Abstract
Chest tubes are utilized to evacuate shed blood after left ventricular assist device (LVAD) implantation, however, they can become clogged, leading to retained blood. We implemented a protocol for active tube clearance (ATC) of chest tubes to determine if this might reduce interventions for retained blood. A total of 252 patients underwent LVAD implantation. Seventy-seven patients had conventional chest tube drainage (group 1), whereas 175 patients had ATC (group 2). A univariate and multivariate analysis adjusting for the use of conventional sternotomy (CS) and minimally invasive left thoracotomy (MILT) was performed. Univariate analysis revealed a 65% reduction in re-exploration (43–15%, p < 0.001), and an 82% reduction in delayed sternal closure (DSC; 34–6%, p <0.001). In a sub-analysis of CS only, there continued to be statistically significant 53% reduction in re-exploration (45% vs. 21%, p = 0.0011), and a 77% reduction in DSC (35% vs. 8%, p < 0.001) in group 2. Using a logistic regression model adjusting for CS versus MILT, there was a significant reduction in re-exploration (odds ratio [OR] = 0.44 [confidence interval {CI} = 0.23–0.85], p = 0.014) and DSC (OR = 0.20 [CI = 0.08–0.46], p <0.001) in group 2. Actively maintaining chest tube patency after LVAD implantation significantly reduces re-exploration and DSC.
Keywords: ventricular assist device, re-exploration, bleeding, chest tube
Postoperative bleeding is the most common complication for patients implanted with left ventricular assist devices (LVADs).1–3 It is not uncommon for patients to require reinterventions for retained blood, such as re-exploration to wash out clot from the mediastinum, delayed primary closures of the sternum, or require additional chest tube placement to drain hemothorax.1,4–6 These patients have increased complications, significantly higher overall mortality and worse 1 year survival after transplantation making this an area with significant unmet clinical needs for process improvement.1,4,5
An often-overlooked variable that may impact the need for a re-exploration for bleeding in patients during early recovery is chest tube patency during the acute bleeding phase. Chest tubes can become clogged while the patient is still bleeding, leading to retained blood around the heart and lungs.7,8 Current methods of clearing chest drainage catheters of clot, such as milking, stripping, and open suction are not beneficial, and can be harmful.8,9 Studies have demonstrated that maintaining chest tube patency with active clearance can reduce the incidence of retained blood, however, protocols of actively maintaining chest tube patency have never been studied in a LVAD population.10–12 The purpose of this study was to evaluate if implementing a protocol to actively maintain chest tube patency reduces retained blood after LVAD implantation.
Methods
This study included 252 consecutive patients from April 2009 to July 2015, undergoing LVAD implantation from the Vanderbilt Advanced Heart Failure Registry collected using the REDCap electronic data capture tool (Vanderbilt Institute for Clinical and Translational Research Grant UL1 TR000445, NCATS/NIH). The study protocol was registered on www.clinicaltrials.gov (NCT02145858) and approved by the Vanderbilt University Institutional Review Board. We analyzed the need for additional interventions for retained blood defined as any re-exploration for bleeding, delayed sternal closure (DSC), pericardial interventions for drainage (pericardial window or pericardiocentesis), and pleural interventions for drainage for hemothorax or bloody effusions.8,12 Patients who had the diagnosis of pleural or pericardial effusion or hemothorax but did not undergo a specific invasive intervention or had conservative medical treatment (i.e., medications) were excluded. Relevant baseline, operative, and postoperative data were obtained. An independent individual blinded to study group assignment collected the data.
Advanced heart failure patients in this study were implanted with either the axial-flow HeartMate II (HM II; St. Jude, St. Paul, MN) or the centrifugal flow HeartWare (HW; Framingham, MA). Specific device selection criteria did not exist in our program; however, both devices were presented to all patients evaluated for LVAD implantation. Most patients were operated through a conventional sternotomy (CS) utilizing cardiopulmonary bypass (CPB). During the study period, we adopted a method of minimally invasive left thoracotomy (MILT) in select patients that included a partial sternotomy, coupled with a left minithoracotomy, utilizing an off pump technique.13,14 After presentation of options, a multidisciplinary committee tailored an informed decision to patient and physician preferences for device and incision selection.6 Delayed sternal closure was used primarily when bleeding risk was perceived to be elevated and occasionally when moderate to severe postoperative right ventricular (RV) dysfunction caused hemodynamic deterioration.
