Abstract
3-Deoxysappanchalcone (3-DSC) has been reported to possess anti-allergic, antiviral, anti-inflammatory and antioxidant activities. In the present study, we investigated the effects of 3-DSC on the proliferation of human hair follicle dermal papilla cells (HDPCs) and mouse hair growth in vivo. A real-time cell analyzer system, luciferase assay, Western blot and real-time polymerase chain reaction (PCR) were employed to measure the biochemical changes occurring in HDPCs in response to 3-DSC treatment. The effect of 3-DSC on hair growth in C57BL/6 mice was also examined. 3-DSC promoted the proliferation of HDPCs, similar to Tofacitinib, an inhibitor of janus-activated kinase (JAK). 3-DSC promoted phosphorylation of β-catenin and transcriptional activation of the T-cell factor. In addition, 3-DSC potentiated interleukin-6 (IL-6)-induced phosphorylation and subsequent transactivation of signal transducer and activator of transcription-3 (STAT3), thereby increasing the expression of cyclin-dependent kinase-4 (Cdk4), fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF). On the contrary, 3-DSC attenuated STAT6 mRNA expression and IL4-induced STAT6 phosphorylation in HDPCs. Finally, we observed that topical application of 3-DSC promoted the anagen phase of hair growth in C57BL/6 mice. 3-DSC stimulates hair growth possibly by inducing proliferation of follicular dermal papilla cells via modulation of WNT/β-catenin and STAT signaling.
Keywords: 3-deoxysappanchalcone, Human hair follicle dermal papilla cells, WNT/β-catenin, STAT3, STAT6, C57BL/6 mice
INTRODUCTION
Hair follicles are composed of cells that possess self-renewal capacity, which can undergo a repetitive regeneration process during hair growth (Yu et al., 2008). The ‘hair growth cycle’ has three phases: the anagen (growth), catagen (regression) and telogen (rest) phases (Stenn and Paus, 2001). During the anagen phase, the pigmented hair shaft is actively generated and the follicle reaches its maximal length and volume. At the end of the anagen phase, the hair follicle enters the catagen phase, during which production of new hair shafts and pigmentation ceases and the club hair starts to form. In the telogen phase, a relatively quiescent state, keratin production ceases and the club hair matures. After completion of the telogen phase, the hair begins to shed and the hair cycle restarts (Paus and Foitzik, 2004). It is known that the regulation of follicular morphogenesis and hair growth partly depends on the interaction between the epithelial and mesenchymal cells in hair follicles. The dermal papilla, a mesenchymal cell population located at the base of the hair follicle, plays an important role in regulating hair growth and cycling (Botchkarev and Kishimoto, 2003). Factors secreted by dermal papilla cells (DPCs) directly promote the surrounding matrix cells either to proliferate and differentiate or to stimulate hair stem cells to initiate a new anagen phase (Kang et al., 2010).
Recent studies in transgenic and knockout mouse models have revealed that the WNT/β-catenin-mediated signaling pathway plays a pivotal role in the regulation of hair follicle morphogenesis, hair shaft differentiation and follicular recycling (Kishimoto et al., 2000; Andl et al., 2002; Kitagawa et al., 2009; Soma et al., 2012; Tsai et al., 2014). Reddy et al. (2001) demonstrated that certain WNT ligands, e.g. WNT-10a and WNT-10b, are overexpressed at the onset of the anagen phase and WNT-5a is selectively expressed in the dermal follicle at a later stage of follicular differentiation in a sonic hedgehog (SHH)-dependent manner. A WNT downstream signaling molecule, β-catenin has established a link between WNT signaling and SHH expression: the stabilization of epidermal β-catenin induced the formation of ectopic hair follicles and SHH expression (Gat et al., 1998), whereas the expression of SHH was lost in the absence of epidermal β-catenin (Huelsken et al., 2001). While the deletion of Wnt/β-catenin reduced the proliferation of hair follicle progenitor cells and induced the early onset of the catagen phase (Reddy et al., 2001), upregulation of WNT/β-catenin signaling resulted in a more extensive hair growth in mice (Andl et al., 2002). Another critical intracellular signaling pathway involved in the regulation of hair cycle is mediated by signal transducer and activator of transcription (STAT) and its upstream regulator, Janus-activated kinase (JAK). A previous study demonstrated that mutation of mouse STAT3 prevents normal progression of telogen follicles into anagen (Sano et al., 2000). Pharmacological inhibition of the JAK-STAT pathway promoted rapid hair regrowth in alopecia areata (AA) in both mice and humans (Xing et al., 2014). Complying with this result, a clinically-approved JAK inhibitor, ruxolitinib, was also reported to reverse AA (Xing et al., 2014). In addition, another JAK inhibitor tofacitinib increases the growth rate of anagen hair shafts (skin grafts and organotypic culture assays) and enhances the inductivity of human dermal papilla spheres (neogenesis assays) (Zeidler et al., 2016). Investigation of the molecular effects of tofacitinib treatment revealed that the treatment causes a molecular restoration of a subset of genes that are disrupted in culture but are present in fully inductive dermal papilla cells (HDPCs) (Harel et al., 2015).
