Abstract
Extracorporeal membrane oxygenation (ECMO) is used as a lifesaving rescue treatment in refractory respiratory or cardiac failure. During venovenous (VV) ECMO, the presence of recirculation is known, but quantification and actions to minimize recirculation after measurement are to date not routinely practiced. In the current study, we investigated the effect of draining cannula design on recirculation fraction (Rf) during VV ECMO; conventional mesh cannula was compared with a multistage cannula. The effect of adjusting cannula position was also studied. Recirculation was measured with ultrasound dilution technique at different ECMO flows and after cannula repositioning. All patients who were admitted to our unit between October 2014 and July 2015 catheterized by the atrio-femoral single lumen method were included. A total of 108 measurements were conducted in 14 patients. The multistage cannula showed significantly less recirculation (19.0 ± 12.2%) compared with the conventional design (38.0 ± 13.7). Pooled data in cases improved from adjustment showing reduced Rf by 7%. In conclusion, the choice of cannula matters, as does adjustment of the draining cannula position during atrio-femoral VV ECMO. By utilizing the ultrasound dilution technique to measure Rf before and after repositioning, effective ECMO flow can be improved for a more effective ECMO treatment.
Keywords: extracorporeal membrane oxygenation, recirculation, cannula, ultrasound dilution
Venovenous extracorporeal membrane oxygenation (VV ECMO) is a life-sustaining salvage therapy in severe refractory respiratory failure, supporting respiratory gas exchange.1,2 Venovenous ECMO, a therapy initially developed in neonatal patients and today used in all ages, is supported by a two randomized, controlled trials, which showed superiority to conventional ventilator therapy.3–5
Different catheterization approaches exist. One dual lumen cannula (DLC) or two single lumen cannulas (SLC) are introduced. In the SLC approach, one cannula is placed via the right internal jugular vein (IJV) with the tip in the upper or mid part of the right atrium (RA). The other cannula is introduced via a femoral vein (FV). The tip can be positioned from the iliac vein to the upper inferior caval vein (IVC) depending on flow direction. When applying a DLC, cannulation is performed via the right jugular vein with the tip positioned according to specific cannula design.
The risk for recirculation is always present in VV ECMO. The recirculation fraction (Rf) is defined as the portion of oxygenated ECMO blood immediately withdrawn by the ECMO circuit without contributing to patient oxygenation. Rf is affected by several factors: supplying and draining cannula design and position, cardiac output, intravascular volume, extracorporeal blood and direction thereof, coronary sinus flow, etc.1,2,6–8 The ELSO guidelines define an SaO2 exceeding 80% to be adequate during VV ECMO.9 To ensure an acceptable SaO2, it is essential to minimize recirculation.
Concerning SLC, disagreement exists in the ECMO community related to recirculation and fluid withdrawal regarding whether to extract blood via the IJV (atrio-femoral direction) or the FV (femoro-atrial direction). The femoro-atrial method is thought to result in less Rf10 because the reintroduced ECMO blood flows with the blood stream to the right chamber since venous blood is drained from the IVC. In contrast, in atrio-femoral VV ECMO, venous blood is drained from the same anatomical space as the returned oxygenated blood passes on the way to the heart.
The conventional formula used to calculate Rf does not apply to VV ECMO.2,7,11–13 In normal physiology, SvO2 is measured in the pulmonary artery. During VV ECMO, the mixed blood in the pulmonary artery does not allow a valid assessment of SvO2,7 nor does the venous blood in the ECMO circuit show a true SvO2 because of partial recirculation.2 The golden standard to estimate SvO2 and recirculation during ECMO is the SvO2 method: the oxygen flow to the oxygenator is stopped, and SvO2 is measured when SpreoxO2 equals SpostoxO2. This maneuver could be hazardous in critically ill ECMO patients. In the alternative CVL method, ScvO2 is measured from a central venous line placed in the lower SVC for approximation of SvO2, and recirculation is calculated using the conventional formula.
