Supplemental Digital Content is Available in the Text.
Key Words: Clinical trial, Prospective study, Meibomian gland dysfunction, Dry eye
Abstract
Objectives:
Thermal pulsation (LipiFlow) has been advocated for meibomian gland dysfunction (MGD) treatment and was found useful. We aimed to evaluate the efficacy and safety of thermal pulsation in Asian patients with different grades of meibomian gland loss.
Methods:
A hospital-based interventional study comparing thermal pulsation to warm compresses for MGD treatment. Fifty patients were recruited from the dry eye clinic of a Singapore tertiary eye hospital. The ocular surface and symptom were evaluated before treatment, and one and three months after treatment. Twenty-five patients underwent thermal pulsation (single session), whereas 25 patients underwent warm compresses (twice daily) for 3 months. Meibomian gland loss was graded using infrared meibography, whereas function was graded using the number of glands with liquid secretion.
Results:
The mean age (SD) of participants was 56.4 (11.4) years in the warm compress group and 55.6 (12.7) years in the thermal pulsation group. Seventy-six percent of the participants were female. Irritation symptom significantly improved over 3 months in both groups (P<0.01), whereas tear breakup time (TBUT) was modestly improved at 1 month in only the thermal pulsation group (P=0.048), without significant difference between both groups over the 3 months (P=0.88). There was also no significant difference in irritation symptom, TBUT, Schirmer test, and gland secretion variables between patients with different grades of gland loss or function at follow-ups.
Conclusions:
A single session of thermal pulsation was similar in its efficacy and safety profile to 3 months of twice daily warm compresses in Asians. Treatment efficacy was not affected by pretreatment gland loss.
Dry eye is a common condition with symptoms that can considerably impact patients' quality of life.1,2 It is perceived to be as distressing as chest pain3,4 and imposes considerable health care (U.S. $0.27 million to U.S. $1.1 million per 1,000 persons annually)5 and productivity costs.2,6 These costs are especially pronounced in Asia, where there is a high prevalence and severity of dry eye.7,8
Meibomian gland dysfunction (MGD) is a major cause of dry eye that affects 46.2% to 69.3% of Asians and 3.5% to 19.9% of whites.9,10 A chronic abnormality of the meibomian glands in the eyelids, MGD, affects tear film stability11 and can cause eye discomfort.12 As a result, it is believed to lead to faster tear evaporation and poorer visual function, exposing the cornea and conjunctiva to more damage.13 Recent opinion, however, suggests that its role may be more accurately described as assisting in tear film spreading14 rather than retarding evaporation.
The cornerstone therapy for MGD is the use of warm compresses.15 Various forms of eyelid-warming therapy have been shown to improve patients' symptoms16–20 and tear film stability,16–21 slow down tear evaporation,20,21 and reduce ocular surface damage.18,22 For more severe MGD, other treatments, such as antibiotics and anti-inflammatory cyclosporin A, are prescribed in addition to warm compresses.15
The recommended regimen for warm compresses is daily treatment.15 However, clinical experience suggests that many patients cannot sustain routine warm compresses for an extended duration of time. Poor patient compliance is further complicated by difficulty in delivering therapeutic range of temperatures to the meibomian glands. To improve patient compliance, more convenient treatment modalities are necessary.
Several eyelid-warming devices have been developed recently with features that improve convenience and deliver heat at safe, calibrated temperatures.17,19,23 Yet, little evidence exists on the efficacy of these devices, especially in their long-term use. Most studies18,19,21–23 lacked control groups for comparison, were short term (up to 3 weeks), or had just one application of lid warming. Among them, only two studies22,23 reported effects in healthy participants.
Currently, there are no data about the benefit of lid warming in patients with a baseline of significant meibomian gland loss. In clinical practice, evaluation of the pretreatment meibographic status may be needed to exclude patients with significant glandular loss from lid warming because they may be unresponsive to treatment, as it is believed that loss is a result of gland atrophy and these glands do not regenerate. As a practical consideration, outcomes of heat treatment for patients with different baseline meibographic status need to be compared.
LipiFlow (TearScience Inc., Morrisville, NC) thermal pulsation, which provides heat and mechanical stimulation to inner eyelids, has been used in clinical settings to treat the blocked meibomian glands. This is a new promising treatment that uses heat to unblock glandular plugging by increasing the temperature of meibomian glands in patients with MGD from the inner surface of the eyelid.
A single LipiFlow treatment has been demonstrated to be efficacious in patients with MGD.19,24–26 In randomized controlled trials (RCTs) conducted in the United States,19 Germany,24 and France,25 patients with MGD who received a 12-min treatment showed significantly improved tear film breakup time and dry eye symptoms. In the U.S. RCT,19 these effects were sustained at even 12 months after treatment in a subgroup of white patients.27 As existing trials have only been conducted for Western populations, there is no published report on the efficacy, safety, and convenience of LipiFlow treatment in an Asian population. As the prevalence of MGD is high in Asia (46.2%–69.3% in Asian studies) compared with the West (3.5%–19.9% in western studies),9,10 the severity of the disease may also be greater in the former ethnic group; thus, it cannot be assumed that equivalent efficacies are achieved in both populations.
To address this issue, we conducted an interventional study of thermal pulsation compared to conventional warm compresses in patients with MGD in Singapore.
METHODS
Overview of Study Design
This is a 3-month single-institution trial comparing two eyelid warming methods in patients with MGD. The study has been approved by the SingHealth Centralised Institutional Review Board and adhered to the Tenets of the Declaration of Helsinki. The study was registered at the clinicaltrials.gov database (NCT01683318).
