Abstract
Objective
To assess the functioning of mesolimbic and fronto-striatal areas involved in reward-based spatial learning in teenaged girls with bulimia nervosa (BN) that might be involved in the development and maintenance of maladaptive behaviors characteristic of the disorder.
Method
We compared functional magnetic resonance imaging blood oxygen level dependent response in 27 adolescent girls with BN to that of 27 healthy, age-matched control participants during a reward-based learning task that required learning to use extra-maze cues to navigate a virtual 8-arm radial maze to find hidden rewards. We compared groups in their patterns of brain activation associated with reward-based spatial learning versus a control condition in which rewards were unexpected because they were allotted pseudo-randomly to experimentally prevent learning.
Results
Both groups learned to navigate the maze to find hidden rewards, but group differences in brain activity associated with maze navigation and reward processing were detected in fronto-striatal regions and right anterior hippocampus. Unlike healthy adolescents, those with BN did not engage right inferior frontal gyrus during maze navigation, activated right anterior hippocampus during the receipt of unexpected rewards (control condition), and deactivated left superior frontal gyrus and right anterior hippocampus during expected reward receipt (learning condition). These patterns of hippocampal activation in the control condition were significantly associated with the frequency of binge-eating episodes.
Conclusion
Adolescents with BN displayed abnormal functioning of anterior hippocampus and fronto-striatal regions during reward-based spatial learning. These findings suggest that an imbalance in control and reward circuits may arise early in the course of BN.
Clinical trial registration information
An fMRI Study of Self-regulation in Adolescents With Bulimia Nervosa; https://clinicaltrials.gov/ct2/show/NCT00345943; NCT00345943.
Keywords: bulimia nervosa, Reward, Learning, fMRI, Virtual Reality
INTRODUCTION
Bulimia nervosa (BN) is characterized by binge-eating episodes that are followed by vomiting or another compensatory means to avoid weight gain. A severe sense of loss of control accompanies the binge-eating episodes.1 Impulsive behaviors are also common in individuals with BN, suggesting the presence of pervasive difficulties in behavioral self-regulation.1 Our previous functional magnetic resonance imaging (fMRI) findings demonstrate that self-regulation is impaired in adults2 and adolescents3 with BN due to their failure to engage fronto-striatal circuits appropriately, thereby likely contributing to their inability to regulate eating behaviors. Other fMRI data suggest that reward processing is also abnormal in individuals with BN due to functional disturbances in ventral aspects of fronto-striatal circuits, insular cortex, and mesolimbic areas during the processing of food4, 5 and monetary6 rewards. These abnormalities implicate the dopaminergic system and likely contribute to the increased drive to eat in binge-eating disorders, thereby contributing, in part, to the development of BN.5 Unclear, however, is whether functional deficits in reward circuits are present in adolescents with BN and how such deficits might alter reward learning processes,7, 8 thereby contributing to the learned associations between food and maladaptive eating behaviors in BN.
In addition to self-regulatory and reward processing deficits, neuropsychological data suggest some impairment on visuo-spatial tasks in adults with BN.9, 10 Data from adolescents with bulimic behaviors suggest impaired performance on a Rey-Osterrieth Complex Figure Test, deficits that were further accentuated with increased cognitive load.11 Those findings suggest difficulty with global integration, an element of central coherence that might be related to the disorganized cognitive style as well as the extreme mood states and impulsivity characteristic of adolescents with BN. Thus, adolescents with bulimic behaviors may also have difficulty with spatial learning, a form of learning that relies on episodic memory and requires binding or integrating mental representations of spatial cues into a coherent scene. To date, neither spatial learning nor its neural correlates have been assessed in adults or adolescents with BN. This type of learning is often assessed in rodents by having them navigate an 8-arm radial maze,12 a paradigm that we adapted to a virtual-reality environment for use with fMRI.13, 14 Both the animal and human tasks require learning to use extra-maze cues to navigate and find hidden rewards. Healthy adults activate temporo-parietal areas when searching the maze, consistent with findings from other studies of healthy individuals performing other spatial navigation tasks.15, 16
Our translational fMRI task also includes a control condition in which the use of spatial cues to find hidden rewards is experimentally disabled, allowing us to assess the neural correlates of reward processing in the absence of spatial learning and thus disentangle the neural correlates of learning and reward. Data from healthy adults show limbic activation (hippocampus and amygdala) when receiving unexpected rewards in the control compared to expected rewards in the learning condition, a finding that may be due to enhanced dopaminergic firing from ventral tegmental area to ventral striatum (VS) and these mesolimbic areas in response to unpredicted rewards.17 Those data were interpreted in terms of learning theory and positive prediction errors (PEs), signals generated when an outcome is better than expected.18 These signals are typically associated with VS activation in humans,19 but rodent electrophysiological data suggest that the hippocampus also generates PE signals during learning, signaling a mismatch between expected and experienced contexts.20 Other fMRI data from healthy adults suggest that lateral prefrontal regions are also involved in spatial learning, signaling when the outcome of a path choice deviates from the predicted outcome.21 Thus, learning-related signals in ventral striatum, limbic and lateral prefrontal areas can be assessed with spatial learning tasks.
We recently reported that adults with obsessive-compulsive disorder (OCD) do not engage limbic areas (VS, anterior hippocampus, or amygdala) when receiving unpredicted rewards on our reward-based spatial learning task, data that we suggested may be attributed to disturbances in the mesolimbic dopamine pathway.22 Similarly, adults with BN show reduced VS responses to unexpected gustatory information during reward learning,23 data suggesting mesolimbic abnormalities akin to those reported in individuals with substance use disorder (SUD).24 Conversely, adults with severe cocaine use disorder over-engage limbic areas (VS and amygdala) when receiving unpredicted monetary rewards on our reward-based spatial learning task.25 Whereas these findings from adults with SUD may be confounded by the chronic effects substance exposure and habitual drug-seeking behaviors,26 the long-term effects of binge-eating and purging on mesolimbic circuits are less clear.5 Thus, we sought to explore the functioning of these circuits in adolescents with BN, early in the course of illness and un-confounded by significant chronicity.
