Abstract
Background
Pragmatic trials comparing standard-of-care interventions may improve the quality of care for future patients, but raise ethical questions about limitations on decisional autonomy. We sought to understand how patients and physicians view and respond to these questions in the contexts of pragmatic trials and of usual clinical care.
Methods
We conducted scenario-based, semi-structured interviews with 32 patients with end-stage renal disease (ESRD) receiving maintenance hemodialysis in outpatient dialysis units and with 24 nephrologists. Each participant was presented with two hypothetical scenarios in which a protocolized approach to hemodialysis treatment time was adopted for the entire dialysis unit as part of a clinical trial or a new clinical practice.
Results
A modified grounded theory analysis revealed three major themes: 1) the value of research, 2) the effect of protocolized care on patient and physician autonomy, and 3) information exchange between patients and physicians, including the mechanism of consent. Most patients and physicians were willing to relinquish decisional autonomy and were more willing to relinquish autonomy for research purposes than in clinical care. Patients’ concerns towards clinical trials were tempered by their desires for certainty for a positive outcome and for physician validation. Patients tended to believe that being informed about research was more important than the actual mechanism of consent, and most were content with being able to opt out from participating.
Conclusions
This qualitative study suggests the general acceptability of a pragmatic clinical trial comparing standard-of-care interventions that limits decisional autonomy for nephrologists and patients receiving hemodialysis. Future studies are needed to determine whether similar findings would emerge among other patients and providers considering other standard-of-care trials.
Keywords: Ethics, Pragmatic Clinical Trials, Comparative Effectiveness Research, Qualitative Research
Introduction
Robust evidence to guide clinical decision-making among multiple treatment approaches is often lacking in routine clinical practice. Although comparative effectiveness research (CER) has the potential to address these widespread gaps in evidence-based knowledge in the usual care setting, evaluation of standard-of-care interventions in randomized clinical trials has recently come under scrutiny from the Department of Health and Human Services’ Office for Human Research Protections, leading to public consultation and debate among bioethics scholars (Anderson et al. 2015; OHRP 2014; Wilfond et al. 2013). Important ethical questions remain regarding traditional regulations for research involving standard-of-care interventions, especially given the challenge of creating uniform regulatory guidelines for a wide range of standard-of-care randomized controlled trials (RCTs) without empirical data to help guide their development (DHHS 2011; Faden et al. 2013; Halpern 2005; Kass et al. 2013; Kim and Miller 2015; Largent et al. 2011; Solomon and Bonham 2013).
In light of the public health benefits that CER offers, the National Institutes of Health, Patient-Centered Outcomes Research Institute, and other funding agencies have recently prioritized “pragmatic” clinical trials, particularly those conducted within “learning health care systems” – that is, within the context of patients’ usual care (Anderson et al. 2015; IOM 2007; IOM 2012). Pragmatic trials are those in which the hypothesis and study design are formulated based on information needed to make clinically relevant decisions (Anderson et al. 2015, Loudon et al. 2015). They seek to produce highly generalizable, real-world information about the effectiveness, benefits, and harms of treatment options to inform clinical decisions and practice guidelines. However, in order to prioritize evaluation of effectiveness over efficacy and generalizability, the designs of pragmatic trials often restrict autonomy of physicians to choose treatments they administer, and of patients to choose treatments they receive. Thus, despite great promise for improving the quality and costs of care, pragmatic trials raise important ethical questions that require reconsideration of traditional research regulations (Cho et al. 2015; Faden et al. 2013; Johnson et al. 2014; Kass et al. 2013; Largent et al. 2011; Menikoff 2013; Solomon and Bonham 2013). Many authorities have called for greater scrutiny of the issues underlying conceptual analyses of pragmatic trials (DHHS 2011; Garber and Tunis 2009; PIPC 2009; Platt et al. 2014; Solomon 2005). Such data may help to identify how patients and physicians address ethical challenges and whether proposals to surmount them align with their views.
A particular challenge in conducting pragmatic clinical trials that compare several approaches falling within the “standard-of-care” is whether informed consent, or simply patient notification that research is being conducted, is required. Participating in such trials does not place patients at risk from the interventions themselves, as the same risks would arise in usual care. However, such trials alter the process by which an individual’s care is selected, thereby limiting patients’ and physicians’ decisional autonomy. Thus, to the extent that patients and physicians may value such decisional autonomy, pragmatic trials may raise concerns for these stakeholders. Maintenance dialysis for patients with end-stage renal disease (ESRD) is a setting that lends itself well to the implementation of pragmatic trials because of the highly standardized approaches to care delivery and clinical monitoring. We conducted qualitative interviews of patients with end-stage renal disease and clinical nephrologists to advance understanding of (1) how patients and physicians value decisional autonomy, (2) how these valuations differ in the domains of research vs. clinical care, and (3) whether limitations on decisional autonomy ought to influence how investigators obtain informed consent or notify participants of the research.
Methods
Data collection occurred between April and November 2014. Two interviewers (one public health professional and one clinical research project manager) trained in qualitative research methods conducted individual, semi-structured interviews lasting approximately 30 minutes with nephrologists and with patients with ESRD receiving maintenance hemodialysis. Purposive, non-probability sampling was used to identify participants who varied in age, gender, race, and level of education. Sampling continued until theoretical saturation was reached – that is, until consecutive new interviews failed to reveal any new information. Eligible patients were 18 years or older, English-speaking, and with ESRD receiving care at one of four outpatient dialysis units in the Philadelphia region. Eligible physicians were practicing nephrologists treating adult patients in outpatient dialysis units in the U.S.
Nephrologists were identified using the American Society of Nephrology (ASN) member list; eligibility was restricted to U.S.-based physicians with an MD, MBBS, or DO degree. Information about the study was provided to nephrologists through email and paper mail, as well as via telephone calls. Interested nephrologists were directed to an online survey to determine eligibility. Eligible nephrologists who provided verbal informed consent participated in individual telephone interviews and received a $50 Amazon gift card for their participation.