Allocation to treatment groups was not randomized (Fig. 1). Recruitments for the thermal pulsation (intervention) group and warm compresses (control) group were commenced at different times, but the evaluation periods greatly overlapped, and the participants were recruited from the same clinic by the same study team under similar recruitment criteria. Nonrandomization was due to a delivery delay of the LipiFlow thermal pulsation machine, together with the LipiView ocular surface interferometer and standardized force meibomian gland evaluator (Details in Supplemental Digital Content 1, http://links.lww.com/ICL/A32).
FIG. 1.
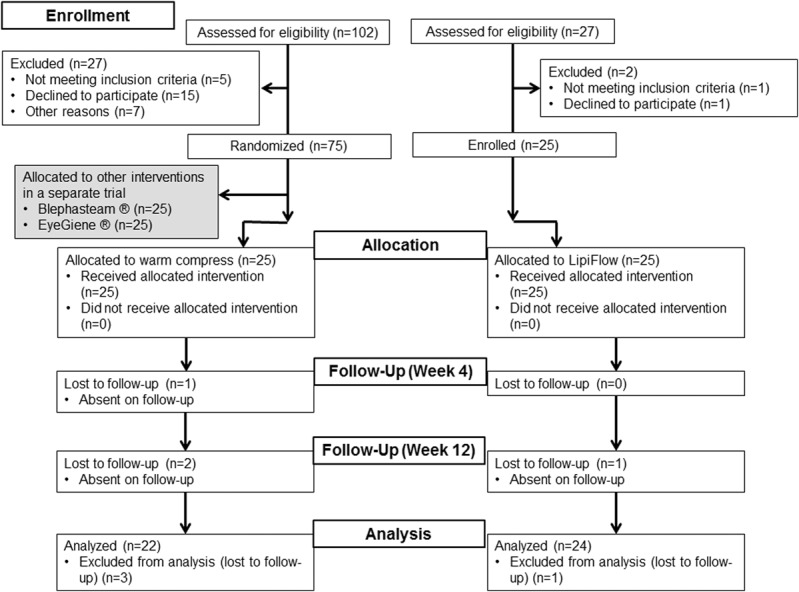
Flowchart showing enrollment and outcomes. The number of participants in the study who were eventually analyzed for the one-month and three-month outcomes is shown in the flowchart. Thermal pulsation group (n=24) was compared to warm compresses group (n=22).
Participants
From February 2012 to March 2013, all patients with dry eye and MGD at the Singapore National Eye Centre dry eye clinic who met the eligibility criteria were briefed about this study and invited for screening. Meibomian gland dysfunction is diagnosed as the presence of dry eye symptoms with visible meibomian gland plugging on examination (exact recruitment criteria shown below). Eligible participants were then enrolled with written informed consent.
Inclusion criteria were the following:
At least one of eight dry eye symptoms (Supplemental Digital Content 2, http://links.lww.com/ICL/A33—questionnaire modified after Schein et al.28,29) was experienced “often” or “all the time”
At least one meibomian gland opening with a visible plugging over the eyelid margin30
No ocular pathology requiring treatment other than eye lubricant and conventional eyelid hygiene within the last month and during the study
Baseline irritation symptom of more than 0 millimeter as reported on the modified Symptom Assessment in Dry Eye (SANDE) visual analog scale (Supplemental Digital Content 3, http://links.lww.com/ICL/A34—in addition to irritation symptom, the modified version also assessed blurring of vision and light sensitivity31,32).
The exclusion criteria were the following:
Known diagnosis of thyroid dysfunction and rheumatoid arthritis and ocular surgery within the previous 6 months
Laser-assisted in situ keratomileusis within the previous year
Central nervous system and hormonal drugs required within the last month and during the study
Active ocular infection or presence of pterygium
Any need to wear contact lens during the study
Any need to use antibiotics, steroid, and anti-inflammatory drugs such as Restasis
Having another household member who also participated in this study.
Baseline Data
For the purpose of characterizing the study population and evaluating treatment efficacy, participants were evaluated for their baseline characteristics before intervention. Dry eye symptoms, namely, irritation, visual blurring, and photophobia, were each assessed for their frequency and severity using two visual analog scales as previously described in a modified SANDE questionnaire.29 A global score was calculated for each symptom by taking the square root of the product of the two scales. For outcome evaluation, only the irritation global score (the only attribute assessed by the original SANDE questionnaire31) was used. The modified questionnaire of Schein et al.28,29 described under the inclusion criteria was only used for screening of eligibility, not for the assessment of study endpoints.
Objective clinical signs, namely, tear breakup time (TBUT), fluorescein corneal staining, and Schirmer I test, were also documented at baseline in both control and thermal pulsation groups.
Tear breakup time was performed as in previous studies.29,33 A wetted fluorescein (1 mg) strip (Fluoret Fluorescein Sodium Ophthalmic Strip; Laboratoire Chauvin, Aubenas, France) was introduced to the inferior palpebral conjunctiva to stain the ocular surface.33 Three readings were taken for each eye, and the average TBUT was calculated.
Corneal fluorescein staining was graded using the previously published Cornea and Contact Lens Research Unit (CCLRU) scheme.34 Each of the five corneal zones was scored between 0 (no staining/scarring) to 4 (severe staining). The components of corneal staining, namely, type, depth, and extent, were assessed individually, and the grade of staining for each eye was determined using the component with the most severe grade. The presence of clinically relevant staining in each corneal zone was taken as a CCLRU staining grade of 1.0 or greater.