Using an ecologically valid navigation task adapted from animal research, we assessed the neural correlates of reward-based spatial learning in adolescent females with BN to understand both general reward learning mechanisms and those specific to spatial learning. Given findings of functional deficits in reward circuits in adults with BN,4, 6, 23 deficits in visuo-spatial and global integration in adolescents with bulimic behaviors11, and our previous report of functional and anatomical abnormalities in inferior frontal cortices in adolescents with BN,3, 27 we tested two specific hypotheses. First, we hypothesized that whereas both groups of adolescents would engage temporo-parietal areas while navigating the maze, participants with BN would not engage inferior frontal cortices during spatial learning. We also hypothesized that the adolescents with BN would not engage VS, anterior hippocampus, or amygdala to the same extent as healthy participants when receiving unpredicted rewards in the control condition, or when predicted rewards were not received in the learning condition. We also explored associations of fronto-striatal and temporo-parietal activations with task performance and the severity of BN symptoms.
METHOD
Participants
Adolescent females with BN (n=27) and healthy comparison (HC) females (n=27), group-matched by age, race and ethnicity, were recruited through flyers, internet, and word-of-mouth. Participants with a history of neurological illness, past seizures, head trauma with loss of consciousness, mental retardation, pervasive developmental disorder, or current Axis I disorders (other than depressive or anxiety disorders for the clinical group), as determined by the Kiddie-Schedule for Affective Disorders and Schizophrenia (SADS)-Present and Lifetime Version.28 HC participants had no lifetime Axis I disorders. Bulimic symptom severity and prior diagnoses of anorexia nervosa were assessed using the Eating Disorders Examination.29 Adolescents in the BN group were included if they engaged in an average of one binge-eating episode (objective or subjective) and one purging episode per week within the past 3 months, with at least one binge-eating and purging episode occurring in the past month. The Institutional Review Board of the New York State Psychiatric Institute approved this study including the informed consent and assent procedures for all participants.
Reward-Based Spatial Learning Paradigm
Our reward-based spatial learning paradigm has been described elsewhere.13, 14 Briefly, the paradigm was set within a virtual reality environment consisting of an 8-arm radial maze surrounded by landscape elements (spatial cues) meant to guide navigation (Figure 1). Prior to scanning, participants were administered a practice navigation session at a computer station. During scanning, non-magnetic goggles were used for stimulus presentation and an MRI-compatible joystick (Current Designs Inc.) was used for navigation. Participants were tasked with navigating the maze environment in search of monetary rewards (denoted with $) that were hidden at the end of the maze arms. They were informed that in each of several task sessions, they could keep any money they found, but that they would lose money if they revisited an arm.
Figure 1.
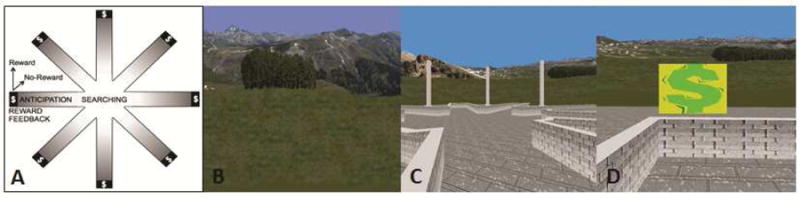
The virtual reality (VR) environment. Note: (A) Schematic of the virtual maze depicting the events modeled: searching, reward anticipation, and reward feedback, reward and no-reward; (B) naturalistic spatial cues in the VR maze; (C) participants’ view of the maze, and (D) the baited area at the end of an arm, with “$” indicating reward receipt.
In the learning condition of the paradigm, each of the 8 maze arms was baited with a monetary reward. Participants could make as many arm visits (trials) as needed to collect all 8 rewards and complete the learning condition (a total of 16 rewards across two sessions of the learning condition). To avoid revisiting arms and the associated monetary loss, they had to learn the layout of the spatial cues. To prevent use of systematic searching strategies in place of spatial learning strategies, each trial began at the center of the maze with the viewing perspective randomly reoriented.
The control condition was designed to provide an experience identical to learning condition but without any possibility of spatial learning. To accomplish this, the spatial configuration of same extra-maze cues used in the learning condition was randomized on each trial. The control condition terminated after participants made the same number of arm visits taken to complete the learning condition in the previous session. For example, if a given participant completed the learning condition in 22 trials (i.e., 8 correct and 14 error trials), the control condition for that participant would also consist of 8 rewarded and 14 error trials delivered randomly across the session. By controlling for all salient features of the learning condition (including lower-order stimulus features and higher-order task features), participants were rewarded without regard to their actual performance and the possibility of spatial learning was therefore experimentally disabled in the control condition. Thus, contrasting neural activity in the learning condition (during spatial learning) and the control conditions (when spatial learning is impossible) reveals the neural correlates of reward-based spatial learning.
The reward-based spatial learning paradigm consisted of two sessions of the learning condition each followed by a session of the control condition for a total of 32 rewarded trials (8 rewards × 2 conditions × 2 runs) and a number of unrewarded trials (errors) that varied for each participant. All participants were paid the same amount of money regardless of performance. Behavioral analyses and image acquisition and processing methods are described in Supplement 1, available online.
Image Analysis
As described previously,22 extraction of subject-level signal differences across the learning and control conditions of the spatial learning task was conducted using general linear models in SPM8. Four regressors corresponding to modeled four events: (i) “searching,” the period between the start of a trial until an arm was selected; (ii) reward “anticipation,” when traversing an arm towards its terminus where the feedback on the trial’s outcome is given; (iii) “reward,” when receiving feedback that the trial was successful thus resulted in a monetary reward; (iv) “no-reward,” at the end of a trial when the receiving feedback that no money was won (Figure 1A). Regressors corresponding to the four events were convolved with a canonical hemodynamic response function and for each, a t-contrast vector was applied to the parameters (beta_j) estimated for each voxel j producing four contrast images for each participant representing each regressor/event (searching, anticipation, reward, no-reward) compared across the two conditions (learning, control).
