Abstract
Cerebrovascular injury while on extracorporeal membrane oxygenation (ECMO) may be caused by excessive brain perfusion during hypoxemic reperfusion. Previous studies have postulated that the most vulnerable period of time for cerebrovascular injury is during the transfer period to ECMO. Therefore, our objective was to compare brain perfusion and hemodynamics in a piglet endotoxic shock ECMO model. The effect of ECMO flow on microcirculation of different brain regions was compared between 10 control pigs and six pigs (7–10 kg) administered IV endotoxin to achieve a drop in mean arterial blood pressure (MAP) of at least 30%. Cardiac output (CO), brain oxygen utilization, and microcirculatory blood flow (BF) were compared at baseline and 2 hours after ECMO stabilization. Matching ECMO delivery with baseline CO in control animals increased perfusion (p < 0.05) in all areas of the brain. In contrast, with endotoxin, ECMO returned perfusion closer to baseline levels in all regions of the brain and maintained brain tissue oxygen consumption. Both control and endotoxic pigs showed no evidence of acute neuronal necrosis in histologic cerebral cortical sections examined after 2 hours of ECMO. Results show that during endotoxic shock, transition to ECMO can maintain brain BF equally to all brain regions without causing overperfusion, and does not appear to cause brain tissue histopathologic changes (hemorrhage or necrosis) during the acute stabilization period after ECMO induction.
Keywords: ECMO, cerebral blood flow, endotoxic shock
Sepsis remains a leading cause of morbidity and mortality in infants and children worldwide. Mortality rates in hospitalized children in the United States remain greater than 10%.1 Venoarterial extracorporeal membrane oxygenation (ECMO) is increasingly utilized in the neonatal population for cardiorespiratory failure to support heart and lung function. Several case reports and retrospective reviews support ECMO for use in septic shock with imminent end organ failure.2,3
One of the known risk factors of ECMO treatment for severe cardiorespiratory failure is cerebrovascular injury. The risk of cerebrovascular injury after ECMO remains fairly high, at 20% with an estimated long-term morbidity of 60% in those cases.4 Brain injury in sepsis has been reported to be associated with lower neonatal ECMO survival (39%) when compared with survival of neonates (80%) without brain injury.1
Animal and human ECMO studies have looked at possible causes of cerebrovascular injury and found that carotid artery ligation and systemic heparinization are not directly responsible.5–7 In both human and animal ECMO models, cerebrovascular injury appears to be more closely associated with reperfusion of ischemic cerebral tissue.8 The porcine central nervous system, more specifically the brain, has been utilized as a proxy human model given similarities in size and anatomic characteristics.9,10 More specifically, the pattern of piglet brain injury on coronary bypass approximates neonatal brain injury patterns and is localized to the neocortex and hippocampus.11
Cerebral perfusion has not been well studied during ECMO. Clinically, ECMO pump flow is set to enable the restoration of mean arterial blood pressure (MAP) at a preshock level. However, it is possible that setting ECMO flow based on achieving a set mean systemic blood pressure may lead to overperfusion of the cerebral circulation.
In this study, we hypothesized that brain perfusion is adequate during early ECMO stabilization in both normotensive and hypotensive states by comparing physiologic parameters and brain metabolism between normal piglets with that of a piglet model of endotoxic shock.
Methods
The study protocol was approved by the Institutional Animal Care and Use Committee at Tripler Army Medical Center. Investigators complied with the policies as prescribed in the USDA Animal Welfare Act and the National Research Council’s “Guide for the Care and Use of Laboratory Animals.” Facilities were fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Animal Preparation
Sixteen Yorkshire cross piglets (7–10 kg body weight of either sex) were sedated and anesthetized with pentobarbital and mechanically ventilated to maintain constant tidal volume and end-tidal CO2. To assess physiologic measurements, the following catheters were inserted: a 6 Fr. Swan Ganz catheter was threaded via the left femoral vein such that the balloon tip was placed in the pulmonary artery for measurement of cardiac output (CO) by thermodilution of cold 5% dextrose bolus injection into the right atrium. An arterial catheter with thermister for CO measurements was placed in the thoracic aorta via left femoral artery access. A left ventricle catheter was placed via the left carotid artery for microsphere delivery for determination of regional blood flow (BF). A left carotid catheter was placed in cephalad direction to obtain oxyhemoglobin measurements of the brain.
