Supplemental Digital Content is available in the text.
Keywords: alirocumab, cardiovascular diseases, clinical trial, genetics, hypercholesterolemia, PCSK9 protein, human
Abstract
Background—
Patients with PCSK9 gene gain of function (GOF) mutations have a rare form of autosomal dominant hypercholesterolemia. However, data examining their clinical characteristics and geographic distribution are lacking. Furthermore, no randomized treatment study in this population has been reported.
Methods and Results—
We compiled clinical characteristics of PCSK9 GOF mutation carriers in a multinational retrospective, cross-sectional, observational study. We then performed a randomized placebo-phase, double-blind study of alirocumab 150 mg administered subcutaneously every 2 weeks to 13 patients representing 4 different PCSK9 GOF mutations with low-density lipoprotein cholesterol (LDL-C) ≥70 mg/dL on their current lipid-lowering therapies at baseline. Observational study: among 164 patients, 16 different PCSK9 GOF mutations distributed throughout the gene were associated with varying severity of untreated LDL-C levels. Coronary artery disease was common (33%; average age of onset, 49.4 years), and untreated LDL-C concentrations were higher compared with matched carriers of mutations in the LDLR (n=2126) or apolipoprotein B (n=470) genes. Intervention study: in PCSK9 GOF mutation patients randomly assigned to receive alirocumab, mean percent reduction in LDL-C at 2 weeks was 62.5% (P<0.0001) from baseline, 53.7% compared with placebo-treated PCSK9 GOF mutation patients (P=0.0009; primary end point). After all subjects received 8 weeks of alirocumab treatment, LDL-C was reduced by 73% from baseline (P<0.0001).
Conclusions—
PCSK9 GOF mutation carriers have elevated LDL-C levels and are at high risk of premature cardiovascular disease. Alirocumab, a PCSK9 antibody, markedly lowers LDL-C levels and seems to be well tolerated in these patients.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique Identifier: NCT01604824.
Autosomal dominant hypercholesterolemia (ADH), which features high levels of low-density lipoprotein cholesterol (LDL-C), is a common monogenic disorder (estimated prevalence 1 in 250–500) that substantially contributes to the worldwide burden of premature cardiovascular disease (CVD).1,2 Plasma levels of LDL-C are regulated primarily by apolipoprotein B–mediated binding of LDL particles to hepatic LDL receptors (LDLRs) followed by cellular internalization and metabolism. Patients with genetic defects in this pathway have high levels of LDL-C and early onset CVD, as evident in patients with LDLR (OMIM #606945) or APOB mutations (OMIM #107730) causing familial hypercholesterolemia (FH) and familial defective apolipoprotein B (FDB), respectively.
Editorial see p 749
Clinical Perspective on p 831
DNA recombinant mapping in families in France and Utah in which ADH did not cosegregate with markers for LDLR or APOB identified 1p34 as the responsible locus.3,4 Shortly thereafter, several gain of function (GOF) mutations in the PCSK9 gene (OMIM #607786) were identified as a third cause of ADH: Ser127Arg and Phe216Leu in 3 French families,5 Asp374Tyr in the Utah family,6 and later in Norwegian and English families.7,8 Additional PCSK9 GOF mutations were later identified in several small studies from various geographical locations.9–12
Proprotein convertase subtilisin/kexin type 9 (PCSK9) regulates serum LDL catabolism by binding and targeting LDLR to lysosomal degradation.13–17 Thus, increased PCSK9 function leads to reduced hepatic LDLR levels and concomitant high plasma LDL-C levels13 and vice versa.18 In several patient populations who cannot achieve target LDL-C levels with currently available lipid-lowering therapies, blockade of PCSK9 with alirocumab, or other human PCSK9 monoclonal antibodies, has demonstrated significant LDL-C reductions.19–23
Despite growing awareness that PCSK9 mutations may cause ADH, no global study has been performed that examines and compares the clinical characteristics of the rare patients with different PCSK9 GOF mutations to each other or to patients with FH and FDB. We report a worldwide comparative compilation of patients known to have varying PCSK9 GOF mutations so as to describe their physical and laboratory manifestations, prevalence of CVD, and lipid response to therapy. We also report results from the first randomized intervention trial in PCSK9 GOF mutation patients treated with alirocumab for which we used a novel randomized placebo-phase study design to enable a double-blinded comparison of alirocumab with placebo (based on differential onset of effect between study arms) and the opportunity for all subjects to receive active study medication and contribute to the analysis of safety and efficacy.24
Methods
Study Designs
The studies were designed by Regeneron Pharmaceuticals Inc in collaboration with one of the authors (J.D. for observational study and P.N.H. for treatment study). The study protocols were approved by the investigational review board at each study center, and all subjects in the treatment study provided written informed consent. Data were collected at the study sites by several of the coauthors and were analyzed by representatives of Regeneron Pharmaceuticals Inc.
Comparative Observational Study
We conducted a retrospective global comparative compilation study in which individuals known to have PCSK9 GOF mutations were categorized so as to associate mutations with lipid profiles, comorbidity, and response to therapy. All of these patients had also been previously characterized for functional mutations in LDLR and APOB exons 26 and 29. Data were collected by supplying the collaborators with a uniform data collection sheet that included untreated and on-treatment lipid profiles; lipid-lowering therapy at the time of treated lipid profiles; the presence of xanthoma, xanthelasma, and arcus lipoides corneae; and occurrence and age of onset of CVD.
We compared lipid profiles and other clinical characteristics of patients with PCSK9 GOF mutations to patients with FH and FDB. For this comparison, we selected molecularly proven carriers of pathological LDLR or APOB mutations from the Dutch Familial Hypercholesterolemia Registry who had untreated lipid levels available.25,26 Each patient with a PCSK9 GOF mutation was matched by sex and age (±2 years) to all available FH and FDB patients from the Dutch Familial Hypercholesterolemia Registry. This approach yielded a cohort with an average of 3 FDB and 16 FH patients for each PCSK9 carrier. LDLR mutations were characterized as defective (missense, small in-frame indel, synonymous with added splice site) or deficient (large or frame-shifting indel, nonsense, splice site, promoter variant). In comparisons of the effect of different PCSK9 GOF mutations on LDL-C, we only performed statistical tests for a particular variant when ≥5 individuals were observed to carry that variant, and we compared that variant with all noncarriers of that particular variant.
Treatment Study
The treatment study was conducted at 3 sites in France and 1 in Utah. We included men and women age aged 18 to 70 years with PCSK9 GOF mutations verified by DNA sequencing and serum LDL-C levels ≥70 mg/dL at screening on a stable lipid-lowering regimen and considered not at goal by the investigator. Subjects had body mass index of 18.0 to 40.0 kg/m2 and no cardiovascular event, heart failure, or uncontrolled diabetes within 6 months of enrollment. Patients continued to take their prestudy lipid-lowering therapies throughout the study. Additional enrollment criteria are provided in the Data Supplement.