A random-effects “omnibus” analysis (F test in SPM8) was then used to test the significance of interactions between group (BN, HC), condition (learning, control), and event (searching, anticipation, reward, no-reward). To reduce the number of statistical tests, we limited the search space to a mask comprised of regions in which we hypothesized, a priori, group differences during spatial learning (inferior frontal cortices) and reward receipt (VS, anterior hippocampus, and amygdala), as defined by the AAL atlas. Monte Carlo simulations (10,000 iterations) were implemented in AFNI (v.16.0.01, Jan 27 2016) 3dClustSim using a spatial auto-correlation function with a mean noise smoothness value (full width at half maximum [FWHM]) of 8.64 estimated in AFNI 3dFWHMx, 1-sided thresholding, and a first-nearest neighbor clustering. This approach generated a cluster extent threshold (k=16) that was then applied with an a priori significance threshold of p<.005 to correct for multiple comparisons (p < .05 family-wise error [FWE] corrected). Interactions identified in the omnibus test were further examined in between-group activation maps (also with a voxel threshold of p<.005 together with k=16) that defined group differences in activation associated with the learning and control conditions for each event. These maps were generated from the four participant-level contrast images that represent each of the four events (searching, anticipation, reward, and no-reward) compared across the learning and control conditions. Parameter estimates for individual participants at the cluster maximum peaks of the statistical map for each contrast were derived by extracting subject-level fMRI signal differences across the learning or control conditions and an implicit global baseline that consisted of the unmodeled task components. In exploratory whole brain analyses, another omnibus analysis followed by additional group comparisons were conducted with a more lenient voxel threshold of p<.01 (uncorrected).
RESULTS
Participants
FMRI scans were acquired from twenty-seven adolescents with bulimic behaviors (BN) and 27 age- and body mass index (BMI)-matched healthy comparison (HC) participants (Table 1). The BN group consisted of 9 outpatients and 11 inpatients who were scanned prior to the initiation of treatment, and 7 adolescents who were symptomatic but not seeking treatment. The mean duration of illness was less than 3 years. Of the BN group, 88% met DSM-5 criteria for BN. The remaining reported subjective bulimic (binge-eating) episodes that were associated with loss of control over eating, which is considered more important than the amount of food consumed in defining bulimic episodes in adolescents.30 All participants with BN engaged in vomiting (96%) or another compensatory behavior to avoid weight gain. Five adolescents with BN met DSM-5 criteria for major depressive disorder (MDD), one for generalized anxiety disorder (GAD), one for social anxiety disorder (SAD), one for specific phobia, one for both MDD and GAD, and one for MDD and multiple anxiety disorders (GAD, SAD, specific phobia, and panic disorder). Nine participants with BN were being treated with selective serotonin reuptake inhibitors at the time of scanning.
Table 1.
Demographic and Clinical Characteristics of Participants
| Characteristic | Bulimia Nervosa | Healthy Control | Analysis | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SD | Mean | SD | t | df | p | |
|
|
|||||||
| Age, years | 16.6 | 1.5 | 16.3 | 2.1 | −0.7 | 52 | .46 |
|
| |||||||
| Height, inches | 63.5 | 2.3 | 63.8 | 3.3 | 0.4 | 52 | .67 |
|
| |||||||
| Weight, lbs. | 126.2 | 17.1 | 126.6 | 20.4 | 0.1 | 52 | .94 |
|
| |||||||
| BMI | 22.03 | 2.0 | 21.98 | 1.9 | −0.1 | 52 | .95 |
|
| |||||||
| Duration of Illness, mos. | 27.5 | 20.2 | … | ||||
|
| |||||||
| WASI IQ Score | |||||||
| Full-4 | 105.4 | 11.6 | 103.0 | 16.4 | −0.6 | 50 | .56 |
| Verbal | 109.2 | 12.9 | 105.8 | 16.0 | −0.8 | 49 | .42 |
| Performance | 99.8 | 9.6 | 97.8 | 15.0 | −0.6 | 49 | .56 |
|
| |||||||
| EDE Ratings | |||||||
| OBEs past 28 days | 15.3 | 18.7 | … | ||||
| SBEs past 28 days | 17.0 | 24.5 | … | ||||
| LOC eating past 28 days | 32.2 | 29.8 | … | ||||
| Vomiting episodes past 28 days | 15.3 | 18.7 | … | ||||
|
| |||||||
| BDIa | 23.9 | 10.6 | 5.1 | 7.1 | −5.8 | 31 | <.01 |
|
| |||||||
| CDI | 50.6 | 2.7 | 52 | … | 0.5 | 4 | .67 |
|
| |||||||
| n | % | n | % | ||||
|
|
|||||||
| Race/Ethnicity | |||||||
| Caucasian | 16 | 59.3 | 9 | 33.3 | |||
| Black | 0 | 0.0 | 4 | 14.8 | |||
| Hispanic | 7 | 25.9 | 9 | 33.3 | |||
| Asian | 3 | 11.1 | 4 | 14.8 | |||
| Other | 1 | 3.7 | 1 | 3.7 | |||
|
| |||||||
| Past AN | 5 | 18.5 | … | ||||
|
| |||||||
| Medication | 9 | 33 | … | ||||
Note: AN = anorexia nervosa; BDI = Beck Depression Inventory; BMI = body mass index; CDI = Children’s Depression Inventory; EDE = Eating Disorders Examination; LOC = loss of control (sum of OBEs and SBEs); OBEs = objective bulimic episodes; SBEs = subjective bulimic episodes; WASI = Wechsler Abbreviated Scale of Intelligence.
BDI scores were collected in n=18 adolescents with bulimia nervosa and n=15 healthy controls. CDI scores were collected in n=9 adolescents with bulimia nervosa and n=12 healthy controls.
Behavioral Performance
Both groups demonstrated learning on the task as evidenced by their significant improvement across runs, taking fewer trials and less time to complete the learning condition in Run 2 compared to Run 1 (Table S1, Supplement 1, available online). There were no significant group differences in trial duration across runs in the learning condition, or in performance across the learning and control conditions (Tables S1 and S2, Supplement 1, available online).
Analysis of Neural Activity
The omnibus analysis revealed significant three-way interactions (group-by-condition-by-event) in right anterior hippocampus and left inferior frontal gyrus (IFG). An exploratory whole-brain analysis revealed additional interactions in bilateral thalamus (k = 57) and superior frontal gyrus (SFG; left: k = 72, right: k = 29), left VS (k = 27), and right IFG (k = 7). These three-way interactions are summarized in Figure 2 and Table 2. Examination of group composite maps (group-by-condition interactions) for each event revealed that the interactions in the omnibus analysis derived from group differences in activations associated with spatial navigation and reward processing (the receipt and non-receipt of rewards) in the learning versus control conditions.
