical analysis to specifically test experimental groups to DTS controls. Points of significance (p≤0.05) are demarcated with an asterisk in applicable figures. Computation was performed in JMP Pro 11 (SAS Institute Inc., USA), Prism (GraphPad Software Inc., USA) and Excel 14 (Microsoft, USA).
Results
Cyclic strain promotes a tenocytic gene expression profile
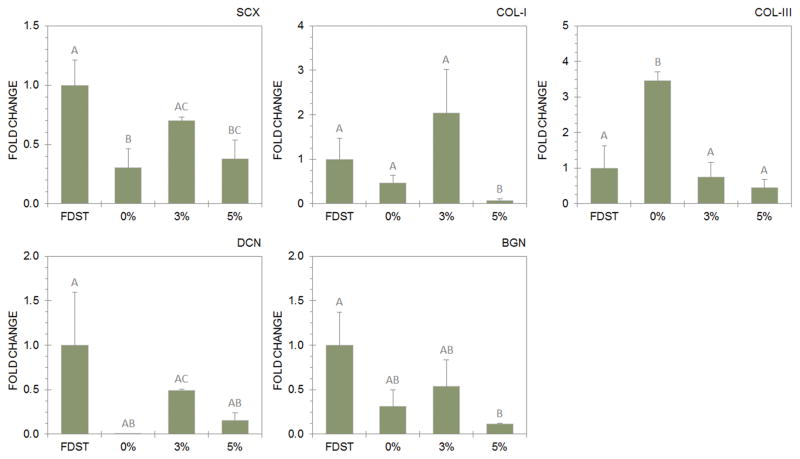
Gene expression data are shown in Figure 3. SCX expression more than doubled from the 0% to the 3% experimental group to 70±15% of FDST, and this difference was significant (p=0.024). SCX in the 5% experimental group fell between the 0% and 3% groups, and was not statistically different from either group. COL-I expression was greatest in the 3% experimental group, with message levels present at 2.09±0.96 times what is observed in FDST: statistically greater than the 5% group (p=0.041). COL-III expression was dramatically upregulated in the 0% experimental group versus FDST (p=0.005). The ratio of relative COL-I to COL-III expression was 1.75 in the 3% experimental group, whereas it was less than or equal to 0.14 in the 0% and 5% experimental groups. DCN expression changed in response to bioreactor protocol, with greatest DCN expression in the 3% experimental group, significantly higher than in the 0% (p<0.001) or 5% (p=0.011) experimental groups. BGN was most expressed in the 3% experimental group, but this difference was not significant.
Figure 3.
Messenger RNA expression profiles of the tenocytic marker genes scleraxis (SCX), collagen types-I/III (COL-I and COL-III), decorin (DCN) and biglycan (BGN) varied by bioreactor protocol – 3% strain induced a phenotype correlated with tenocytic differentiation and development. Data is reported by fold-change with respect to FDST. Data points that share a letter are not significantly different.
3%-strained constructs mimic the mechanical properties of native FDST
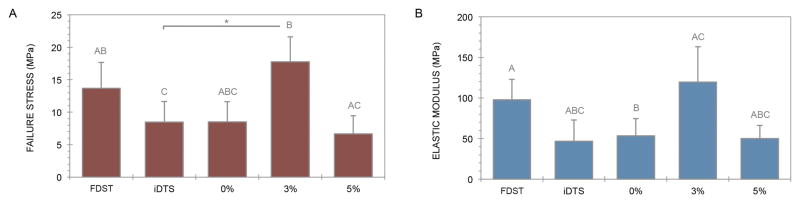
Constructs in the 3% experimental group failed at a mean stress of 17.7±3.8MPa, which is more than double that of iDTS (p=0.041) (Figure 4A). Constructs in the 0% and 5% experimental groups failed at significantly lower stresses than those in the 3% experimental group (p=0.009 and p=0.043, respectively). Constructs in the 0% and 5% experimental groups had significantly lower elastic moduli than FDST (p=0.034 and p=0.019, respectively), while this difference only approached significance for iDTS (p=0.090) (Figure 4B). Relative to iDTS, the 3% experimental group exhibited a 2.56-fold increase in elastic modulus to 119±44MPa, a value within 25% of the elastic modulus of matched native tendons (98±25MPa), and without statistical significance between the two.