Patients were recruited while receiving hemodialysis treatment and interviewed in-person. Dialysis unit facility administrators and nurse managers were contacted prior to the scheduled visit to approve or decline recruitment of individual patients, and to confirm that potential participants were English speaking and had cognitive capacity. Patients provided written informed consent, completed a demographic questionnaire, and participated in a scenario-based interview. Patients received $25 cash in exchange for their time and participation.
This study was approved by the University of Pennsylvania Institutional Review Board, the dialysis provider organization, and the dialysis unit medical directors. For interviews with patients and physicians, interviewers read aloud two standardized hypothetical scenarios: 1) a clinical trial in which dialysis units would be randomized to a standardized duration of hemodialysis sessions falling within the distribution of standard-of-care, and 2) a clinical care scenario in which the same standardized duration of hemodialysis sessions would be implemented unit-wide (Table 1). These two scenarios were created in light of current practice in the United States, in which the length of dialysis treatment sessions is highly variable across dialysis centers and physicians. Comparable language regarding the rationale and evidence base for the standardized duration of hemodialysis was presented to participants in both the research and clinical care scenarios, allowing participants’ views on the care delivered in each setting to be directly compared. The scenarios directed toward physicians contained higher-level medical terminology. Each scenario also included the provision that an alternate hemodialysis session duration could be prescribed for an individual patient if the physician disagreed with the standardized treatment time. The scenarios were modeled after the NIH-funded “Time to Reduce Mortality in End-Stage Renal Disease” (TiME) trial (NCT02019225) with three key differences, namely: 1) the TiME trial is restricted to incident patients, 2) the standardized length of hemodialysis sessions in the TiME trial is 4.25 hours (as opposed to 4.5 hours in the scenarios used for this study), and 3) for the TiME trial a telephone call is required in order for patients to opt out of sharing their data, but opting out of the intervention can occur through discussions with the treating nephrologist, while in the hypothetical scenario there is no distinction made between opting out of the intervention and opting out of data sharing.
Table 1.
Standardized Research and Clinical Care Scenario
| Scenario | Patient Population | Physician Population |
|---|---|---|
| Research Scenario | Currently there is no standard length for hemodialysis sessions in the U.S. Some small research studies suggest that longer dialysis sessions may help some people, but doctors really don’t know. I’d like you to imagine that doctors are now doing a large study. They are hoping to find out if slightly longer hemodialysis sessions help patients to live longer and feel better than shorter hemodialysis sessions. In this research study, there will be two groups of dialysis clinics. In one type of clinic, patients will receive care as they usually do, with doctors deciding the length of their dialysis sessions. In the other clinics, all patients will receive dialysis sessions of at least 4 ½ hours unless their doctor disagrees. Let’s imagine that you are just starting hemodialysis for the first time, and the clinic you go to is one of several hundred clinics participating in this study. On your first visit to the dialysis clinic, you are told about the research study. You are given information about the study and a number to call if you don’t want to participate. You do not yet know which treatment group your clinic is in. This will be decided by the doctors doing the research so that all doctors can learn how long dialysis sessions should be. |
Currently there is no standard length for hemodialysis sessions in the U.S. Some small research studies suggest that longer dialysis sessions may help some patients, but the data are inconclusive. I’d like you to imagine that investigators are now conducting a large research study, involving several hundred outpatient hemodialysis facilities. The investigators are hoping to find out if slightly longer hemodialysis sessions, designed to remove fluid more slowly, help patients to live longer and feel better than shorter hemodialysis sessions. In this research study, dialysis facilities will be randomized to two groups. In some facilities, patients will receive care as they usually do, with nephrologists deciding the length of their dialysis sessions. In the other facilities, all patients will receive dialysis sessions of at least 4 ½ hours unless their nephrologist disagrees. Let’s imagine that the outpatient dialysis facility where you treat patients is participating in this study. On admission to the dialysis facility, patients are provided with written notification about the study and given a number to call if they don’t want to participate. In this IRB-approved study, patients do not provide written informed consent. |
| Clinical Care Scenario | Currently there is no standard length for hemodialysis sessions in the U.S. Some small research studies suggest that longer dialysis sessions may help some people, but doctors really don’t know. Let’s imagine that you are just starting hemodialysis for the first time, and the doctors at the clinic you go to have just started a new practice. On your first visit to the dialysis clinic, your health care team tells you about how the clinic’s practice has changed. Until now, individual doctors decided how long patients’ sessions would be. But now, all new patients in the clinic will receive dialysis for a set amount of time - at least 4½ hours each session unless their doctor disagrees. This new practice was chosen because doctors think it will help most of their patients feel better and live longer, although that might not be true for everyone. Remember, the reason the doctors are doing this new practice is not to help find out whether longer sessions help dialysis patients. Rather, they are doing this new practice because they think it will help patients treated at this clinic. |
Currently there is no standard length for hemodialysis sessions in the U.S. Some small research studies suggest that longer hemodialysis sessions, designed to remove fluid more slowly, may help some patients to live longer and feel better, but the data are inconclusive. Let’s imagine that the outpatient hemodialysis facility where you treat patients has just started a new clinic practice. Until now, individual nephrologists decided how long patients’ sessions would be. But now, all new patients are told on admission to your facility that they will receive dialysis for a set amount of time - at least 4½ hours each session unless their nephrologist disagrees. Experts believe that this new practice will help most patients feel better and live longer, although that might not be true for everyone. Remember, this is a new clinic practice and not a research study. |
The order in which the two scenarios were presented to participants was randomized to mitigate potential ordering effects. The interviewer asked all participants to share their initial thoughts and reactions to the scenario presented, followed by open-ended questions exploring both positive and negative features of the scenario (e.g., features they liked and disliked, potential benefits or concerns, positive and negative aspects of patient notification, etc.). After eliciting each participant’s viewpoints, the interviewer followed with targeted probes.