The Schirmer I test without anesthesia was performed with 5-mm wide Schirmer Tear Test strips (4701001; Clement Clarke International, Harlow, Essex, United Kingdom) as described earlier.35 The length of wetting after 5 min was measured from the notch of the strip.30
The meibomian gland morphology was also evaluated using noncontact infrared meibography equipment modified from Topcon slit lamp SL-D7 (Topcon Corporation, Tokyo, Japan)32 and was graded as healthy, intermediate, or unhealthy based on the amount of glandular loss and glandular characteristics (Supplemental Digital Content 4, http://links.lww.com/ICL/A35—grading of meibomian gland).32 Two trained optometrists graded each image independently, and in the case of a disagreement, a third investigator was involved in determining the grade.
As part of the exploratory outcomes in this study, the baseline tear lipid layer thickness (LLT) and the number of glands expressing liquid secretion were measured with the LipiView ocular surface interferometer (TearScience Inc., Morrisville, NC)36 and the standardized force meibomian gland evaluator (TearScience Inc.). Using a standardized force meibomian gland evaluator (TearScience Inc.), the number of glands yielding liquid secretion was counted in the lower lid under slit-lamp microscopy. The number of plugged glands was also counted in both upper and lower lids under slit-lamp microscopy. These meibomian gland secretion variables were only assessed in the thermal pulsation group.
Interventions
In the thermal pulsation group, on the first visit, participants underwent a single 12-min treatment session of LipiFlow thermal pulsation after instillation of topical local anesthetic as instructed by the manufacturer. In the control group, participants received a towel on the first visit and were instructed to use it for twice daily warm compresses lasting 10 min per session. They were instructed to warm the towel in warm water before placing it over closed eyes and to rewarm it once it cools. Patients in both intervention groups were asked to do lid hygiene, which consisted of daily manual lid massage and lid cleaning with Blephagel (Spectrum Théa Pharmaceuticals Limited, Fernbank House, Cheshire, United Kingdom). Warm compresses (in the warm compresses group) and lid hygiene (in both groups) were performed for the entire study duration (12 weeks).
To monitor treatment compliance, participants from both groups were given a diary to record details of eye lubricant use, lid hygiene with Blephagel, and warm compresses (the latter two for control group only).
Outcomes
Participants were assessed before treatment (baseline) and also 4 weeks and 12 weeks after the start of treatment. At week 4, SANDE symptom score and TBUT were evaluated, whereas at week 12, SANDE symptom score, TBUT, and Schirmer I test were evaluated. The primary outcome measure was the SANDE global score for irritation symptom one month after treatment. As a secondary outcome, irritation score changes after treatment over the period of 12 weeks were evaluated.
The other secondary outcome measures were (1) the change in the TBUT and (2) Schirmer I test results after treatment over the period of 12 weeks.
The exploratory outcomes, namely, (1) LLT, (2) number of meibomian glands yielding liquid secretion, and (3) number of plugged meibomian glands, were evaluated at week 4 and week 12 in the thermal pulsation group only.
Safety was assessed through a prespecified safety outcome measure (best-corrected spectacle visual acuity [VA] changes after 3 months of treatment). The best-corrected spectacle VA was tested at 4 meters using a distance VA number test (cat. no. C 102; Lighthouse International, New York, NY). The test tool was presented in Snellen format and then converted to the corresponding logMAR equivalent for statistical analysis. A smaller logMAR value represents better vision. At all visits, other safety outcomes were documented if present, including signs of ocular and periocular irritations or other complaints.
Sample Size Calculation
We endeavored to detect a 20% difference in the primary outcome of improved global symptom score between thermal pulsation and control groups. Based on an online calculator, 21 participants in each study arm were required for 80% power and a two-sided significance level of 5%. We aimed to recruit 25 participants per arm to allow for 4 cases of lost to follow-up or withdrawals per arm.
Statistical Analyses
Data were checked for normality with the skewness and kurtosis test to determine the appropriate parametric or nonparametric tests. To test for differences among groups for baseline characteristics and the various outcomes, we used the relevant chi-square test, independent t test, two-sample t test, and Wilcoxon rank-sum test.
Linear mixed model analyses were conducted to analyze the repeated measures taken at baseline, 4 weeks, and 12 weeks after treatment. A fixed effect model was used to compare the thermal pulsation and control groups using compound, autoregressive (AR(1)), and unstructured variance–covariance structures to select the best model fit using information criterion (based on the smallest Akaike information criterion [AIC] and Bayesian information criterion [BIC] values). The AIC and BIC are measures of the relative quality of a model compared to other models for a particular data set, and the AIC/BIC values are a trade-off between the goodness-of-fit and complexity (number of terms) of the model.37 Interaction terms, along with age and sex adjustments, were also modeled. Bonferroni correction was applied for multiple comparisons.
In subgroup analysis focusing on the thermal pulsation group, we evaluated the TBUT, Schirmer test results, LLT, and number of glands with liquid secretion at the two time points (weeks 4 and 12) stratified by baseline meibographic status.
Statistically significant difference was defined as an alpha of 0.05. All analyses were performed with SPSS, version 21 (SPSS Inc., Chicago, IL).
RESULTS
Baseline Characteristics of Participants
Twenty-five patients were recruited into the thermal pulsation group, and 25 patients were recruited into the warm compresses group. Four participants were lost to follow-up as shown in Figure 1. The study population was 56.2 (mean) ± 11.7 (SD) years, 24.5% male (12 participants), and 89.1% Chinese (41 participants) (Table 1). This was similar to the previously reported profile of patients from the dry eye clinic at the Singapore National Eye Center.35 We present only the data for the right eye (baseline TBUT, Schirmer I test, LLT, and number of glands with liquid secretion were not significantly different from the left and right eyes [P>0.05]).
TABLE 1.