We used a novel double-blind, randomized, placebo-phase design instead of an open-label nonrandomized study design to enable a double-blinded comparison of alirocumab with placebo (Figure I in the Data Supplement). This study design also provided on-drug treatment data for all subjects in this small group of unique patients.24 All participants received a single-blind dose of placebo at week–2. After subsequent randomization, group A received alirocumab (150 mg SC) at weeks 0, 2, 4, 6, and 10 and placebo at weeks 8, 12, and 14; group B received alirocumab at weeks 2, 4, 6, 8, and 12 and placebo at weeks 0, 10, and 14 (Figure I in the Data Supplement). Follow-up visits were conducted at weeks 16, 18, 20, and 22. Accordingly, the number of alirocumab doses was equal in the 2 groups, but the dosing schedule for group B was shifted by 2 weeks compared with group A.
The primary end point was a comparison in percent change of measured serum LDL-C from pretreatment to 2 weeks between group A (single alirocumab dose) and group B (placebo). Secondary efficacy end points included changes in other lipids at week 2 and changes in lipid measures from baseline to each study visit. Safety assessments included a physical examination, the evaluation of vital signs, electrocardiography, and blood tests. Further details and the schedule of assessments are provided in the Data Supplement.
Statistical Analysis
Comparative Observational Study
For the comparative observational study, we used ANOVA to assess differences in mean lipoprotein levels between each of the individual PCSK9 mutations and all other PCSK9 GOF mutations combined. This methodology was also used to compare lipoprotein levels in all patients with PCSK9 GOF mutations (without LDLR mutations) combined and patients with FH and FDB. To determine the effect of medication, a paired t test was performed on lipoprotein levels before and after treatment.
Treatment Study
Power analysis for the treatment study were based on previous efficacy data and suggested that ≈6 patients per dose group in this rare patient population would provide at least 80% power to detect a treatment difference of 30% (SD, 15%) versus placebo for the primary end point at a 5% significance level. Continuous primary and secondary efficacy variables were analyzed using ANCOVA model with treatment arm as the fixed effect and using the relevant baseline value as a covariate. The rank-based ANCOVA was used for triglycerides and lipoprotein (a) (Lp[a]). The results for the remaining lipid parameters were also confirmed using a nonparametric method (Kruskal–Wallis). There were no missing data points.
Results
Comparative Observational Study
During 2012, 200 lipid specialty centers around the world were contacted, and 164 patients (83 men and 81 women, aged <1 to 79 years) heterozygous with previously identified PCSK9 GOF mutations were compiled from 12 centers in 8 countries (Table 1). The patients carried 16 different missense mutations, 6 of which were previously undescribed (Table 1). Individual PCSK9 GOF mutations generally had restricted geographic distributions and were found in a small number of pedigrees (Figure II in the Data Supplement). Examples include 22 patients with Arg215His found only in 2 pedigrees in Norway and 12 patients with Val4Ile and 30 patients with Glu32Lys found only in Japan. Obligate carrier founders in the Utah pedigree were migrants from the United Kingdom. For pooled PCSK9 GOF mutation patients, mean untreated total and LDL-C were 359 and 272 mg/dL, respectively. Eleven patients were double heterozygotes for mutations in PCSK9 and LDLR; these patients tended to have higher untreated lipids compared with patients with the same GOF mutation alone, as previously reported for the 3 Glu32Lys double-heterozygote patients.12
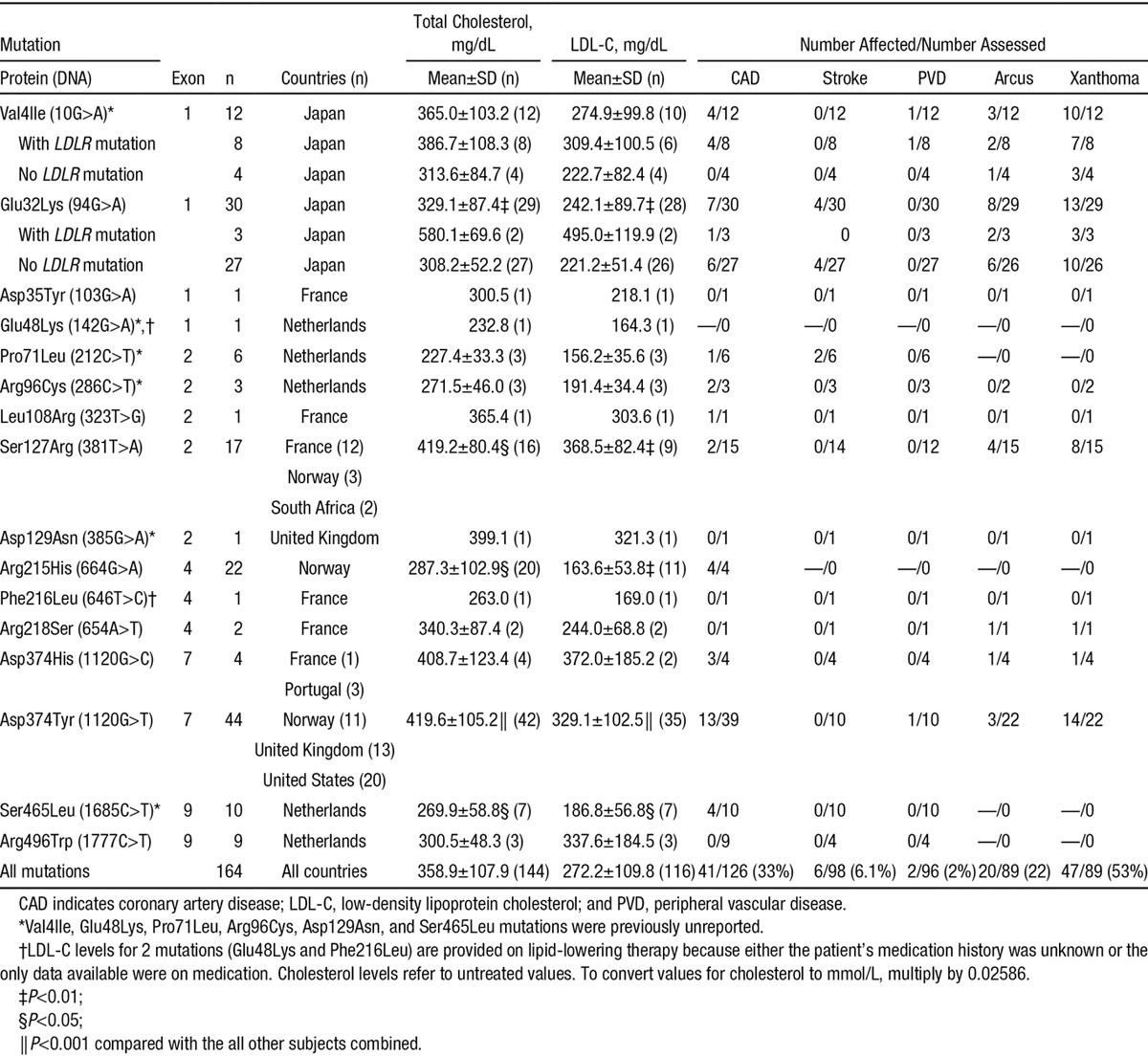
Table 1.