All interviews were audio recorded, professionally transcribed verbatim, and verified for accuracy. De-identified transcripts were imported into NVivo10, which was used to store and organize data, code content and track emerging themes. Using a modified grounded theory approach and open-coding, the study team developed a coding structure and coding dictionary for patients and physicians. The first three transcripts for both patient and physician interviews were coded by two independent coders and analyzed for inter-rater (inter-coder) reliability. Coding agreement below 85% was evaluated and adjudicated and the study team discussed discrepancies. Double coding ensued for every fourth interview. Additionally, two independent coders reviewed all interview transcripts for the gestalt directional response (positive, mixed, or negative) to each hypothetical scenario and agreement was evaluated and adjudicated (κ= .75). We achieved theoretical saturation on all major themes, and analyzed the data for recurring themes and significant thematic links.
Results
Of the 35 eligible patients approached, 32 patients (91%) consented to be interviewed. Of the 470 ASN nephrologists who were cold contacted through paper mail and email, 12 agreed to be interviewed following initial contact. Subsequent telephone follow-ups resulted in 36 direct contacts (13 interested, 12 more interviewed, 11 declined), 247 indirect messages (voicemail, receptionist, etc.), 61 found ineligible, and 50 non-contactable telephone numbers. Sixty-four nephrologists were not contacted by telephone as theoretical saturation had been reached at 24 interviews. Thus, a total of 56 open-ended semi-structured interviews were analyzed (32 patients, 24 nephrologists). Tables 2 and 3 describe the demographic composition of the sample.
Table 2.
Patient Demographics
| Gender | N (%) |
|---|---|
| Male | 16 (50%) |
| Female | 16 (50%) |
| Age | |
| 30–49 | 6 (18%) |
| 50–69 | 13 (41%) |
| 70+ | 13 (41%) |
| Race | |
| Black | 20 (63%) |
| White | 11 (34%) |
| Other | 1 (3%) |
| Ethnicity | |
| Hispanic | 2 (6%) |
| Non-Hispanic | 30 (94%) |
| Education | |
| < High School | 4 (13%) |
| High School/GED | 10 (31%) |
| Some College | 9 (28%) |
| College | 6 (19%) |
| Post College | 3 (9%) |
| Marital Status | |
| Married | 13 (41%) |
| Not married | 19 (59%) |
| Employed | |
| Full-time | 1 (3%) |
| Part-time | 5 (16%) |
| Retired/Not employed | 26 (81%) |
| Dialysis Session Duration | |
| < 4 hours | 8 (25%) |
| 4 hours | 22 (69%) |
| > 4 hours | 2 (6%) |
| Years of Dialysis Treatment | |
| <1 year | 10 (31%) |
| 1–3 years | 9 (28%) |
| 4–6 years | 8 (25%) |
| 6+ years | 5 (16%) |
| Hospitalized in Previous Year | |
| None | 13 (41%) |
| 1 time | 9 (28%) |
| 2 times | 3 (9%) |
| 3–5 times | 5 (16%) |
| > 5 times | 2 (6%) |
| ICU in Previous Year | |
| Yes | 3 (9%) |
| No | 29 (91%) |
Table 3.
Physician Demographics
| Gender | N (%) |
|---|---|
| Male | 19 (79%) |
| Female | 5 (21%) |
| Age | |
| 30–39 | 13 (54%) |
| 40–49 | 6 (25%) |
| 50–59 | 3 (13%) |
| 60–69 | 2 (8%) |
| Race | |
| Black | 0 (0%) |
| White | 11 (46%) |
| Asian | 11 (46%) |
| Other | 2 (8%) |
| Ethnicity | |
| Hispanic | 3 (12%) |
| Non-Hispanic | 21 (88%) |
| Years Since Fellowship | |
| < 5 years | 10 (42%) |
| 5–10 years | 7 (29%) |
| > 10 years | 7 (29%) |
| % Time in Outpatient Dialysis Setting | |
| 20% or less | 5 (21%) |
| 21–40% | 10 (41%) |
| 41–60% | 4 (17%) |
| 61–80% | 4 (17%) |
| 81–100% | 1 (4%) |
| Practice Setting | |
| Academic practice | 12 (50%) |
| Community practice | 12 (50%) |
| Practice Location | |
| Urban | 15 (62%) |
| Suburban | 5 (21%) |
| Rural | 3 (13%) |
| Missing | 1 (4%) |
| Practice Size (# Nephrologists) | |
| Small (1–4) | 6 (25%) |
| Medium (5–9) | 8 (33%) |
| Large (10 or more) | 9 (38%) |
| Missing | 1 (4%) |
| Research Experience (#Trials as an Investigator) | |
| 0 | 7 (29%) |
| 1–2 | 5 (20%) |
| 3–5 | 6 (25%) |
| 6–10 | 3 (13%) |
| More than 10 | 3 (13%) |
We identified emergent codes for patients and nephrologists and organized them into the three major themes: 1) research value, 2) protocolized care and patient/physician autonomy, and 3) information exchange between patients and physicians and the mechanism of consent. The sections below summarize the findings from patients, followed by findings from physicians. We have included a subset of the most salient themes, and not an exhaustive list of all themes that arose. Primary data are utilized to support the summative statements, and the scenario (research or clinical) is identified in parentheses following all quotations.
Patient Findings
Research Value
Few patients had participated in research studies previously, yet almost all patients saw research as valuable and many said they would like to participate in research (either in the hypothetical study presented, or other research studies) as a form of altruism. Participants expressed hope that this hypothetical research study, or other research studies in general, would improve patient care and outcomes. However, patients also conveyed various qualifying factors for their own participation such as certainty of personal benefit or the absence of harm. A minority expressed concern about motivations behind research, such as the promotion of interests of insurance companies or other financial drivers of care, but these were commonly unrelated to the hypothetical research scenario presented to them. Almost no patients spontaneously offered their perspectives on data collection and privacy.