Extracorporeal Circuit
Extracorporeal membrane oxygenation cannulae were placed for venous-arterial (VA) ECMO delivery with a 12–14 Fr venous cannula aligned with the right atrium via right jugular vein access, 8–10 Fr cannula tip placed in the aortic arch via left carotid artery access, and 8–10 Fr cephalad catheter placed via the right jugular vein. Extracorporeal membrane oxygenation circuits were primed via CO2 introduction, 0.9% saline solution, and then filled with whole blood harvested within 24 hours from donor Sus scrofa 50 kg pigs. Hundred units of heparin, calcium chloride, and sodium bicarbonate were then added. The total prime volume was 450 ml. Extracorporeal membrane oxygenation flow was achieved via centrifugal pump (Jostra-Rotaflow HL20, Maquet, Rastatt, Germany) through a hollow fiber membrane oxygenator (Quadrox, Josta, Maquet, Rastatt, Germany). All piglets received an initial heparin bolus about 75–100 units/kg before cannulation. Activated clotting time (ACT) samples were drawn periodically and additional heparin boluses were given to maintain ACT between 150 and 200 seconds. Brain venous oxyhemoglobin measurements were obtained from samples collected from the cephalad venous ECMO catheter.
Oxygen Saturation Measurements
Blood samples were obtained for oxygen utilization measurements determined from blood gases and co-oximetry. Brain oxygen extraction was then calculated from the arterial-venous difference in oxygen content in samples collected from the brain catheters. Near infrared spectroscopy (NIRS; INVOS 5100, Somanetics, Troy, MI) was used in the endotoxin group to monitor real-time changes in brain oxygen saturation for descriptive purposes as a noninvasive device to correlate with oxygen utilization.12–14
Hemodynamic Measurements
Physiologic parameters assessed included CO, wedge pressure, central venous pressure, mean arterial pressure, cerebral arterial pressure, and cephalad vein pressure. Hemodynamic measurements were recorded continuously throughout the experiment. Regional microcirculatory BF assessments were obtained by injecting different colored microspheres into the left ventricle for each specified time period.
The percent of total perfusion delivered by the native heart while on ECMO was determined by measuring CO via a Swan Ganz catheter thermistor placed in the abdominal aorta and cold thermodilution vehicle injected into the ECMO circuit immediately at the flow delivery site into the aortic arch. The difference between this measured total flow and ECMO pump flow was attributed to native heart CO.
Experimental Protocol
After catheters were placed, a 90 min baseline period was allowed. After baseline measurements were obtained, pigs were randomized to either a control group or an endotoxin-induced hypotension shock group.
In the control group (n = 10), ECMO measurements were taken at an early phase of ECMO (within 30 min of ECMO initiation) and a later phase after ECMO steady state was established for 2 hours. Extracorporeal membrane oxygenation flow rates (0.7–1.0 L/min) were adjusted to maintain MAP and CO at baseline levels.
In the endotoxin-induced septic shock group (n = 6), baseline measurements were first obtained. Endotoxic shock was induced with an IV injection of 3–30 mg/kg of Escherichia coli endotoxin to achieve a consistent hypotension with at least a 30% decrease in blood pressure.
All animals were euthanized and tissues harvested for microsphere data and brain specimens were collected for histology. Tissues were harvested for determination of brain regional BF in the cerebral cortex, cerebellum, brainstem, and midbrain. Histologic sections from the cerebral cortex, hippocampus, deep grey nuclei, and cerebellum were examined for acute neuronal necrosis (acidophilic neurons) in pigs treated with ECMO.