Summary of Clinical Data of Patients With a Familial GOF Mutation in PCSK9
GOF mutations were found in all structural protein domains and 5 of 9 coding exons (Figure 1) and were associated with varying degrees of lipid abnormalities (Table 1). Untreated lipid levels associated with each mutation were compared with the entire PCSK9 GOF mutation population: Asp374Tyr and Ser127Arg carriers had severe dyslipidemia, whereas Glu32Lys, Arg215His, and Ser465Leu carriers were comparatively mild, although substantial variation was present in patients carrying the same mutation (Figure 1).
Figure 1.
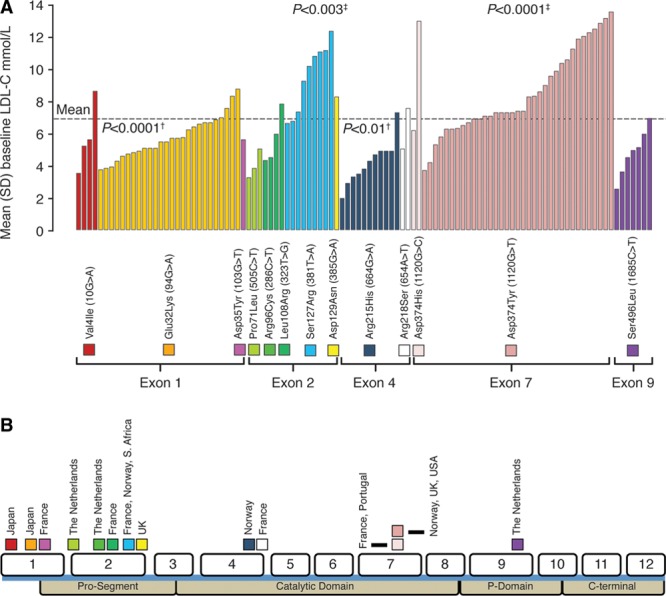
Distribution of untreated low-density lipoprotein cholesterol (LDL-C) for patients with familial GOF mutations in PCSK9 without LDLR mutations (A) and position of the mutations and the 12 exons of the PCSK9 gene relative to the protein domains (B). †P value indicates reduction for mutation versus overall mean. ‡P value indicates increase for mutation versus overall mean. Dotted line represents mean LDL-C level of all PCSK9 mutation carriers from whom untreated LDL-C levels were available. 1.81 mmol/L=70 mg/dL; 2.59 mmol/L=100 mg/dL.
The physical stigmata of elevated cholesterol were frequent (Table 1), with prevalence similar to previous reports for FH and FDB (Table I in the Data Supplement). Also similar to FH and FDB,3,27 44% of patients had a history of CVD. Coronary artery disease was the most prevalent manifestation (33%) with an average age of onset of 49.4±13.8 years (Table 1; Table I in the Data Supplement).
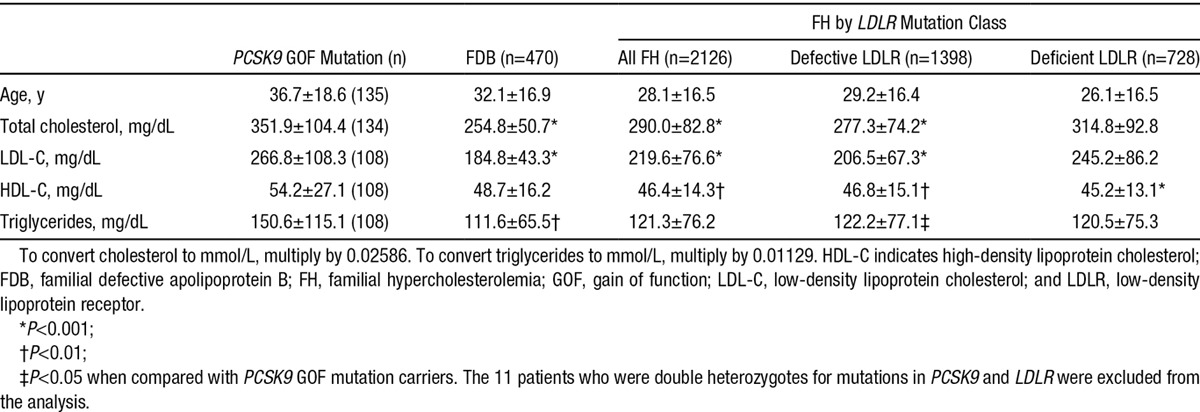
In a comparison with FH and FDB patients drawn from the Dutch Hypercholesterolemia Registry, PCSK9 GOF mutation patients had the highest, and FDB patients the lowest, mean untreated LDL-C levels (Table 2). Among patients with FH, those with deficient mutations had higher untreated LDL-C levels than those with defective mutations (Table 2). Although lipid-lowering therapy (primarily statins; Figure III in the Data Supplement) improved lipid profiles, a substantial proportion failed to achieve guideline LDL-C levels (Figure III in the Data Supplement).
Table 2.