Chest tubes were placed in the operating room at the end of the procedure, just before closure. All the patients were implanted with 32 Fr chest tubes connected to drainage canisters set on −20 cm H2O suction, with one in each open spaces (pericardial, pleural). Aspirin, thienopyridines, and warfarin were generally held 5 to 7 days before the operation. Preoperative bridging using a heparin drip was used only on patients from whom warfarin was withdrawn and who had a mechanical valve.
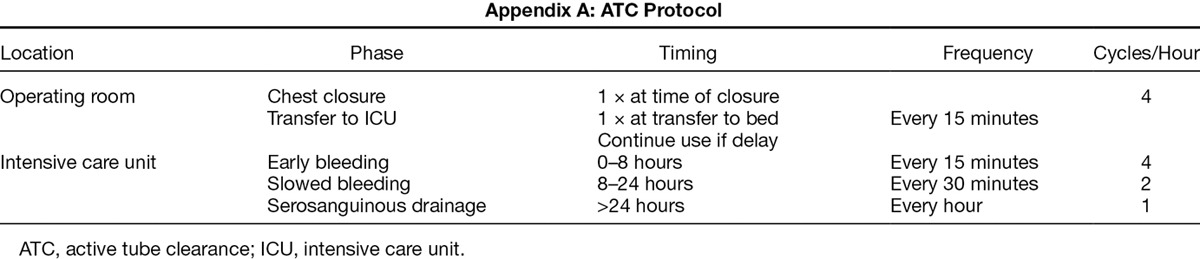
For this analysis, patients were divided into those who received conventional chest tubes only group (group 1, from April 1, 2009 to May 2013), and a cohort treated with active clearance of chest drains via a defined intensive care unit (ICU) use protocol (group 2, June 2013 to July 2015). A total of 77 LVAD implantation procedures were performed in group 1 using conventional chest tubes. There was no crossover between groups. In group 1, when clots were identified, stripping, milking, or fan folding was carried out on an as needed basis to break up clot in the visible portion of the chest tube when clotting was noticed in the chest tube. Chest tubes were not open suctioned to clear clot. There were 175 patients in group 2 who were treated with up to three 32 Fr active tube clearance (ATC) systems using the PleuraFlow Active Clearance Technology (ClearFlow Inc., Anaheim, CA). One system was placed in each open space (pericardial or pleural). The PleuraFlow is a chest tube clearance apparatus with a mechanism to actively keep the entire inner lumen of the chest tube clear of obstructing blood clot or fibrinous debris.15,16 Care providers periodically advanced a guide wire with an open loop back and forth within the chest tube to break up clot. A protocol was utilized that instructs more frequent use in the acute phase, when chest tube outputs tend to be higher, and less so in subsequent hours, as well as on an as needed basis was instituted12,17 (Appendix A). An educational in-service was conducted before universal implementation of the protocol for training of all nurses and physicians in the ICU.
Statistical Analysis
Comparisons of categorical variables used Fisher’s exact test, whereas comparisons of continuous variables used Welch’s test if the variable was symmetrically distributed and Wilcoxon’s rank sum test if the variable had a skewed distribution. Unadjusted odds ratios were calculated from logistic regression models with a single predictor for group 2 versus group 1. Adjusted odds ratios were calculated from logistic regression models with predictors for group, incision type (CS vs. MILT), and CPB. All logistic regression models were built using the glm function from R version 3.0.2 for the Mac, and 95% confidence intervals (CIs) were calculated using the confint method.