Ultrasound dilution technique (UDT) has been available in clinical practice for the measurement of recirculation in atrio-venous fistulas in dialysis,14 but it has not been available for ECMO use before. Ultrasound dilution technique measures the sound velocity in blood. With blood dilution, a velocity reduction is measured in both ECMO tubes, and dilution curves are calculated. Ultrasound dilution technique has been validated and is considered reliable. van Heijst et al.7 showed a stronger correlation between UDT and the SvO2 method than between the CVL method and the SvO2 method in lambs. Darling et al.13 reported a strong correlation between UDT and the SvO2 method in pigs. Thermo and lithium dilution have been proposed but not validated during VV ECMO in humans.15,16 In clinical practice, estimations of recirculation can be made by measuring SpreoxO2 and SaO2.17 An increase in SpreoxO2 and a decrease in SaO2 indicate increased recirculation, an insensitive but always available method using a blood gas analyzer. The naked eye may detect high recirculation by color equilibration between the ECMO tubes.2
The aims of this investigation were to evaluate whether drainage cannula design affects recirculation during VV ECMO and whether repositioning of the cannula could reduce recirculation.
Materials and Methods
All patients admitted to ECMO Center Karolinska between October 2014 and July 2015 and catheterized according to the atrio-femoral SLC method with good biventricular function were included. The study was approved by the Central Ethical Review Board in Stockholm, with ethical review number: 2014/945-31.
Two different cannula designs were evaluated regarding recirculation as well as the feasibility of cannula adjustment to decrease recirculation and improve ECMO treatment. Recirculation was measured at different ECMO flows and also after cannula reposition.
Equipment and Cannulation
The ECMO circuit was composed of a CentriMag centrifugal pump (Thoratec Europe Ltd., Cambridgeshire, United Kingdom) and a hollow fiber oxygenator (HILITE LT, Medos Medizintechnik AG, Stolberg, Germany). Catheterization was performed peripherally for VV ECMO according to atrio-femoral flow direction. For drainage of venous blood, a single lumen multistage cannula (25 Fr/38 cm Maquet HLS, Maquet Nordic AB, Solna, Sweden) or a conventional mesh cannula (23 Fr/25 cm Bio-Medicus, Medtronic, MN), Figure 1, was introduced through the right IJV and advanced to the RA. Oxygenated blood was reintroduced by a 17, 19, or 21/18 cm Bio-Medicus cannula via either FV. The draining cannula position was evaluated by chest RX or echocardiography.
Figure 1.

The different tip designs of the multistage 25 Fr/38 cm (upper cannula) and the conventional mesh 23 Fr/25 cm cannulas. The latter design drains blood through a mesh of holes at the most distal 38 mm (1½″). The multistage cannula has side holes in five segments along the most distal 102 mm (4″).
Measurements
For baseline measurements, an echocardiogram was performed to estimate IVC size, right atrium size, and the cannula position. Blood gases were sampled from the patient’s radial arterial line, before and after the oxygenator, and analyzed with a Radiometer ABL800 Flex (Radiometer Medical ApS, Copenhagen, Denmark). Recirculation fraction was measured with ELSA (Transonic Systems Inc., Ithaca, NY) as described by van Heist et al.7 Two ultrasonic flow probes were connected to the ECMO tubes, one on the draining “venous” tube in close proximity to the patient, the other transducer after the oxygenator, termed “arterial.” The probes measure the blood flow rate by Doppler technique. A rapid (<3 s) bolus of 15–20 ml saline was injected in the ECMO circuit before the oxygenator. A transient dilution-correlated reduction in ultrasound velocity is first registered by the “arterial” flow probe, and shortly thereafter the recirculation part of the bolus will be sensed by the “venous” probe. The respective ultrasound velocity data are processed by the ELSA device. The area under the curve quotient is considered the recirculation fraction. Rf was measured twice, and the mean value was calculated. The ECMO flow was increased or decreased, and a new measurement was executed. After returning to initial ECMO flow, the cannula was moved manually 20 ± 10 mm (depending on echocardiographic, chest RX findings, patient size), and another measurement was performed. The cannula was then maintained in the best position regarding Rf.