Comparison of Untreated Lipid Profiles (Mean±SD) of Heterozygous Patients With Familial GOF Mutation in PCSK9, FDB, and Defective and Deficient LDLR Mutations in FH
Treatment Study
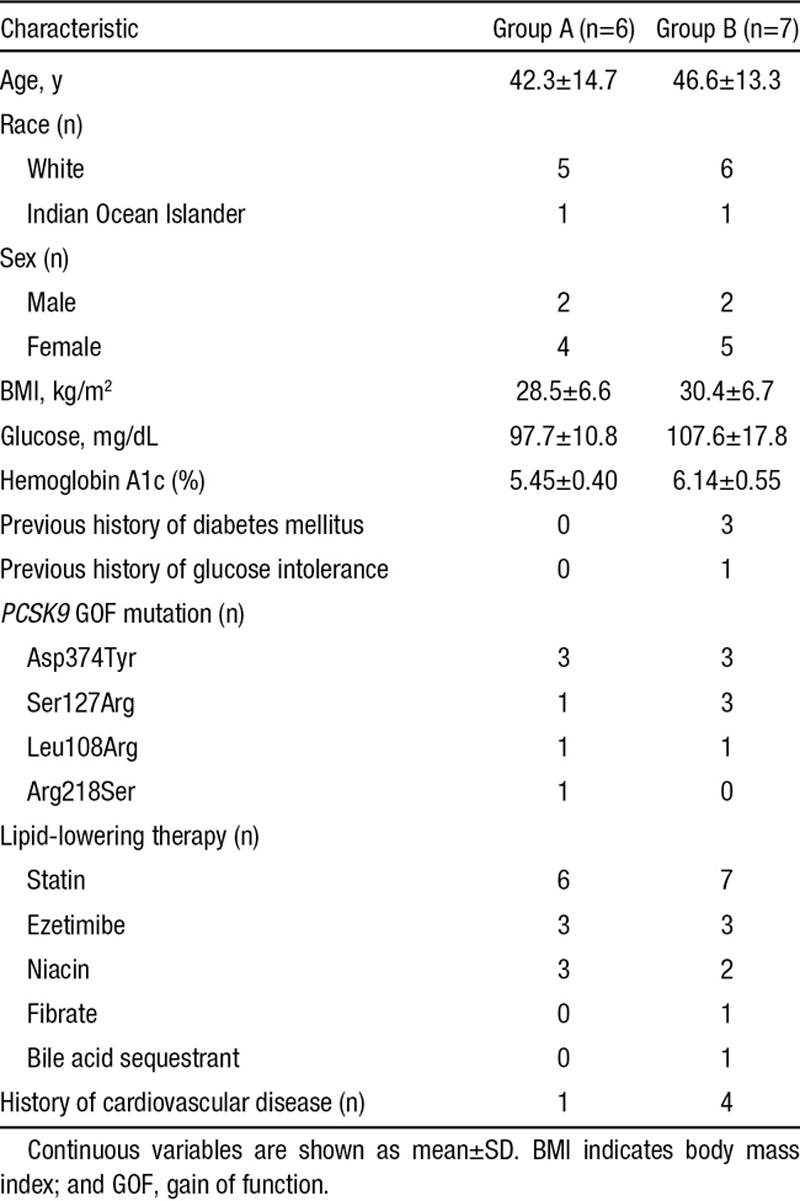
Six Asp374Tyr mutation carriers were enrolled in Utah, and 4 Ser127Arg, 2 Leu108Arg, and 1 Arg218Ser carriers in France (Figure IV in the Data Supplement). Baseline characteristics of the subjects in groups A and B were mostly similar (Table 3) although some differences are apparent.
Table 3.
Baseline Characteristics of Patients With Familial GOF Mutation in PCSK9 in the Randomized Alirocumab 150 mg Study
Lipid and Lipoprotein Response
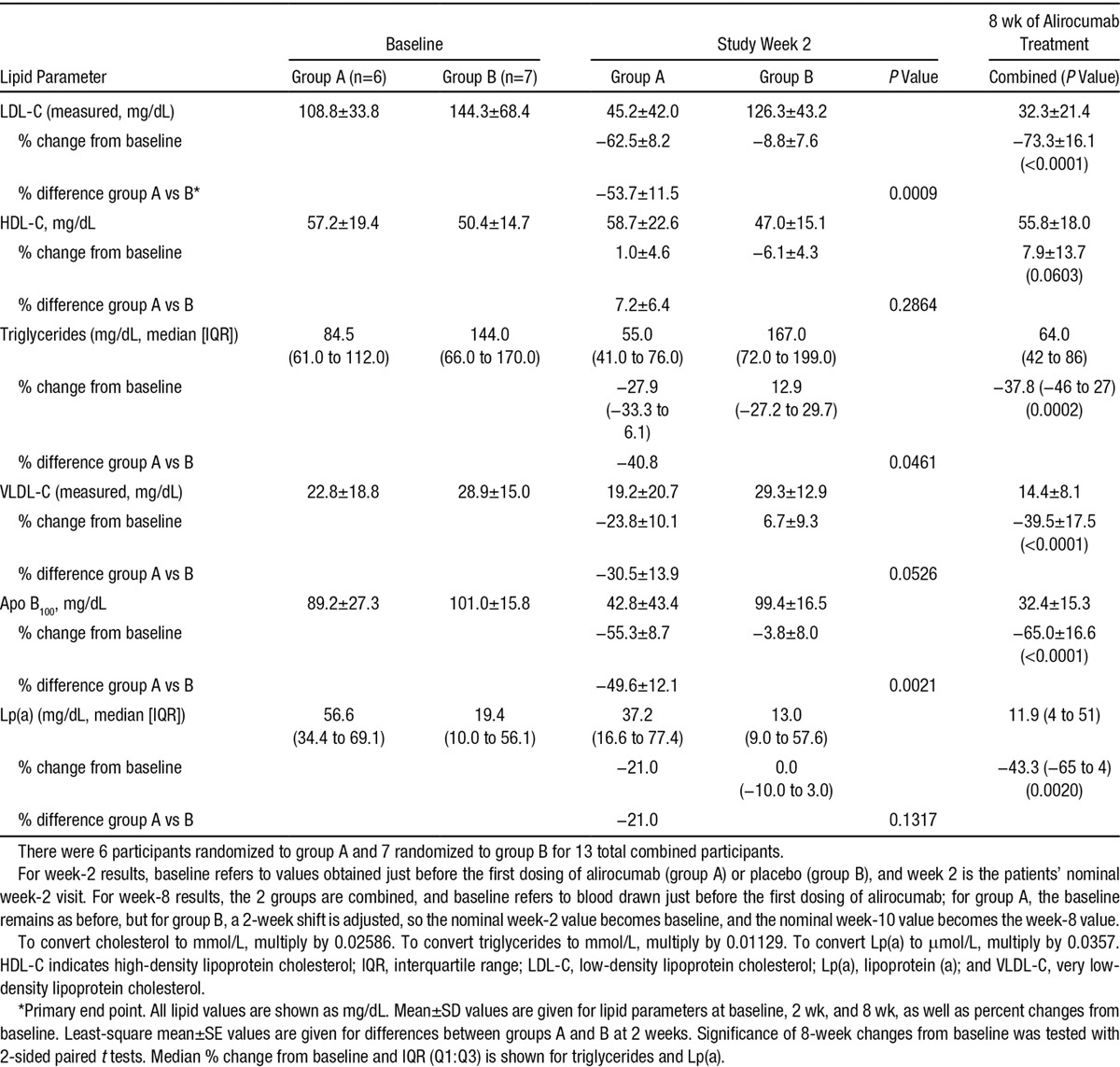
In PCSK9 GOF mutation patients randomly assigned to receive alirocumab, mean percent reduction in LDL-C at 2 weeks was 62.5% (P<0.0001) from baseline and 53.7% compared with control PCSK9 GOF mutation patients treated with placebo for 2 weeks (P=0.0009; primary end point). Changes in LDL-C levels in response to alirocumab were similar but temporally delayed by 2 weeks in group B compared with group A because of the placebo-phase study design (Figure 2A). After 8 weeks of alirocumab treatment, mean percent change in LDL-C was 73.3% (P<0.0001), and 12 of 13 subjects achieved an LDL-C level of <70 mg/dL (Table 4). Reductions of LDL-C in the 2 groups were temporally related to reductions of free PCSK9 (Figure 2B). In a pooled analysis of 8-week lipid changes apolipoprotein B, triglycerides, very low-density lipoprotein cholesterol, and Lp(a) were significantly reduced (Table 4). In an exploratory analysis, we examined changes in levels of LDL-C and free PCSK9 from baseline as a function of PCSK9 GOF genotype. Alirocumab treatment resulted in marked reductions in LDL-C levels from baseline in all patients with all PCSK9 genotypes (Figure 2C). Potential differences in the rate of LDL-C reduction between the genotypes seemed to correlate with kinetics of free PCSK9 reduction (Figure 2D).