I don’t know why they haven’t done this before. To me it’s really ridiculous for people to be getting treatments and they don’t know, if the doctor doesn’t know the difference between a 4 hour session and the lesser. I don’t understand that. (Research)
I would want to know how this research is going to affect me, good or bad. If it’s good I want to go with it. If it’s bad I might have some reservations about it. But it all depends on if I have to suffer to make someone else’s life better then maybe I might consider it. (Research)
Here’s the thing about this. People complain. They don’t want to be on the machine but yet they don’t want to die. So research study is to show them what they actually have to do if they want to be alive. The doctors will find out exactly what’s going to keep us alive. (Research)
Protocolized Care and Decisional Autonomy
Both the research and clinical care scenarios included a protocolized approach to hemodialysis duration with the potential to limit patient and physician autonomy in medical decision-making. As patients reflected on the two scenarios, they expressed a range of views in response to the concept of protocolized care and its sequelae. Several patients stated that setting a protocolized time for dialysis treatment in either the research or clinical context would not deviate substantially from current practice, stating that their dialysis unit already implemented protocols for many aspects of care. Those who expressed this also believed that protocolized care would not dramatically affect patients or physicians.
I’m very used to things being standard with dialysis. (Clinical)
[Setting the length of the dialysis sessions] doesn’t matter to me. I don’t have a problem with that. I don’t think any doctor should have a problem with that either, really. It doesn’t affect them at all. (Research)
Several others (and some of the same patients) expressed concerns about the use of a protocol. A majority of these concerns were connected to the absence of individualized treatment and lack of consideration of patient-level factors such as age, size, or medical condition. Most patients were also concerned about the potential impacts or burdens on their life such as increased fatigue, logistical consequences of increasing time, and lifestyle limitations. A minority of these concerns pertained to systems-level factors such as insurance companies or other financial drivers of care. A majority of patients believed that physicians should treat patients individually according to their specific needs and preferences, and that physicians should have the ability to modify care on an individual basis.
I just don’t like the fact that everybody is just lumped into the same basket. Patients are individuals. I think that whatever they’re doing should be tailored to them. Dialysis lengths are related to sometimes possibly to what race you are, what sex you are, how much you weigh, how you’re responding. So how can they say everybody is going to go on the same thing? (Clinical)
Patients described trust as mitigating factor for their concerns about the loss of decisional autonomy. Trust in their nephrologist, and the healthcare system in general, together with a desire for physicians to have autonomy in decision-making about their care led most patients to accept protocolized care as described in the scenarios should their physician agree with and justify its use. Conversely, patients said they would not accept protocolized care in either research or clinical scenarios if they did not receive their individual physician’s validation. A minority of patients made specific mention of their own autonomy in decision-making about their care. However, of those who did, most said they would relinquish their own autonomy to follow their individual physicians’ recommendations.
If [the doctor] says that I think you should go on 4 ½ hours then I would say fine. But I would definitely go with [the doctor’s] advice rather than the clinic’s advice or policy, whatever they have. (Clinical)
Once my doctor okays it and he evaluates it and says well it wouldn’t hurt then I probably would participate. But I must be informed by my doctor. I don’t do anything against his wishes. (Research)
Information Exchange and Mechanism of Consent
Patients discussed their informational needs, most of which included a desire to be informed of potential risks and/or benefits in either research or clinical care scenarios. Patients also discussed the importance of who would provide information, when it would be provided, and the method of delivery. While few patients voiced concerns about specific medical risks in these scenarios, patients usually expressed a desire for physician assurance that the treatment might benefit them directly and not cause harm. Many patients also wanted more information about the reasons behind the treatment time, such as existing evidence that motivated the research or clinical change.
Well, I think that the doctor would, someone would have to give you a reason why it’s 4 ½ hours instead of 4. I don’t know whether it’s, I guess the facility would have the information to let you know but I would think you would want your doctor to be informed or told by and then he or she can explain it to the patient. (Clinical)
This is only a half hour so it’s not a big deal but I think people should know and the person should know both the positive and the negative sides of it. (Research)
I would be willing to do the research. It puts patients at actual risk but it’s not known whether they provide any extra benefits. If you don’t really know if it’s going to, but that’s what research is all about is to find out, I guess. Yeah, I would go along with it. (Research)
In an effort to elicit objective responses to the hypothetical scenarios, the interviewer asked open-ended questions without explicitly soliciting thoughts about the method of consent. However, many patients addressed this on their own accord, and for these patients, the interviewer systematically probed further. Most of these patients were less concerned about how consent procedures took place as long as they were given information about the research study and the opportunity to decline treatment assignment (opt-out).
Some participants described feeling comfortable with the hypothetical scenarios and did not question the means by which patients were notified; others thought they might have reservations about specific aspects of information exchange, including their ability to decline the standardized hemodialysis treatment time in both scenarios. Some participants wanted to know the degree to which a protocolized approach might limit their options. For instance, some participants questioned whether they would be forced into care they did not want or effectively be forced to transfer to a different dialysis unit. These sentiments were expressed more frequently in the clinical care scenario, as the majority of patients expressed satisfaction with the mechanism to opt-out of the research study if they so desired (by calling a telephone number).
I would want to know what are the benefits and what are the downfalls of an extra half hour and just that a clinic decides that there’s another half hour would not be overwhelmingly convincing. (Clinical)
I’ve been coming for 3 hours and now you’re going to change it for 4 ½? I mean what the heck is going on here? I would want a reason for that more than them just saying this is how we’re going to do things from now on. That’s really how they do things a lot in this system. (Clinical)
That’s a good option [to call a number if they don’t want to participate in the study] also because, again, it tells them, hey, it’s nothing that you are stuck to do. If you don’t feel like it you don’t have to do it and that’s a good thing. I don’t know when you call that number what happens but I think it’s a good thing, yes. It’s like informed. (Research)
I think its fine because I think you’re given an out if you want it. It’s not forced. (Research)
I have a problem with being put in something and I’m not aware of it. You know what I mean. Just putting me in it and I didn’t know I was in it and I would feel like that they wasn’t being real with me. But putting me in and I’m aware of it, yes, I have no problem with it. I’ve got to know that I’m being researched or something. (Research)
Nephrologist Findings
Research Value
There was near consensus regarding the evidence to support increased dialysis time and many nephrologists cited specific studies to suggest that longer dialysis leads to improved patient outcomes. Therefore, many stated that they did not foresee harm in providing a protocolized hemodialysis treatment time of 4.5 hours in either the research or clinical care context. Moreover, a majority believed that the hypothetical research study if conducted could provide valuable data and potentially incentivize patients to adhere to their prescribed dialysis time, thereby improving patient care and outcomes.