Baseline Characteristics of Clinical Trial
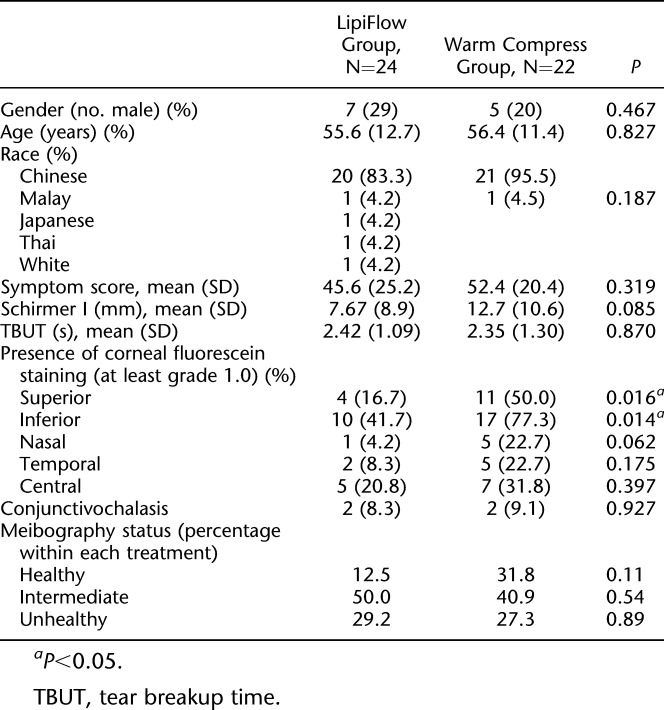
As both groups were recruited at different time points, the baseline characteristics of their participants were checked for any significant differences (Table 1). In the superior and inferior corneal zones, the presence of fluorescein staining was significantly more common in the control group than in the thermal pulsation group. All other baseline characteristics were not significantly different between both groups.
Symptom Assessment
The SANDE irritation global score was significantly reduced at 4 weeks from baseline for both groups (Fig. 2A). However, there was no significant difference between both groups at 4 weeks (P=0.30) (Table 2).
FIG. 2.
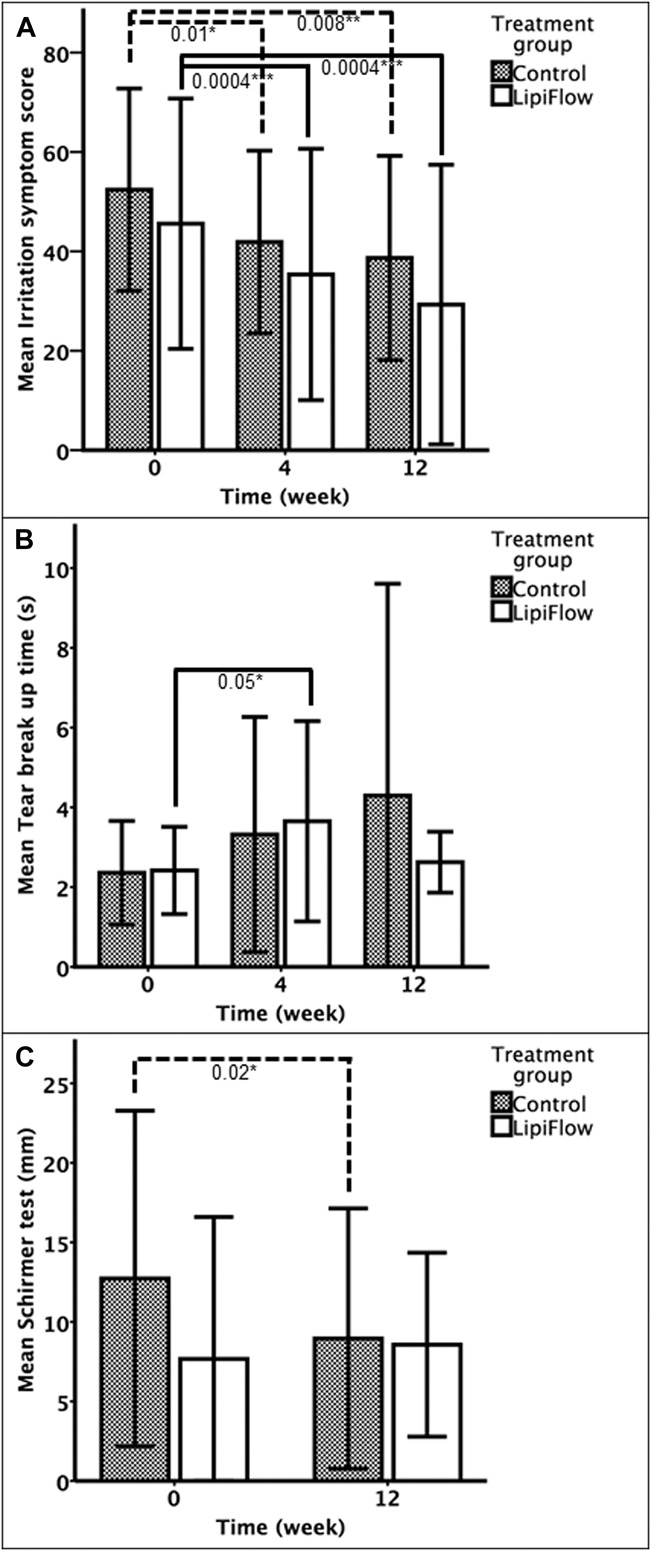
Primary (A) and secondary (B–C) outcome measures at various time intervals after the initiation of treatment. Heights of bars indicate mean values, and error bars indicate ±1 SD. *P<0.05; **P<0.01; ***P<0.001.
