Abstract
Objective:
To evaluate the efficacy of a cleansing eyelid wipe in reducing the microbiota present on the ocular surface before cataract surgery.
Methods:
A single-center, prospective, single-blind phase IV study was conducted at the University Complutense of Madrid. Forty-five adult patients who were scheduled for ocular surgery after treatment with commercially available eyelid wipes were consecutively enrolled. The study lasted 5 days and the patients were examined at day 0 (D0), day 3 (D3), and day 5 (D5). They received instructions to apply the eyelid wipe only to the eye subject to surgery, using the other eye as a control with no treatment. Lid and conjunctival swabs were taken on each day and microbes identified. Ocular surface microbiota was estimated by measuring the area of the agar plate occupied by the grown colonies with respect to the total available area.
Results:
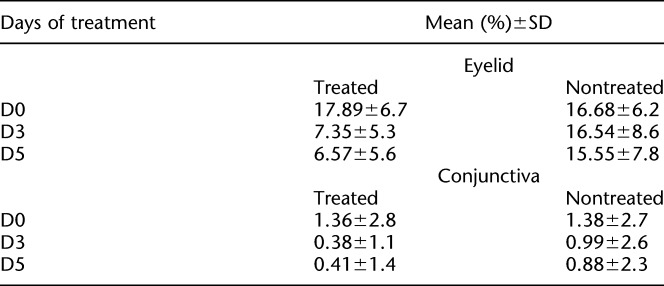
Measurements at D3 and D5 showed a percent reduction of 58% and 63%, respectively, in the microbial load on the eyelid in the treated eyes (P=0.0011). There was also a reduction, although nonsignificant, in the microbiota of the conjunctiva of 72% and 69% on D3 and D5, respectively.
Conclusions:
The degree of microbiota reduction was comparable with that obtained after topical application of antibiotics in other studies. The results suggest the use of these eyelid wipes as a complementary prophylactic method before any ocular surgery.
Key Words: Ocular surface microbiota, Eyelid wipe, Cataract surgery, Ocular surgery
Intraocular postoperative infections are a permanent concern for every eye surgeon. Although postoperative endophthalmitis is infrequent, with an incidence rate of 0.06% to 0.09% in the last decade,1–7 it represents a devastating complication in ophthalmic surgery.8 For this reason, the search for optimal procedures that will help reduce its incidence is a constant challenge.
Although the incidence of postoperative infections has a low rate, an increasing number of cases are now occurring after intravitreal injections of antivascular endothelial growth factor (VEGF) medications for choroidal neovascularization related to age-related macular degeneration,9–11 diabetic cystoid macular edema,12,13 and retinal vein occlusion.14 In fact, current protocols for the treatment of neovascular age-related macular degeneration dictate monthly or near-monthly injections of anti-VEGF.15 The risk of endophthalmitis reported after intravitreal injections is between 0.022% and 0.16%.16,17
The etiologic agents of acute postoperative endophthalmitis are generally microorganisms of the eyelid margin, conjunctiva, and tear film.18 The normal microbiota from the eyelid and conjunctiva has already been described.19 The most common bacteria isolated from the eyelids, conjunctiva, and tears are gram-positive bacteria, mostly coagulase-negative Staphylococcus spp. Depending on the study, the frequency of isolation of bacteria ranges from 16% to 100%, with microbial growth shown in approximately 50% of swabs from the conjunctiva and tears, and more than 50% of swabs from eyelids.19 The most commonly isolated microbes are Staphylococcus epidermidis, Corynebacterium spp, Staphylococcus aureus, Pseudomonas spp, or Proteus spp, which also have been isolated from intraocular infections. Bacteria present on the eyelid are responsible for acute postoperative endophthalmitis.18,20
To prevent the onset of postoperative endophthalmitis, several prophylactic strategies have been used. These approaches, which often feature use of topical antibiotics, are focused on reducing the bacterial load on the ocular surface assuming this could reduce the risk of endophthalmitis.8,21 The most commonly used treatments are perioperative topical antibiotics, preoperative topical antibiotics, intracameral antibiotics, antibiotics at the end of the surgery, and postoperative topical antibiotics.22 In addition to antibiotics, the antiseptic povidone–iodine (PVI) 5% to 10% is topically applied to the cornea, the conjunctival sac, and to the periorbital area. Several studies have demonstrated that PVI reduces the microbiota in the conjunctiva.7,8,21,23
This study tested the efficacy of lid hygiene using commercially available cleansing wipes to reduce the ocular surface microbiota in patients before ocular surgery.