Figure 2.
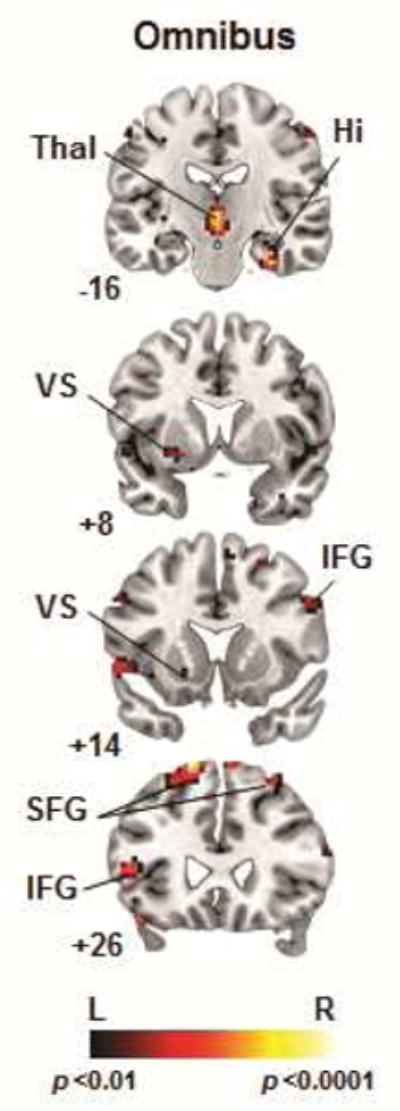
Whole-brain analysis indicating 3-way interactions (diagnosis-by-condition-by-event). Note: Interactions were detected in right (R) hippocampus (Hi), bilateral thalamus (Thal), and fronto-striatal regions including left (L) ventral striatum (VS), bilateral inferior, and superior frontal gyri (p<.01, uncorrected). IFG = inferior frontal gyrus; SFG = superior frontal gyrus.
Table 2.
Group Differences in Neural Activity Associated With Spatial Navigation and Reward Processing Across the Learning and Control Conditions
| Region | Size | # of Voxels | MNI Coordinates x y z |
Statistic |
|---|---|---|---|---|
| Omnibus Test | ||||
| Hippocampus (anterior)a | R | 36 | 33 −16 −29 | 5.63 |
| Thalamus | L, R | 57 | 0 −16 −2 | 5.81 |
| Striatum (ventral) | L | 27 | −30 −1 −8 | 3.94 |
| Superior Frontal Gyrus | L | 72 | −15 26 64 | 6.36 |
| R | 29 | 30 32 55 | 4.65 | |
| Inferior Frontal Gyrusa | L | 66 | −48 26 10 | 4.55 |
| R | 7 | 51 14 34 | 3.85 | |
|
| ||||
| Spatial Navigation (Searching) | ||||
| Inferior frontal gyrusa | R | 50 | 57 20 37 | −3.13 |
|
| ||||
| Reward Receipt | ||||
| Hippocampus (anterior) | R | 73 | 36 −19 −20 | −3.40 |
| Striatum (ventral) | L | 3 | −27 8 −8 | −2.48 |
| Superior Frontal Gyrus | L | 25 | −15 26 64 | −3.50 |
|
| ||||
| No Reward | ||||
| Thalamus | L, R | 23 | 0 −13 1 | −3.15 |
| Superior Frontal Gyrus | L | 20 | −21 32 55 | −2.89 |
| Inferior Frontal Gyrus | L | 55 | −54 17 −2 | −2.89 |
Note: F statistics are reported for the omnibus analysis, and T statistics are reported for group differences in activations associated with the learning and control conditions for each event (p<.01, uncorrected). Montreal Neurological Institute (MNI) coordinates are provided for the peak (maxima) voxel within each cluster.
Clusters significant at p<.005 after correction for multiple comparisons (p<.05 family wise error corrected).
Between-Group Analyses of Neural Activity
Neural Activity During Spatial Navigation
A significant group-by-condition interaction in right IFG derived from its activation in the HC but not the BN group when navigating the maze and searching for rewards in the learning compared to control conditions (p<.005, corrected; Table 2, Figure 3). An exploratory whole-brain analysis revealed that both groups activated temporal and parietal regions during spatial navigation in the learning condition, including hippocampus, lingual and fusiform gyri, and superior parietal lobule (p<.01, uncorrected).
Figure 3.
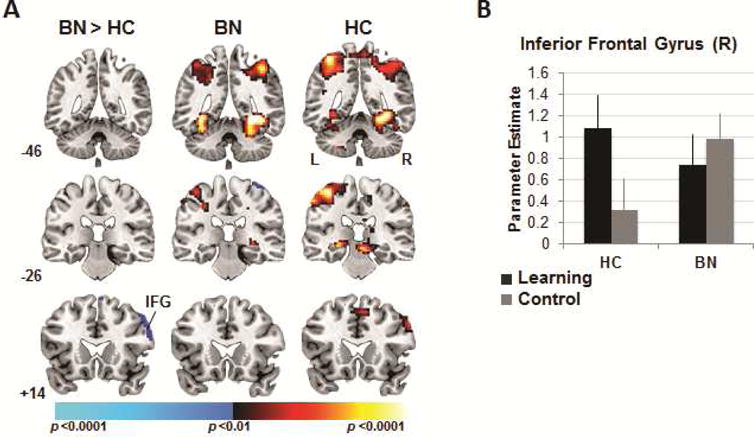
Neural activity during spatial navigation. Note: (A) Group differences (left column) in activations associated with searching the maze in the learning versus the control condition detected in right inferior frontal gyrus. Group average activations for the adolescents with bulimia nervosa (BN; center) and healthy (HC; right) adolescents with increases in signal during searching in the learning vs. control condition in hot colors, and increases during searching in the control vs. learning condition in cool colors. These maps are thresholded at a two-sided significance threshold (p<.01, uncorrected). (B) Parameter estimates at the peak voxel of labeled right inferior frontal gyrus (IFG; 57 20 37) cluster in both conditions and for both groups. Error bars represent ± 1 standard error of the mean.