Data Analysis
Two-way Analysis of variance (ANOVA) with repeated measures over time was used to compare mean values of MAP, CO, oxygen delivery to the brain, brain oxygen extraction, and brain oxygen consumption between and within groups (JMP 4.0.4 program, SAS Institute, Cary, NC). Regional BF was measured at baseline, during endotoxic shock, and during ECMO. ANOVA was followed by posthoc comparisons with the Tukey’s HSD test. For all statistical tests, a p < 0.05 was considered significant.
Results
Effects of Extracorporeal Membrane Oxygenation on Brain Perfusion and Tissue Histology
Extracorporeal membrane oxygenation delivered approximately 70% of total arterial BF versus the native heart (30%). In the control group, CO and MAP were closely matched to baseline during ECMO. The endotoxin group had decreased MAP and CO that never returned to baseline levels even with maximal ECMO flows (Figures 1A, B). This is likely due to systemic vasodilation caused by endotoxin. However, brain microcirculatory flow remained stable on ECMO (Figure 2A) in all four regions of the brain (cerebrum, cerebellum, brainstem, and midbrain) despite endotoxin whereas in the control group, brain microcirculatory flow increased in all four regions of the brain compared with baseline while controlling for CO and blood pressure (Figure 2B). Cerebral hippocampal H&E stains from both control and endotoxin groups demonstrated healthy nuclei without eosinophilic necrosis or apoptosis.
Figure 1.
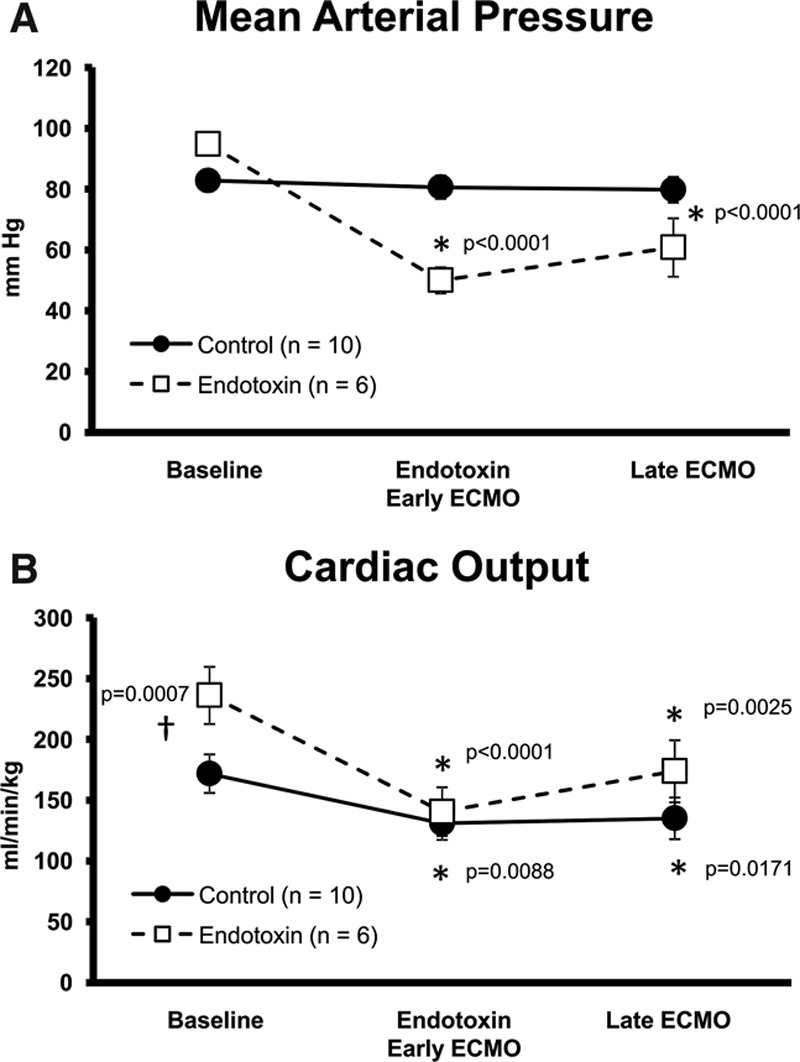
ECMO effects on hemodynamics. A: Mean arterial blood pressures and (B) cardiac output in control and endotoxin animals at baseline, endotoxin/early ECMO, and late ECMO (*different from baseline, p < 0.05, †different between groups, p < 0.05). ECMO, extracorporeal membrane oxygenation.