Results
A cohort of 252 patients underwent LVAD implantation during the study period. No patients during this time period were excluded from analysis. The mean age was 53 ± 12, of which 202 (80%) were male. Seventy-seven consecutive patients underwent LVAD implantation in group 1 with conventional chest tube drainage and 175 underwent LVAD implantation with the ATC protocol in group 2. There were no differences between groups in terms of age, sex, weight, diabetes, hypertension, cardiogenic shock, class of heart failure, preop atrial arrhythmias, and use of preop intra-aortic balloon pumps or creatinine (Table 1). There was no difference in terms of percent of first time cardiac surgery, or type of device inserted. Patients in group 2 were more likely to have had a MILT, more likely to have a HW device, had a higher percentage of destination therapy, and were less likely to have had CPB (Table 2).
Table 1.
Preoperative Demographic Variables
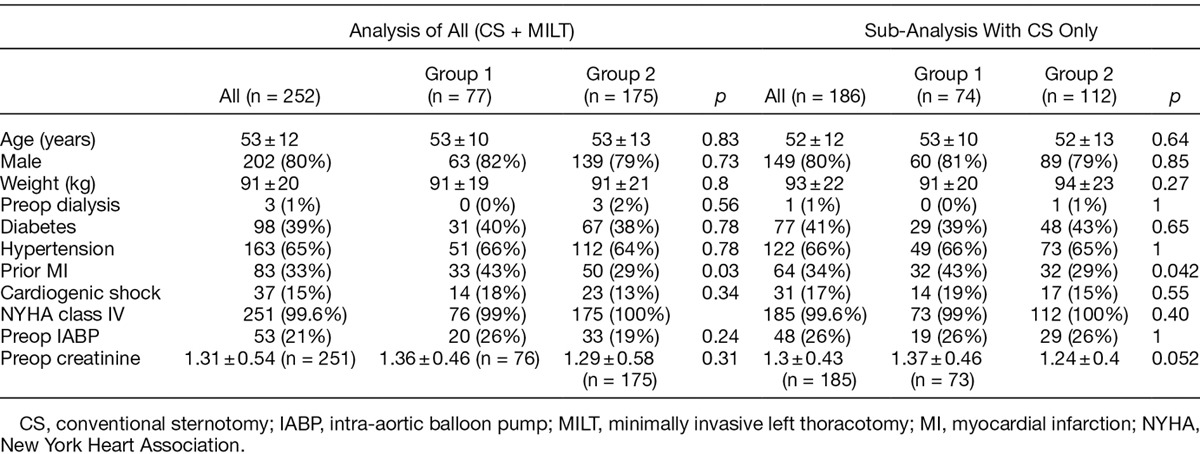
Table 2.
Intraoperative Demographic Factors
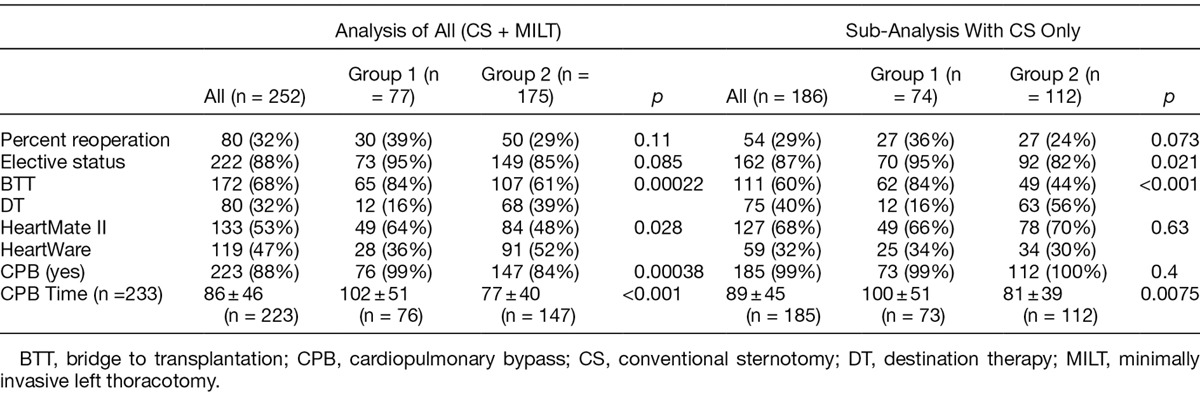
Considered as a composite of patients with any form of retained blood (re-exploration, DSC, thoracentesis, pericardiocentesis, and supplemental chest tube placement), patients in group 2 had an overall reduction of 39% in retained blood (51% in group 1 vs. 31% in group 2, p = 0.0044). This included a 65% reduction in re-explorations in patients in group 2 (43% vs. 15%, p <0.001; Table 3). The unadjusted odds ratio for re-exploration was 0.24, indicating a 76% reduction in the odds of a re-exploration between groups (95% CI = 0.13–0.45, p < 0.001). In addition, there was an 82% reduction in the use of delayed primary closure in group 2 (34% vs. 6%, p < 0.001). The unadjusted odds ratio for DSC in PleuraFlow versus control was 0.12, indicating an 88% reduction in the odds of a DSC for the PleuraFlow group (95% CI = 0.05–0.26, p <0.001). Patients in group 2 had a 57% reduction in the need for supplemental chest tube placements in the postoperative period for fluid collections. The unadjusted odds ratio for an additional chest tube in PleuraFlow versus control was 0.36, indicating a 64% reduction in the odds of an additional chest tube for the PleuraFlow group (95% CI = 0.17–0.77, p = 0.0083). There was no difference between groups in the need for drainage of pleural effusions (10% vs. 13%, p = 0.52) or pericardial effusions (5% vs. 4%, p = 1).
Table 3.
RBS Results
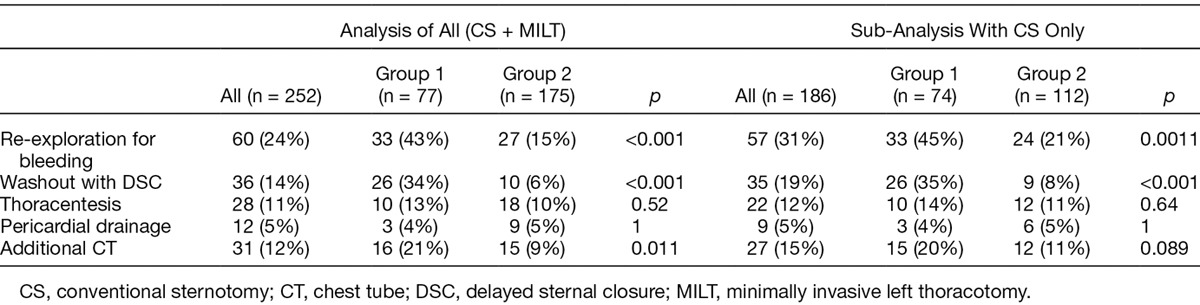
To evaluate the impact of MILT or off pump technique, a sub-analysis was completed of only patients operated through a CS utilizing CPB (excluding MILT without CPB). Even when we eliminated the patients with MILT without CPB, the reduction in re-exploration for bleeding persisted. Comparing only patients who had CS and CPB in group 2, there was a 53% reduction in the need for re-exploration for bleeding (21% vs. 45%, p =0.0011). Likewise, there remained a reduction in the use of delayed primary closure of 77% (8% vs. 35%, p < 0.001). Patients with CS and CPB noted a 45% reduction in the need for supplemental chest tubes, however this was not statistically significant (11% vs. 20%, p =0.089; Table 3).