I think we would definitely want to contribute to that body of literature and then also help to affirm our clinical suspicion and judgment that we’ve been doing. (Research)
I believe that we have four decades of observational data showing that outcomes are better with longer treatment times…The reason that it would be useful to do an RCT would be to have clear data to convince insurance companies to pay for longer treatment times. (Research)
I think that more time is better and I would want to offer that to my patients. Also, if it were an opportunity to get them to a longer time so maybe being a part of research and they would be doing it for a different reason other than just because I asked them. So they might be more likely to sign up for that if it had a bit of a shine on it because it was a research study as opposed to just my request. (Research)
I think if that’s the new clinic practice they’d better have the research information and data to say this is our new clinic practice and this is why. It’s because you’re going to feel better, you’re going to live longer, you’re not going to end up in the hospital. There’s got to be a good reason why. (Clinical)
Protocolized Care and Decisional Autonomy
Like patients, nephrologists expressed a range of responses to protocolized care as described in the hypothetical scenarios. While some nephrologists expressed concerns about limitations on physician autonomy and the need for individualized treatment, a majority of nephrologists said they felt comfortable with the standardization of treatment time, and were largely supportive of protocolized care so long as some flexibility to individualize remained. Many nephrologists gave examples of existing protocols in their dialysis units, including length of treatment and other standardized treatment practices, and several nephrologists discussed the potential benefits of creating such protocols.
I prefer individualized care because I believe that humans are not like laboratory animals. We’re not all the same. I feel that protocols are good to use as guidelines if needed but I think each patient’s care should be individualized. So I’m not a big fan of having protocols without giving, following protocols blindly without taking into account each patient’s individual condition. (Clinical)
I know there is some academic evidence suggesting a longer dialysis would be better. I like to keep my patients minimum of four hours from the beginning unless they make a choice not to do that. I try to keep it at four hours….I think it’s okay not to offer the patient consent because it’s pretty much doing the standard-of-care we are just lengthening some hours and they have the choice not to do that. (Research)
Many physicians believed that a major challenge to longer dialysis in a clinical or research setting was that patients may not be willing to increase dialysis time. Most nephrologists discussed how this type of protocolized care could be useful, particularly for new patients as depicted in the hypothetical scenarios, to gain patient acceptance and agreement to increase dialysis time, and consequently improve quality of care. Concerns revolved mainly around treating patients who might need individualized care due to age, gender, BMI, or other individual characteristics. However, of the nephrologists who expressed these concerns, nearly all believed that protocolized care in the context of the research or clinical scenario would be beneficial to most of their patients. A majority stated that they would be comfortable participating in either the research or clinical scenario so long as physicians and patients were provided some flexibility or mechanism to opt-out.
I think it’s a wonderful idea because all of us try to push for a longer dialysis but most of the patients do not like it. (Research)
I agree that longer treatment times would be better. However, I feel there would be a lot of resistance with patients. It’s difficult to convince people to stay that long. So I feel like while it definitely might be best for some patients I think it’s going to have some difficulty with some patients accepting that. (Clinical)
Information Exchange and Mechanism of Consent
Physicians expressed a range of views with regard to the exchange of information in both scenarios. Most physicians said patients should be adequately notified and provided information about the rationale for implementing a protocolized approach to hemodialysis time in both research and clinical contexts.
I think there are certain studies I think you need to have written notification and you need to be more vigilant and explain more and things. But 4 ½ hours of dialysis is not truly so far away from what the standard-of-care is. Really you’re not going to hurt anyone. (Research)
I think notifying the patients and as long as the doctor is convinced it’s the right thing to do for the patients I don’t see any ethical dilemma with telling them you need 4 ½ hours and giving them notice and the ability to sort of not do it, which they have for any treatment we offer. Every patient has the right to deny. I don’t see why that would be a problem. (Research)
As long as they [patients] know that you’re pulling for them then you can do anything and you can explain anything to them. They have to know that you’re going to keep showing up and then you work with them and you talk about it. (Clinical)
The fact that this is just a new practice rather than a clinical trial it’s kind of difficult to stomach that because you don’t necessarily know if what you’re doing really is of benefit or not. So I think that you would have to inform the patient… Inform them that we’re doing things for a longer duration without knowing if it’s of benefit or not and then the ability to tailor to patients who may need it would be great but then what parameters are you using to tailor it exactly. (Clinical)
Most nephrologists believed that if an appropriate opt-out mechanism were in place for both physicians and patients, as described in the scenarios, then there would be no ethical concerns.
I think that just gives a patient a feel that they’re not bound to any decision they have made, that they have the choice to walk away if they choose. So I think it gives them a lot more comfort and it might help to enroll patients that they can feel it’s easy enough to opt out of this study if they choose to rather than feel that they’ve signed some consent and then they feel obligated to have to stay in the study reluctantly. (Research)
A minority of physicians, however, believed that defaulting patients into treatment in either research or clinical scenario could be unethical or impractical.