Neural Activity During Reward Processing
A significant group-by-condition interaction in right anterior hippocampus (p<.005, corrected) and an additional interaction in left SFG (k=25; p<.01, uncorrected) derived from group differences across the learning and control conditions during the receipt of rewards (Table 2, Figure 4). In HC adolescents, activation of these regions was greater in response to receiving rewards in the learning compared to control conditions. Conversely, in adolescents with BN, deactivation of right anterior hippocampus (along with left SFG) was detected in the learning condition (when receiving expected rewards) and accompanied by activation of right anterior hippocampus in the control condition (when receiving unexpected rewards).
Figure 4.
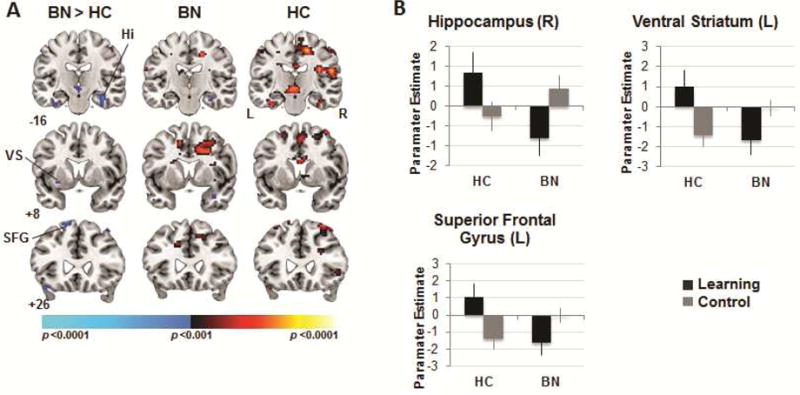
Neural activity during reward receipt. Note: (A) Group differences (left column) in activations associated with reward receipt in the learning versus control conditions detected in right hippocampus (Hi), left ventral striatum (VS), and bilateral superior frontal gyrus (SFG). Group average brain activations for the adolescents with bulimia nervosa (BN; center) and healthy (HC; right) participants with increases in signal associated with reward receipt in the learning vs. control condition in hot colors, and increases in the control vs. learning condition in cool colors. These maps are thresholded at a two-sided significance threshold (p<.01, uncorrected). (B) Parameter estimates at the peak voxel of labeled, hippocampal (36 −19 −20), VS (−27 8 −8), and superior frontal clusters (L: −15 26 64) in both conditions for both groups. Error bars represent ± 1 standard error of the mean. L = left; R = right.
Our exploratory whole-brain analysis also revealed group-by-condition interactions in bilateral thalamus (k = 23), left SFG (k = 20), and IFG (k = 55) that were produced by group differences across the learning and control conditions when rewards were not received (p<.01, uncorrected; Table 2, Figure 5). Specifically, these interactions derived from greater activation of these regions in the learning relative to control condition in HC adolescents, and from greater activation in the control relative to learning condition in participants with BN.
Figure 5.
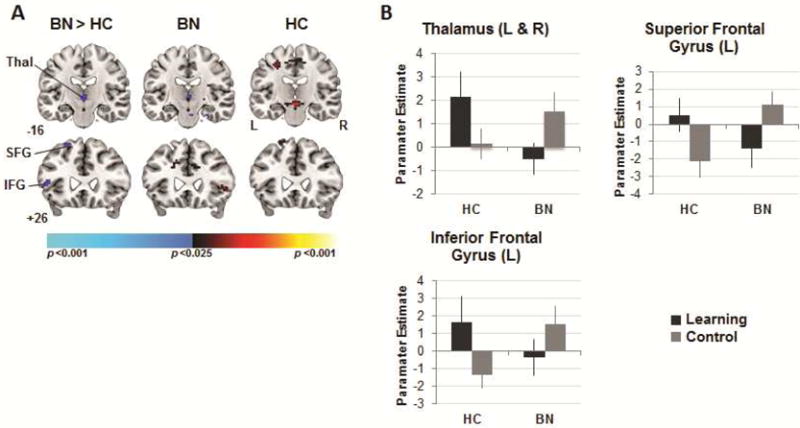
Neural activity during no-reward receipt. Note: (A) Group differences (left column) in activations associated with no reward receipt in the learning versus control conditions detected in bilateral thalamus (Thal), left inferior and superior gyri. Group average activations for the adolescents with bulimia nervosa (BN; center) and healthy (HC; right) participants with increases in signal associated with no reward receipt in the learning vs. control condition in hot colors, and increases in the control vs. learning condition in cool colors. These maps are thresholded at a two-sided significance threshold (p<.01, uncorrected). (B) Parameter estimates at the peak voxel of labeled thalamic (left [L] and right [R]: 0 −13 1), inferior (L: −54 17 −2) and superior (L: −21 32 55) frontal clusters. IFG = inferior frontal gyrus; SFG = superior frontal gyrus; VS = ventral striatum. Error bars represent ± 1 standard error of the mean.
Clinical Correlates
The frequency of bulimic behaviors was significantly associated with activation of right anterior hippocampus during reward processing (Figure S1, Supplement 1, available online). Both binge-eating and vomiting episodes over the past 28 days correlated positively with activation of right anterior hippocampus (p < .05) during the receipt of rewards in the control condition.. Thus, the adolescents with the most severe bulimic symptoms activated left anterior hippocampus in response to receiving unexpected rewards (control condition).
Potential Confounding Effects
A comparison of the map generated from our a priori omnibus analysis with maps generated in omnibus analyses excluding adolescents with BN taking medications, with concurrent MDD, with concurrent anxiety, or lifetime AN suggests that these potential confounds did not contribute to the group differences in brain activations associated with reward-based spatial learning (Figure S2, Supplement 1, available online). Likewise, excluding the adolescents with BN who did not meet DSM-5 criteria for BN or were not seeking treatment in our clinic did not alter these findings.