Statistics
IBM SPSS Statistics (IBM Corp., Armonk, NY) was used for statistical analysis. Comparison concerning Rf between the different cannulas was performed using Mann–Whitney U test. The Rf attained at first measurement and after optimization of position was analyzed using a paired T test. Quadratic regression was used for correlation of ECMO flow to effective flow. Datasets were checked for normal distribution using Shapiro–Wilks test, and correlations were tested according to Pearson. A p value <0.05 was considered significant. Data are presented as mean ± SD and fractions in percent.
Results
The study group contained 14 patients as displayed in Table 1. A total of 108 measurements were performed. Thirty-one measurements were carried out with the conventional and 77 with the multistage cannula.
Table 1.
Display Patient Characteristics, Risk Scores at Admission, Cannulas, Days on ECMO, Survival to Discharge from Ward, and 1 Year Survival
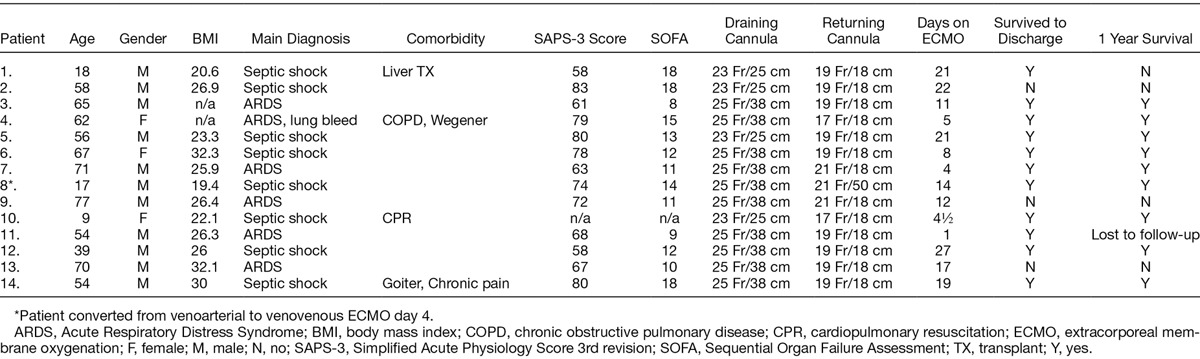
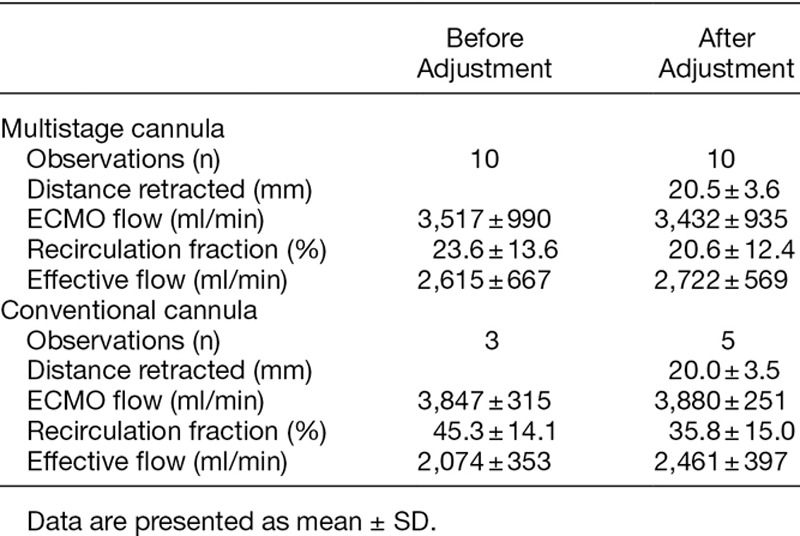
Pooled data from all measurements regarding the multistage cannula showed an Rf of 19.0 ± 12.2%. The corresponding data for the conventional cannula were significantly higher, 38.0 ± 13.7% (p < 0.001). In nine of the patients supported with the multistage cannula, 10 adjustments were performed of which a reduction in Rf of 10% or more was observed in two cases. For the conventional cannula, five adjustments were performed in three patients, and an improvement was attained in three of these. When an improvement of 5% was chosen instead, the numbers were four out of 10 and four out of five, respectively.