You can’t demand something of them and say, okay, now you’ve got to call this phone number or we’re going to force you to have 4 ½ hours. They shouldn’t have to call a phone number. They don’t have to do anything. That is against ethics. (Research)
I think it’s going to be very hard to enforce a policy and patients are going to go to other clinics so that they don’t have to do that time. You can’t enforce or force a patient. Just like patients come off their dialysis machines before they want, you can’t enforce them to that they have to stay for their full time. They’ll sign off AMA [against medical advice]. ‘Doc, nurse, get me off’. (Clinical)
Research vs. Clinical Care
All patients and physicians were directly asked to compare the research and clinical care scenarios and discuss similarities, differences and their perspectives on each. The vast majority of patients stated that they felt “the same” about the two hypothetical scenarios and tended to respond sparsely to the question of differentiation. The issue of increased time was routinely identified as the main similarity between the two scenarios, but almost no patients discussed any substantive differences arising from standardizing hemodialysis times by virtue of a clinical protocol as opposed to routine clinical care. Physicians on the other hand, readily discussed the differences between the two scenarios, with or without prompting. A majority of physicians had preference for the research scenario over the clinical care scenario, even though many still had some concerns about both, as described above. All physicians who preferred the research scenario believed that rigorous empirical data could be used to improve patient care and gain acceptance for increased dialysis treatment time. Most of these physicians also preferred the research scenario because of the opt-out procedures, which they favored over a clinical “mandate” that might diminish autonomy for both patients and physicians.
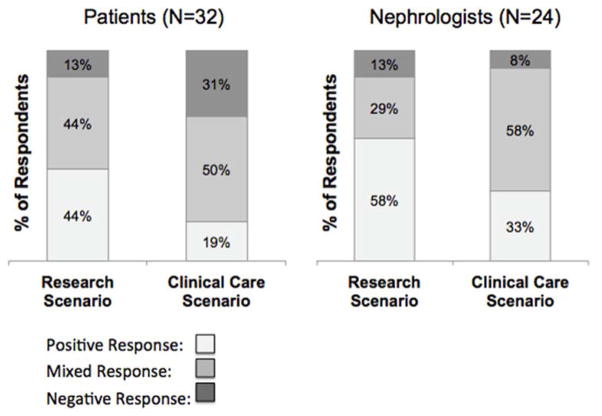
To assess and compare the acceptability of each scenario, full transcripts were coded for the gestalt directional response to the research scenario and to the clinical care scenario. Each patient and physician was identified as having a positive, mixed, or negative response to each scenario, and coded as such by two independent coders. As demonstrated in Figure 1, a minority of patients and physicians expressed resolutely negative responses to the research or clinical care scenarios, and fewer patients responded negatively to the research scenario than the clinical scenario. For both patients and physicians, a higher percentage of participants responded positively to the research scenario, as compared to the clinical care scenario. Illustrative quotations that demonstrate these response categories can be found in Table 4.
Figure 1.
Directional Response to Research and Clinical Care Scenarios
Table 4.
Illustrative Quotes for Directional Response Categories (Positive, Mixed, Negative)
| Directional Response | Scenario | Quote | Patient or Physician |
|---|---|---|---|
|
| |||
| Positive Response | Research Scenario | I would be willing to, really excited kind of, that they’re actually doing a study. | Patient |
| I think definitely I would want to include [my patients] because I feel it theoretically makes sense that longer dialysis may be beneficial for slower fluid removal. I would like to know what subset of patients that did make sense for, if it made sense for anyone, and was actually clinically beneficial. I think enrolling patients in this study would help. | Physician | ||
|
| |||
| Mixed Response | Research Scenario | When I think about the length of the dialysis I mean there are pluses and there are minuses. The pluses is to live longer and feel better and the minus is the excessive amount of time. | Patient |
| That would be the only thing that I would be hesitant if there was not a provision to decrease dialysis then maybe I would be hesitant. But honestly I still think I would do it. | Physician | ||
|
| |||
| Negative Response | Research Scenario | No, because like I said, being in this study they don’t really know. I’m not going to say you’re going to be a guinea pig but (laughs) that’s almost like being a guinea pig. | Patient |
| You can’t say you’re participating in a study and all of you are now going to go to 4 ½ hours. Believe me, the first thing they would do was say I what? No, no, no, no. I’m going to another unit, sorry. You’d have a mass defection. | Physician | ||
|
| |||
| Positive Response | Clinical Scenario | I think it would really be benefitting me. Number one, when I first got on it was 4 ½ hours and I didn’t question why. But then I began to do some research myself. So in doing the research it has enhanced my longevity because of when you are getting this pulled off you your heart is in better condition. | Patient |
| I think that that’s ultimately much more beneficial. The longer and slower you can remove the fluid I think is generally more beneficial for cardiac stunning. So I think that it’s a very good concept and I would like to try to do that with patients. | Physician | ||
|
| |||
| Mixed Response | Clinical Scenario | It may be able to process more waste, maybe take more fluid off, get your numbers a little bit more balanced. Those would probably be the biggest things that I could see. I know they can adjust this machine so you’re already going to be set for a certain amount of waste, certain amount of fluid. So I don’t know would the 4 ½ hours be beneficial? It would just be more time on this machine, away from your family. So I wouldn’t, I don’t know about that part. | Patient |
| I don’t think there is data to support it so the fact that this is just a new practice rather than a clinical trial it’s kind of difficult to stomach that because you don’t necessarily know if what you’re doing really is of benefit or not. | Physician | ||
|
| |||
| Negative Response | Clinical Scenario | I don’t like the fact that they just sort of foist this upon you. | Patient |
| I wouldn’t be very happy about it especially if it’s coming from the clinic itself not from the doctors. I would want to know why. That would raise my suspicions. Are they looking for a couple extra bucks? | Patient | ||
| I feel like our clinical judgment would be compromised with using this protocol. | Physician | ||
| You just cannot show up at the dialysis unit and he is being told by the dialysis nurses and the dialysis staff that this is what should be a good practice. So it should all start with the physician. So the physician will decide if it’s going to be 4 ½ hours or not. | Physician | ||
Discussion
The ethical oversight of comparative effectiveness research conducted within “learning health care systems” is a topic of considerable interest given the need to more efficiently study key questions in healthcare delivery and the new options for doing so with the advent of electronic health records (Largent et al. 2011; Platt et al. 2014; Solomon and Bonham 2013). Several authors have called for revising traditional ethics regulations to accommodate the challenges and opportunities of CER within learning health care systems (Faden et al. 2013; Kass et al. 2013) and have proposed frameworks for evaluating studies that directly compare interventions falling within the standard-of-care (Faden et al. 2013; Kass et al. 2013; Kim and Miller 2015; Largent et al. 2011; Solomon and Bonham 2013). However, these models have largely been developed without evidence of the perspectives of key stakeholders.