3-Deoxysappanchalcone (3-DSC) is a naturally-occurring chalcone compound (Fig. 1A) isolated from Caesalpinia sappan L. (Leguminosae). C. sappan it is commonly used as a herbal medicine to reduce inflammation and improve blood circulation (Shen et al., 2007; Liu et al., 2009; Yodsaoue et al., 2009). Several studies have demonstrated that 3-DSC exerts several biological properties, including anti-allergic (Liu et al., 2009), anti-influenza virus (Yang et al., 2012), anti-inflammatory (Yodsaoue et al., 2009), and antioxidant activities (Youn et al., 2011). In an effort to identify novel natural products that might promote hair growth, we observed that 3-DSC exerts stimulatory effects on hair growth in mice. Our study also demonstrates the potential molecular mechanisms of action of 3-DSC in the proliferation of HDPCs with a special focus on modulation of STAT and WNT/β-catenin signaling.
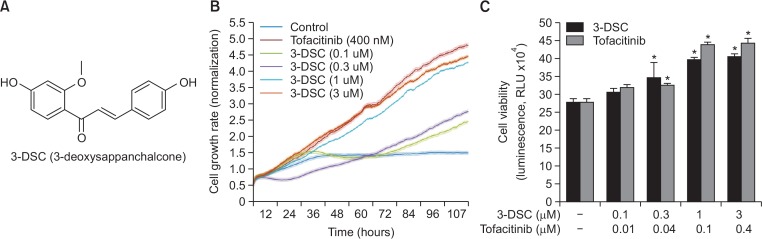
Fig. 1.
Effects of 3-DSC on hair cell growth. (A) Chemical structures of 3-DSC. Effects of 3-DSC on hair cell growth was examined by (B) real-time xCELLigence system and (C) CellTiter-Glo® luminescent cell growth assay as described in the Methods. All experiments were performed in triplicate. The asterisk indicates a significant statistical significance (*p<0.05).
MATERIALS AND METHODS
Cell culture
The human hair follicle dermal papilla cells (HDPCs; Promo cell: ABM Inc., Richmond, BC, Canada) were cultured in Pri-GrowIII (ABM Inc.) medium supplemented with 10% fetal bovine serum (FBS) and 100 U/ml penicillin and 100 μg/ml streptomycin. WNT reporter NIH3T3 cells lines were obtained from Enzo Life Sciences (Farmingdale, NY, USA) for TCF/LEF transcription factor to activate Wnt target gene expression. Stable STAT3 Luciferase-(LUCPorter™) reporter gene-expressing HEK293 cell lines were purchased from Novus (Littleton, CO, USA). Cells were incubated in accordance with the product manual and maintained in an incubator in a humidified atmosphere of 5% CO2 at 37°C.