DISCUSSION
We investigated the neural correlates of reward-based spatial learning in adolescents with BN using a translational fMRI paradigm. In the learning condition, participants had to learn to use cues in the virtual environment to navigate the maze and find hidden rewards. In the control condition, randomization of the scene experimentally disabled learning by making it impossible to use the cues for navigation. Both the healthy and BN groups exhibited spatial learning, similarly taking less time and fewer trials to find all rewards across task runs. However, we detected group differences in activation of cortical, limbic, and subcortical regions associated with spatial navigation and reward processing. Our a priori analyses revealed that only healthy adolescents engaged right IFG when searching the maze and right anterior hippocampus when receiving (expected) rewards in the learning condition. In contrast, adolescents with BN engaged right anterior hippocampus when receiving (unexpected) rewards in the control condition – especially those with the most severe BN symptoms – and deactivated this region upon receipt of (expected) rewards in the learning condition. Our exploratory whole-brain analyses revealed that both groups of adolescents activated temporo-parietal areas typically engaged during maze navigation.16 Unlike their healthy counterparts, adolescents with BN did not engage left SFG and VS when receiving (expected) rewards or bilateral thalamus and left IFG when such rewards were not received in the learning condition. Only adolescents with BN engaged bilateral thalamus, left SFG, and IFG when not receiving (or expecting) rewards in the control condition. Together, these findings describe abnormal functioning of anterior hippocampus and fronto-striatal circuits during reward-based spatial learning in adolescents with BN.
Unlike healthy adolescents, those with BN did not engage right IFG when navigating the maze and searching for rewards in the learning condition, a finding consistent with the failure of adolescents3 and adults2 with BN to engage inferior frontal cortices during self-regulation, and with findings of smaller local volumes within these cortices in BN compared to healthy individuals.31 Right IFG supports self-regulatory capacities, as evidenced by its activation during successful response inhibition in healthy individuals.32, 33 Although we did not formally measure self-regulation here, maze navigation indeed requires the mobilization of attentional resources within frontal cortices in healthy individuals.34 Activation of right IFG was detected during navigation in the learning versus control conditions and correlated with performance speed in healthy adolescents. Thus, deficient IFG activation during navigation in adolescents with BN further suggests that deficits in this inferior frontal attentional35 and regulatory system are likely involved in the pathogenesis of BN.
Adolescents with BN engaged anterior hippocampus when receiving unexpected rewards in the control condition and deactivated anterior hippocampus and VS when receiving expected rewards in the learning condition. Anterior hippocampus is intrinsically connected to VS,36 and healthy adults activate these regions in response to unexpected reward receipt on this task13, 37, data interpreted in light of positive PEs (i.e., signals generated when an outcome is better than expected) typically associated with VS activation in healthy individuals.19 In contrast, expected value (EV) signals are generated when actual and expected outcomes match.38 Both PE and EV signals within lateral prefrontal cortex, striatum, hippocampus, and midbrain have been detected in healthy individuals.39, 40 Thus increased activation of anterior hippocampus, VS, and SFG upon receipt of unexpected rewards may suggest increased sensitivity to positive PEs in adolescents with BN, while their deactivation of anterior hippocampus and VS during the receipt of expected rewards may suggest insensitivity to EVs.
In contrast to previous findings from adults, healthy adolescents activated neither VS nor anterior hippocampus in the control condition, and instead engaged these regions when receiving rewards in the learning condition, perhaps suggesting that healthy adolescents are insensitive to positive PEs, consistent with data suggesting their increased sensitivity to negative PEs41 (i.e., when expected rewards are omitted). Whereas healthy adolescents are sensitive to negative PEs, adolescents with BN may be more sensitive to positive PEs (i.e., receiving unexpected rewards). Moreover, activation of anterior hippocampus during unexpected reward receipt in the control condition was greatest in the adolescents who engaged in the most frequent binge-eating behaviors, suggesting that these adolescents may be most sensitive to positive PEs.
Data suggest that adults with BN are less sensitive than their healthy counterparts in their reward circuit responses to both positive and negative PEs when learning associations between conditioned visual and unconditioned taste stimuli.23 Women with BN showed reduced activation of bilateral amygdala, left insula, and OFC in response to the unexpected receipt of unconditioned taste stimuli, and reduced activation of bilateral amygdala, insula, and right VS in response to the unexpected omission of those stimuli. Although these findings cannot be compared directly with ours, given the differences across tasks and ages of our study samples, they converge in suggesting abnormal functioning of reward circuitry during learning in BN. We can also speculate that such functional abnormalities may be greater in adults with persistent BN, especially in the context of food-related stimuli.
BN is conceptualized as a “food addiction” based on behavioral and neurobiological features common across BN and substance use disorders (SUDs).42 Dopaminergic dysfunction in reward circuits is documented in adults with both SUDs24 and BN.43 Cocaine-dependent men also show altered functioning of reward circuitry on our spatial task,25 further pointing to dopaminergic, reward-circuit dysfunction in both disorders. Such functional abnormalities may influence the initial learning of binge-purge behaviors, consistent with the role of VS in the early stages of reinforcement-based learning8 (in drug addiction7). Thus, dysfunction in the VS target of the midbrain dopaminergic system may contribute to neural changes that lead to BN. Given the differences between study samples (i.e., age, gender, illness chronicity, cocaine exposure), future studies should assess the functioning of the dopaminergic reward system transdiagnostically and developmentally, over the course of these illnesses.
Together with the documented functional/anatomical abnormalities in lateral PFC in BN, our findings may reflect an imbalance in the circuits that support top-down (attentional and regulatory) and more bottom-up (reward) processes that characterizes eating disorders.44 Longitudinal studies are required to determine whether this imbalance is associated with the developmental trajectory of BN or may be a target for the development of novel treatments (or additions to evidence-based treatments) aimed, for example, at enhancing control over disordered eating behaviors or diminishing the salience of food rewards. Brain stimulation techniques, such as repetitive transcranial magnetic stimulation, targeting reward circuits via striatal, hippocampal, or prefrontal regions might achieve this goal. In addition, a better understanding of reward learning in BN could inform future research aimed at assessing how reward circuit function might change following treatments based on the principles of conditioning and learning (i.e, cue exposure with response prevention of binge-eating or vomiting).45
This study is limited by the modest sample size and inclusion of adolescents with BN and comorbid depression and anxiety disorders. However, the presence of these comorbidities did not contribute significantly to our findings, and inclusion of these cases is consistent with the true presentation of BN in adolescents in the population.46 Although inclusion of adolescents who did not meet DSM-5 criteria for BN may have decreased our ability to detect group differences in brain activations, our inclusion criteria were consistent with those used in other studies of adolescents11, 47 and consistent with data suggesting that the loss of control during eating is more definitive of binge episodes for adolescents than the amount of food consumed.30 Moreover, our detection of significant group differences in activations with this sample suggests that the functional abnormalities reported herein are likely robust and relevant to adolescents with subclinical BN. We did not control for hunger/satiety or menstrual status. Hunger might impact attentional and executive processes48 that are required for maze navigation but unlikely involved in reward processing. Although menstrual status may affect reward-related neural functioning,49 we have no reason to believe that menstrual status differed across the BN and healthy adolescents in this study. Finally, findings from our exploratory analyses must be interpreted with caution since they were not corrected for multiple comparisons.