Figure 2.
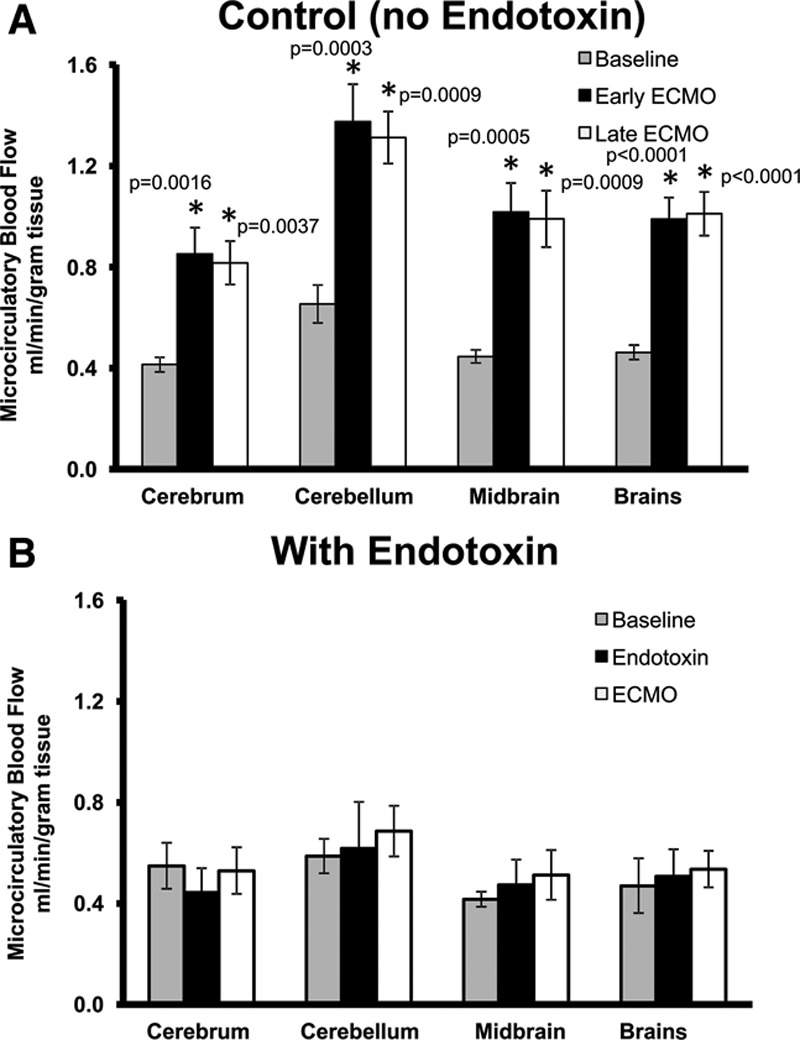
Brain microcirculatory blood flow. A: Endotoxin and (B) control at baseline, endotoxin/early ECMO, and late ECMO (*different from baseline, p < 0.05). ECMO, extracorporeal membrane oxygenation.
Effects of Extracorporeal Membrane Oxygenation on Oxygen Utilization
Although there were no sustained differences in systemic oxygen utilization among control and endotoxin groups, changes were observed in brain oxygen utilization. In the control subjects, there was a significant decrease in brain oxygen delivery and consumption immediately after initiation of ECMO despite keeping MAP constant with ECMO delivery that was restored to baseline levels by the 2 hour stabilization period. ECMO ameliorated the endotoxin effect of decreased oxygen delivery and improved brain tissue oxygen utilization close to preendotoxin levels (Figure 3A–C).