METHODS
This study adhered to the Helsinki Declaration and the Good Clinical Practice (GCP) guidance and was approved by the Research Ethics Committee of the Hospital Clínico San Carlos (Madrid, Spain). All patients gave written informed consent.
This single-center, prospective, single-blind phase IV study included adults of either sex, who were scheduled for ocular surgery after the treatment. Exclusion criteria included any kind of ocular or systemic disease, past or present, and any ocular and/or systemic treatments/antibiotics within the last 6 months.
The study lasted 5 days and the patients were examined at day 0 (D0), day 3 (D3), and day 5 (D5). The 5-day treatment was applied to only 1 eye that is, the eye selected for surgery; the other eye served as control with no treatment applied. Patients were instructed to apply heat over the eye for 4 min. A small towel was heated in a microwave on full power for 10 to 15 sec (which rises towel temperature to approximately 40°C) which was repeated every 2 min. It was applied to the closed eye area with sufficient pressure to ensure that the towel remained in contact with the eyelids. Then patients massage, applying a gently pressure, the upper lid “inward–downward” and the lower lid “inward–upward,” to mobilize the debris adhered to the eyelashes and meibum, and finally they use a sterile eyelid wipe and slide it across the eyelid from the inside to the outside, to clean the upper and lower lids. The Blephaclean (Laboratoires Théa, Clermont-Ferrant, France) sterile wipes contain a solution of hyaluronic acid, capryloyl glycine, iris florentine, and centella asiatica. Hyaluronic acid can influence cell proliferation, differentiation, and dermal tissue repair.24 Capryloyl glycine and iris florentine extracts are natural emollients show antibacterial activity and present a sebum-regulator effect and centella asiatica acts repairing dermal tissue, increasing collagen production, and activating blood circulation.25
They were used twice-a-day for 5 days in the selected eye. Visual acuity was measured at D0 and D5, and a slit-lamp examination was performed in each visit, to ensure there was no adverse reaction on the treated tissues.
Procedures and Assessments
Microbiota samples were taken from the surface of the lower lid and the inferior conjunctival sac fundus of both eyes at D0, D3, and D5 using sterile swabs moistened with saline solution without administering topical anesthesia. The nontreated eye was used as a control and microbiota samples were obtained in the same way. Sterile gloves were used when taking the samples.
Samples were cultured in specific media on blood and chocolate agar plates and incubated aerobically for 48 hr at 37°C. Sterile cotton swabs (Materlab SL, Madrid, Toledo, Spain) were used wet with 50 µL of saline solution. The volume of saline was controlled and applied by a pipette. Initially a sample from the eyelid was taken and spread onto 1 semipart of the dish and, by the same procedure, another cotton swab was taken from the conjunctival sac of the conjunctiva and seeded onto the other semipart of the dish. Dishes were divided in two semiparts; the left side corresponding to the eyelid culture and the right side to the conjunctiva. Grown colonies were photographed with a high-resolution digital camera, and the total area of microbial growth on the plates was analyzed by ImageJ software.
The percentage of the area occupied by the colonies was determined as follows: (1) The original image of the plate, shown on the left side of Figures 1 and 2, is a full RGB TIFF, which was converted to 8-bit grayscale. Figure 2 shows full images of the dishes. The upper plates belong to the right eye and the lower plates to the left eye. (2) A mask was created by marking out the region where the colonies may grow. The mask is a semicircle covering half the plate, corresponding to the region in which the colonies from the lid or the colonies from the conjunctiva were grown. (3) The selected region is transformed to 1-bit image by selecting an adequate threshold. The contrast between the colonies and the background (culture medium) was very good and a sharp contour of the colonies was achieved. The final image is shown in Figure 1 (right). Black pixels correspond to colonies, and white pixels correspond to the culture medium. (4) The ratio of the area occupied by the colonies and the total available growing region was obtained as the ratio of the number of pixels set to 0 and the number of pixels set to 1.