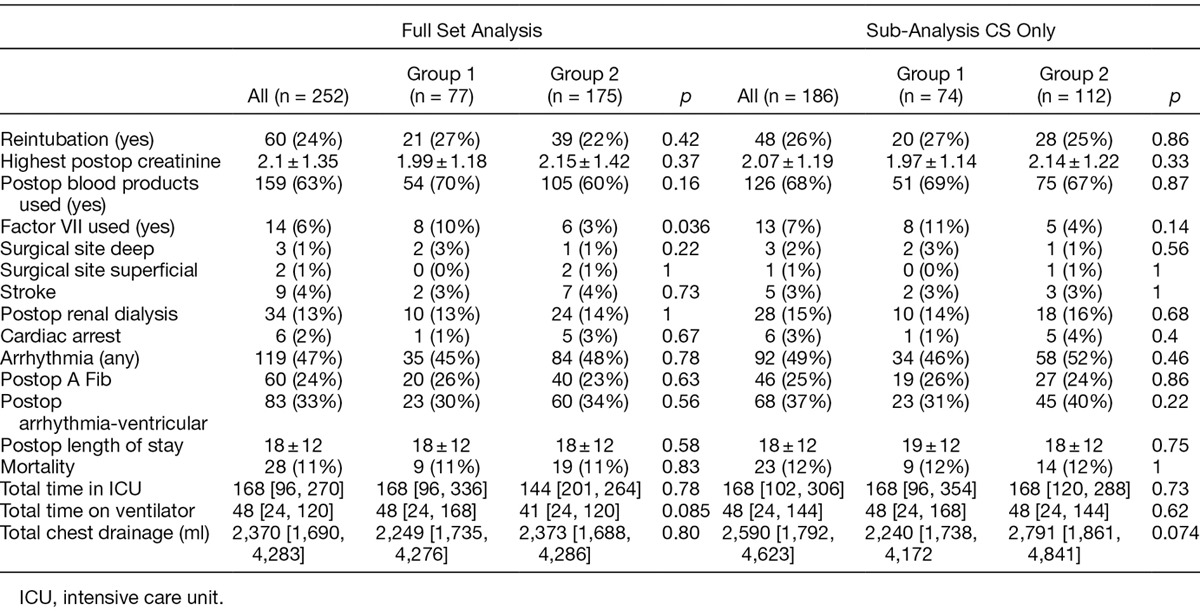
To further account for the confounding variables, multivariate regression analysis was performed. After adjusting for incision type and CPB, the odds ratio for re-exploration was 0.44, indicating a 56% reduction in the odds of a re-exploration in group 2 compared with group 1 (95% CI = 0.23–0.85, p = 0.014). Similarly after adjusting for incision and CPB, the odds ratio for DSC in group 2 versus group 1 was 0.20, indicating an 80% reduction in the odds of a DSC with ATC after adjusting for incision and CPB. (95% CI = 0.08–0.46, p = 0.00025.) After adjusting for incision type and CPB, the odds ratio for an additional chest tube in PleuraFlow versus control was 0.51, indicating a 49% reduction in the odds of an additional chest tube in the PleuraFlow group, however, this was not statistically significant (Table 4). Outcomes were otherwise similar between groups (Table 5).
Table 4.
Adjusted ORs Calculated from Logistic Regression Models with Predictors for Group Incision Type (CS vs. MILT) and CPB
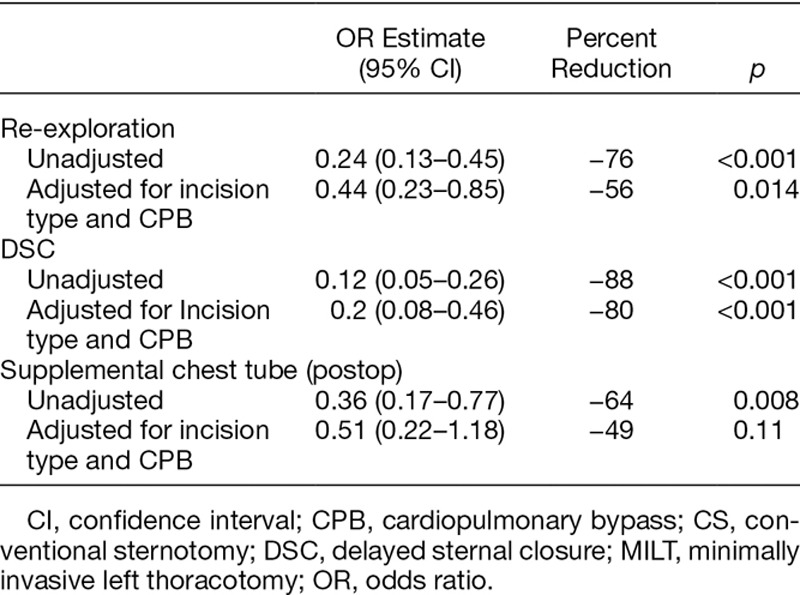
Table 5.