Figure 3.
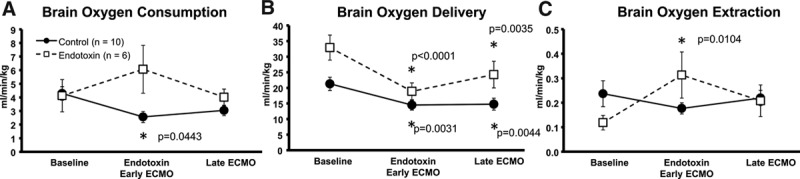
ECMO effects on brain oxygen utilization during baseline, endotoxic shock/early ECMO, and late ECMO in control and endotoxin animals. A: Oxygen consumption; (B) oxygen delivery; (C) oxygen extraction (*different from baseline, p < 0.05). ECMO, extracorporeal membrane oxygenation.
Effects of Extracorporeal Membrane Oxygenation on Co-Oximetry Analyses
Co-oximetry blood gas and oxygen saturation monitoring were used additionally to calculate oxygen utilization. Brain fractional oxygen extraction ratios (FOER) from both PaO2 and co-oximetry calculation methods showed no differences in both control subjects and endotoxin groups after 2 hour long stabilization on ECMO, but endotoxin did increase the FOER ratio demonstrating preferential cerebral oxygen utilization during endotoxic stress (Figure 4).
Figure 4.
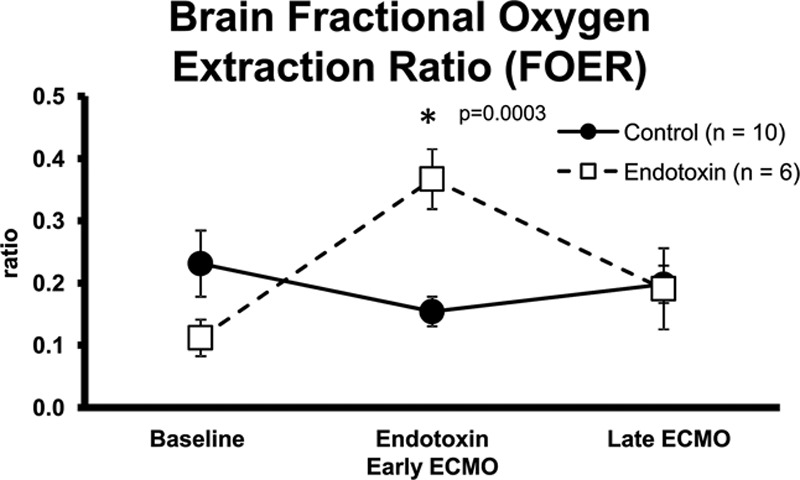
Brain FOER from PaO2 in control and endotoxin animals during baseline, endotoxic shock/early ECMO, and late ECMO (*different from baseline, p < 0.05). ECMO, extracorporeal membrane oxygenation; FOER, fractional oxygen extraction ratio.
Effects of Extracorporeal Membrane Oxygenation on Hematocrit
Whole blood transfusions were administered to the ECMO circuit with the intent of maintaining stable hematocrits. Results are reported as mean ± standard error of the mean (SEM). Control and endotoxin groups had similar baseline hematocrits, 31.9 ± 1.5% and 31.6 ± 0.6%, respectively. In the endotoxin group, hematocrit remained stable during early ECMO at 34.4 ± 2.2%, declined to 29.9 ± 2.3% at late ECMO, but these values were not statistically significant. In the control group, hematocrit decreased during early and late ECMO periods, 25.8 ± 0.7% (p = 0.001) and 25.1 ± 0.6% (p = 0.0003), respectively.