In summary, we assessed the neural correlates of spatial learning and reward processing in adolescents with BN using a translational fMRI paradigm that we previously used in studies of adults with OCD22 and with SUDs.25 These findings from adolescents with BN point to functional abnormalities within anterior hippocampus and fronto-striatal regions associated with reward-based learning and suggest that an increased sensitivity to positive PEs, together with deficient engagement of inferior frontal cortices, may contribute to the purported imbalance between top-down control and more bottom-up reward circuits that characterizes eating disorders.44 Indeed, abnormal activation of anterior hippocampus and VS during the receipt of unexpected rewards (and increased sensitivity to positive PEs) in adolescents with BN suggests altered bottom-up representations of action–outcome associations that may contribute to the learned associations between food cues and maladaptive binge-eating behaviors. These functional abnormalities also suggest deficits in the integration of contextual information during spatial learning and reward-processing that are likely related to the cognitive disorganization, extreme mood states, and impulsivity characteristic of adolescents with BN.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health (NIMH) grant R01MH090062 (R.M.).
Dr. Wang served as the statistical expert for this research.
The authors thank Mark Packard, PhD, of Texas A & M University, Dongrong Xu, PhD, of the New York State Psychiatric Institute, and Bradley S. Peterson, MD, of the Keck School of Medicine of University Southern California, for their efforts in the development of the virtual reality paradigm described in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This article is discussed in an editorial by Dr. Guido K.W. Frank on page xx.
Supplemental material cited in this article is available online.
Disclosure: Drs. Cyr, Wang, Tau, Friedl, Marsh, Mr. Zhao, Ms. Stefan, and Ms. Terranova report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Marilyn Cyr, Division of Child and Adolescent Psychiatry, the New York State Psychiatric Institute and the College of Physicians and Surgeons, Columbia University, New York.
Zhishun Wang, Division of Translational Imaging, the New York State Psychiatric Institute and College of Physicians and Surgeons, Columbia University.
Gregory Z. Tau, Division of Child and Adolescent Psychiatry, the New York State Psychiatric Institute and the College of Physicians and Surgeons, Columbia University, New York.
Guihu Zhao, Division of Child and Adolescent Psychiatry, the New York State Psychiatric Institute and the College of Physicians and Surgeons, Columbia University, New York; School of Information Science and Engineering, Central South University, Changsha 410083, China.
Eve Friedl, Division of Child and Adolescent Psychiatry, the New York State Psychiatric Institute and the College of Physicians and Surgeons, Columbia University, New York.
Mihaela Stefan, Division of Child and Adolescent Psychiatry, the New York State Psychiatric Institute and the College of Physicians and Surgeons, Columbia University, New York.
Kate Terranova, Division of Child and Adolescent Psychiatry, the New York State Psychiatric Institute and the College of Physicians and Surgeons, Columbia University, New York.
Rachel Marsh, Division of Child and Adolescent Psychiatry, the New York State Psychiatric Institute and the College of Physicians and Surgeons, Columbia University, New York.
References
- 1.Kaye W, Strober M, Jimerson DC. The neurobiology of eating disorders. In: Charney D, Nestler EJ, editors. The neurobiology of mental illness. New York: Oxford Press; 2004. pp. 1112–1128. [Google Scholar]
- 2.Marsh R, Steinglass JE, Gerber AJ, et al. Deficient activity in the neural systems that mediate self-regulatory control in bulimia nervosa. Arch Gen Psychiatry. 2009;66:51–63. doi: 10.1001/archgenpsychiatry.2008.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh R, Horga G, Wang Z, et al. An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am J Psychiatry. 2011;168:1210–20. doi: 10.1176/appi.ajp.2011.11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohon C, Stice E. Reward abnormalities among women with full and subthreshold bulimia nervosa: A functional magnetic resonance imaging study. Int J Eat Disord. 2011;44:585–595. doi: 10.1002/eat.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank GK. Altered brain reward circuits in eating disorders: chicken or egg? Curr Psychiatry Rep. 2013 Oct;15(10):396–406. doi: 10.1007/s11920-013-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner A, Aizenstein H, Venkatraman VK, et al. Altered striatal response to reward in bulimia nervosa after recovery. Int J Eat Disord. 2010 May;43(4):289–294. doi: 10.1002/eat.20699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009 Apr 12;199(1):89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Atallah HE, McCool AD, Howe MW, Graybiel AM. Neurons in the ventral striatum exhibit cell-type-specific representations of outcome during learning. Neuron. 2014;82:1145–1156. doi: 10.1016/j.neuron.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weider S, Indredavik MS, Lydersen S, Hestad K. Neuropsychological function in patients with anorexia nervosa or bulimia nervosa. Int J Eat Disord. 2015;48:397–405. doi: 10.1002/eat.22283. [DOI] [PubMed] [Google Scholar]
- 10.Lopez CA, Tchanturia K, Stahl D, Treasure J. Central coherence in women with bulimia nervosa. Int J Eat Disord. 2008 May;41(4):340–347. doi: 10.1002/eat.20511. [DOI] [PubMed] [Google Scholar]
- 11.Darcy AM, Fitzpatrick KK, Manasse SM, et al. Central coherence in adolescents with bulimia nervosa spectrum eating disorders. Int J Eat Disord. 2015 Jul;48(5):487–493. doi: 10.1002/eat.22340. [DOI] [PubMed] [Google Scholar]
- 12.Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci. 1989;9(5):1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsh R, Hao X, Xu D, et al. A Virtual Reality-Based FMRI Study of Reward-Based Spatial Learning. Neuropsychologia. 2010;48(10):2912–2921. doi: 10.1016/j.neuropsychologia.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu D, Hao X, Wang Z, et al. A Virtual Radial Arm Maze for the Study of Multiple Memory Systems in a Functional Magnetic Resonance Imaging Environment. Int J Virtual Real. 2012 Jun;11(2):63–76. [PMC free article] [PubMed] [Google Scholar]