This qualitative study of a diverse sample of nephrologists and ESRD patients identified several themes related to ethical considerations in implementing CER in usual care settings. Several key findings emerged from our exploration of how patients and physicians view limitations on decisional autonomy, whether these views differ in the domains of research versus clinical care, and whether limitations on decisional autonomy ought to influence how investigators obtain informed consent or notify participants of research.
First, we found that patients and physicians are willing to relinquish autonomy around dialysis time, at least as much in the context of research as in clinical care, and that they would allow some limitations on individual-patient decision-making in research studies. For both patients and physicians, this comfort seemed to stem, in large part, from the perceived value of research and the belief that the knowledge to be gained justified such limitations. For patients, the level of trust they had in their physician and health system also seemed to be an important contributor. It is possible that the acceptability to ESRD patients and physicians of limiting decisional autonomy is related to the extent to which many dialysis units already protocolize several aspects of patient care. For example, many physicians believed that the trial and clinical scenarios presented would have a negligible impact on their clinical practice and would still allow for independent decision-making. However, such familiarity with protocolized care in the clinical setting would not be expected to preferentially influence views regarding similar limitations in the research setting.
Second, although most patients indicated a desire for individualized treatment, patients and physicians alike expressed greater overall acceptance of protocolized care delivered in a research context versus a clinical context. Indeed, few patients or providers expressed negative views of the proposed research study despite its limitations on individualized patient treatments and lack of written informed consent. Together, these findings suggest that although patients value individualized treatment when all else is equal, clinical trials that employ pragmatic designs for a specific purpose may be quite acceptable to patients and physicians alike. Further, these data suggest that research participants may not perceive such studies as generating higher risks than ordinary care (Faden 2013; Platt 2014).
Third, we found that patients and providers often differed in some of their views regarding the role and mechanism of informed consent. Patients expressed a desire to receive notification and information about their treatment for both research and clinical care. This is noteworthy in light of the dramatic difference in current disclosure in research activities versus clinical practice. However, patients did not display strong preferences regarding the mechanism of consent and, because they retained their rights to decline participation, did not oppose an opt-out model of consent. Future research is needed to determine whether this general acceptance of opt-out consent was specific to the scenario posed in this study or may be extrapolated to other standard-of-care interventions. A recent nationwide survey found that more members of the public stated a preference for specific consent rather than notification (Nayak et al. 2015). However, these results are difficult to interpret because the logistical and other tradeoffs required in pursuing informed consent were not revealed to participants, and because the participants were individuals with generally good health rather than patients who would be eligible for standard-of-care treatment trials.
For some physicians, the lack of traditional, prospective, opt-in consent was seen as a barrier to research conduct. Nonetheless, several of the physicians who expressed concerns about opt-out approaches also readily described the potential benefits of pragmatic trials, and most said they would participate in and encourage such research despite their concerns. These seemingly divergent findings may be attributable to physicians believing that traditional research regulations are too stringent to be applied to standard-of-care research, while also feeling bound to uphold such perceived requirements. Further research among physicians about their views on notification and consent should therefore clarify that the proposed studies are acceptable to an IRB so as to enable physicians to more freely indicate their views.
These findings should be interpreted in light of several limitations. First, these data were collected within the context of a single hypothetical scenario (changing the duration of hemodialysis sessions) with samples of ESRD patients and nephrologists. Although this focus enabled participants to articulate views on matters that were most salient to them, the generalizability of these findings to other clinical contexts is uncertain. Second, participants’ responses to the hypothetical scenarios used in this study may not precisely reflect their views if truly facing these same research and clinical scenarios. Third, although patients’ familiarity with hemodialysis enabled them to speak informatively, it may have influenced certain responses. Indeed, many physicians and patients suggested that new, or “incident,” hemodialysis patients might be more receptive to the longer prescribed treatment times discussed in the research and clinical scenarios than were patients who were already familiar with somewhat shorter treatment sessions.
Fourth, when asked directly, most patients did not identify substantive distinctions between the research and clinical scenarios. Although many of their comments suggested that their views of the two scenarios were similar because the most important elements to them, such as the dialysis duration, were indeed the same in both, we cannot rule out the possibility that some patients may have misunderstood the differences in the goals of research and clinical care. Indeed, patients’ lack of exposure to research may have impaired their ability to distinguish between the two scenarios. Fifth, although we sampled purposefully within the participating dialysis units to ensure that age and ethnicity were representative of patients on dialysis in the same regional network (age: 62.5 vs. 62.3 years; ethnicity: 6.0% vs 4.6% Hispanic or Latino), the race profile of respondents reflects the demographics of a mainly inner-city dialysis population. Additionally, although we sought a nationally representative sample of nephrologists with a range of research experience, and from a variety of practice settings, the physician results may be biased due to self-selection into our study.
Sixth, this study did not afford the opportunity to explore the level of risk patients would accept in participating in usual care trials. The observation that few patients even mentioned risk as a relevant consideration does not mean risk is irrelevant to patients, but did diminish our opportunities to probe its importance. Finally, like most qualitative studies, this work was designed to be hypothesis-generating and to identify the range of factors affecting willingness to participate in research studies. As such, we cannot quantify the relative importance of each factor identified on patients’ or physicians’ views. However, this investigation has informed a larger study currently underway using conjoint analysis to assess the relative influences and importance of different trial elements on participants’ willingness to participate in CER.