Discussion
The brain can tolerate a longer period of ischemia suggested up to 20 min with proper restoration of BF.15 Extracorporeal membrane oxygenation should be able to provide adequate BF to the brain during reperfusion albeit with nonpulsatile flow.16 Currently, brain injury is a major complication associated with ECMO. In response to hypoxia, the brain regulatory system causes a massive dilation of vascular beds, which may itself be the principle cause of injury versus reperfusion.17 Combined aggregate factors associated with cerebrovascular injury include severe hypoxia, hypercapnia, systemic heparinization, decreased cerebral venous outflow, and ligation of right internal carotid artery, but none independently have been associated with brain injury.6,7,15,18Although a potential for an ischemic period after right carotid artery ligation exists, several studies reported no differences in cerebral BF or perfusion between right and left hemispheres6,7,18 and restoration of cerebral oxygenation after ECMO cannulation.19
The effects of ECMO on cerebral perfusion in the acute period during transfer and ECMO stabilization remain to be defined. It is not clear whether injury occurs sooner such as within the first few hours of instituting ECMO or whether it occurs during the latter period, as a complication that manifests after prolonged reperfusion with ECMO. Short et al.5 reported a 25% reduction in cerebral BF with 30% reduction of oxygen delivery during ECMO induction in a lamb model. Their target flow of 100 ml/kg/hr was lower than our targeted flow of close to 160 ml/kg/hr, which we found was needed to maintain baseline MAP and likely contributed to our increased cerebral BF in control, nonhypotensive animals. In contrast, another study utilizing a pig model to compare cerebral BF on ECMO between normoxemic and hypoxemic pigs found increased cerebral BF in both groups at 60 and 120 min at flow rates between 65 and 100 ml/kg/min along with a significant decrease in pulsatility ratio and concluded that hyperperfusion was most likely related to the ECMO procedure.17 Cerebral vascular resistance was high in another lamb ECMO model, which could be a response to change in pulsatility and ECMO flow adjustments.20 These authors speculated that prolonged hypoxia combined with cerebral hyperperfusion renders the venous-capillary endothelium vulnerable to injury.20 Interestingly, although we report similarly increased brain perfusion on ECMO, we present no evidence for brain tissue injury by H&E staining in the immediate period after ECMO induction under both normoxic/normotensive and hypoxic/hypotensive conditions. Our data is one of the first to describe changes in cerebral perfusion in normotensive and severe shock conditions in the 2 hour period after ECMO induction.
Our data suggest that ECMO restores cerebral BF and brain tissue metabolism similar to baseline. Without ECMO support in a setting of severe endotoxic shock requiring vasopressor use, cerebral BF is decreased.21 Among our control animals, microcirculatory BF to the brain increased supporting the theory that in a nonhypotensive animal with constant baseline mean arterial pressure and CO, ECMO induction hyperperfuses brain tissue. In our model using endotoxin to induce hypoxemia, we showed a restoration of brain microcirculatory flow and brain tissue oxygen metabolism with ECMO. Setting the pump flow to match baseline CO and blood pressure may not be the optimal indicator to set adequate ECMO BF and oxygen delivery.
The lack of real-time brain oxygen delivery monitoring can contribute to brain injury.22 NIRS has been used and validated as a method to measure adequacy of cerebral tissue oxygenation during ECMO.23,24 Oxygen utilization would be an indicator of how well oxygen delivery is being met with the pump flow. Further study is warranted using real-time NIRS technology as a guide for establishing ECMO pump flow rates as well as measuring oxygen delivery during ECMO for longer time periods.
There are limitations to our study. The aim of our study was to characterize changes in cerebral perfusion in the acute stabilization period after ECMO induction. Therefore, our data are unable to characterize outcomes from longer ECMO time spans beyond 2 hours and limit its applicability in the clinical realm. Additionally, our outcomes focused on hemodynamic and perfusion markers with histologic comparison during acute transitions that do not necessarily correlate with long-term potential central nervous system injury. From a hematocrit preservation perspective, our experimental protocol underwent interim process refinement, which reflect improved hematocrits in the endotoxin group but affected our control group data. Finally, our NIRS monitoring capabilities began mid-way thru experimental protocol and thus, we had data from the endotoxin group only and therefore were not able to report comparative data from both groups.