Chemicals and antibodies
3-DSC (purity >98%) was purchased from AK Scientific, Inc (Union City, CA, USA). CellTiter-Glo®Luminescent Cell Viability Assay kit was purchased from Promega Corporation (Madison, WI, USA). DMEM and fetal bovine serum (FBS) were procured from Invitrogen (Carlsbad, CA, USA). Interleukin (IL)-6 and IL-4 were purchased from R&D systems (Minneapolis, MN, USA). β-actin antibody was obtained from Sigma-Aldrich (St. Louis, MO, USA). Polyclonal antibodies against total β-catenin, phospho-specific β-catenin (Thr41/Ser45), total STAT3, STAT6, phospho-specific STAT3 (Tyr705) and STAT6 (Tyr641) were purchased from Cell Signaling Technology (Beverly, MA, USA). All other chemicals used in our experiments were molecular biology grade.
Real-time cell analyzer (RTCA) system
The xCELLigence System (ACEA Biosciences; San Diego, CA, USA) allows for label-free and real-time monitoring of cellular processes, such as cell proliferation, cytotoxicity, adhesion, viability, invasion, and migration, using the electronic cell sensor array technology (Ke et al., 2011). Electrode impedance, which is displayed as cell index (CI) values, was used to provide quantitative information about the biological status of cells, including cell number, viability, and morphology. Changes in the cell status, such as cell morphology, cell adhesion, or cell viability led to a change in the CI, which is a quantitative measure of the number of cells present in a well. Subsequently, 150 μl of cell culture media at room temperature was added to each well of E-plate 8 in the xCELLigence System. After that, the E-plate 8 was connected to the system and checked in the cell culture incubator for proper electrical contacts and the background impedance was measured during 24 hrs. Meanwhile, the HDPCs were resuspended in cell culture medium and adjusted to a cell number of 20,000 cells/well. Cell suspension (50 μl) was added to the 150 μl medium-containing wells in E-plate 8, in order to determine the optimum cell concentration. After 30-min incubation at room temperature, E-plate 8 was placed in the cell culture incubator. Then, adhesion, growth and proliferation of the cells were monitored every 1 h for a period of up to 24 h via the incorporated sensor electrode arrays in the E-Plate 8. After 24 hr, 0–3 μM of 3-DSC was added to 200 μl cell culture medium and live cells were monitored every 15 min for a period of up to 96 hr. Electrical impedance was measured by the RTCA-integrated software of the xCELLigence system as a dimensionless parameter termed CI.
CellTiter-Glo® luminescent cell growth assay
Cell proliferation and cytotoxicity were assessed using a CellTiter-Glo® Luminescent Cell Viability Assay Kit (Promega Corporation), which is a homogeneous method to determine the number of viable cells in culture based on quantitation of the ATP present. Briefly, cells were seeded for 24 h in a 96-well plate (10,000 cells/well) and then attached cells were treated with 3-DSC (0–3 μM) in serum free medium for 48 h. A volume of CellTiter-Glo® Reagent equal to the volume of cell culture medium present in each well was added and incubated at room temperature for 10 minutes to stabilize the luminescent signal. Amounts of ATP were determined by recording luminescence on a LuBi microplate luminometer (Micro Digital Ltd., Seoul, Republic of Korea).