Figure 2.
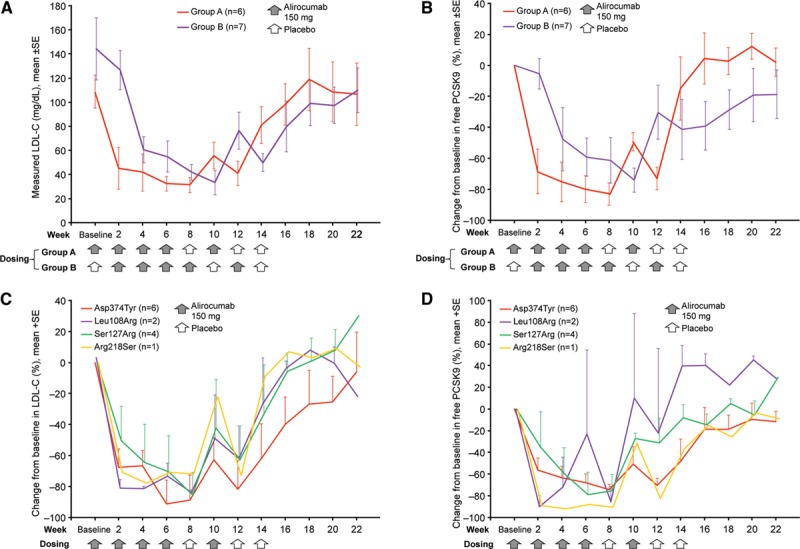
Change in low-density lipoprotein cholesterol (LDL-C) and free PCSK9 for patients with familial gain of function mutation in PCSK9 in the randomized alirocumab study. (A) Mean (±SE) LDL-C values and (B) mean (±SE) percent change from baseline in free plasma PCSK9 are shown by study group together with an indication of the dosing schedules. Mean (+SE) percent change from baseline in LDL-C (C) and free plasma PCSK9 (D) are shown by PCSK9 GOF mutation. C and D, Results from groups A and B were combined by shifting group A visits forward 2 weeks, thereby aligning the dosing schedule in the 2 groups.
Table 4.
Lipid Parameters in Patients in the Randomized Study at Baseline, at Week 2, and After 8 weeks of Alirocumab 150 mg Treatment
Safety
No patient discontinued early from the study for any reason. The most common treatment-emergent adverse events were infections and included nonserious upper and lower respiratory tract infections and gastroenteritis (Table II in the Data Supplement). No patient experienced an elevation of hepatic enzymes or creatinine kinase 3-fold above the upper limit of normal; no trends were observed in hepatic enzymes, creatinine kinase, or fasting blood glucose over the course of the study. Five patients experienced ≥1 fasting blood glucose levels >126 mg/dL during the course of the trial. All of these patients had a history of abnormal fasting blood glucose or an elevated level at screening. One subject experienced a serious adverse event of chest pain. Evidence for myocardial infarction was not found, and a follow-up stress test did not reveal cardiac ischemia.
Discussion
PCSK9 GOF mutations are a third, rare cause of ADH, but knowledge of the clinical attributes of mutation carriers and their response to therapy have heretofore been limited. In an observational study, we characterized the PCSK9 GOF mutation phenotype. Compared with FH or FDB, these patients had similarly frequent physical stigmata and premature CVD but higher LDL-C levels. Although we report evidence that these patients respond to available lipid-lowering treatments, most did not attain optimal lipid profiles on their current regimen of statins plus other lipid-lowering therapies, thus establishing the need for additional therapies. We then demonstrated in a clinical intervention trial that patients with 4 different PCSK9 GOF mutations achieved a marked additional reduction in LDL-C (≤73%) after the addition of alirocumab to their current regimen, and nearly all attained the goal of 70 mg/dL. The results of this small, randomized, placebo-phase trial suggest that PCSK9 antibodies may become a specific and effective treatment for PCSK9 GOF mutation patients.
Our observational study demonstrated that PCSK9 GOF variants had mostly restricted geographical distributions, were found in a limited number of pedigrees, and exhibited significant phenotypic variability in associated disease severity. Although GOF mutations were found throughout the PCSK9 coding sequence, our study confirms that carriers of either Asp374Tyr or Ser127Arg mutations had significantly higher untreated LDL-C levels than the other PCSK9 GOF mutation carriers. This result is not unexpected given that these 2 mutations were among the first to be described and extends the results from a smaller study suggesting that the Asp374Tyr variant may be associated with a severe form of ADH.7 However, because most mutations were reported in a limited number of pedigrees, in our comparison of the different variants, we are unable to define the portion of the phenotype contributed by background genetics. The geographic isolation of GOF variants suggests that they are likely due to private mutations in different populations and is consistent with a relatively recent origin of many or all of them. As cascade screening28 was used to enrich for the presence of PCSK9 mutations, we are unable to obtain a true prevalence of GOF mutations in the general population. However, extensive efforts were undertaken to define the genetic architecture of ADH in Holland and Japan, but no overlap in the variants was found, supporting the geographical isolation of these mutations.