TABLE 2.
Relative Change in Symptom Score, TBUT, and Schirmer
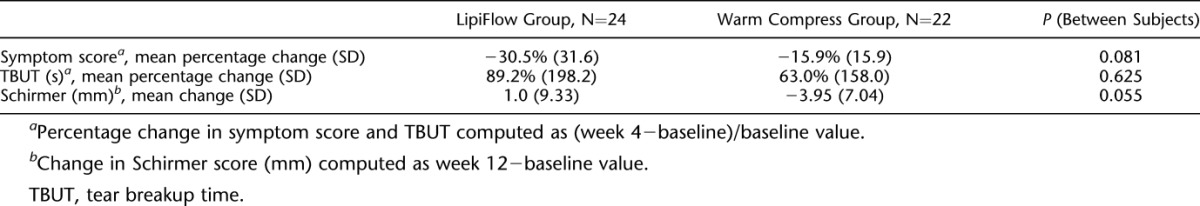
Based on a linear mixed model with an AR(1) structure (AIC: 1137; BIC: 1142) adjusted for age and sex, irritation score significantly improved over the 12 weeks (P<0.01), whereas the difference in score improvement was not significantly different between treatment groups (P=0.22). There was no significant interaction between treatment group and time (P=0.79).
Assessment of Clinical Signs of Dry Eye
At 4 weeks, TBUT significantly improved from baseline (P=0.015) on the whole. When the treatment groups were examined (Fig. 2B—showing absolute values of TBUT), TBUT significantly increased from baseline in subjects who received thermal pulsation (P=0.048), but it was not significantly changed in the warm compresses group (P=0.14). However, the difference between both groups at 4 weeks was not statistically significant (P=0.66) (Table 2—showing relative change in TBUT over time). At week 12, TBUT changes in both groups were not statistically significant from baseline.
For the change in TBUT, a mixed model with an unstructured structure (AIC: 627; BIC: 645) was used and adjusted for age and sex. There was a significant trend of TBUT improvement over the 12 weeks (P=0.035) with no significant difference between treatment groups (P=0.88). At week 4, TBUT modestly improved by 1.10 sec (95% CI: 0.11–2.10), whereas at week 12, the improvement in TBUT was not significantly different from baseline (mean difference: 1.03; 95% CI: −0.29 to 2.36). Age was also found to be significantly associated with TBUT (0.028 sec decrease in TBUT for every year older, P=0.043) in the mixed model. There was no significant interaction between treatment group and time or between treatment group and age (P>0.05).
For the Schirmer test (Fig. 2C), the control group had a significant change of −3.95 mm (95% CI: −7.16 to −0.75; P=0.018), whereas the thermal pulsation group had a nonsignificant improvement of 1.00 mm (mean difference [mm]: 1.00; 95% CI: −3.03 to 5.03; P=0.612) at week 12.
Meibomian Gland Secretion Variables (Exploratory Outcomes)
The meibomian gland secretion variables were only assessed in the thermal pulsation group. For the change in the number of plugged meibomian glands (Fig. 3A), a mixed model with a compound structure (AIC: 302; BIC: 307) was used and adjusted for age and sex. Over the 12 weeks, there was a significant and decreasing trend in the number of plugged meibomian glands (P<0.01) in the thermal pulsation group.
FIG. 3.
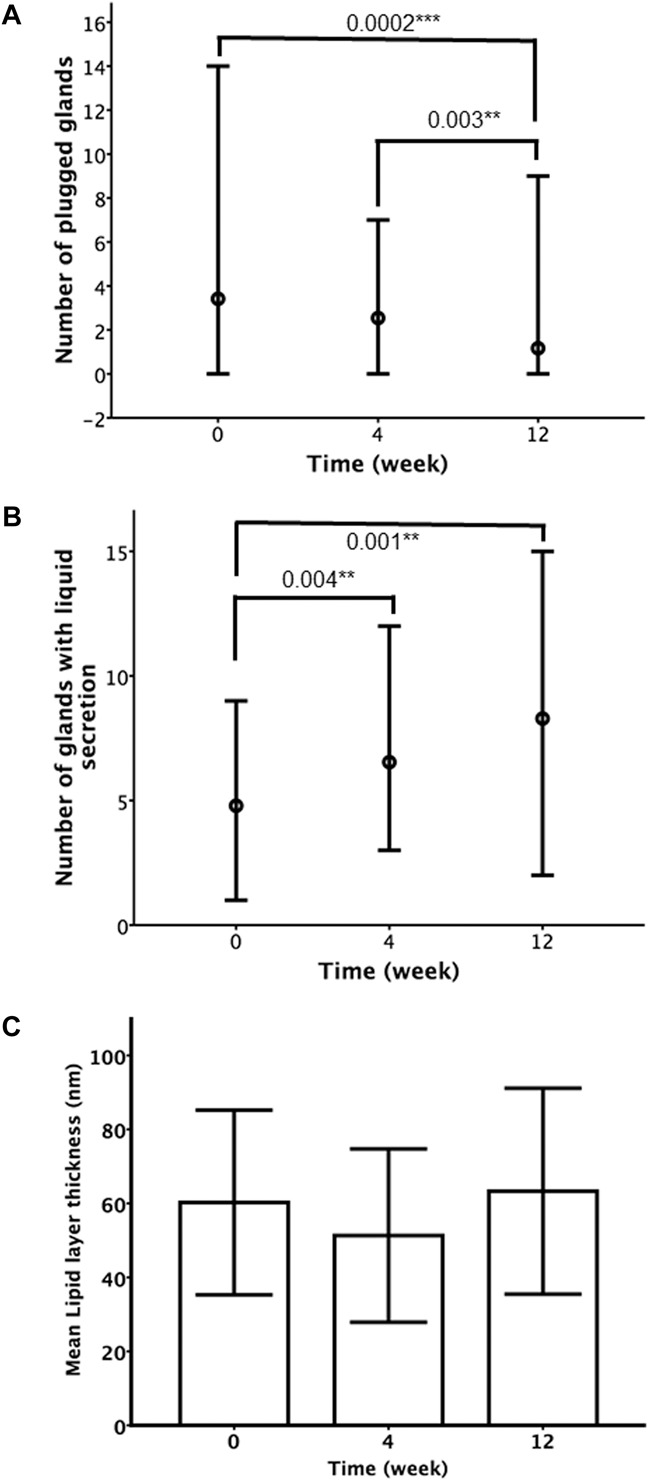
Meibomian gland secretion variables (exploratory outcomes) in the thermal pulsation group (A–C) at various intervals after the initiation of treatment. Dots represent median values, and error bars indicate minimum and maximum (A–B). Heights of bars represent mean values, and error bars indicate ±1 SD (C). *P<0.05; **P<0.01; ***P<0.001.