RNA isolation and Quantitative real-time PCR (qPCR)
Cells were seeded for 24 h and then attached cells were treated with 3-DSC (0–3 μM) in a serum free medium for 48 h. For mRNA quantification, total RNA was extracted using NucleoSpin® RNA Kit (Macherey-Nagel Gmbh & Co., Düren, Germany). cDNA was synthesized using iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. Briefly, 2 μg of total RNA was used for cDNA preparation. The synthesized cDNA was amplified separately using primers for β-catenin, Lef/TCF, STAT3, STAT6, cyclin-dependent kinase (CDK)-4, fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF) and GAPDH using GeneAmp PCR 9700 thermocycler (Thermo Fisher Scientific, Waltham, MA, USA). PCR products were analyzed by 1% agarose gel using 1X TAE buffer. Relative mRNA levels were quantified using myECL imager analysis software (Thermo Fisher Scientific). Quantitative real-time PCR was performed using the iQ™ SYBR® Green Supermix (Bio-Rad) specific for each gene. All reverse transcription reactions were run on a CFX96™ Real-Time System (Bio-Rad) using the following steps: 3 min at 95°C, 42 cycles of 10 s at 95°C, 15 s at 55°C, 30 s at 72°C, and then 10 s at 95°C. Relative expression levels were determined using the Bio-Rad CFX Manager 3.0 (Bio-Rad). The expression of target genes was normalized to that of GAPDH. The primer pairs for RT-PCR were as follows: β-catenin forward 5′-CCCACTAATGTCCAGCGTTT-3′, reverse 5′-AACCAAGCATTTTCACCAGG-3′; glycogen synthase kinase (GSK)-3β forward 5′-AACTGCCCGACTAACAACAC-3′, reverse 5′-ATTGGTCTGTCCACGGTCTC-3′; lymphoid enhancer factor (Lef)-1/T Cell factor (TCF) forward 5′-AATCATCCCGGCCAGC A-3′, reverse 5′-TGTCGT GGTAGGGCTCCTC-3′; BAX forward 5′-GTTGTCGCCCTTTT CTACT-3′, reverse 5′-GAAGTCCAATGTCCAGCC-3′; BCL2 forward 5′-CACCAGAATCA AGTGTTCC-3′, reverse 5′-GCTATTTTATTGGATGTGCTTTG-3′, STAT1 forward 5′-ACA TCATTCGCAATTACAAAGTC-3′, reverse 5′-TCAAGTTCCATTGGCTCTG-3′; STAT3 for ward 5′-GTTATTGTTGTTGTTGTTCTTAGAC-3′, reverse 5′-AATGCCAGGAGTATGTAG C-3′; STAT4 forward 5′-AACCTACTCTTGATACACAATCTAA-3′, reverse 5′-TCTCCTCT CTTCCCTTAAACA-3′; STAT5A forward 5′-CTTTGCCCTCCTAAGAGAGA-3′, reverse 5′-TGAATCGGTTACATCAACACAT-3′, STAT5B forward 5′-TATTCTCTCTTTGTCCTCT CTCC-3′, reverse 5′-CGGCATTGGCACTGTAAG-3′; STAT6 forward 5′-CCAGGATGGCT CTCCACAG-3′, reverse 5′-CATGGAGGAATCAGGGGC-3′; Axin forward 5′-GCAACTCA GTAACAGCCCGA-3′, reverse 5′-AAGTCAGCAGGGGCTCATCT-3′; SOX9 forward 5′-AGACCTTTGGGCTGCCTTAT-3′, reverse 5′-TAGCCTCCCTCACTCCAAGA-3′; BMP4 forward 5′-CACTGGCTGACCACCTCAAC-3′, reverse 5′-GGCACCCACATCCCTCTACT -3′; FGF forward 5′-GCTCTTAGCAGACATTGGAAG-3′, reverse 5′-GTGTGTGCTAAC CGTTACCT-3′, VEGF forward 5′-GGAGAGATGAGCTTCCTACAG-3′, reverse 5′-TCACC GCCTTGGCTTGTCACA-3′; CDK4 forward 5′-ACCTGAGATGGAGGAGTC-3′, reverse 5′-AAGGCAGAGATTCGCTTG-3′, and GAPDH forward 5′-TGGCAAATTCCATGCAC-3′, reverse 5′-CCATGGTGGTGAAGACGC-3′.
Measurement by luciferase-reporter assay
WNT reporter NIH3T3 cells permanently transfected with TCF/LEF-luciferase constructor and HEK293 cells stably transfected with STAT3-luciferase constructs were seeded at 2×104 cells in a 96-well plate and maintained in DMEM media containing puromycin (3 μg/ml) and 5% FBS for 24 h. WNT reporter cells were then exposed to WNT3a and/or 3-DSC (0.01–10 μM) for 24 h. The stable STAT3-luciferase-expressing HEK293 cells were seeded in a 96-well plate and treated with IL-6 (10 ng) alone or in combination with 3-DSC (0.1–10 μM) for 24 h. HEK293 cells were permanently transfected with IL-4Rsite-TKluc/STAT6 containing the IL-4 receptor site (5′-AGCTTCTTCATCTGAAAAGGG-3′) (Kotanides and Reich, 1996). Cells were seeded at 1×104 cells in each well of a 96-well plate in DMEM containing 5% FBS for 24 h. Cells were then treated with IL-4 (10 ng) alone or in combination with 3-DSC (0.1–10 μM) for 24 h. The supernatant was discarded and passive lysis buffer was added and incubated for 10 min in an orbital shaker. The luciferase activity was measured by LuBi microplate luminometer (Micro Digital Ltd.). All experiments were repeated at least three times and the average values together with standard deviations are depicted.