Despite variability in disease severity of individual mutations, pooled analyses revealed significantly greater LDL-C levels in PCSK9 GOF mutation patients compared with patients with FH or FDB. FH patients are found worldwide, and LDLR variants causing FH are distributed throughout the gene (>1700 reported) with greater disease severity associated with individual mutations. In contrast, FDB is also found worldwide, although a single APOB variant (Arg3527Gln), found primarily in northern Europeans, is responsible for the vast majority of FDB cases (>95%). For our comparison, we matched the PCSK9 GOF mutation patients with FH and FDB patients from the Dutch Familial Hypercholesterolemia Registry, the largest such resource in the world. It is possible that these patients have more or less severe disease than patients from other parts of the world because of genetics or shared environment, and additional comparisons with other large collections of patients will be of interest. However, because this registry includes a large number of patients identified by cascade screening, it may better reflect the phenotype of patients with FH and FDB in a population-based sample than many other registries that consist mostly of index patients and their first-degree relatives. Although relative severity of these patients bear future investigation, it is clear that the severity of the PCSK9 GOF phenotype warrants maximizing lipid-lowering therapies in these patients.
In our intervention study, alirocumab administration significantly reduced LDL-C levels in all patients enrolled, and this was temporally correlated with free PCSK9 reductions (Figure 2B and 2D). The magnitude of LDL-C reduction was similar to that observed in previous studies of PCSK9 monoclonal antibodies administered to different patient populations. By using a randomized placebo-phase design,24 each patient contributed to the safety and efficacy data while still enabling the comparison of alirocumab administration to placebo. During the 2-week placebo-controlled portion of the trial, alirocumab administration also significantly reduced apolipoprotein B and triglycerides. Although some difference in the baseline LDL-C levels and other characteristics was present between groups A and B (not unexpected given the small size and international design of the study), a post hoc pooled analysis of all subjects after 8 weeks of alirocumab treatment revealed statistically significant reductions of LDL-C, apolipoprotein B, triglycerides, Lp(a), and very low-density lipoprotein cholesterol levels. We conclude that inhibition of PCSK9 in patients with PCSK9 GOF mutations greatly reduces LDL-C levels. Although all mutation carriers responded to treatment, our results suggest that the rate of reduction in LDL-C may differ in patients carrying different GOF mutations, and this may correlate with the rate of free PCSK9 reduction after alirocumab administration, providing interesting future avenues of research into the biochemical mechanisms of PCSK9.
Our study has limitations. Although we endeavored to obtain all available PCSK9 GOF mutation carriers from a wide selection of lipid research and specialty clinics around the world, we think that additional PCSK9 GOF mutations will be found. Furthermore, the collection of clinical information on the PCSK9 GOF mutation carriers was necessarily limited by the retrospective study design. Although additional information on other coronary artery disease risk factors and course of lipid management would be desirable, such data may best be collected in the setting of prospective follow-up. Although we found that carriers of either Asp374Tyr or Ser127Arg mutations had higher LDL-C levels than carriers of other mutations as a whole, our analysis was constrained by available sample size, which may limit the generalizability of our findings. Finally, we note some clinical differences between the 2 randomized intervention groups, a result not surprising given the relatively small number of mutation carriers included in the intervention trial. Although imbalance in baseline factors may have had some unexpected effect on lipid response at 2 weeks (the time for the placebo-controlled primary end point determination), the large and essentially universal change from baseline at 8 weeks makes an important contribution to responses at 2 weeks less likely.
In conclusion, patients with PCSK9 GOF mutations are characterized by a high prevalence of premature CVD and higher untreated LDL-C levels than FH and FDB. Intervention in these patients with alirocumab, a monoclonal antibody against PCSK9, was well tolerated and resulted in marked reductions in LDL-C levels, suggesting that PCSK9 antibodies may become an important targeted treatment option for these patients.
Acknowledgments
We thank Michael Livingston, Director of the FH International Foundation, under whose auspices we performed the characterization study. We thank Anthony Wierzbicki, Véronique Maire, and Akihiro Inazu for their contributions to the characterization study. Editorial assistance was provided by Brandy Bennett (Regeneron Pharmaceuticals Inc). Technical assistance with the article was provided by an employee of the Prime Medica Group Ltd and funded by Regeneron Pharmaceuticals Inc and Sanofi.
Sources of Funding
This was study sponsored by Regeneron Pharmaceuticals Inc and Sanofi.
Disclosures
P.N. Hopkins reports research support from Sanofi/Regeneron Pharmaceuticals Inc, Merck, and Takeda, and consultancy fees from Regeneron Pharmaceuticals Inc in 2013. J. Defesche reports personal fees from consultancy, outside the submitted work; and consultancy fees from Regeneron in 2013. E. Bruckert reports grants from Danone and Amgen, and personal fees from Aegerion, Genfit, MSD, Sanofi/Regeneron Pharmaceuticals Inc, AstraZeneca, and Unilever. Bertrand Cariou reports personal fees for advisory boards or lectures from Amgen, Eli-Lilly, Janssen, Novartis, Novo Nordisk, MSD, and Sanofi/Regeneron Pharmaceuticals Inc, and fees for consultancy from Genfit. M.H. Shiba reports grants from Kaneka Medics, MSD, and Astellas Pharma Inc. H. Mabuchi reports grants and advisory board fees from Sanofi K.K. outside the submitted work. J.-P. Rabès reports grants from the International FH Foundation during the conduct of the study. In addition, he has had patent EP 1 471 152 A1 Mutations in the human PCSK9 gene associated with hypercholesterolemia issued. A. Carrié reports lecture fees from Roche Diagnostics and a partnership with Multiplicom for the development of molecular diagnostic testing kits for familial hypercholesterolemia. M. Farnier reports having received grants, consulting fees or honoraria and delivering lectures for Abbott, Amgen, AstraZeneca, Boehringer-Ingelheim, Genzyme, Kowa, Merck and Co, Novartis, Pfizer, Recordati, Roche, Sanofi/Regeneron Pharmaceuticals Inc, and SMB. Y.P. Teoh reports personal fees from Sanofi/Regeneron Pharmaceuticals Inc. M. Bourbon reports grants from the Portuguese Science and Technology Foundation (a government institution) during the conduct of the study and grants from Portuguese Cardiology Society (through a Pfizer grant) and congress support from Praxis Pharmaceutical outside the submitted work. A. Nohara reports grants and advisory board fees from Sanofi K.K. outside the submitted work. H. Soran reports fees for advisory board, consultancy, and educational events from Sanofi/Regeneron Pharmaceuticals Inc and Amgen, outside the submitted work. D. Marais reports personal fees from Amgen for advisory boards, grants from Sanofi/Regeneron Pharmaceuticals Inc, and grants from Medical Research Council of South Africa, outside the submitted work. M. Abifadel reports personal fees from Amgen outside the submitted work. In addition, he has had patent Mutations in the PCSK9 gene associated to hypercholesterolemia WO2004/097047 issued. C. Boileau reports personal fees from Sanofi/Regeneron Pharmaceuticals Inc and personal fees from Amgen during the conduct of the study. In addition, he has had patent Mutations in the PCSK9 gene associated to hypercholesterolemia WO2004/097047 issued. G. Lambert reports grants and personal fees from Sanofi/Regeneron Pharmaceuticals, Inc, outside the submitted work. S. Mellis, G.D. Yancopoulos, N. Stahl, J. Mendoza, Y. Du, S. Hamon, and G.D. Swergold are employees and stockholders of Regeneron Pharmaceuticals, Inc. M. Krempf reports grants and personal fees from Sanofi/Regeneron Pharmaceuticals Inc during the conduct of the study, personal fees from Abbott, grants and personal fees from MSD, personal fees from BMS, and grants and personal fees from Novo Nordisk, outside the submitted work. The other authors report no conflicts.