FIG. 1.
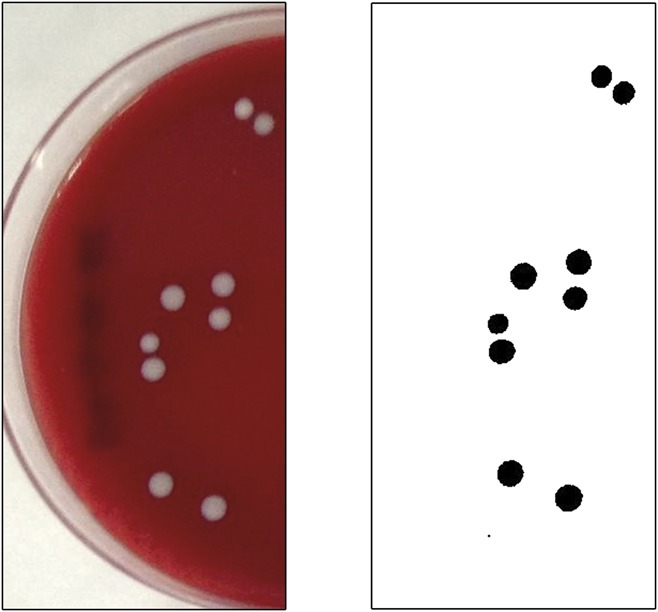
Counting mask. Detail of eyelid colonies. Image corresponds to patient #39.
FIG. 2.
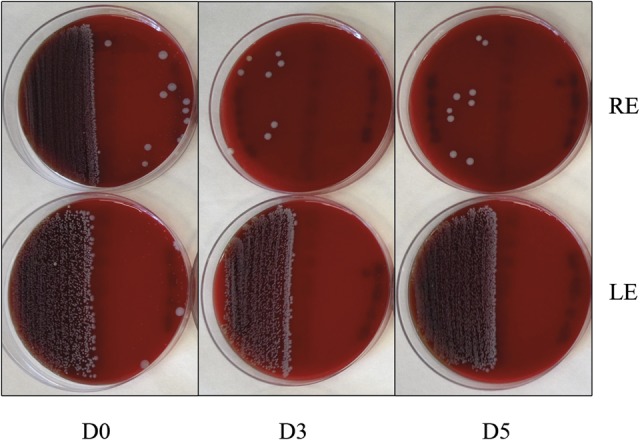
Dishes are divided in two semiparts: the left sides correspond to the eyelid culture and the right sides to the conjunctiva, the upper plates belong to the right eye and the lower ones to the left. Image corresponds to patient #39.
Once the photographs of the dishes were taken, microorganisms were identified by Gram staining, catalase, coagulase, DNase, and mannitol fermentation tests.
Statistical Analyses
Data are presented as mean±SD. Differences in microbiota numbers between D0, D3, and D5 were assessed using the Student t test, assuming a significance level of 0.05.
RESULTS
The study included 45 consecutive patients (17 men, 28 women) with a mean age of 53.8±6.3 years (range 45–73 years). Visual acuity in the treated eye was in a range between 1.0 and 0.54 logMAR and remained stable during the treatment, and the slit-lamp examination showed no adverse reaction on the treated tissues.
Microorganisms Present in the Eyelid and Conjunctiva
At D0, microbial analysis demonstrated similar profiles for eyelid and conjunctiva. Identified microbiota is shown in Table 1. The eyelid showed the presence of S. epidermidis and Corynebacterium spp (94.7% and 32.98% of the plates, respectively) as the most frequent microorganisms. In a smaller percentage, S. aureus, Micrococcus spp, and Bacillus spp (6.38%, 8.51%, and 1.06%, respectively) were isolated. From the conjunctival swabs, S. epidermidis and Corynebacterium spp (54.26% and 38.29%) were identified as well as S. aureus and Micrococcus spp (5.32% and 1.06%).
TABLE 1.