The initial ECMO flows to maintain acceptable peripheral saturation did not differ, 3,436 ± 964 ml/min for the conventional cannula and 3,515 ± 654 ml/min for the multistage. Recirculation was lower for the multistage compared with the conventional cannula (p < 0.01, Figure 2). The effective ECMO flow was higher for the multistage at comparable total ECMO flow (p < 0.05, Figure 3). Because of few observations, adjustment flow data were pooled for both catheters comparing recirculation. As displayed in Figure 4, over all manipulation of cannula position reduced Rf by almost 7% (p = 0.05). In Table 2, the actual recirculation data are displayed both before and after cannula adjustment.
Figure 2.
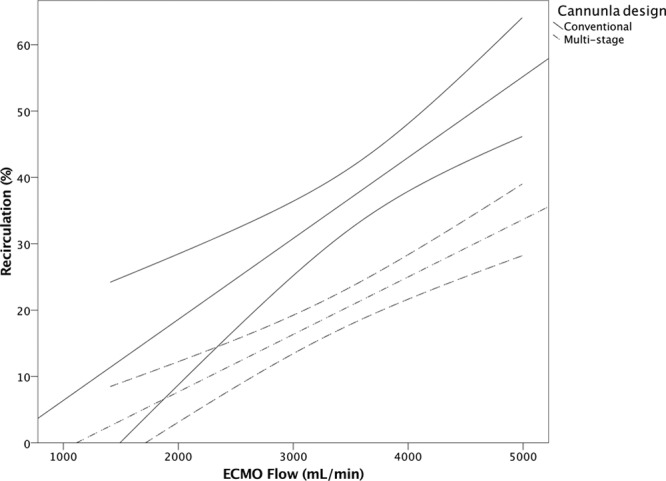
Recirculation fraction to ECMO flow for the conventional (⁃⁃, R2 = 0.581, n = 18) and the multistage cannula (▪⁃▪, R2 = 0.515, n = 40), p < 0.001.
Figure 3.
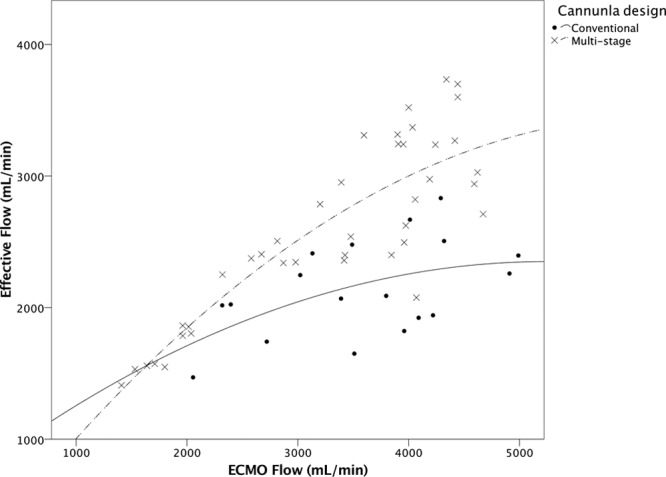
Effective ECMO to total ECMO flow from all measurements of the conventional (•/⁃⁃, R2 = 0.263, n = 18) and the multistage cannula (x/▪⁃▪, R2 = 0.750, n = 40), p < 0.05.