- 15.Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37(5):877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 16.Spiers HJ, Maguire EA. A navigational guidance system in the human brain. Hippocampus. 2007;17(8):618–626. doi: 10.1002/hipo.20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 18.Schultz W. Reward functions of the basal ganglia. J Neural Transm (Vienna) 2016;123:679–93. doi: 10.1007/s00702-016-1510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage. 2006;31:790–5. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Mizumori SJ. Context prediction analysis and episodic memory. Front Behav Neurosci. 2013;7:132. doi: 10.3389/fnbeh.2013.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiers HJ, Maguire EA. Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage. 2006 Jul 15;31(4):1826–1840. doi: 10.1016/j.neuroimage.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 22.Marsh R, Tau GZ, Wang Z, et al. Reward-Based Spatial Learning in Unmedicated Adults with Obsessive-Compulsive Disorder. Am J Psychiatry. 2015;172(4):383–392. doi: 10.1176/appi.ajp.2014.13121700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank GK, Reynolds JR, Shott ME, O’Reilly RC. Altered temporal difference learning in bulimia nervosa. Biological psychiatry. 2011 Oct 15;70(8):728–735. doi: 10.1016/j.biopsych.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011 Sep 13;108(37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tau GZ, Marsh R, Wang Z, et al. Neural correlates of reward-based spatial learning in persons with cocaine dependence. Neuropsychopharmacology. 2014;39(3):545–555. doi: 10.1038/npp.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol. 2013 Aug;23(4):615–624. doi: 10.1016/j.conb.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Marsh R, Stefan M, Bansal R, Hao X, Walsh BT, Peterson BS. Anatomical Characteristics of the Cerebral Surface in Bulimia Nervosa. Biol Psychiatry. 2015;77:616–23. doi: 10.1016/j.biopsych.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufman J, Birmaher B, Brent D, et al. The Schedule for Affective Disorders and Schizophrenia for School Aged Children: Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Cooper Z, Fairburn CG. The Eating Disorder Examination: a semistructured interview for the assessment of the specific psychopathology of eating disorders. Int J Eat Disord. 1987;6:1–8. [Google Scholar]
- 30.Fitzsimmons-Craft EE, Ciao AC, Accurso EC, et al. Subjective and objective binge eating in relation to eating disorder symptomatology, depressive symptoms, and self-esteem among treatment-seeking adolescents with bulimia nervosa. Eur Eat Disord Rev. 2014;22:230–236. doi: 10.1002/erv.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsh R, Horga G, Parashar N, Wang Z, Peterson BS, Simpson HB. Altered activation in fronto-striatal circuits during sequential processing of conflict in unmedicated adults with obsessive-compulsive disorder. Biol Psychiatry. 2014 Apr 15;75(8):615–622. doi: 10.1016/j.biopsych.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsh R, Zhu H, Schultz RT, et al. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006 Nov;27(11):848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–46. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Arnold AE, Burles F, Bray S, Levy RM, Iaria G. Differential neural network configuration during human path integration. Front Hum Neurosci. 2014;8:263. doi: 10.3389/fnhum.2014.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002 Mar;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 36.Kahn I, Shohamy D. Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus. 2013;23(3):187–192. doi: 10.1002/hipo.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsh R, Tau GZ, Wang Z, et al. Reward-based spatial learning in unmedicated adults with obsessive-compulsive disorder. Am J Psychiatry. 2015 Apr 1;172(4):383–392. doi: 10.1176/appi.ajp.2014.13121700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutton RS, Barto AG. Reinforcement Learning: An Introduction. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- 39.Spiers HJ, Gilbert SJ. Solving the detour problem in navigation: a model of prefrontal and hippocampal interactions. Front Hum Neurosci. 2015;9:125. doi: 10.3389/fnhum.2015.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizumori SJ, Tryon VL. Integrative hippocampal and decision-making neurocircuitry during goal-relevant predictions and encoding. Prog Brain Res. 2015;219:217–242. doi: 10.1016/bs.pbr.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Hauser TU, Iannaccone R, Walitza S, Brandeis D, Brem S. Cognitive flexibility in adolescence: neural and behavioral mechanisms of reward prediction error processing in adaptive decision making during development. NeuroImage. 2015 Jan 1;104:347–354. doi: 10.1016/j.neuroimage.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meule A, von Rezori V, Blechert J. Food addiction and bulimia nervosa. Eur Eat Disord Rev. 2014 Sep;22(5):331–337. doi: 10.1002/erv.2306. [DOI] [PubMed] [Google Scholar]
- 43.Broft A, Shingleton R, Kaufman J, et al. Striatal dopamine in bulimia nervosa: a PET imaging study. Int J Eat Disord. 2012 Jul;45(5):648–656. doi: 10.1002/eat.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wierenga CE, Ely A, Bischoff-Grethe A, Bailer UF, Simmons AN, Kaye WH. Are Extremes of Consumption in Eating Disorders Related to an Altered Balance between Reward and Inhibition? Front Behav Neurosci. 2014;8:410–420. doi: 10.3389/fnbeh.2014.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt U, Marks IM. Exposure plus prevention of bingeing vs. exposure plus prevention of vomiting in bulimia nervosa. A crossover study. J Nerv Ment Dis. 1989;177(5):259–266. doi: 10.1097/00005053-198905000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714–723. doi: 10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray SB, Anderson LK, Cusack A, et al. Integrating Family-Based Treatment and Dialectical Behavior Therapy for Adolescent Bulimia Nervosa: Preliminary Outcomes of an Open Pilot Trial. Eat Disord. 2015;23(4):336–344. doi: 10.1080/10640266.2015.1044345. [DOI] [PubMed] [Google Scholar]
- 48.Kemps E, Tiggemann M, Marshall K. Relationship between dieting to lose weight and the functioning of the central executive. Appetite. 2005;45(3):287–294. doi: 10.1016/j.appet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007 Feb 13;104(7):2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