Microbiota Profiles for Eyelid and Conjunctiva Before Treatment

Microbiota Reduction
The microbial area of growth on the plates was measured according to the method described in the methods section. A photograph of a typical petri dish, along with the mask used for measuring the area occupied by the colonies is shown in Figure 1. The colonies' area analysis yielded a higher microbial load from the eyelids (17.9%) compared with the conjunctiva (1.4%) in most of the samples, as it is seen in Figure 2. Percent reduction in microbial load on the eyelid in the treated eyes was 58% at D3 and 63% at D5 63% (P=0.001) (Fig. 3). The microbiota of the conjunctiva was lower than that of the eyelid. There was a reduction, although nonsignificant, of 72% and 69% at D3 and D5, respectively (Fig. 4) in the numbers of microbes on the conjunctiva. Table 2 and Figures 1 and 2 show the microbiota growing areas from eyelid and conjunctiva in all groups. There were no differences by sex.
FIG. 3.
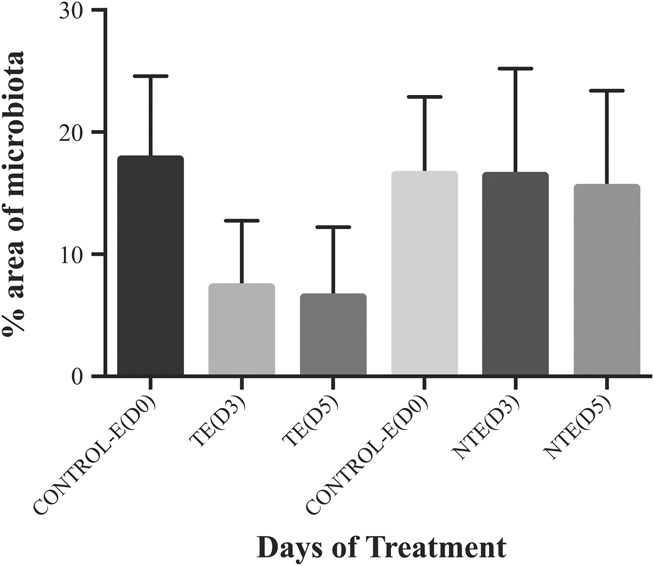
Eyelid microbiota throughout the study (D0, D3, and D5) for the treated eye (TE) and the nontreated eye (NTE). Bars represent mean±SD. Differences between TE and NTE were statistically significant (P<0.0001).
FIG. 4.
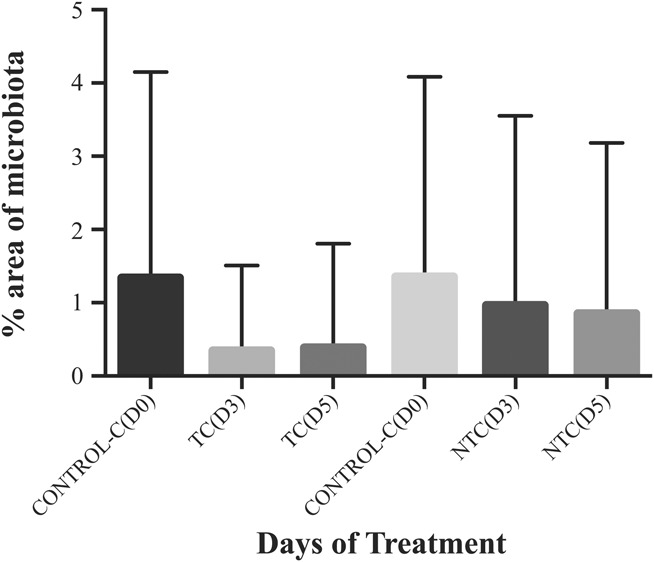
Conjunctiva microbiota throughout the study (D0, D3, and D5) for the treated eye (TC) and the nontreated eye (NTC). Bars represent mean±SD. No statistically significant differences were found between TC and NTC.
TABLE 2.