Appendix
From the Division of Cardiovascular Genetics, University of Utah, Salt Lake City, UT (P.N.H.); Departments of Vascular Medicine (J.D., S.W.F., B.S.) and Medical Biochemistry (S.W.F.), Academic Medical Center, Amsterdam, the Netherlands; Hôpital de la Pitié-Salpêtrière, Paris, France (E.B., V.C.); Université de Lille 2, Lille, France (G. Luc); Department of Endocrinology, Metabolic Diseases and Nutrition, l’Institut du Thorax, Nantes University Hospital, Nantes, France (B. Cariou, M.P., Y.Z., M. Krempf); Oslo University Hospital, Oslo, Norway (T.P.L.); National Cerebral and Cardiovascular Center Research Institute, Osaka, Japan (M.H.-S., I.K., H. Makino, Y.M.); Department of Lipidology, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Japan (H. Mabuchi, A.N.); Laboratoire de Biochimie et de Génétique Moléculaire, Hôpitaux Universitaires Paris Ile-de-France Ouest (APHP/UVSQ), Site Ambroise Paré, Boulogne-Billancourt, France (J.-P.R.); Hôpital de la Pitié-Salpêtrière, Groupe Hospitalier Universitaire Pitié-Salpêtrière/Charles-Foix and Université Pierre et Marie Curie, Paris, France (A.C.); Aintree University Hospital, Liverpool, United Kingdom (C.v.H.); Point Médical, Dijon, France (M.F.); Wrexham Maelor Hospital, Wrexham, United Kingdom (Y.P.T.); Instituto Nacional de Saúde Dr. Ricardo Jorge and Centre for Biodiversity, Functional and Integrative Genomics, Faculty of Sciences, University of Lisbon, Lisbon, Portugal (M.B.); Department of Cardiovascular Medicine, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Japan (M. Kawashiri, H.T.); Cardiovascular Research Group, School of Biomedicine, University of Manchester, Manchester, United Kingdom (H.S.); Cardiovascular Trials Unit, University Department of Medicine, Central Manchester University Hospitals NHS Foundation Trust, Manchester, United Kingdom (H.S.); Chemical Pathology and MRC Cape Heart Group, University of Cape Town Health Science Faculty, Cape Town, South Africa (A.D.M.); Laboratory for Vascular Translational Science, INSERM U1148, Hôpital Bichat-Claude Bernard, Paris, France (M.A.); Université Saint-Joseph, Beirut, Lebanon (M.A.); Départment de Génétique & INSERM U1148, Hôpital Xavier Bichat, Paris (C.B.); Department of Endocrinology, Diabetes and Nutrition, Jean Verdier Hospital, Bondy, France (B. Chanu); Department of Cardiology, Toyama Red Cross Hospital, Toyama, Japan (S.K.); Faculté de Médecine, Université de Nantes, Nantes, France (G. Lambert); Medical Education Research Center (K.Y.), Division of Cardiovascular Medicine (M.Y.), Kanazawa University Graduate School of Medical Science, Kanazawa, Japan; Regeneron Pharmaceuticals Inc, Tarrytown, NY (S.M., G.D.Y., N.S., J.M., S.H., G.D.S.); and Regeneron Pharmaceuticals Inc, Basking Ridge, NJ (Y.D.)
Supplementary Material
Footnotes
Drs Hopkins and Defesche contributed equally to this work.
The Data Supplement is available at http://circgenetics.ahajournals.org/lookup/suppl/doi:10.1161/CIRCGENETICS.115.001129/-/DC1.
CLINICAL PERSPECTIVE
Gain of mutation mutations in the PCSK9 gene lead to a rare form of autosomal dominant hypercholesterolemia. However, clinical data on this condition are scarce. Our article presents the results of 2 studies. Firstly, we identified 16 different PCSK9 gain of mutation mutations from 164 patients in a multinational observational study and compared them with age- and sex-matched patients with familial hypercholesterolemia and familial defective apolipoprotein B from the Dutch Registry. The PCSK9 mutations were associated with varying degrees of low-density lipoprotein cholesterol (LDL-C) elevations, but mean untreated LDL-C levels were higher compared with those seen in patients with familial hypercholesterolemia caused by LDLR or APOB mutations. Overall, 44% of patients had cardiovascular disease, and most still presented with elevated LDL-C even after treatment with statins plus other lipid-lowering drugs. Hence, we assessed that additional treatment for these patients would be beneficial. In a second study, we subcutaneously administered the anti-PCSK9 monoclonal antibody alirocumab 150 mg 5× every 2 or every 4 weeks to patients with 4 different PCSK9 gain of mutation mutations. The randomized placebo-phase study design maximized the safety and efficacy data that could be obtained from a study with only 13 subjects. Treatment with alirocumab was well tolerated and resulted in LDL-C reductions of ≤73%. Our results suggest that anti-PCSK9 antibodies may become a targeted and effective additional treatment for these patients.