Postoperative Outcomes
Discussion
Postoperative bleeding is a common complication in LVAD patients, requiring re-exploration and washout of retained blood in approximately one in three patients during early recovery after device implantation.1,5 Beyond re-exploration for bleeding, additional complications of retained blood can occur, such as the need for DSC, supplemental chest tubes to drain hemopericardium, hemothorax, and bloody effusions. We have previously detailed that LVAD patients generally drain over 3 L through their chest tubes in early recovery from LVAD implantation, 90% of which occurs in the first several days.6 This is a critical time when chest tubes have a high potential to clot and impair drainage.7,8 This led us to form the hypothesis that the high volume of early bleeding combined with a potentially high rate of chest tube clogging may contribute to the elevated need for reinterventions for retained blood in this population. We carried out this analysis to determine whether a dedicated protocol to keep chest tubes patent during this time period might help reduce the need to re-explore and wash out or drain retained blood in patients recovering after LVAD implantation. Our data for the first time suggest that the implementation of the ATC reduces the need for retained blood, primarily through a reduction in the need for re-exploration, DSC, and the need for supplemental chest tubes.
There are several important observations in this study. The decision to re-explore a patient for bleeding is complex and involves a team assessment of numerous variables such as the presence or absence of tamponade, the volume trends of bleeding, and often, an assessment of if the chest tubes are still adequately draining the shed blood in the mediastinum during this timeframe. One mechanism might be that a system to keep chest tubes patent gave the team more confidence to manage some patients without re-exploration, even in the presence of brisk coagulopathic bleeding. Ultimately the team has to decide if they think there is a surgical source of bleeding or if there is coagulopathic bleeding that can be managed without return to the operating room for re-exploration. Maintaining chest tube patency may allow patients with coagulopathic bleeding to be managed in the ICU in some patients without re-exploration by keeping the chest tubes patent while the coagulopathy is treated. Actively clearing chest tubes has been documented to reverse tamponade and avoid the need for re-exploration in patients with nonsurgical bleeding sources.16 Because an important factor in deciding to re-explore is the development of acute tamponade, perhaps the use of ACT prevented tamponade in some patients. Further studies are needed to track the hemodynamics of patients during this period of recovery, echocardiographic findings of tamponade, as well as operative findings at re-exploration such as the presence or absence of surgical bleeding and the amount of retained blood encountered at re-exploration to better understand the mechanism by which active clearance of chest tubes might reduce the need for re-exploration.
Delayed sternal closure is utilized primarily for patients bleeding too much to safely close, leading to the need to return to the operating room to wash out the mediastinum before definitive closure.4,18 It is also used when there is concern for hemodynamic compromise from RV compression, either because of swelling of the heart, compression from unevacuated retained blood or both. These data suggest a strong trend toward reduced need for DSC after ATC was instituted. One explanation could be that our team developed more confidence closing the sternum of patients after LVAD implantation for those receiving ACT.
We further observed a trend toward a decrease need for supplemental chest tubes for drainage in the ICU in the ATC group. Early effusions are typically bloody, inflammatory, and exudative, suggesting that retained blood may be a contributing factor.19,20 These data suggest that by having more complete drainage in the acute phase of chest tube drainage with ATC, we had to reinsert fewer chest tubes postoperatively less often. The number of patients having thoracentesis and pericardiocentesis were too small for statistical comparison.
