Abstract
Background
Gastric stimulation via high-frequency, low-energy pulses can provide an effective treatment for gastric dysmotility; however, the current commercially available device requires surgical implantation for long-term stimulation and is powered by a nonrechargeable battery.
Objective
To test and describe endoscopic implantation techniques and testing of stimulation of a novel, wireless, batteryless, gastric electrical stimulation (GES) device.
Design
Endoscopic gastric implantation techniques were implemented, and in vivo gastric signals were recorded and measured in a non-survival swine model (n = 2; 50-kg animals).
Intervention
Five novel endoscopic gastric implantation techniques and stimulation of a novel, wireless, batteryless, GES device were tested on a non-survival swine model.
Main Outcome Measurements
Feasibility of 5 new endoscopic gastric implantation techniques of the novel, miniature, batteryless, wireless GES device while recording and measurement of in vivo gastric signals.
Results
All 5 of the novel endoscopic techniques permitted insertion and securing of the miniaturized gastrostimulator. By the help of these methods and miniaturization of the gastrostimulator, successful GES could be provided without any surgery. The metallic clip attachment was restricted to the mucosal surface, whereas the prototype tacks, prototype spring coils, percutaneous endoscopic gastrostomy wires/T-tag fasteners, and submucosal pocket endoscopic implantation methods attach the stimulator near transmurally or transmurally to the stomach. They allow more secure device attachment with optimal stimulation depth.
Limitations
Non-survival pig studies.
Conclusion
These 5 techniques have the potential to augment the utility of GES as a treatment alternative, to provide an important prototype for other dysmotility treatment paradigms, and to yield insights for new technological interfaces between non-invasiveness and surgery.
Use your mobile device to scan this QR code and watch the author interview. Download a free QR code scanner by searching ‘QR Scanner’ in your mobile device’s app store.
Gastric dysmotility, a common feature of such GI disorders as gastroparesis, is marked by debilitating, socially isolating symptoms that often progress to chronic status, with a devastating impact on a patient’s ability to persevere in economic, familial, and social activities. A cure for motility disorders currently eludes the collaborative and independent efforts of global experts; thus symptom relief for these patients is of paramount importance.1
In gastroparesis, gastric electrical activities, such as slow wave frequency and/or amplitude, become abnormal.2 The application of high-frequency, low-energy stimulation of the gastric muscles can effectively moderate gastric dysmotility symptoms.3-5 However, owing to its size (60 mm × 55 mm × 10 mm), a lengthy surgical implantation under general anesthesia is required for implantation of the Enterra (Medtronic Inc, Minneapolis, Minn) gastric electrical stimulation (GES) device, a neurostimulator approved for humanitarian use by the U.S. Food and Drug Administration.6 Even temporary stimulation, which now relies on endoscopically placed transnasal leads attached to the Enterra, carried in a belt or a pocket, has significant, obvious drawbacks owing to device wires.7-10
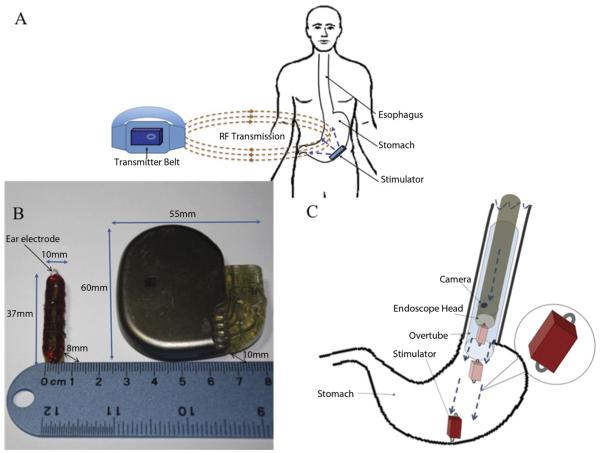
We recently developed a novel, wirelessly powered, miniature gastrostimulator, and this breakthrough technology, the result of significant form factor reductions, eliminates the need for any battery and for conventionally wired leads (Fig. 1). Most importantly, the device is sufficiently small to circumvent surgery, rendering GES a potential treatment option for dysmotility patients who are not strong surgical candidates. Here we describe the concomitant development testing in an experimental model of 5 novel endoscopic techniques. The first 4 of these innovative techniques provide alternatives for implantation and attachment of the device under varying anatomic and/or physiologic conditions. The fifth involves the endoscopic creation of a submucosal pocket, sized to accommodate and positioned to secure the miniature gastro-stimulator within the gastric submucosa.