References
- 1.Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, et al. European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–3490. doi: 10.1093/eurheartj/eht273. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sjouke B, Kusters DM, Kindt I, Besseling J, Defesche JC, Sijbrands EJ, et al. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J. 2015;36:560–565. doi: 10.1093/eurheartj/ehu058. doi: 10.1093/eurheartj/ehu058. [DOI] [PubMed] [Google Scholar]
- 3.Varret M, Rabès JP, Saint-Jore B, Cenarro A, Marinoni JC, Civeira F, et al. A third major locus for autosomal dominant hypercholesterolemia maps to 1p34.1-p32. Am J Hum Genet. 1999;64:1378–1387. doi: 10.1086/302370. doi: 10.1086/302370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt SC, Hopkins PN, Bulka K, McDermott MT, Thorne TL, Wardell BB, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089–1093. doi: 10.1161/01.atv.20.4.1089. [DOI] [PubMed] [Google Scholar]
- 5.Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 6.Timms KM, Wagner S, Samuels ME, Forbey K, Goldfine H, Jammulapati S, et al. A mutation in PCSK9 causing autosomal-dominant hypercholesterolemia in a Utah pedigree. Hum Genet. 2004;114:349–353. doi: 10.1007/s00439-003-1071-9. doi: 10.1007/s00439-003-1071-9. [DOI] [PubMed] [Google Scholar]
- 7.Leren TP. Mutations in the PCSK9 gene in Norwegian subjects with autosomal dominant hypercholesterolemia. Clin Genet. 2004;65:419–422. doi: 10.1111/j.0009-9163.2004.0238.x. doi: 10.1111/j.0009-9163.2004.0238.x. [DOI] [PubMed] [Google Scholar]
- 8.Sun XM, Eden ER, Tosi I, Neuwirth CK, Wile D, Naoumova RP, et al. Evidence for effect of mutant PCSK9 on apolipoprotein B secretion as the cause of unusually severe dominant hypercholesterolaemia. Hum Mol Genet. 2005;14:1161–1169. doi: 10.1093/hmg/ddi128. doi: 10.1093/hmg/ddi128. [DOI] [PubMed] [Google Scholar]
- 9.Allard D, Amsellem S, Abifadel M, Trillard M, Devillers M, Luc G, et al. Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Hum Mutat. 2005;26:497. doi: 10.1002/humu.9383. doi: 10.1002/humu.9383. [DOI] [PubMed] [Google Scholar]
- 10.Homer VM, Marais AD, Charlton F, Laurie AD, Hurndell N, Scott R, et al. Identification and characterization of two non-secreted PCSK9 mutants associated with familial hypercholesterolemia in cohorts from New Zealand and South Africa. Atherosclerosis. 2008;196:659–666. doi: 10.1016/j.atherosclerosis.2007.07.022. doi: 10.1016/j.atherosclerosis.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Miyake Y, Kimura R, Kokubo Y, Okayama A, Tomoike H, Yamamura T, et al. Genetic variants in PCSK9 in the Japanese population: rare genetic variants in PCSK9 might collectively contribute to plasma LDL cholesterol levels in the general population. Atherosclerosis. 2008;196:29–36. doi: 10.1016/j.atherosclerosis.2006.12.035. doi: 10.1016/j.atherosclerosis.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi T, Katsuda S, Kawashiri MA, Tada H, Nohara A, Inazu A, et al. The E32K variant of PCSK9 exacerbates the phenotype of familial hypercholesterolaemia by increasing PCSK9 function and concentration in the circulation. Atherosclerosis. 2010;210:166–172. doi: 10.1016/j.atherosclerosis.2009.11.018. doi: 10.1016/j.atherosclerosis.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50:S172–S177. doi: 10.1194/jlr.R800091-JLR200. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo Surdo P, Bottomley MJ, Calzetta A, Settembre EC, Cirillo A, Pandit S, et al. Mechanistic implications for LDL receptor degradation from the PCSK9/LDLR structure at neutral pH. EMBO Rep. 2011;12:1300–1305. doi: 10.1038/embor.2011.205. doi: 10.1038/embor.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J Lipid Res. 2012;53:2515–2524. doi: 10.1194/jlr.R026658. doi: 10.1194/jlr.R026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Huang Y, Hobbs HH, Cohen JC. Molecular characterization of proprotein convertase subtilisin/kexin type 9-mediated degradation of the LDLR. J Lipid Res. 2012;53:1932–1943. doi: 10.1194/jlr.M028563. doi: 10.1194/jlr.M028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seidah NG, Awan Z, Chrétien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res. 2014;114:1022–1036. doi: 10.1161/CIRCRESAHA.114.301621. doi: 10.1161/CIRCRESAHA.114.301621. [DOI] [PubMed] [Google Scholar]
- 18.Maxwell KN, Breslow JL. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci U S A. 2004;101:7100–7105. doi: 10.1073/pnas.0402133101. doi: 10.1073/pnas.0402133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–1118. doi: 10.1056/NEJMoa1105803. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- 20.Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. doi: 10.1016/S0140-6736(12)60771-5. doi: 10.1016/S0140-6736(12)60771-5. [DOI] [PubMed] [Google Scholar]
- 21.Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, et al. LAPLACE-TIMI 57 Investigators. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380:2007–2017. doi: 10.1016/S0140-6736(12)61770-X. doi: 10.1016/S0140-6736(12)61770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308:2497–2506. doi: 10.1001/jama.2012.25790. doi: 10.1001/jama.2012.25790. [DOI] [PubMed] [Google Scholar]
- 23.Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–2417. doi: 10.1161/CIRCULATIONAHA.112.144055. doi: 10.1161/CIRCULATIONAHA.112.144055. [DOI] [PubMed] [Google Scholar]
- 24.Feldman B, Wang E, Willan A, Szalai JP. The randomized placebo-phase design for clinical trials. J Clin Epidemiol. 2001;54:550–557. doi: 10.1016/s0895-4356(00)00357-7. [DOI] [PubMed] [Google Scholar]
- 25.Fouchier SW, Kastelein JJ, Defesche JC. Update of the molecular basis of familial hypercholesterolemia in the Netherlands. Hum Mutat. 2005;26:550–556. doi: 10.1002/humu.20256. doi: 10.1002/humu.20256. [DOI] [PubMed] [Google Scholar]
- 26.Umans-Eckenhausen MA, Defesche JC, Sijbrands EJ, Scheerder RL, Kastelein JJ. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet. 2001;357:165–168. doi: 10.1016/S0140-6736(00)03587-X. doi: 10.1016/S0140-6736(00)03587-X. [DOI] [PubMed] [Google Scholar]
- 27.Haddad L, Day IN, Hunt S, Williams RR, Humphries SE, Hopkins PN. Evidence for a third genetic locus causing familial hypercholesterolemia. A non-LDLR, non-APOB kindred. J Lipid Res. 1999;40:1113–1122. [PubMed] [Google Scholar]
- 28.Leren TP. Cascade genetic screening for familial hypercholesterolemia. Clin Genet. 2004;66:483–487. doi: 10.1111/j.1399-0004.2004.00320.x. doi: 10.1111/j.1399-0004.2004.00320.x. [DOI] [PubMed] [Google Scholar]