Figure 4.
Construct elastic modulus and ultimate tensile strength were increased to native physiological levels by bioreactor culture at 3% strain. Data points that share a letter are not significantly different. Asterisks demarcate t-test significance from iDTS.
MSCs integrate into DTS and modulate scaffold composition
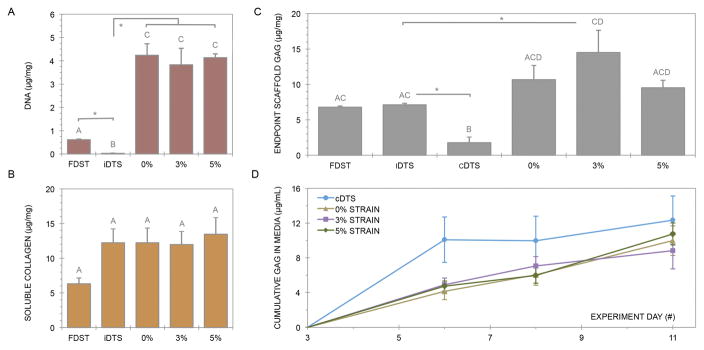
Decellularization eliminated 95% of DNA from FDST, to 0.03μg/mg in DTS (p<0.001). All MSC-seeded bioreactor constructs had significantly more DNA than native tendon (p<0.001), equating to 6.6±0.2 times the value of FDST (Figure 5A). There were no statistical differences in DNA content between the 0%, 3% and 5% experimental groups. Soluble collagen production from the 3% experimental group after 11 days was 12.0±1.9μg/mg (Figure 5B). There were no significant differences in soluble collagen between groups.
Figure 5.
Endpoint scaffold content of DNA (A), soluble collagen (B) and GAG (C) were quantified by spectrophotometric assays. Cumulative GAG release into cell culture media was similarly assessed (D). Data points that share a letter are not significantly different as determined via one-way MANOVA. Asterisks demarcate t-test significance from iDTS.
Endpoint GAG composition in the 3% experimental group increased by a factor of 2.14 relative to iDTS to 13.5±3.1μg/mg (p=0.050), while unseeded cDTS released 74% of GAG content into the culture media (p=0.004) (Figure 5C). GAG release into culture media was tracked cumulatively, and it was found that cDTS lost 10.1±2.6μg/mL of GAG in the first three days (Figure 5D). In contrast, the mean GAG release of the 0%, 3% and 5% experimental groups was 4.6±0.26μg/mL three days into the bioreactor culture period. There was no further GAG release between days 8–11 in the 3% experimental group (p=0.106).
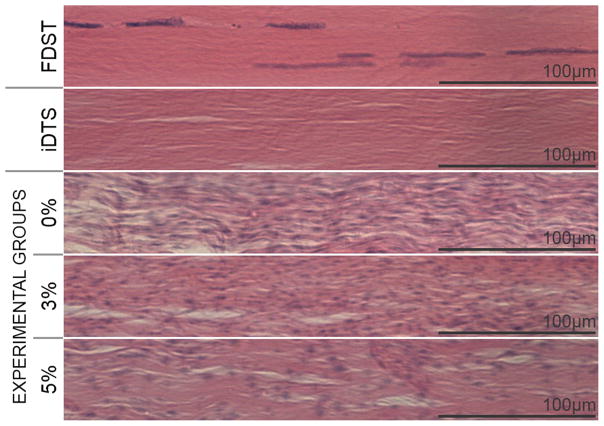
Histological examination confirmed the high cellularity of MSC-seeded constructs relative to native FDST (Figure 6). Cells integrated at least 200μm deep into DTS by 11 days. Cells also established an anisotropic phenotype, elongating parallel to the DTS collagen fibers.
Figure 6.
Scaffolds were successfully decellularized and reseeded at supraphysiological density relative to FDST. MSCs integrated into DTS and adopted a tenocytic phenotype which did not change relative to strain amplitude.