Figure 1.
A, Endoscopically implantable wireless stimulator system. B, Size comparisons of the wireless stimulator and the commercial stimulator device. C, Stimulator through an overtube.
These techniques can help individual patients and their physicians assess the utility of GES therapy without fear of surgical consequences where symptoms of dysmotility are medically refractory. The techniques moreover have the potential to augment GES as a treatment alternative and to provide an important prototype for gastric dysmotility treatment paradigms.
METHODS
Design
The miniature stimulator system (Fig. 1A) consists of a batteryless stimulator and an external transmitter, which powers the stimulator by transmitting electromagnetic energy into the body via radio frequency. This external transmitter contains 2 lithium-ion batteries, a signal generator producing a 1.3-MHz square waveform, and a power amplifier. The waveform is fed and amplified by a class-E amplifier and passed to a transmitter antenna. The signal is received by a receiving coil antenna, then amplified and rectified by a charge pump that powers the preprogrammed peripheral interface controller so as to generate stimulation pulse cycles.
Take-home Message.
For patients with gastric dysmotility such as gastroparesis, surgical implantation of a large stimulator providing gastric electrical stimulation is an effective treatment option. The device is endoscopically implantable and has the potential to eliminate surgical implantation and replacement.
The authors’ miniature gastrostimulator and its associated novel attachment techniques will likely change the way patients are approached with gastric dysmotility and gastroparesis.
Complete mathematical modeling and computer simulation procedures were performed to optimize the turn numbers and to determine the minimum transmitter size able to transmit the required power to the stimulator. During stimulation, the transmitter, which can be worn on a belt, must be kept close to the stimulator. Moving the transmitter away from the body turns off the stimulator instantaneously. The wireless stimulator measures 37 mm × 10 mm × 8 mm, less than one-tenth the size (area) of the commercial device (Fig. 1C).
Animals
The University of Mississippi Medical Center Institutional Animal Care and Use Committee approved this study. We used two 5-month-old male pigs (50-kg each).
Endoscopic insertion and attachment of device
The procedures through which to implant the device and facilitate its attachment were tested as follows. A diagnostic gastroscope (Olympus GIF-Q160; Olympus America, Center Valley, Pa) was used for endoscopic visualization and device attachment. An overtube (00711147 Guardus; US Endoscopy, Mentor, Ohio) was gently pushed into the esophagus, over the endoscope, for subsequent endoscopic esophageal reintubation (Fig. 1D). After endoscopic examination of the stomach, the miniature stimulator was easily deployed through the overtube into the esophagus and pushed by endoscope into the stomach.
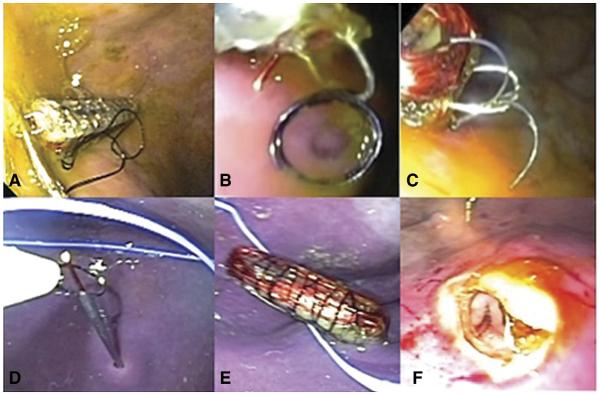
We first inserted the miniature gastrostimulator and attached its electrodes to the gastric wall by using commercially available endoclips (Resolution clip; Boston Scientific, Natick, Mass) (Fig. 2A). Next, we endoscopically achieved a transmural attachment by using round prototype endoscopic spring coils (Cook Medical, Winston-Salem, NC) (Fig. 2C) (Video 1, along with Videos 2 and 3, is available online at www.giejournal.org) delivered through a catheter. We then were able to attain a neartransmural attachment of the gastrostimulator to the gastric wall by the use of prototype endoscopic tacks (Cook Medical) (Fig. 2B) (Video 1).11
Figure 2.