Figure 4.
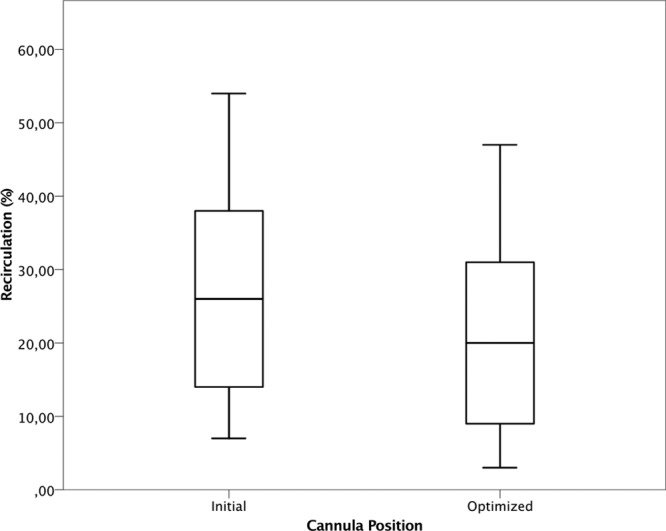
Pooled distribution data on recirculation fraction at initial 28.5 ± 15.6% (mean ± SD) and after optimization 21.3 ± 13.6% of the draining cannula independent of design (n = 13), p = 0.05.
Table 2.
Recirculation Data Obtained Before and After Adjustment of Cannula Position for the Multistage and Conventional Cannula, Respectively
Discussion
The patients in this study were included independently of cause for ECMO. All showed normal biventricular function, i.e., VV ECMO was a supportive therapy for respiratory gas exchange. The resolution of problems derived from recirculation phenomena depends on understanding the cause by knowledge in integrated physiology and physics.
If the drainage and return cannulas are positioned end to end, a very high recirculation fraction is expected as for example during cardiac arrest. In low cardiac output, a higher degree of recirculation occurs than in high cardiac output situations. Most ECMO patients have systemic inflammation syndrome with a certain degree of overhydration. The severely water-soaked, nonaerated lung loses compliance, and the diaphragm moves in a cephalad direction. Both the change in diaphragmatic resting level and alterations of intrathoracic organ interrelationship may affect the flow pattern around the cannula. These examples illustrate clinical observations affecting central flow characteristics and thereby relate to recirculation. Some adverse effects of recirculation can easily be dealt, whereas others need time or intervention. Reducing Rf increases effective ECMO blood flow; more oxygen is delivered at a constant pump flow. Factors related to ECMO physics contribute to the destruction of red blood cells, and high ECMO flows augment these problems. Concerning the centrifugal pump, at the vane edges negative pressure pockets may induce bubble formation and secondary hemolysis.1,18 The lowest venous pressure noted in the current study was −93 mm Hg, and in all cases with pressures below −35 mm Hg the conventional cannula was used. Hemolysis increases free plasma hemoglobin and free heme, which ravages the physiologic effect of nitric oxide (NO), thus increasing inflammation, coagulation, and vasoconstriction.19–22
In our practice, atrio-femoral flow direction is used for VV ECMO (SLC), a strategy used by a minority of the ELSO members. The “Red Book”1 recommends femoro-atrial management. Rich et al.10 showed that higher ECMO flows were reached by draining from the IVC. In our experience, in septic adults and adolescents with fluid overload, we dare not to adapt to this because a strict fluid withdrawal strategy is crucial. Consequently, the IVC is subjected to dramatic changes in dimension by preload variation. With applied femoro-atrial approach, pumping problems often occur as the draining IVC cannula starts to chatter and clinch to the vascular wall. This is usually resolved by adding fluids and could lead to prolonged time to weaning. It has been theorized that the femoro-atrial method has a lower Rf because venous blood is predominantly drained in the IVC, where abdominal organs empty. However, maintaining a high fluid withdrawal with a concomitant decrease in preload/IVC diameter which in sequence might form a funnel down from the decreased sized RA and thereby moving the draining point closer toward the diaphragm. In this situation, an increased recirculation is obvious. In the atrio-femoral approach, drainage occurs in a larger capacitance cavity, and adequate flow will be maintained longer despite ambitious fluid withdrawal. However, the closer proximity between draining and delivery points can increase the probability for recirculation compensated for by maintained ECMO flow as fluid withdrawal is continued.