For the number of glands with liquid secretion in the patients treated with thermal pulsation (Fig. 3B), a mixed model with an unstructured structure (AIC: 341; BIC: 354) showed that there was a significant increase over the 12 weeks (P<0.01) in the number of glands with liquid secretions, and age was also significantly associated with a smaller number of glands (P=0.005), but there was no significant interaction between age and time (P>0.05).
Lipid layer thickness (Fig. 3C) did not significantly change over time in the thermal pulsation group over the 12 weeks (P=0.088) in a mixed model with a compound structure (AIC: 589; BIC: 593).
Outcomes Stratified by Baseline Meibography
The irritation improvement was not different between the baseline meibography classifications (P=0.98) at 4 weeks and 12 weeks (P=0.98). At 4 weeks, TBUT (P=0.60), number of glands yielding liquid secretion (P=0.53), and LLT (P=0.90) outcomes were not different between patients with varying meibographic severities. At 12 weeks, TBUT (P=0.78) and Schirmer test (P=0.90), and number of glands yielding liquid secretion (P=0.30) and LLT (P=0.44) were not influenced. None of the patients who underwent thermal pulsation had total loss of meibomian glands before treatment.
In the thermal pulsation group, participants were stratified based on their baseline meibomian gland function (defined as number of meibomian glands with liquid secretion ≥5 or <5) and analyzed for differences in the relative improvements in symptom score, TBUT, and Schirmer I score at 4 weeks. These treatment outcomes were not significantly different between both groups (P=0.60, 0.19, and 0.30, respectively, for between-group comparisons for symptom score, TBUT, and Schirmer I score).
Safety Outcomes
Participants who received LipiFlow thermal pulsation all had transient redness in the eyes, and also mild puffiness of the eyelids, for a few minutes after treatment. Over the 12 weeks, there was no significant change in the best-corrected VA in either group (P>0.05). There was also no report of unexpected adverse event either related or unrelated to the study treatment.
DISCUSSION
Our study found that a single session of LipiFlow thermal pulsation improved dry eye outcomes (irritation symptom and modest TBUT improvements) and was similar in efficacy as twice daily warm compresses over the 12 weeks. The improvements in symptoms and TBUT were not affected by the baseline meibographic status or meibomian gland function (assessed by the number of meibomian glands with liquid secretion). Both forms of treatments were found to be safe and well tolerated.
LipiFlow thermal pulsation is a relatively novel treatment option for patients with MGD. As such, there are limited data on its efficacy at the moment. When our study was conducted, there were only one 3-month noncontrolled trial26 and 1 multicenter controlled trial with crossover at 1 month19 conducted in the United States. In the latter trial, after crossing over, the participants were reassessed at 3 months19 and recruited for two longer duration follow-up studies.27,38
During the course of our study, two 3-month RCTs, conducted by Finis et al. (2014)24 in Germany and Baumann et al. (2014)25 in France, respectively, have also been published. The study by Finis et al. compared the one-month and 3-month outcome of LipiFlow against conventional lid warming and massage, whereas the study by Baumann et al. compared the 3-month outcome of LipiFlow against a commercial lid warming eye mask (MeiboPatch). These studies found that participants of both groups experienced symptomatic improvement at 1 month, and the effect was sustained up to a period of 3 months.24,25 In our study, we found a similar trend of symptomatic improvement at 1 month with sustained effect at 3 months in both groups. In addition, the study by Finis et al. showed that symptomatic improvement, measured with the Ocular Surface Disease Index, was significantly greater in the LipiFlow group than the conventional lid warming group at one and 3 months. In our study, symptomatic improvement tended to be greater in the thermal pulsation group, although this difference was not statistically significant.
In terms of the thermal pulsation group alone, all aforementioned trials and this study showed a significant posttreatment improvement in symptoms at 1 month, which was sustained at 3 months.19,24,25,38
In terms of objective clinical signs, after thermal pulsation treatment, although TBUT was significantly improved, similar to the findings of most previous trials,19,25,38 it should be noted that the increase in TBUT in our study was modest and may not be clinically significant. The U.S. multicenter RCT also demonstrated an increase in TBUT of 1.9 sec at 1 month.19 In the study by Finis et al.,24 the improvement in TBUT at 1 month was significant but not sustained to three months.