Mean Values of Microbiota Growing Areas From Eyelid and Conjunctiva for Days 0, 3, and 5
DISCUSSION
In addition to the classical procedures such as cataract surgery, the number of intraocular interventions is increasing because of the development of new therapies. Endophthalmitis caused by microorganisms present on the ocular surface may occur as a consequence of these treatments. The results obtained in this study demonstrated a significant reduction in the microbiota present on the lids and conjunctiva after 3 days of rubbing the lids with the wipes, demonstrating the importance of lid hygiene before ocular surgery or an intraocular procedure, as a complementary prophylactic approach.
The percentages of microorganisms existing on the eyelids and conjunctiva in the study were similar to that described previously with the most commonly isolated bacteria being coagulase-negative staphylococci and, more specifically, S. epidermidis followed by Corynebacterium spp Between 20% and 80% of swabs from the conjunctiva and between 30% and 100% of swabs from the lids showed growth of the bacteria S. epidermidis.19 Carron et al.26 found the most common bacteria were coagulase-negative staphylococci (66.7%), followed by Corynebacterium spp (11.5%) from the conjunctiva, and Li et al.27 described a prevalence of 62.9% and 32.4% for S. epidermidis and Corynebacterium spp, respectively, from the conjunctiva. Vasavada et al.28 detected between 90.4% and 94.4% of Staphylococcus species from the conjunctiva, of which 71% were S. epidermidis. The rate of isolation of the same bacteria in the study by Höfling-Lima et al.29 ranged between 50% and 80% for the conjunctiva and between 76% and 100% for the eyelids.
The microbial isolation of the conjunctiva was reduced on presurgical prophylaxis of wiping the eye after briefly heating the eye. Hueso Abancens et al.30 presented a similar study analyzing the use of palpebral cleansing solution with capryloyl glycine over the conjunctiva microbiota. A clinical reduction was seen from the third day of applying the cleansing solution that lasted until day 5. In the study of Hueso Abancens et al.,30 the maximum reduction obtained after 5 days of treatment was approximately 10% and the main bacteria was Staphylococcus spp coagulase-negative. The smaller reduction found by Hueso Abancens et al.30 than this study could be due to the hygiene protocol used to clean the lids. In the study by Hueso Abancens et al.,30 the patients were not told to apply heat and massage the lids before using the cleansing solution.
In addition, many studies have been conducted on the reduction of eyelid and conjunctiva microbiota after the application of different antibiotics on their own or in combination with PVI. Höfling-Lima et al.29 examined the efficacy of topical lomefloxacin 0.3% and tobramicin 0.3% 3 days before cataract surgery in reducing biota, and showed statistical reductions in conjunctiva of 66.7% and in lid biota of 3.9% with lomefloxacin, and of 75% and 34.5%, respectively, with tobramicin.
Carron et al.26 described a 4.3% reduction in conjunctiva biota after the administration of ciprofloxacin 0.3% 1 day before cataract surgery that increased to 60.9% after the application of PVI. The use of PVI before cataract surgery has become a standard of care and a mandatory step in reducing ocular surface microbiota. Apt et al.31 showed a 91% reduction in the number of colonies of the conjunctiva after the application of PVI. Halachmi-Eyal et al.32 reported a 38% reduction after the application of PVI 3 min before surgery and a 34% reduction with the topical application of moxifloxacin 0.5% 2 hr before surgery and PVI 3 min before surgery.
The percentage reductions in the microbiota from this study are comparable in some ways to those obtained after the application of topical antibiotics. Eyelid hygiene did not sterilize the tissue, it just decreased the number of bacteria present in the external tissue of the eye without modifying the composition of saprophytic biota on both eyelids and conjunctiva. The reason for these reduced values is probably due to the hygiene protocol that patients followed of cleaning their eyelids after the application of heat and the massage.
Although microbiota is present in eyelids and conjunctiva, the percentage of area covered by colonies shows an important microbial load from the eyelids, which highlights the need to maintain preoperative and postoperative asepsis of both eyelid and conjunctiva.
Results obtained from bacteria cultures can indicate the type of antibiotic prophylaxis to use for minimizing ocular postoperative infections: broad-spectrum antibiotics or, preferably, antibiotics with specific activity against gram-positive bacteria.
Eyelid hygiene cannot replace general preoperative procedures focused on preventing contamination, such as the use of PVI solutions for eyelids and conjunctiva or antibiotics. Nevertheless, eyelid hygiene can be used as a complementary prophylactic approach to prevent endophthalmitis.