To reduce and quantify, recirculation with UDT is an easy bedside tool to improve draining cannula position. To achieve low recirculation, the choice of drainage cannula would be the first step before commencing atrio-femoral VV ECMO. The multistage cannula showed consistently lower Rf and less negative pressures than the conventional design. Additionally, by calculating effective ECMO flow [Qeff = QECMO (1 − Rf)], it was possible to objectify the amount of oxygenated blood delivered to the patient by the ECMO support. The multistage cannula showed a higher effective ECMO flow. The advantage of the multistage cannula is explained by design; the location of the most proximal holes of the multistage cannula drains a larger fraction deoxygenated blood from the upper body and less from the junction of RA. This is probably the reason why only minor gain in Rf was seen after adjustment in contrast to the conventional cannula. The latter drains predominantly closer to its tip, i.e., at or very close to the atrium, where more oxygenated ECMO blood is mixed with venous. If the conventional cannula is retracted in cephalad direction, recirculation is likely to decrease. However, this maneuver might lead to chattering. Thus, it is difficult to minimize recirculation with this design. Our results for measured Rf correlate with measurements and estimates by others.17
Larger recirculation is suggested using SLC than DLC.1,7,23 The pinnacle of DLC design, the Avalon Elite (Maquet), exhibits low Rf including unpublished data of our own. However, severe cannulation complications have been reported such as placement in the right ventricle and pericardial perforations.24 The design calls for a precise positioning because simultaneous draining from the IVC and SVC is the main advantage. Considerations should be taken whether assessment suggests a risk for developing cardiac failure. It is more complicated and higher risk to convert any DLC patient to VA ECMO than from SLC, where atrio-femoral is the most favorable.
This study is the first study to apply UDT in a clinical setting to measure Rf. Significant reduction in recirculation was reached by adjustment of cannula position. After catheterization, the placement was verified by plain chest RX in all cases and often also by echocardiography. Bedside UDT was the most important factor in attaining best position. In this study, the multistage cannula showed a more desirable recirculation profile and might need fewer adjustments.
The alternative methods to assess Rf are all inferior to UDT. “The Physicians eye,” looking at tubing color, and trend SpreoxO2 and SaO2 over time have inborn limitations. To “homogenize,” a bolus of cold saline before the oxygenator/heater would destroy the tracer from start, and the method is not validated on ECMO. The SvO2 method may be the more correct method, but use is limited in our critically ill patient group. Finally, looking at the CVL method, it is very uncertain to “best sample” and running atrio-femoral mode, and multistage cannulas will probably increase the bias of this method. It has also been suggested that the UDT method correlates better with the SvO2 method than the CVL method.7
As shown in earlier studies, there is a positive correlation between flow and recirculation.7,16 At pump rates exceeding CO, superfluous flow will be drained, and the effective flow decreases.25 In one case, ECMO flow was increased from 4,190 to 5,540 ml/min, but SpO2 declined from 97% to 92%. Measurements showed effective ECMO flow to decrease from 2,960 to 1,885 ml/min as Rf increased from 30 to 66%. Thus, increasing ECMO flow per se does not always increase DO2, as discussed by Abrams et al.17 In most situations, a significant recirculation can be detected by a trained physician and dealt with using echocardiography and blood gases.2,17 In difficult cases, however, UDT is a tool, which can guide the physician in improving medical care and assist in understanding physiologic problems not only concerning recirculation but also indirectly in assessing cardiac function over time, etc.