A, Endoclips. B, Prototype endoscopic spring coil. C, Prototype endoscopic tacks. D, Percutaneous endoscopic gastrostomy needles. E, Stimulator implanted by percutaneous endoscopic gastrostomy method. F, Endoscopic submucosal pocketing and device implantation.
To test our fourth method, we inserted the gastrostimulator by a different endoscopic technique. A hollow PEG needle, without endoscopic T-tag fasteners (T-bars), was used to inject one end of a coiled metal wire transmurally into the stomach through the skin of the belly. A rat-tooth forceps, inserted endoscopically, was used to pull this wire up through the mouth. A second identical injection was performed approximately 3 cm below the initial one, and that wire also was pulled through the mouth in a similar manner (Figs. 2D and E) (Video 2).12 The PEG needles were removed, and the metal wires extending from the mouth were then sutured to the device electrodes, and the wires protruding from the belly were pulled outside and used to guide the gastrostimulator to the stomach wall and attach it.
Our fifth endoscopic innovation consisted of the creation of an endoscopic submucosal pocket (ESP) and endoscopic implantation, positioning, and securing of the gastrostimulator within it (ESPI) (Fig. 2F) (Video 3). After submucosal injection with diluted epinephrine (1:10,000), we made an entry cut with a needle-knife (Olympus America) to permit ESPI. An endoscopic biliary stone extraction balloon (Cook Medical) was inserted through the entry cut into the submucosal space to create an initial submucosal pocket. The inflation target of the balloon was 15 mm. Optionally, the entry opening can be dilated with an endoscopic biliary or through-the-scope dilating balloon (Cook Medical). The gastroscope was then further advanced into the submucosal pocket, a limited submucosal dissection with biopsy forceps performed to sufficiently enlarge this pocket to accommodate the gastrostimulator, and the device implanted into the submucosal pocket. The implanted device can be secured by placing endoclips at the pocket opening; with tissue healing, the embedded stimulator will be firmly positioned in contact with the muscular layer of the gastric wall.
Gastric signal acquisition
In this study, a data acquisition system based on an interface card DAQ-USB-6210 (National Instruments, Austin, Tex) was used to measure the miniature gastrostimulator’s voltage output. The electrical current delivered into the tissues was computed from the voltage readings and the measured direct current impedances. The electrogastrogram recordings were analyzed by signal averaging for mean frequencies and amplitudes as well as for frequency-to-amplitude ratios.
Electrogastrogram measurements were not primarily obtained to compare abnormal and normal signals but to confirm stimulation effects on such gastric activities as the rhythm (regular or irregular), mean frequency, frequency range, amplitude (equal or unequal), mean amplitude, and amplitude range of the electrogastrogram signals. Control measurements were obtained with the stimulators turned off, during which intervals no stimulation was applied.
RESULTS
The mean value of the measured mucosal impedance we recorded in our studies was 815Ω, with voltage output delivered at 2.5 V and calculated current at 3.06 mA. The Enterra device uses a stimulation pulse train of 14 Hz frequency with 5 mA amplitude of current, less than 1% duty cycle, 0.1 second in on-time and 5 seconds off-time electrogastrogram signal frequencies and mean amplitudes varied at different stimulation settings.13,14 Recorded frequency-to-amplitude ratios indicated significant changes in gastric activities during stimulation as compared with control measurements. Amplitude activity became more unequal during baseline measurement than during stimulation. An anomaly in the electrogastrogram baseline pattern resulted from minute gastric disturbances that were caused during endoscopic implantation and the placement of the leads for electrogastrogram measurement.