Discussion
There is an immediate clinical demand for tendon graft material as well as a long-term need to better understand the tendon differentiation process. The purpose of this study was to establish a tissue culture technique for investigating tenogenesis and engineering tendon graft material. A novel approach was taken to this end, applying biologically-inspired mechanical stimulation protocols to natural decellularized scaffolds laden with matched cells ex vivo. Cyclic linear strain of 3% at 0.33Hz for up to 60 minutes per day was found to promote tendon differentiation in bone marrow-derived MSCs on syngeneic DTS.
Although there is no single marker gene of the tenocyte phenotype, SCX and COL-I are highly expressed in tendon26 and are responsive to exercise27. SCX is a basic-helix-loop-helix transcription factor acting on the COL1A1 promoter28–29, the expression of which characteristically demarcates tendon from surrounding tissues beginning during fetal development30–31. SCX is instrumental in the MSC to tendon progenitor transition32, as well as in adaptability to injury27. SCX was expressed in all constructs, suggesting that DTS promotes tenogenesis even in the absence of mechanical stimulation, but was highest in the 3% strain group. COL-I represents 75% of the dry mass of tendon33, while COL-III is more highly expressed in degenerate tendon33 as well as in early tendon healing34. COL-I was most highly expressed in 3%-strained constructs with COL-III expressed at normal levels: a result concurrent with physiologically normal tendon anabolism. The ratio of COL-III to COL-I was greater than 5.0 in the 0% and 5% experimental groups, indicating that under- or over-stimulation results in atypical collagen gene expression.
DCN and BGN are small leucine-rich proteoglycans (SLRPs). DCN is the most abundant non-collagenous ECM protein in tendon35, and among other functions modulates fibrillogenesis36. DCN was most heavily upregulated in the 3% experimental group. The results of this study are consistent with a previous observation that DCN expression is increased following exercise37. BGN is similarly important for directing assembly of collagens38, yet it is also notable for its essential role in the maintenance of the putative tendon stem cell niche39. BGN transcription was not statistically different from FDST in the 0% or 3% groups, but was downregulated in the 5% group (p=0.027). DCN and BGN expression exhibit temporal dependence during the tendon healing process, with BGN peaking earlier in development40. Therefore, it may be of interest in future studies to follow trends in SLRP expression over a variety of time points.
Failure testing confirmed one of the most critical factors in tendon graft candidacy: that bioreactor-cultured constructs were mechanically robust relative to FDST. The elastic modulus and ultimate tensile strength of constructs in the 3% experimental group increased versus iDTS and were not statistically different from matched FDST. Thus over- or under-stimulation decreases the efficacy of MSC differentiation toward tendon and/or inhibits ECM anabolism in our model. The material properties of tendons are dependent on cross-sectional area41. Whole tissues in vivo may therefore have elastic moduli and ultimate tensile strengths approximately an order of magnitude greater than what is reported here6. For future tissue augmentation applications, several layers of constructs may potentially be combined to achieve the properties desired42.
GAGs are functional side chains of proteoglycans, the concentrations of which are quantifiable measures of tendon structure and function43. GAGs facilitate collagen fibril sliding under load44, and influence microenvironments and mechanotransduction on a cellular level19. Decellularization results in GAG loss, but MSC-seeded DTS regained or exceeded native composition. Indeed, GAG levels detected in the 3% experimental group may be considered supraphysiological for equine FDST12, though similar results in the horse were observed in response to low-intensity exercise45. BGN, with two GAG chains per molecule, modulates tenogenic signaling pathways and enhances stem cell differentiation toward tendon39. Thus, supraphysiological concentrations of GAG may naturally coincide with supraphysiological concentrations of resident stem cells.
Turnover of GAGs is a hallmark of tendon homeostasis37, hence GAG release into culture media was observed. However, MSC seeding appeared to prevent the rapid and substantial GAG loss observed in cDTS, suggesting an immediate and sustained influence of these cells on maintaining ECM integrity. This corroborates a recent study reporting a cell type-dependent reduction in GAG loss in equine tendon explants46.
Histological examination confirmed MSC integration into scaffolds at high density, as well as the adoption of a tenocytic morphology1, in line with results observed following MSC cell therapy in vivo8 and in the cultivation of other tissue engineered tendons in vitro14. DNA quantification supported subjective microscopic assessment of cellularity, validating the high cytocompatibility of DTS reported previously12. Despite its relatively low porosity, DTS accommodates cells exceeding physiological concentrations. Morphology, cellularity and soluble collagen content were not influenced by bioreactor strain amplitude.