Conclusions
Recirculation fraction during atrio-femoral VV ECMO can be significantly reduced by choice of cannula. The multistage cannula used for draining showed less recirculation than the conventional design cannula. Adjusting cannula position might decrease recirculation further. The use of ultrasound dilution technology is simple, noninvasive and provides bedside feedback regarding recirculation fraction. Less recirculation means a more effective ECMO therapy. Maintaining a highly effective ECMO flow leads to secondary benefits such as reduced risk of hemolysis and reduced morbidity.
Acknowledgment
The authors thank Dr. Peter Radell, Department of Pediatric Anesthesia and Intensive Care, Karolinska University Hospital, Stockholm, Sweden, for language revision.
Footnotes
Disclosures: After finalization the draft Mikael Broman became a member of the Advisory Board of Eurosets srl., Medolla, Italy. This has no implication on this study. No conflict of interest concerning the other authors is to report.
References
- 1.Annich GM, Lynch W, MacLaren G, Wilson J, Bartlett R, editors. ECMO Extracorporeal Cardiopulmonary Support in Critical Care. Ann Arbor, MI: Extracorporeal Life Support Organization; 2012. [Google Scholar]
- 2.Short BL, Williams L, editors. ECMO Specialist Training Manual. Ann Arbor, MI: Extracorporeal Life Support Organization; 2010. [Google Scholar]
- 3.Bennett CC, Johnson A, Field DJ, Elbourne D UK Collaborative ECMO Trial Group. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation: Follow-up to age 4 years. Lancet. 2001;357:1094–1096. doi: 10.1016/S0140-6736(00)04310-5. [DOI] [PubMed] [Google Scholar]
- 4.Field D, Davis C, Elbourne D, Grant A, Johnson A, Macrae D. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. UK Collaborative ECMO Trail Group. Lancet. 1996;348:75–82. [PubMed] [Google Scholar]
- 5.Peek GJ, Mugford M, Tiruvoipati R, et al. CESAR Trial Collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 6.Körver EP, Ganushchak YM, Simons AP, Donker DW, Maessen JG, Weerwind PW. Quantification of recirculation as an adjuvant to transthoracic echocardiography for optimization of dual-lumen extracorporeal life support. Intensive Care Med. 2012;38:906–909. doi: 10.1007/s00134-012-2534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Heijst AF, van der Staak FH, de Haan AF, et al. Recirculation in double lumen catheter veno-venous extracorporeal membrane oxygenation measured by an ultrasound dilution technique. ASAIO J. 2001;47:372–376. doi: 10.1097/00002480-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Broman LM, Hultman J. Double lumen catheter placement during VV ECMO in an infant with persistent left superior vena cava-important considerations. ASAIO J. 2014;60:603–605. doi: 10.1097/MAT.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 9.ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support. Ann Arbor, MI, USA: Publ: Extracorporeal Life Support Organization; 2013. Nov, Version 1.3 Available at: www.elsonet.org. Accessed May 30, 2016. [Google Scholar]
- 10.Rich PB, Awad SS, Crotti S, Hirschl RB, Bartlett RH, Schreiner RJ. A prospective comparison of atrio-femoral and femoro-atrial flow in adult venovenous extracorporeal life support. J Thorac Cardiovasc Surg. 1998;116:628–632. doi: 10.1016/S0022-5223(98)70170-9. [DOI] [PubMed] [Google Scholar]