In conclusion, in both control and endotoxin-exposed piglets, ECMO is able to maintain oxygen delivery to the brain in the setting of microcirculatory hyper-perfusion. Although some studies have suggested that cerebrovascular injury may occur during the immediate period after ECMO initiation, our data may suggest otherwise and highlight the value of additional inquiries into ECMO’s effect on cerebral circulation. Furthermore, our data indicate that optimal perfusion settings for ECMO are very different in the normotensive and hypotensive states. Additionally, our results suggest that perhaps ECMO perfusion settings should be adjusted according to brain BF and oxygen delivery versus systemic pressure criteria. Further studies, particularly incorporating longer ECMO treatment periods, are needed to more fully characterize the effects of ECMO under both normoxic/normotensive and hypoxic/hypotensive states on brain perfusion and injury.
Acknowledgment
The authors are very grateful for the expert technical assistance of Aileen Sato, Claudia Hernandez, Wayne Ichimura, Glenn Hashiro, and the Department of Clinical Investigation Animal Research Section Staff.
Footnotes
S. G. Batts and T. S. Mu contributed equally to this article and share co-first authorship.
Disclosures: The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of the Army or the Department of Defense or the US Government.
Supported by US Army Medical Research and Materiel Command Congressionally Directed Medical Research Program Grant W23RYX6200N602.
References
- 1.Fortenberry JD, Paden ML. Extracorporeal therapies in the treatment of sepsis: Experience and promise. Semin Pediatr Infect Dis. 2006;17:72–79. doi: 10.1053/j.spid.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 2.MacLaren G, Butt W. Extracorporeal membrane oxygenation and sepsis. Crit Care Resus. 2007;9:76–80. [PubMed] [Google Scholar]
- 3.Maclaren G, Butt W, Best D, Donath S, Taylor A. Extracorporeal membrane oxygenation for refractory septic shock in children: One institution’s experience. Pediatr Crit Care Med. 2007;8:447–451. doi: 10.1097/01.PCC.0000282155.25974.8F. [DOI] [PubMed] [Google Scholar]
- 4.Hardart GE, Fackler JC. Predictors of intracranial hemorrhage during neonatal extracorporeal membrane oxygenation. J Pediatr. 1999;134:156–159. doi: 10.1016/s0022-3476(99)70408-7. [DOI] [PubMed] [Google Scholar]
- 5.Short BL, Walker LK, Traystman RJ. Impaired cerebral autoregulation in the newborn lamb during recovery from severe, prolonged hypoxia, combined with carotid artery and jugular vein ligation. Crit Care Med. 1994;22:1262–1268. doi: 10.1097/00003246-199408000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Perlman JM, Altman DI. Symmetric cerebral blood flow in newborns who have undergone successful extracorporeal membrane oxygenation. Pediatrics. 1992;89:235–239. [PubMed] [Google Scholar]
- 7.Raju TN, Kim SY, Meller JL, Srinivasan G, Ghai V, Reyes H. Circle of Willis blood velocity and flow direction after common carotid artery ligation for neonatal extracorporeal membrane oxygenation. Pediatrics. 1989;83:343–347. [PubMed] [Google Scholar]
- 8.Fukuda S, Aoyama M, Yamada Y, et al. Comparison of venoarterial versus venovenous access in the cerebral circulation of newborns undergoing extracorporeal membrane oxygenation. Pediatr Surg Int. 1999;15:78–84. doi: 10.1007/s003830050521. [DOI] [PubMed] [Google Scholar]
- 9.Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: A model for human infectious diseases. Trends Microbiol. 2012;20:50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogdanski R, Blobner M, Becker I, Hänel F, Fink H, Kochs E. Cerebral histopathology following portal venous infusion of bacteria in a chronic porcine model. Anesthesiology. 2000;93:793–804. doi: 10.1097/00000542-200009000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Priestley MA, Golden JA, O’Hara IB, McCann J, Kurth CD. Comparison of neurologic outcome after deep hypothermic circulatory arrest with alpha-stat and pH-stat cardiopulmonary bypass in newborn pigs. J Thorac Cardiovasc Surg. 2001;121:336–343. doi: 10.1067/mtc.2001.112338. [DOI] [PubMed] [Google Scholar]