The use of cleansing eyelid wipes may help in the case of undiagnosed blepharitis before cataract surgery or other kinds of ocular surgery, as they could also reduce the risk of postoperative endophthalmitis.
Further studies are needed regarding the convenience of the medical regimen of use 3 and 5 days before surgery; the time the microbiota takes to recover itself after the use of the eyelid wipes; and what occurs if the patient uses the wipes more than 5 days. Another aspect worthy of study is the usefulness of applying the eyelid wipes as a postoperative prophylaxis or the medical regimen of use. This study demonstrated that the application of heat and a massage to the eyelids before the use of the eyelid wipe improves the reduction in the microbiota present in the ocular surface in 5 days, and eyelid hygiene can be use as a complementary prophylactic approach to prevent ocular infections.
Footnotes
The authors have no conflicts of interest to disclose.
Supported by the eyelid wipes were supplied by Thea Laboratories (Barcelona, Spain).
Presented at the Association for Research in Vision and Ophthalmology (ARVO) 2014 Annual Meeting, May 4–8, 2014, Orlando, FL.
REFERENCES
- 1.Keay L, Gower EW, Cassard SD, et al. Postcataract surgery endophthalmitis in the United States: analysis of the complete 2003 to 2004 Medicare database of cataract surgeries. Ophthalmology 2012;119:914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lalitha P, Rajagopalan J, Prakash K, et al. Postcataract endophthalmitis in South India incidence and outcome. Ophthalmology 2005;112:1884–1889. [DOI] [PubMed] [Google Scholar]
- 3.Jambulingam M, Parameswaran SK, Lysa S, et al. A study on the incidence, microbiological analysis and investigations on the source of infection of postoperative infectious endophthalmitis in a tertiary care ophthalmic hospital: an 8-year study. Indian J Ophthalmol 2010;58:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong TY, Chee SP. The epidemiology of acute endophthalmitis after cataract surgery in an Asian population. Ophthalmology 2004;111:699–705. [DOI] [PubMed] [Google Scholar]
- 5.Mollan SP, Gao A, Lockwood A, et al. Postcataract endophthalmitis: incidence and microbial isolates in a United Kingdom region from 1996 through 2004. J Cataract Refract Surg 2007;33:265–268. [DOI] [PubMed] [Google Scholar]
- 6.Lundstrom M, Wejde G, Stenevi U, et al. Endophthalmitis after cataract surgery: a nationwide prospective study evaluating incidence in relation to incision type and location. Ophthalmology 2007;114:866–870. [DOI] [PubMed] [Google Scholar]
- 7.Barry P, Cordoves L, Gardner S. ESCRS Guidelines for Prevention and Treatment of Endophthalmitis Following Cataract Surgery: Data, Dilema and Conclusions. Dublin, Ireland, The European Society for Cataract & Refractive Surgeons, Temple House, Temple Road, Blackrock, Co, 2013. Available at: http://www.escrs.org/endophthalmitis/guidelines/ENGLISH.pdf. Accessed December 6, 2015. [Google Scholar]
- 8.Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology 2002;109:13–24. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–1431. [DOI] [PubMed] [Google Scholar]
- 10.Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 2006;113:363–372. [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, Kobayakawa S, Sotozono C, et al. Evaluation of the incidence of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor. Ophthalmologica 2011;226:145–150. [DOI] [PubMed] [Google Scholar]
- 12.Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina 2006;26:999–1005. [DOI] [PubMed] [Google Scholar]
- 13.Chun DW, Heier JS, Topping TM, et al. A pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edema. Ophthalmology 2006;113:1706–1712. [DOI] [PubMed] [Google Scholar]