Western blot
Cells treated with 3-DSC (0–3 μM) for 48 h in a serum free medium were homogenized with a cell lysis buffer (100 mM Tris pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 5 mM DTT, 0.1 mM PMSF, 10% Glycerol, protease inhibitor) and lysed with 2 h incubation on ice. The cell lysate was centrifuged at 13,000 rpm for 15 min at 4°C. Equal amounts of proteins (20 μg) were separated on a SDS/8%-polyacrylamide gel, and then transferred to a polyvinylidene difluoride (PVDF) membrane (Thermo Scientific). Blots were blocked for 1 h at room temperature with 5% (w/v) non-fat dried milk in Tris-Buffered Saline Tween-20 [TBS-T: 10 mM Tris (pH 8.0) and 150 mM NaCl solution containing 0.05% Tween-20]. After a short washing in TBST, the membranes were immunoblotted with specific antibodies. To detect target proteins, specific antibodies against stat3, stat6, phospho-stat3, phospho-stat6, β-catenin, phospho-β-catenin (1:1000, Cell Signaling Technology) and β-actin (1:5000, Sigma-Aldrich) were used. The blots were then incubated with the corresponding conjugated goat anti-rabbit or goat anti-mouse or donkey anti-goat IgG-horse-radish peroxidase (HRP) (1:5000; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) secondary antibodies. Immunoreactive proteins were detected with an enhanced chemiluminescence western blotting detection system (myECL imager, Thermo Scientific).
Hair growth activity in mice
Seven-week-old female C57BL mice were purchased from Oriental Bio Co (Seoul, Republic of Korea). After a 7 day acclimation period for being automatically maintained at 21–25°C and a relative humidity of 45–65% with a controlled light-dark cycle, the animals were divided into 2 randomized groups (n=4) to investigate the hair growth promoting activity of 3-DSC. Two hundred microliters of 3-DSC (3 μM) were applied twice daily for 15 days. Reagents used for the hair growth test were dissolved in a vehicle containing 50% ethanol. All animals were cared for by using protocols approved by the Institutional Animal Care and Use Committee (Chungbuk, Republic of Korea).
RESULTS
The effects of 3-DSC on hair follicle dermal papilla cell growth
Since 3-DSC has been reported to elicit the activation of cell survival pathways (Kim et al., 2014), we examined the effects of 3-DSC on growth of HDPCs. Analysis of real-time cell proliferation using xCELLigence system revealed that the proliferation of HDPCs was promoted by 3-DSC treatment in a concentration-dependent manner (Fig. 1B). Likewise, 3-DSC resulted in a dose-dependent increase in the viability of HDPCs (Fig. 1C). Tofacitinib, which has been reported to promote growth of the hair shaft (Harel et al., 2015), was used as a positive control.