The strategy of implanting LVADs without a full sternotomy and without the use of CPB was introduced during this study.13,14 In our reported experience, MILT patients do not have measurable reductions in the need for transfusions, the amount of bleeding, or re-exploration when compared with those with CS.21 To account for the potential impact of MILT in this study, we further sub-analyzed just those patients who had a CS and again saw the same statistical trends of reduced re-exploration for bleeding and DSC. Further, regression analysis was carried out which showed the odds of requiring a re-exploration for bleeding and DSC were lower in the ATC group after accounting for the impact of sternotomy or CPB. Thus while MILT may impact the need for re-exploration in some patients, our analysis suggests the reductions in the need for re-exploration and DSC were not impacted in large part by this variable, allowing us to conclude that the ATC protocol may account for a large degree of these improved outcomes. The number of patients who had MILT in the baseline group 1 were too small to allow a direct comparison in only MILT patients, but this can be considered in future studies.
It is worthy of note that we evaluated ATC not as a bail out device used selectively, but as a prophylactic device used to proactively maintain chest tube patency in all patients. This required adoption of a protocol by the entire ICU team to optimally improve outcomes because management of chest drainage involves nearly every member of the care team. We recognized a team effort was indicated to develop and test this protocol to maximize blood evacuation and thus carried out this study in the context of a real world, pragmatic continuous quality improvement framework.
Several limitations of this study are acknowledged. This was a retrospective analysis of prospectively collected single site data. We acknowledge that because this was a historical controlled study design, there may have been an effect of time as the team improved its experience. Overall outcomes, however, were generally consistent over the course of the study. We acknowledge that it is possible not all patients who required re-exploration or DSC had inadequate chest tube drainage. Imaging studies establishing chest tube patency, as well as hemodynamics, indications, and findings at re-exploration and DSC were not collected. This study design and size did not allow for in-depth analysis of chest tube outputs between each group, which is an area of interest that deserves further analysis. However, based on the data from this first of its kind study in this population, we conclude it is worthy to aspire to keep chest tubes patent during the acute period of bleeding for this challenging patient population. Further studies will be beneficial in validating these findings with larger patient numbers and across several institutions. In addition, long-term follow-up may be useful to determine how this impacts outcomes after discharge, as there may be additional benefits of avoiding retained blood realized later in recovery.
Conclusion
This study demonstrates benefits of an institutional clinical protocol to actively maintain chest tube patency during the acute phase of high output chest tube drainage of bleeding after LVAD implantation.
Appendix A:
ATC Protocol
Footnotes
Disclosures: E.B. is a consultant and equity holder in ClearFlow, Inc. S.M. is the recipient of a grant from ClearFlow, Inc, and a Clinical Trial Educator for Heartware. For the remaining of authors, none were declared. ClearFlow, Inc, had no role in data collection, analysis or manuscript preparation nor did it have final approval rights over the conclusions.
This study was funded in part by a grant from ClearFlow, Inc.
Presented at the International Society for Heart and Lung Transplantation, Washington DC, April 27–30, 2016.
References
- 1.Miller LW, Pagani FD, Russell SD, et al. HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS. Hematologic effects of continuous flow left ventricular assist devices. J Cardiovasc Transl Res. 2010;3:618–624. doi: 10.1007/s12265-010-9222-6. [DOI] [PubMed] [Google Scholar]
- 3.Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010;90:1263–9; discussion 1269. doi: 10.1016/j.athoracsur.2010.04.099. [DOI] [PubMed] [Google Scholar]