The limited extracellular matrix expression and biomechanical properties observed in the 5% group was somewhat surprising considering the similar cellularity between groups. A potential explanation for this phenotype is that ECM damage resulted in stress-deprived microenvironments within the 5%-strained constructs47, resulting in a gene expression profile that in many ways more closely resembled the 0% group than the 3% group. Differences in collagen gene expression did not translate to detectable differences in endpoint soluble collagen after 11 days. It would be interesting to further determine temporal characteristics of the collagen remodeling process and test for the degradation products in the media. Finally, while biomechanical testing confirmed the functionality of incorporating a bioreactor conditioning protocol, the clinical relevance of these changes in gene expression is unknown.
The results of this study suggest that a tuned, bioreactor-based conditioning protocol may assist in the cultivation of functional tendon graft material and serve as a platform for in vitro testing. A protocol applying 3% cyclic strain at 0.33Hz for up to an hour daily for 11 days promoted tenocytic differentiation of MSCs on DTS and improved construct material properties. This bioreactor platform could uniquely address questions of relative tenogenic efficacy of various types of stem cells, or be used to test the influence of small molecule or protein supplementation on pathways relevant to tendon differentiation and regeneration.
Acknowledgments
Partial funding for this study was provided by the Virginia Tech Institute for Critical Technology and Applied Science (ICTAS), the Morris Animal Foundation, and the Tufts University Tissue Engineering Resource Center (TERC) [NIH P41 EB002520]. DWY also gratefully acknowledges support from the Grayson-Jockey Club Research Foundation via the 2013 Storm Cat Career Development Award. Shannon L. Smith, MS and Jonathan A. Kluge, PhD designed the bioreactor system, provided training and facilitated helpful discussions at Tufts University.
Footnotes
Author Contributions Statement
Research study design: DWY, JGB; data acquisition, analysis and interpretation: DWY, IR, DLK, JGB; drafting manuscript: DWY, JGB; critical revisions: DWY, IR, DLK, JGB; final approval: DWY, IR, DLK, JGB.
Contributor Information
Daniel W. Youngstrom, Program in Biomedical and Veterinary Sciences, Marion duPont Scott Equine Medical Center, Virginia-Maryland College of Veterinary Medicine, Virginia Tech, Leesburg, Virginia, United States of America
Ibtesam Rajpar, Program in Biomedical and Veterinary Sciences, Marion duPont Scott Equine Medical Center, Virginia-Maryland College of Veterinary Medicine, Virginia Tech, Leesburg, Virginia, United States of America.
David L. Kaplan, Department of Biomedical Engineering, Tissue Engineering Resource Center, Tufts University, Medford, Massachusetts, United States of America
Jennifer G. Barrett, Department of Large Animal Clinical Sciences, Marion duPont Scott Equine Medical Center, Virginia-Maryland College of Veterinary Medicine, Virginia Tech, Leesburg, Virginia, United States of America
References
- 1.Riley G. Chronic tendon pathology: molecular basis and therapeutic implications. Expert Rev Mol Med. 2005;7:1–25. doi: 10.1017/S1462399405008963. [DOI] [PubMed] [Google Scholar]
- 2.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 3.Wang JHC, Guo Q, Li B. Tendon Biomechanics and Mechanobiology—A Minireview of Basic Concepts and Recent Advancements. J Hand Ther. 2012;25:133–141. doi: 10.1016/j.jht.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koob TJ. Biomimetic approaches to tendon repair. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1171–1192. doi: 10.1016/s1095-6433(02)00247-7. [DOI] [PubMed] [Google Scholar]
- 5.Muttini A, Salini V, Valbonetti L, Abate M. Stem cell therapy of tendinopathies: suggestions from veterinary medicine. Muscles Ligaments Tendons J. 2012;2:187–192. [PMC free article] [PubMed] [Google Scholar]