Endoscopic attachment of the miniature gastrostimulator was successfully performed with all 5 proposed methods and devices. However, the duration of stimulator attachment achieved via currently available endoclips is likely to be limited, because these endoclips permit attachment to the gastric mucosa only. The other attachment devices, including the endoscopic tacks, spring coils, and PEG wires/T-tag fasteners attach the stimulator near transmurally or transmurally and so allow secure device attachment with theoretically optimal stimulation depth; they also can conduct electrical current and so function as extension electrodes. For the transmural placement, the minimization of and abdominal wall stimulation was accomplished by careful placement of leads. The placement of the endoclip, prototype spring coil, and prototype endoscopic tack each took <10 minutes. The PEG method took approximately 20 minutes including the time required for insertion of the wireless miniature stimulator through the overtube. Implanting the miniature stimulator with the submucosal pocket endoscopic procedure took approximately 30 minutes. The GES with each of the endoscopic techniques was recorded for 30 minutes.
During this acute animal study, there was no intraprocedural complication such as perforation or bleeding. Because our study was non-survival, the risks of infection, delayed bleeding, and perforation associated with these methods are unknown. Based on reported experience with endoscopic submucosal dissection and endoscopic suturing, these potential risks are expected to be minimal (Table 1).
TABLE 1.
Electrogastrogram results*
| Stimulation methods | Current (mA) |
Frequency range (bpm) |
Mean frequency (bpm) |
Amplitude type |
Amplitude range (mV) |
Mean amplitude (mV) |
FAR (bpm/V) |
|---|---|---|---|---|---|---|---|
| Baseline 1 | 0 | 2.0-4.0 | 2.667 | UA | 0.625-0.600 | 0.146 | 18.288 |
|
| |||||||
| Stimulation by PEG method | 3.06 | 5.0-6.0 | 5.333 | EA | 0.122-0.280 | 0.201 | 26.561 |
|
| |||||||
| Baseline 2 | 0 | 4.0-5.0 | 4.667 | UA | 0.625-0.875 | 0.717 | 6.509 |
|
| |||||||
| Stimulation by prototype tack method |
3.06 | 5.0-6.0 | 5.667 | EA | 0.15-0.35 | 0.233 | 24.320 |
|
| |||||||
| Baseline 2 | 0 | 4.0-6.0 | 5.333 | UA | 0.8-1.0 | 0.933 | 5.713 |
FAR, Frequency-to-amplitude ratio; UA, unequal amplitude; PEG, percutaneous endoscopic gastrostomy; EA, equal amplitude.
Regular rhythm.
DISCUSSION
Submucosal injection with dissection is an established clinical method for the removal of mucosal neoplasms. However, to the best of our knowledge, ESP has not been previously reported in the literature. ESPI mimics a surgical approach and methods, permitting both secure stimulator implantation and direct contact of the surface electrodes with the muscular and submucosal layers. Depending on the size and depth of the submucosal pocket, the miniature gastrostimulator can be partially, subtotally, or completely implanted or embedded, facilitating tailoring according to clinical indications and device exchange needs. The attachment devices and methods we describe also can be combined variously. With improved endoscopic suturing devices and methods, the gastrostimulator could be sutured to the gastric wall.
Previously, we demonstrated the clinical feasibility and utility of temporary GES by using an external surgical gastrostimulator and endoscopic attachment of transnasal electric leads to the gastric wall by using endoclips.10-11 We believe that the evolution of this novel, miniature gastrostimulator, in conjunction with the aforementioned innovative methods and devices for attachment, will permit a paradigm shift toward minimal invasiveness in the treatment of gut dysmotility disorders.13
The wirelessly powered, batteryless, miniature gastrostimulators that we developed can provide either a short or long-term platform for GES. Our studies in an experimental model further demonstrated the capacity of our newly developed endoscopic techniques to permit successful delivery, pocketing, and securing of these devices. After implantation and attachment through these novel methods, the miniature gastrostimulators were able to successfully modulate gastric myoelectrical activities. We believe that the refinement of these innovative endoscopic techniques can eliminate the need for the surgeries now associated with GES therapy.