- 14.Rabena MD, Pieramici DJ, Castellarin AA, et al. Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina 2007;27:419–425. [DOI] [PubMed] [Google Scholar]
- 15.Hoevenaars NE, Gans D, Missotten T, et al. Suspected bacterial endophthalmitis following intravitreal anti-VEGF injection: case series and literature review. Ophthalmologica 2012;228:143–147. [DOI] [PubMed] [Google Scholar]
- 16.Fintak DR, Shah GK, Blinder KJ, et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina 2008;28:1395–1399. [DOI] [PubMed] [Google Scholar]
- 17.Durand ML. Endophthalmitis. Clin Microbiol Infect 2013;19:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callegan MC, Engelbert M, Parke DW, et al. Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin Microbiol Rev 2002;15:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willcox MD. Characterization of the normal microbiota of the ocular surface. Exp Eye Res 2013;117:99–105. [DOI] [PubMed] [Google Scholar]
- 20.Speaker MG, Milch FA, Shah MK, et al. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology 1991;98:639–649. [DOI] [PubMed] [Google Scholar]
- 21.Ta CN, Egbert PR, Singh K, et al. Prospective randomized comparison of 3-day versus 1-hour preoperative ofloxacin prophylaxis for cataract surgery. Ophthalmology 2002;109:2036–2040. [DOI] [PubMed] [Google Scholar]
- 22.Chang DF, Braga-Mele R, Mamalis N, et al. Prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2007 ASCRS member survey. J Cataract Refract Surg 2007;33:1801–1805. [DOI] [PubMed] [Google Scholar]
- 23.Gawronska M, Kaluzny J, Mikucka A, et al. Bacterial flora of conjunctival sac in patients with cataract. Methods of disinfection and evaluation of their efficiency. Klin Oczna 2005;107:408–413. [PubMed] [Google Scholar]
- 24.Girardeau-Hubert S, Teluob S, Pageon H, et al. The reconstructed skin model as a new tool for investigating in vitro dermal fillers: increased fibroblast activity by hyaluronic acid. Eur J Dermatol 2015;25:312–322. [DOI] [PubMed] [Google Scholar]
- 25.Bylka W, Znajdek-Awiżeń P, Studziñska-Sroka E, et al. Centella asiatica in dermatology: an overview. Phytother Res 2014;28:1117–1124. [DOI] [PubMed] [Google Scholar]
- 26.Carron A, Samudio M, Laspina F, et al. Efficacy of topical 0.3% ciprofloxacin application in reducing the conjunctival biota of patients undergoing cataract extraction. Arch Soc Esp Oftalmol 2013;88:345–351. [DOI] [PubMed] [Google Scholar]
- 27.Li B, Nentwich MM, Hoffmann LE, et al. Comparison of the efficacy of povidone-iodine 1.0%, 5.0%, and 10.0% irrigation combined with topical levofloxacin 0.3% as preoperative prophylaxis in cataract surgery. J Cataract Refract Surg 2013;39:994–1001. [DOI] [PubMed] [Google Scholar]
- 28.Vasavada AR, Gajjar D, Raj SM, et al. Comparison of 2 moxifloxacin regimens for preoperative prophylaxis: prospective randomized triple-masked trial. Part 2: residual conjunctival flora. J Cataract Refract Surg 2008;34:1383–1388. [DOI] [PubMed] [Google Scholar]
- 29.Höfling-Lima A, Farah M, Montenegro L. Changes in conjunctival and lid flora after topical use of lomefloxacin and tobramycin in cataract and refractive surgery. Arq Bras Oftalmol 2002;65:21–29. [Google Scholar]
- 30.Hueso Abancens JR, Mengual VE, Schargel PK, et al. Modification of the conjuntival flora with cleaning palpebral solutions. Arch Soc Esp Oftalmol 2004;79:617–621. [DOI] [PubMed] [Google Scholar]
- 31.Apt L, Isenberg S, Yoshimori R, et al. Chemical preparation of the eye in ophthalmic surgery. III. Effect of povidone-iodine on the conjunctiva. Arch Ophthalmol 1984;102:728–729. [DOI] [PubMed] [Google Scholar]
- 32.Halachmi-Eyal O, Lang Y, Keness Y, et al. Preoperative topical moxifloxacin 0.5% and povidone-iodine 5.0% versus povidone-iodine 5.0% alone to reduce bacterial colonization in the conjunctival sac. J Cataract Refract Surg 2009;35:2109–2114. [DOI] [PubMed] [Google Scholar]