- 6.Dowling BA, Dart AJ. Mechanical and functional properties of the equine superficial digital flexor tendon. Vet J. 2005;170:184–192. doi: 10.1016/j.tvjl.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Burk J, Badylak SF, Kelly J, Brehm W. Equine cellular therapy--from stall to bench to bedside? Cytometry A. 2013;83:103–113. doi: 10.1002/cyto.a.22216. [DOI] [PubMed] [Google Scholar]
- 8.Godwin EE, Young NJ, Dudhia J, et al. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012;44:25–32. doi: 10.1111/j.2042-3306.2011.00363.x. [DOI] [PubMed] [Google Scholar]
- 9.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdick JA, Mauck RL, Gorman JH, 3rd, Gorman RC. Acellular biomaterials: an evolving alternative to cell-based therapies. Sci Transl Med. 2013;5:176ps174. doi: 10.1126/scitranslmed.3003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: a systematic review. Br Med Bull. 2010;94:165–188. doi: 10.1093/bmb/ldp051. [DOI] [PubMed] [Google Scholar]
- 12.Youngstrom DW, Barrett JG, Jose RR, Kaplan DL. Functional characterization of detergent-decellularized equine tendon extracellular matrix for tissue engineering applications. PLoS One. 2013;8:e64151. doi: 10.1371/journal.pone.0064151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Y, Liu H, Li H, et al. A proteomic analysis of engineered tendon formation under dynamic mechanical loading in vitro. Biomaterials. 2011;32:4085–4095. doi: 10.1016/j.biomaterials.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 14.Yang G, Rothrauff BB, Lin H, et al. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials. 2013;34:9295–9306. doi: 10.1016/j.biomaterials.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayer ML, Schjerling P, Herchenhan A, et al. Release of tensile strain on engineered human tendon tissue disturbs cell adhesions, changes matrix architecture, and induces an inflammatory phenotype. PLoS One. 2014;9:e86078. doi: 10.1371/journal.pone.0086078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitlock PW, Seyler TM, Northam CN, et al. Effect of cyclic strain on tensile properties of a naturally derived, decellularized tendon scaffold seeded with allogeneic tenocytes and associated messenger RNA expression. J Surg Orthop Adv. 2013;22:224–232. doi: 10.3113/jsoa.2013.0224. [DOI] [PubMed] [Google Scholar]
- 17.Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol. 1999;521(Pt 1):307–313. doi: 10.1111/j.1469-7793.1999.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butcher MT, Hermanson JW, Ducharme NG, et al. Contractile behavior of the forelimb digital flexors during steady-state locomotion in horses (Equus caballus): an initial test of muscle architectural hypotheses about in vivo function. Comp Biochem Physiol A Mol Integr Physiol. 2009;152:100–114. doi: 10.1016/j.cbpa.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Screen HRC. Hierarchical approaches to understanding tendon mechanics. J Biomech Sci Eng. 2009;4:481–499. [Google Scholar]
- 20.Stewart AA, Barrett JG, Byron CR, et al. Comparison of equine tendon-, muscle-, and bone marrow-derived cells cultured on tendon matrix. Am J Vet Res. 2009;70:750–757. doi: 10.2460/ajvr.70.6.750. [DOI] [PubMed] [Google Scholar]
- 21.Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 22.Riekstina U, Cakstina I, Parfejevs V, et al. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev Rep. 2009;5:378–386. doi: 10.1007/s12015-009-9094-9. [DOI] [PubMed] [Google Scholar]
- 23.Matheson LA, Jack Fairbank N, Maksym GN, et al. Characterization of the Flexcell™ Uniflex™ cyclic strain culture system with U937 macrophage-like cells. Biomaterials. 2006;27:226–233. doi: 10.1016/j.biomaterials.2005.05.070. [DOI] [PubMed] [Google Scholar]
- 24.Joshi SD, Webb K. Variation of cyclic strain parameters regulates development of elastic modulus in fibroblast/substrate constructs. J Orthop Res. 2008;26:1105–1113. doi: 10.1002/jor.20626. [DOI] [PubMed] [Google Scholar]
- 25.Woon CY, Kraus A, Raghavan SS, et al. Three-dimensional-construct bioreactor conditioning in human tendon tissue engineering. Tissue Eng Part A. 2011;17:2561–2572. doi: 10.1089/ten.TEA.2010.0701. [DOI] [PubMed] [Google Scholar]