- 4.Schaffer JM, Arnaoutakis GJ, Allen JG, et al. Bleeding complications and blood product utilization with left ventricular assist device implantation. Ann Thorac Surg. 2011;91:740–7; discussion 747. doi: 10.1016/j.athoracsur.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter MS, Rogers JG, Milano CA, et al. HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 6.Haglund NA, Davis ME, Tricarico NM, et al. Perioperative blood product use: A comparison between HeartWare and HeartMate II devices. Ann Thorac Surg. 2014;98:842–849. doi: 10.1016/j.athoracsur.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Karimov JH, Gillinov AM, Schenck L, et al. Incidence of chest tube clogging after cardiac surgery: A single-centre prospective observational study. Eur J Cardiothorac Surg. 2013;44:1029–1036. doi: 10.1093/ejcts/ezt140. [DOI] [PubMed] [Google Scholar]
- 8.Boyle EM, Jr, Gillinov AM, Cohn WE, Ley SJ, Fischlein T, Perrault LP. Retained blood syndrome after cardiac surgery: A new look at an old problem. Innovations (Phila) 2015;10:296–303. doi: 10.1097/IMI.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 9.Day TG, Perring RR, Gofton K. Is manipulation of mediastinal chest drains useful or harmful after cardiac surgery? Interact Cardiovasc Thorac Surg. 2008;7:888–890. doi: 10.1510/icvts.2008.185413. [DOI] [PubMed] [Google Scholar]
- 10.Arakawa Y, Shiose A, Takaseya T, et al. Superior chest drainage with an active tube clearance system: Evaluation of a downsized chest tube. Ann Thorac Surg. 2011;91:580–583. doi: 10.1016/j.athoracsur.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Shiose A, Takaseya T, Fumoto H, et al. Improved drainage with active chest tube clearance. Interact Cardiovasc Thorac Surg. 2010;10:685–688. doi: 10.1510/icvts.2009.229393. [DOI] [PubMed] [Google Scholar]
- 12.Sirch J, Ledwon M, Püski T, Boyle EM, Pfeiffer S, Fischlein T. Active clearance of chest drainage catheters reduces retained blood. J Thorac Cardiovasc Surg. 2016;151:832–8.e1. doi: 10.1016/j.jtcvs.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Maltais S, Davis ME, Haglund N. Minimally invasive and alternative approaches for long-term LVAD placement: The Vanderbilt strategy. Ann Cardiothorac Surg. 2014;3:563–569. doi: 10.3978/j.issn.2225-319X.2014.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner CE, Bick JS, Kennedy J, et al. Minimally invasive thoracic left ventricular assist device implantation; case series demonstrating an integrated multidisciplinary strategy. J Cardiothorac Vasc Anesth. 2015;29:271–274. doi: 10.1053/j.jvca.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Shalli S, Boyle EM, Saeed D, Fukamachi K, Cohn WE, Gillinov AM. The active tube clearance system: A novel bedside chest-tube clearance device. Innovations (Phila) 2010;5:42–47. doi: 10.1097/IMI.0b013e3181cf7ce3. [DOI] [PubMed] [Google Scholar]
- 16.Vistarini N, Gabrysz-Forget F, Beaulieu Y, Perrault LP. Tamponade relief by active clearance of chest tubes. Ann Thorac Surg. 2016;101:1159–1163. doi: 10.1016/j.athoracsur.2015.10.098. [DOI] [PubMed] [Google Scholar]
- 17.Perrault LP, Pellerin M, Carrier M, et al. The PleuraFlow Active Chest Tube Clearance System: Initial clinical experience in adult cardiac surgery. Innovations (Phila) 2012;7:354–358. doi: 10.1097/IMI.0b013e31827e2b4d. [DOI] [PubMed] [Google Scholar]
- 18.Stulak JM, Romans T, Cowger J, et al. Delayed sternal closure does not increase late infection risk in patients undergoing left ventricular assist device implantation. J Heart Lung Transplant. 2012;31:1115–1119. doi: 10.1016/j.healun.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Labidi M, Baillot R, Dionne B, Lacasse Y, Maltais F, Boulet LP. Pleural effusions following cardiac surgery: Prevalence, risk factors, and clinical features. Chest. 2009;136:1604–1611. doi: 10.1378/chest.09-0689. [DOI] [PubMed] [Google Scholar]
- 20.Guha A, Munjampalli S, Bandi V, Loebe M, Noon G, Lunn W. Pleural effusion after ventricular assist device placement: Prevalence and pleural fluid characteristics. Chest. 2008;134:382–386. doi: 10.1378/chest.07-2777. [DOI] [PubMed] [Google Scholar]
- 21.Sileshi B, Haglund NA, Davis ME, et al. In-hospital outcomes of a minimally invasive off-pump left thoracotomy approach using a centrifugal continuous-flow left ventricular assist device. J Heart Lung Transplant. 2015;34:107–112. doi: 10.1016/j.healun.2014.09.023. [DOI] [PubMed] [Google Scholar]