In summary, these data from patients and physicians augment understanding of how these stakeholders view and respond to restrictions on decisional autonomy in research and clinical care. Although there has been an increase in the conduct and evaluation of CER, important questions remain regarding regulatory guidance and the variability of oversight (Anderson et al. 2015). These data may usefully inform the development of such regulations (DHHS 2011; Faden et al. 2013) by suggesting that, at least in the context of standard-of-care trials among patients receiving dialysis, patients are generally accepting of restrictions on their decisional autonomy and, when necessary to achieve the goals of the study, agreeable to simple notification rather than formal consent. Future research is needed to explore the generalizability of our findings in other clinical populations and contexts, and to quantify how stakeholders view the potential tradeoffs between the rigor of consent and the value of research in low-risk settings. Nonetheless, the current findings are consistent with a growing body of evidence that suggests that new regulations for standard-of-care research may permit different standards of consent than required in higher-risk studies such research promotes improved quality and costs of care.
Contributor Information
Ashley Kraybill, Email: akray@mail.med.upenn.edu.
Laura M. Dember, Email: Laura.Dember@uphs.upenn.edu.
Steven Joffe, Email: joffes@exchange.upenn.edu.
Jason Karlawish, Email: Jason.Karlawish@uphs.upenn.edu.
Susan S. Ellenberg, Email: sellenbe@mail.med.upenn.edu.
Vanessa Madden, Email: vmadden@mail.med.upenn.edu.
References
- 1.Anderson ML, Califf RM, Sugarman J. Ethical and regulatory issues of pragmatic cluster randomized trials in contemporary health systems. Clinical Trials. 2015;12(3):276–86. doi: 10.1177/1740774515571140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho MK, Magnus D, Constantine M, et al. Attitudes toward risk and informed consent for research on medical practices: A cross-sectional survey. Annals of Internal Medicine. 2015;162(10):690–6. doi: 10.7326/M15-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Health and Human Services (DHHS) Human subjects research protections: Enhancing protections for research subjects and reducing burden, delay, and ambiguity for investigators. 2011 Available at: http://www.gpo.gov/fdsys/pkg/FR-2011-07-26/pdf/2011-18792.pdf.
- 4.Faden RR, Kass NE, Goodman SN, Pronovost P, Tunis S, Beauchamp TL. An ethics framework for a learning health care system: A departure from traditional research ethics and clinical ethics. Hastings Center Report. 2013;43(1):S16–S27. doi: 10.1002/hast.134. [DOI] [PubMed] [Google Scholar]
- 5.Garber AM, Tunis SR. Does comparative-effectiveness research threaten personalized medicine? New England Journal of Medicine. 2009;360(19):1925–1927. doi: 10.1056/NEJMp0901355. [DOI] [PubMed] [Google Scholar]
- 6.Halpern SD. Towards evidence based bioethics. British Medical Journal. 2005;331(7521):901–903. doi: 10.1136/bmj.331.7521.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine (IOM) The learning health care system workshop summary. Washington, DC: National Academies Press; 2007. Available at: http://www.ncbi.nlm.nih.gov/books/NBK53494/ [PubMed] [Google Scholar]
- 8.Institute of Medicine (IOM) Best care at lower cost: the path to continuously learning health care in America. Washington, DC: National Academies Press; 2012. Available at: http://www.iom.edu/Reports/2012/Best-Care-at-Lower-Cost-The-Path-to-Continuously-Learning-Health-Care-in-America.aspx. [PubMed] [Google Scholar]
- 9.Johnson KE, Tachibana C, Coronado GD, et al. A guide to research partnerships for pragmatic clinical trials. British Medical Journal. 2014;349:g6826. doi: 10.1136/bmj.g6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kass NE, Faden RR, Goodman SN, Pronovost P, Tunis S, Beauchamp TL. The research-treatment distinction: A problematic approach for determining which activities should have ethical oversight. Hastings Center Report. 2013;43(1):S4–S15. doi: 10.1002/hast.133. [DOI] [PubMed] [Google Scholar]
- 11.Kim SYH, Miller FG. Varieties of standard-of-care treatment randomized trials, ethical implications. Journal of the American Medical Association. 2015;313(9):895–896. doi: 10.1001/jama.2014.18528. [DOI] [PubMed] [Google Scholar]
- 12.Largent EA, Joffe S, Miller FG. Can research and care be ethically integrated? Hastings Center Report. 2011;41(4):37–46. doi: 10.1002/j.1552-146x.2011.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 13.Loudon K, Shaun T, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. British Medical Journal. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 14.Menikoff J. The unbelievable rightness of being in clinical trials. Hastings Center Report. 2013;43:S30–S31. doi: 10.1002/hast.136. [DOI] [PubMed] [Google Scholar]
- 15.Nayak RK, Wendler D, Miller FG, Kim SYH. Pragmatic randomized trials without standard informed consent?: A national survey. Annals of Internal Medicine. 2015 doi: 10.7326/M15-0817. E-pub ahead of print. Available at: http://annals.org/article.aspx?articleID=2398906. [DOI] [PMC free article] [PubMed]
- 16.OHRP and standard-of-care research. New England Journal of Medicine. 2014;371:2125–2126. doi: 10.1056/NEJMe1413296. [DOI] [PubMed] [Google Scholar]
- 17.Partnership to Improve Patient Care (PIPC) Individualized patients, personalized care. 2009 Available at: http://www.pipcpatients.org/issues.php?tab=4.
- 18.Platt R, Kass NE, McGraw D. Ethics, regulation, and comparative effectiveness research: Time for a change. Journal of the American Medical Association. 2014;311(15):1497–1498. doi: 10.1001/jama.2014.2144. [DOI] [PubMed] [Google Scholar]
- 19.Solomon MZ. Realizing bioethics’ goals in practice: Ten ways “is” can help “ought”. Hastings Center Report. 2005;35(4):40–7. [PubMed] [Google Scholar]
- 20.Solomon MZ, Bonham AC. Ethical oversight of research on patient care. Hastings Center Report. 2013;43(SI):S2–S3. doi: 10.1002/hast.132. [DOI] [PubMed] [Google Scholar]
- 21.Wilfond BS, Magnus D, Antommaria AH, et al. The OHRP and SUPPORT. New England Journal of Medicine. 2013;368(25):e36. doi: 10.1056/NEJMc1307008. [DOI] [PubMed] [Google Scholar]