The achievement of our immediate study aims with respect to endoscopic innovations, however, has ushered in an entirely new direction for GES, one that can be applied more universally and comprehensively to treat gastric dysmotility. These novel techniques, in tandem with increasing size reductions in biomedical devices and an expanding armamentarium, demonstrate the rapidly growing contribution of expert endoscopic manipulation and research to new arenas of medical management. Their refinement will further the emergence of increasingly applicable endoscopic interventions between that which is now deemed noninvasive and the clearly surgical.
Supplementary Material
ACKNOWLEDGMENT
We wish to thank Julie Easter, Tyler McLawhorn, and Cook Medical for providing the prototype endoscopic tacks and spring coils devices. We also express our sincere appreciation to the entire staff of the GI Division and Endoscopy Unit at University of Mississippi Medical Center for their help with this study and to Jo Anne Fordham for her assistance with manuscript production.
Abbreviations
- ESP
endoscopic submucosal pocketing
- ESPI
endoscopic submucosal pocketing and device implantation
- GES
gastric electrical stimulation
- PEG
percutaneous endoscopic gastrostomy
Footnotes
DISCLOSURE: T. Abell is an investigator, speaker, consultant, and licensor for Medtronic Inc. Certain aspects of this technology are covered in intellectual property filed by the University of Texas Arlington and the University of Mississippi. J. Chiao is a consultant for Alcon. J. Easter and T. McLawhorn are employees of Cook Medical. No other financial relationships relevant to this publication were disclosed.
REFERENCES
- 1.Chang J, Rayner CK, Jones KL, et al. Diabetic gastroparesis—backwards and forwards. J Gastroenterol Hepatol. 2011;26(supp 1):46–57. doi: 10.1111/j.1440-1746.2010.06573.x. [DOI] [PubMed] [Google Scholar]
- 2.O’Grady G, Egbuji JU, Du P, et al. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World J Surg. 2009;33:1693–701. doi: 10.1007/s00268-009-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–8. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 4.Eagon JC, Kelly KA. Effect of electrical stimulation on gastric electrical activity, motility and emptying. Neurogastroenterol Motil. 1995;7:39–45. doi: 10.1111/j.1365-2982.1995.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Ross RA, McCallum RW, et al. Two-channel gastric pacing with a novel implantable gastric pacemaker accelerates glucagon-induced delayed gastric emptying in dogs. Am J Surg. 2008;195:122–9. doi: 10.1016/j.amjsurg.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:2578. doi: 10.1111/j.1572-0241.2003.08681.x. [DOI] [PubMed] [Google Scholar]
- 7.Ayinala S, Batista O, Goyal A, et al. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc. 2005;61:455–61. doi: 10.1016/s0016-5107(05)00076-3. [DOI] [PubMed] [Google Scholar]
- 8.Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496–503. doi: 10.1016/j.gie.2011.05.022. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daram SR, Tang SJ, Vick K, et al. Novel application of GI electrical stimulation in Roux stasis syndrome (with video) Gastrointest Endosc. 2011;74:683–6. doi: 10.1016/j.gie.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daram SR, Tang SJ, Abell TL. Video: temporary gastric electrical stimulation for gastroparesis: endoscopic placement of electrodes (ENDOstim) Surg Endosc. 2011;25:3444–5. doi: 10.1007/s00464-011-1710-5. [DOI] [PubMed] [Google Scholar]
- 11.Bhat YM, Hegde S, Knaus M, et al. Transluminal endosurgery: novel use of endoscopic tacks for the closure of access sites in natural orifice transluminal endoscopic surgery (with videos) Gastrointest Endosc. 2009;69:1161–6. doi: 10.1016/j.gie.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 12.Sallam HS, Chen JD, Pasricha PJ. Feasibility of gastric electrical stimulation by percutaneous endoscopic transgastric electrodes. Gastrointest Endosc. 2008;68:754–9. doi: 10.1016/j.gie.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 13.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–8. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 14.Jalilian E, Onen D, Neshev E, et al. Implantable neural electrical stimulator for external control of gastrointestinal motility. Med Engineer Physics. 2007;29:238–52. doi: 10.1016/j.medengphy.2006.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.