Effects of 3-DSC on the expression of genes involved in hair growth regulation
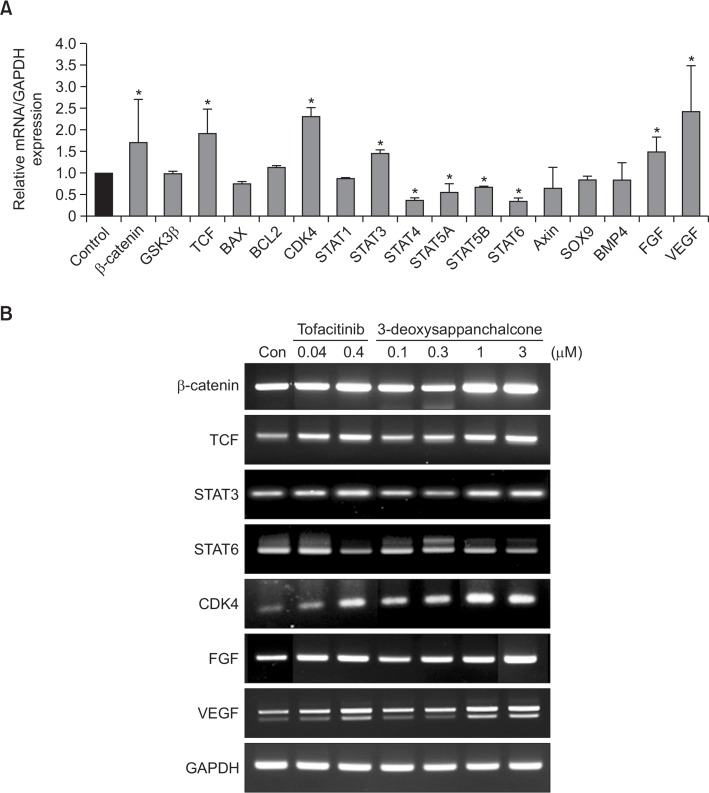
To investigate the effect of 3-DSC on hair growth regulation factors, we analyzed the transcriptional expression changes in various genes, using a conventional and quantitative reverse-transcriptase PCR (RT-PCR). As a result, 3-DSC caused transcriptional activation of β-catenin, tcf, fgf, vegf, cdk4 and stat3, whereas it decreased the level of stat4, stat5A/B and stat6 mRNAs (Fig. 2A). We observed that the expression of Bax, Bcl-2, Sox-9 and BMP4 mRNAs was unaltered. This finding was further confirmed by conventional RT-PCR analysis and tofacitinib elicited a similar pattern of gene expression changes in HDPCs (Fig. 2B).
Fig. 2.
Effects of 3-DSC on hair growth regulating gene expression. (A) The gene expression of hair growth regulating factors was detected by real-time qPCR using specific primers in HDPCs. (B) The level of hair growth regulating factors was detected by gel electrophoresis using specific primers in HDPCs. GAPDH was used as an internal control. All experiments are presented three independent experiments. The asterisk indicates a significant statistical significance (*p<0.05).
Effects of 3-DSC on the transcriptional activity of TCF, STAT3 and STAT6 in HDPCs
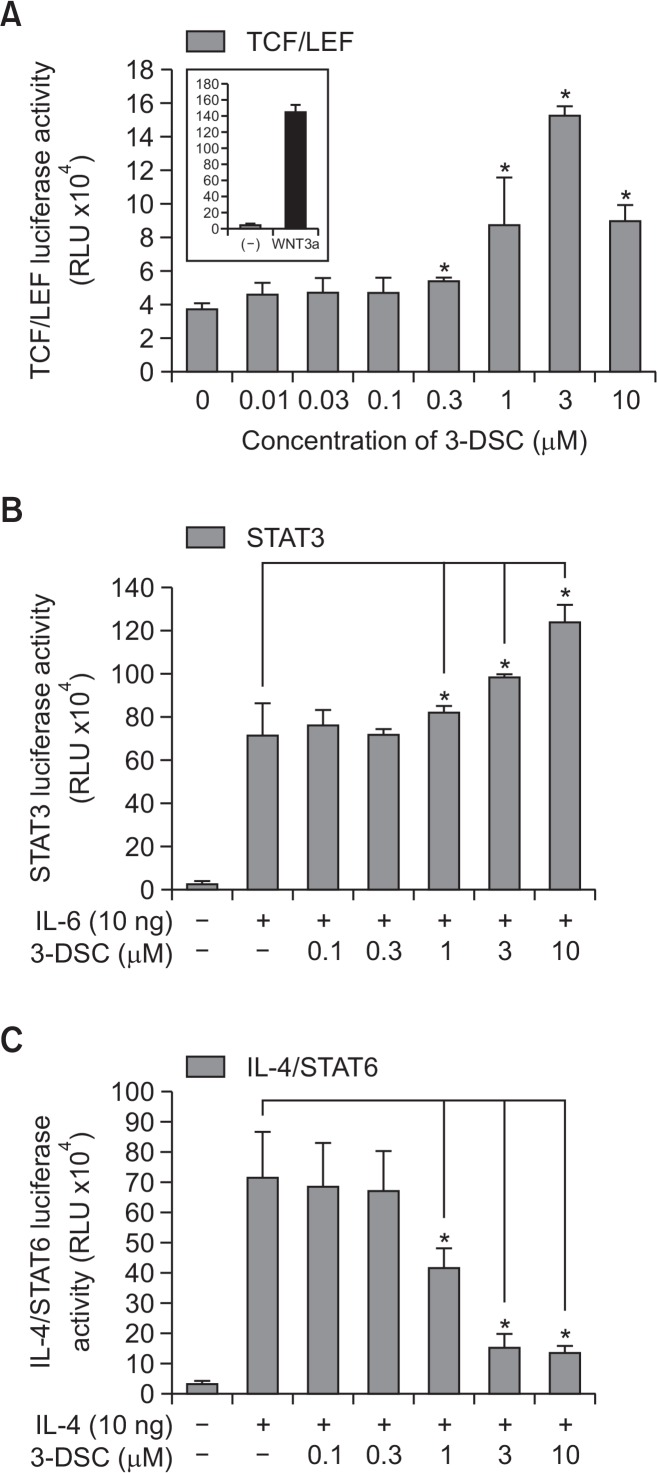
Next, we analyzed the effects of 3-DSC on TCF/LEF-mediated reporter activity, using NIH3T3-WNT-luciferase cells (Fig. 3A). As a result, we observed that 3-DSC resulted in TCF/LEF luciferase activation. In order to determine the effects of 3-DSC on STAT3-mediated transcriptional activity, we exposed the cells to 3-DSC and measured the STAT3-mediated luciferase activity in HEK293 cells that stably expressed a STAT3-regulated luciferase reporter plasmid (Fig. 3B). As