With respect to our exploratory outcomes, the number of meibomian glands yielding liquid secretion improved at 1 month in our study and in all previous studies.19,24,25,38 However, the median number of meibomian glands yielding liquid secretion only increased by 2 to 3 across the follow-up period and may not be clinically significant. We did not assess this in the warm compresses group. The magnitude of increase was much higher in the U.S. noncontrolled trial (by a mean of 5.4 at 1 month).26
The improvement in symptoms and meibomian gland function may be attributed to the novel design of the LipiFlow device. The advantages of thermal pulsation may be its use of the right temperature and site of heating, as heat is applied from both inner and outer surfaces of the eyelids, and the mechanical massage of glands helps relieve obstruction. Further mechanistic findings after lid warming/thermal pulsation, that is, tear evaporation rates and lipid changes related to this trial, will be published separately.
The main strength of our study was the high follow-up rates on reassessment of participants. Furthermore, we included parameters such as the number of plugged glands in the upper and lower eyelids, which have not been reported previously. The use of a recording diary also reinforced adherence to intervention and aided monitoring of treatment compliance. Standardized force expression of meibomian gland is not routinely assessed in most ophthalmology clinics; thus, symptom assessment, which is more commonly used by eye practitioners, was used as the main outcome of this study.
The limitations of this study were nonrandomization of interventions, nonblinding of assessors and participants, and lack of meibomian gland secretion evaluation in the control group (Supplemental Digital Content 1, http://links.lww.com/ICL/A32—describing late delivery of LipiFlow and evaluation equipment). Given the lack of randomization, there is a potential bias in the patient selection in this study. As participants could not be masked to the type of treatment, it is possible that their perception of symptom severity or frequency was affected by this knowledge. Nevertheless, these issues are of greater concern had the study results show a significant difference between groups, which is not the case.
A clinical implication of this study is that evaluation of MGD should consider the severity of inflammation and fibrosis, and not just glandular obstruction. Appropriate treatment for inflammation and fibrosis should be provided in combination with lid warming.39,40 Another implication is that regardless of the amount of meibomian glands (provided that there is no total loss), treatment with lid warming will still be effective.
In MGD treatment, sustained long-term improvement in symptoms and tear function is desired. It was suggested that a retreatment strategy with thermal pulsation, scheduled at 9 to 12 months after the first session, might sustain both symptomatic and objective tear function improvements.27,38 Studies using more than 1 thermal pulsation treatment may therefore be useful in the future.
Equipment and consumables for LipiFlow are overwhelmingly more expensive than lid warming. Nevertheless, as the effects of a single treatment may be sustained for months, thermal pulsation is more convenient to patients and may encourage better compliance as compared with daily lid warming. Therefore, future studies on long-term efficacy of LipiFlow and cost effectiveness of thermal pulsation treatment are necessary.
CONCLUSIONS
This trial demonstrated that in Asian patients, LipiFlow thermal pulsation had clinical efficacy in improving patient symptoms and produced modest increase in TBUT and meibomian gland function. In general, one session of thermal pulsation was comparable to three months of twice daily lid warming. In addition, the benefit from both forms of therapy may extend to cases with glandular loss, and if thermal pulsation is performed, there may be improvement in glandular function. However, affordability of thermal pulsation treatment will influence its degree of adoption in routine clinical practice.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Htoon Hla Myint for his help in statistical analyses. In addition to the authors, CORIM also consists of Markus R Wenk and Manfred Rada from National University of Singapore; Lee Hwee Kuan from Singapore Bioinformatics Institute (Agency of Science and Technology Singapore); Guanghou Shui and Sin Man Lam from Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, China; Sim Hui Shan from Duke-NUS Medical School, Singapore; and Cynthia SK Boo, Samantha SY Lee, and Cheah Loon Too from Singapore Eye Research Institute.
Footnotes
The authors have no conflicts of interest to disclose.
Supported by National Medical Research Council, Singapore (NMRC/CSA/045/2012), Biomedical Research Council, Singapore (BMRC(TCRP)10/1/35/19/670 R828), and MOE Innovation Fund TIF 11105.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.eyeandcontactlensjournal.com).