- 12.Maldonado Y, Singh S, Taylor MA. Cerebral near-infrared spectroscopy in perioperative management of left ventricular assist device and extracorporeal membrane oxygenation patients: Curr Opin Anesthesia. 2014;27:81–88. doi: 10.1097/ACO.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 13.Papademetriou MD, Tachtsidis I, Leung TS, Elliott MJ, Hoskote A, Elwell CE. Cerebral and peripheral tissue oxygenation in children supported on ECMO for cardio-respiratory failure. Adv Exp Med Biol. 2010;662:447–453. doi: 10.1007/978-1-4419-1241-1_64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redlin M, Koster A, Huebler M, et al. Regional differences in tissue oxygenation during cardiopulmonary bypass for correction of congenital heart disease in neonates and small infants: Relevance of near-infrared spectroscopy. J Thorac Cardiovasc Surg. 2008;136:962–967. doi: 10.1016/j.jtcvs.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 15.Jonas RA. The effect of extracorporeal life support on the brain: Cardiopulmonary bypass. Semin Perinatol. 2005;29:51–57. doi: 10.1053/j.semperi.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Brown KL, Goldman AP. Neonatal extra-corporeal life support: Indications and limitations. Early Hum Dev. 2008;84:143–148. doi: 10.1016/j.earlhumdev.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Liem KD, Kollée LA, Klaessens JH, De Haan AF, Oeseburg B. The influence of extracorporeal membrane oxygenation on cerebral oxygenation and hemodynamics in normoxemic and hypoxemic piglets. Pediatr Res. 1996;39:209–215. doi: 10.1203/00006450-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Van Heijst A, Liem D, Hopman J, Van Der Staak F, Sengers R. Oxygenation and hemodynamics in left and right cerebral hemispheres during induction of veno-arterial extracorporeal membrane oxygenation. J Pediatr. 2004;144:223–228. doi: 10.1016/j.jpeds.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Liem KD, Hopman JC, Oeseburg B, de Haan AF, Festen C, Kollée LA. Cerebral oxygenation and hemodynamics during induction of extracorporeal membrane oxygenation as investigated by near infrared spectrophotometry. Pediatrics. 1995;95:555–561. [PubMed] [Google Scholar]
- 20.Hunter CJ, Blood AB, Bishai JM, et al. Cerebral blood flow and oxygenation during venoarterial and venovenous extracorporeal membrane oxygenation in the newborn lamb. Pediatr Crit Care Med. 2004;5:475–481. doi: 10.1097/01.pcc.0000130992.73123.bc. [DOI] [PubMed] [Google Scholar]
- 21.Holt DB, Delaney RR, Uyehara CF. Effects of combination dobutamine and vasopressin therapy on microcirculatory blood flow in a porcine model of severe endotoxic shock. J Surg Res. 2011;171:191–198. doi: 10.1016/j.jss.2009.11.739. [DOI] [PubMed] [Google Scholar]
- 22.Wahr JA, Tremper KK, Samra S, Delpy DT. Near-infrared spectroscopy: Theory and applications. J Cardiothorac Vasc Anesth. 1996;10:406–418. doi: 10.1016/s1053-0770(96)80107-8. [DOI] [PubMed] [Google Scholar]
- 23.Moravec R, Neitzel T, Stiller M, et al. First experiences with a combined usage of veno-arterial and veno-venous ECMO in therapy-refractory cardiogenic shock patients with cerebral hypoxemia. Perfusion. 2014;29:200–209. doi: 10.1177/0267659113502832. [DOI] [PubMed] [Google Scholar]
- 24.Davies DJ, Su Z, Clancy MT, et al. Near-infrared spectroscopy in the monitoring of adult traumatic brain injury: A review. J Neurotrauma. 2015;32:933–941. doi: 10.1089/neu.2014.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]