- 26.Taylor SE, Vaughan-Thomas A, Clements DN, et al. Gene expression markers of tendon fibroblasts in normal and diseased tissue compared to monolayer and three dimensional culture systems. BMC Musculoskelet Disord. 2009;10:27. doi: 10.1186/1471-2474-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendias CL, Gumucio JP, Bakhurin KI, et al. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res. 2012;30:606–612. doi: 10.1002/jor.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lejard V, Brideau G, Blais F, et al. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J Biol Chem. 2007;282:17665–17675. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- 29.Maeda T, Sakabe T, Sunaga A, et al. Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr Biol. 2011;21:933–941. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 31.Liu CF, Aschbacher-Smith L, Barthelery NJ, et al. Spatial and temporal expression of molecular markers and cell signals during normal development of the mouse patellar tendon. Tissue Eng Part A. 2012;18:598–608. doi: 10.1089/ten.tea.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alberton P, Popov C, Pragert M, et al. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells and Development. 2012;21:846–858. doi: 10.1089/scd.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birch HL, Bailey AJ, Goodship AE. Macroscopic ‘degeneration’ of equine superficial digital flexor tendon is accompanied by a change in extracellular matrix composition. Equine Vet J. 1998;30:534–539. doi: 10.1111/j.2042-3306.1998.tb04530.x. [DOI] [PubMed] [Google Scholar]
- 34.Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res. 2005;23:84–92. doi: 10.1016/j.orthres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Thorpe CT, Birch HL, Clegg PD, Screen HR. The role of the non-collagenous matrix in tendon function. Int J Exp Pathol. 2013;94:248–259. doi: 10.1111/iep.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe T, Hosaka Y, Yamamoto E, et al. Control of the collagen fibril diameter in the equine superficial digital flexor tendon in horses by decorin. J Vet Med Sci. 2005;67:855–860. doi: 10.1292/jvms.67.855. [DOI] [PubMed] [Google Scholar]
- 37.Heinemeier KM, Bjerrum SS, Schjerling P, Kjaer M. Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise. Scand J Med Sci Sports. 2013;23:e150–161. doi: 10.1111/j.1600-0838.2011.01414.x. [DOI] [PubMed] [Google Scholar]
- 38.Ameye L, Aria D, Jepsen K, et al. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. Faseb j. 2002;16:673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- 39.Bi YM, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 40.Zhang G, Ezura Y, Chervoneva I, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 41.Qin TW, Chen Q, Sun YL, et al. Mechanical characteristics of native tendon slices for tissue engineering scaffold. J Biomed Mater Res B Appl Biomater. 2012;100:752–758. doi: 10.1002/jbm.b.32508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran RT, Thevenot P, Zhang Y, et al. Scaffold Sheet Design Strategy for Soft Tissue Engineering. Nat Mater. 2010;3:1375–1389. doi: 10.3390/ma3021375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parkinson J, Samiric T, Ilic MZ, et al. Involvement of proteoglycans in tendinopathy. J Musculoskelet Neuronal Interact. 2011;11:86–93. [PubMed] [Google Scholar]
- 44.Rigozzi S, Muller R, Stemmer A, Snedeker JG. Tendon glycosaminoglycan proteoglycan sidechains promote collagen fibril sliding-AFM observations at the nanoscale. J Biomech. 2013;46:813–818. doi: 10.1016/j.jbiomech.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 45.Birch HL, Wilson AM, Goodship AE. Physical activity: does long-term, high-intensity exercise in horses result in tendon degeneration? J Appl Phys. 2008;105:1927–1933. doi: 10.1152/japplphysiol.00717.2007. [DOI] [PubMed] [Google Scholar]
- 46.Garvican ER, Dudhia J, Alves AL, et al. Mesenchymal stem cells modulate release of matrix proteins from tendon surfaces in vitro: a potential beneficial therapeutic effect. Regen Med. 2014;9:295–308. doi: 10.2217/rme.14.7. [DOI] [PubMed] [Google Scholar]
- 47.Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol. 2007;88:217–226. doi: 10.1111/j.1365-2613.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]