REFERENCES
- 1.The epidemiology of dry eye disease: Report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:93–107. [DOI] [PubMed] [Google Scholar]
- 2.Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care 2008;14(3 Suppl):S102–S106. [PubMed] [Google Scholar]
- 3.Buchholz P, Steeds CS, Stern LS, et al. Utility assessment to measure the impact of dry eye disease. Ocul Surf 2006;4:155–161. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman RM, Walt JG, Jacobsen G, et al. Utility assessment among patients with dry eye disease. Ophthalmology 2003;110:1412–1419. [DOI] [PubMed] [Google Scholar]
- 5.Clegg JP, Guest JF, Lehman A, et al. The annual cost of dry eye syndrome in France, Germany, Italy, Spain, Sweden and the United Kingdom among patients managed by ophthalmologists. Ophthalmic Epidemiol 2006;13:263–274. [DOI] [PubMed] [Google Scholar]
- 6.Le Q, Zhou X, Ge L, et al. Impact of dry eye syndrome on vision-related quality of life in a non-clinic-based general population. BMC Ophthalmol 2012;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jie Y, Xu L, Wu YY, et al. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye (Lond) 2009;23:688–693. [DOI] [PubMed] [Google Scholar]
- 8.Lin PY, Tsai SY, Cheng CY, et al. Prevalence of dry eye among an elderly Chinese population in Taiwan: The Shihpai Eye Study. Ophthalmology 2003;110:1096–1101. [DOI] [PubMed] [Google Scholar]
- 9.Schaumberg DA, Nichols JJ, Papas EB, et al. The international workshop on meibomian gland dysfunction: Report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci 2011;52:1994–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea 2004;23:762–770. [DOI] [PubMed] [Google Scholar]
- 11.Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci 2011;52:1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol 1995;113:1266–1270. [DOI] [PubMed] [Google Scholar]
- 13.Bron AJ, Tiffany JM, Gouveia SM, et al. Functional aspects of the tear film lipid layer. Exp Eye Res 2004;78:347–360. [DOI] [PubMed] [Google Scholar]
- 14.Millar TJ, Schuett BS. The real reason for having a meibomian lipid layer covering the outer surface of the tear film—A review. Exp Eye Res 2015;137:125–138. [DOI] [PubMed] [Google Scholar]
- 15.Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: Report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci 2011;52:2050–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson MC, Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact Lens 2003;29:96–99. [DOI] [PubMed] [Google Scholar]
- 17.Mori A, Shimazaki J, Shimmura S, et al. Disposable eyelid-warming device for the treatment of meibomian gland dysfunction. Jpn J Ophthalmol 2003;47:578–586. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto Y, Dogru M, Goto E, et al. Efficacy of a new warm moist air device on tear functions of patients with simple meibomian gland dysfunction. Cornea 2006;25:644–650. [DOI] [PubMed] [Google Scholar]
- 19.Lane SS, DuBiner HB, Epstein RJ, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea 2012;31:396–404. [DOI] [PubMed] [Google Scholar]
- 20.Goto E, Monden Y, Takano Y, et al. Treatment of non-inflamed obstructive meibomian gland dysfunction by an infrared warm compression device. Br J Ophthalmol 2002;86:1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearce EI, Pentland A, Shabbir S. Changes in visual acuity following Meibomian gland heat therapy. Abstract TFOSVA 2007. [Google Scholar]
- 22.Pult H, Riede-Pult BH, Purslow C. A comparison of an eyelid-warming device to traditional compress therapy. Optom Vis Sci 2012;89:E1035–E1041. [DOI] [PubMed] [Google Scholar]
- 23.Purslow C. Evaluation of the ocular tolerance of a novel eyelid-warming device used for meibomian gland dysfunction. Cont Lens Anterior Eye 2013;36:226–231. [DOI] [PubMed] [Google Scholar]
- 24.Finis D, Hayajneh J, Konig C, et al. Evaluation of an automated thermodynamic treatment (LipiFlow(R)) system for meibomian gland dysfunction: A prospective, randomized, observer-masked trial. Ocul Surf 2014;12:146–154. [DOI] [PubMed] [Google Scholar]
- 25.Baumann A, Cochener B. Meibomian gland dysfunction: A comparative study of modern treatments [in French]. J Fr Ophtalmol 2014;37:303–312. [DOI] [PubMed] [Google Scholar]
- 26.Friedland BR, Fleming CP, Blackie CA, et al. A novel thermodynamic treatment for meibomian gland dysfunction. Curr Eye Res 2011;36:79–87. [DOI] [PubMed] [Google Scholar]
- 27.Greiner JV. Long-term (12-month) improvement in meibomian gland function and reduced dry eye symptoms with a single thermal pulsation treatment. Clin Exp Ophthalmol 2013;41:524–530. [DOI] [PubMed] [Google Scholar]
- 28.Schein OD, Tielsch JM, Munõz B, et al. Relation between signs and symptoms of dry eye: A population-based perspective. Ophthalmology 1997;104:1395–1401. [DOI] [PubMed] [Google Scholar]
- 29.Sim HS, Petznick A, Barbier S, et al. Controlled treatment trial of eyelid-warming therapies in meibomian gland dysfunction. Ophthalmol Ther 2014;3:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: Report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci 2011;52:2006–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaumberg DA, Gulati A, Mathers WD, et al. Development and validation of a short global dry eye symptom index. Ocul Surf 2007;5:50–57. [DOI] [PubMed] [Google Scholar]
- 32.Koh YW, Celik T, Lee HK, et al. Detection of meibomian glands and classification of meibography images. J Biomed Opt 2012;17:086008. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro A, Merin S. Schirmer test and break-up time of tear film in normal subjects. Am J Ophthalmol 1979;88:752–757. [DOI] [PubMed] [Google Scholar]
- 34.Barr JT, Schechtman KB, Fink BA, et al. Corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: Baseline prevalence and repeatability of detection. Cornea 1999;18:34–46. [PubMed] [Google Scholar]
- 35.Tong L, Chaurasia SS, Mehta JS, et al. Screening for meibomian gland disease: Its relation to dry eye subtypes and symptoms in a tertiary referral clinic in singapore. Invest Ophthalmol Vis Sci 2010;51:3449–3454. [DOI] [PubMed] [Google Scholar]
- 36.Yang Z, Tan C, Tong L. Intra-rater and inter-rater repeatability of ocular surface interferometer in measuring lipid layer thickness. BMC Ophthalmol 2015;15:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akaike H, Parzen E, Tanabe K, et al. Selected Papers of Hirotugu Akaike. Springer Series in Statistics1998. New York, NY: Springer, viii, 434. [Google Scholar]
- 38.Greiner JV. A single LipiFlow(R) Thermal Pulsation System treatment improves meibomian gland function and reduces dry eye symptoms for 9 months. Curr Eye Res 2012;37:272–278. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki T, Teramukai S, Kinoshita S. Meibomian glands and ocular surface inflammation. Ocul Surf 2015;13:133–149. [DOI] [PubMed] [Google Scholar]
- 40.Pflugfelder SC, Karpecki PM, Perez VL. Treatment of blepharitis: Recent clinical trials. Ocul Surf 2014;12:273–284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