- 11.Walker JL, Gelfond J, Zarzabal LA, Darling E. Calculating mixed venous saturation during veno-venous extracorporeal membrane oxygenation. Perfusion. 2009;24:333–339. doi: 10.1177/0267659109354790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker J, Primmer J, Searles BE, Darling EM. The potential of accurate SvO2 monitoring during venovenous extracorporeal membrane oxygenation: An in vitro model using ultrasound dilution. Perfusion. 2007;22:239–244. doi: 10.1177/0267659107083656. [DOI] [PubMed] [Google Scholar]
- 13.Darling EM, Crowell T, Searles BE. Use of dilutional ultrasound monitoring to detect changes in recirculation during venovenous extracorporeal membrane oxygenation in swine. ASAIO J. 2006;52:522–524. doi: 10.1097/01.mat.0000237589.20935.a4. [DOI] [PubMed] [Google Scholar]
- 14.Little MA, Conlon PJ, Walshe JJ. Access recirculation in temporary hemodialysis catheters as measured by the saline dilution technique. Am J Kidney Dis. 2000;36:1135–1139. doi: 10.1053/ajkd.2000.19821. [DOI] [PubMed] [Google Scholar]
- 15.Linton R, Turtle M, Band D, O’Brien T, Jonas M. In vitro evaluation of a new lithium dilution method of measuring cardiac output and shunt fraction in patients undergoing venovenous extracorporeal membrane oxygenation. Crit Care Med. 1998;26:174–177. doi: 10.1097/00003246-199801000-00035. [DOI] [PubMed] [Google Scholar]
- 16.Sreenan C, Osiovich H, Cheung PY, Lemke RP. Quantification of recirculation by thermodilution during venovenous extracorporeal membrane oxygenation. J Pediatr Surg. 2000;35:1411–1414. doi: 10.1053/jpsu.2000.16402. [DOI] [PubMed] [Google Scholar]
- 17.Abrams D, Bacchetta M, Brodie D. Recirculation in venovenous extracorporeal membrane oxygenation. ASAIO J. 2015;61:115–121. doi: 10.1097/MAT.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda T, Funakubo A, Fukui Y. An investigation of blood damage induced by static pressure during shear-rate conditions. Artif Organs. 2002;26:27–31. doi: 10.1046/j.1525-1594.2002.06786.x. [DOI] [PubMed] [Google Scholar]
- 19.Ulatowski JA, Nishikawa T, Matheson-Urbaitis B, Bucci E, Traystman RJ, Koehler RC. Regional blood flow alterations after bovine fumaryl beta beta-crosslinked hemoglobin transfusion and nitric oxide synthase inhibition. Crit Care Med. 1996;24:558–565. doi: 10.1097/00003246-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Vogel WM, Dennis RC, Cassidy G, Apstein CS, Valeri CR. Coronary constrictor effect of stroma-free hemoglobin solutions. Am J Physiol. 1986;251(2 pt 2):H413–H420. doi: 10.1152/ajpheart.1986.251.2.H413. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR., Jr Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 22.Vaughn MW, Kuo L, Liao JC. Effective diffusion distance of nitric oxide in the microcirculation. Am J Physiol. 1998;274(5 pt 2):H1705–H1714. doi: 10.1152/ajpheart.1998.274.5.H1705. [DOI] [PubMed] [Google Scholar]
- 23.Bermudez CA, Rocha RV, Sappington PL, Toyoda Y, Murray HN, Boujoukos AJ. Initial experience with single cannulation for venovenous extracorporeal oxygenation in adults. Ann Thorac Surg. 2010;90:991–995. doi: 10.1016/j.athoracsur.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Chimot L, Marqué S, Gros A, et al. Avalon© bicaval dual-lumen cannula for venovenous extracorporeal membrane oxygenation: Survey of cannula use in France. ASAIO J. 2013;59:157–161. doi: 10.1097/MAT.0b013e31827db6f3. [DOI] [PubMed] [Google Scholar]
- 25.Nunes LB, Mendes PV, Hirota AS, et al. ECMO Group. Severe hypoxemia during veno-venous extracorporeal membrane oxygenation: Exploring the limits of extracorporeal respiratory support. Clinics (Sao Paulo) 2014;69:173–178. doi: 10.6061/clinics/2014(03)05. [DOI] [PMC free article] [PubMed] [Google Scholar]