a result, we observed that IL-6 stimulated STAT3 transcriptional activity in a concentration-dependent manner. In order to determine whether the inhibitory effects of 3-DSC on STAT6 phosphorylation can be ascribed to the attenuation of the STAT6 transcriptional response, we analyzed the effects of 3-DSC on IL-4 receptor site-mediated reporter gene expression using a stably transfected HEK293 cell line that expresses the IL-4R site-TKluc/STAT6 regulated luciferase gene after treatment with IL-4 (Fig. 3C). 3-DSC decreased the IL-4R site-TKluc/STAT6 luciferase activity in a dose-dependent manner. It seems that 3-DSC increased the TCF/LEF activity by stabilizing β-catenin through the inhibition of β-catenin phosphorylation. 3-DSC also decreased IL-4-induced phosphorylation of STAT6 via interfering with the JAK1/3 pathway, and it activated phosphorylation of STAT3 via the IL-6-induced JAK2 pathway. These results indicate that 3-DSC-induced hair growth promotion is caused by regulating the molecular target of hair dermal follicular papilla cells.
Fig. 3.
Effects of 3-DSC on reporter gene assay. WNT reporter NIH3T3 cells permanently transfected with TCF/LEF-luciferase construct or HEK293 cells stably transfected with STAT3-luciferase constructs were seeded at 2×104 cells in 96-well plate and HEK293 cells permanently transfected with IL-4R site-TKluc/STAT6 contained IL-4 receptor site were seeded at 1×104 cells in each well of a 96-well plate in DMEM containing 5% FBS for 24 h. (A) TCF/LEF reporter gene assay: cells were treated with 3-DSC (0.01–10 μM) for 24 h. (B) IL-6 induced STAT3. (C) IL-4 induced STAT6 reporter gene assay as determined by luciferase activity. Each assay is representative for 3 experiments. The asterisk indicates a significant statistical significance (*p<0.05).
Effects of 3-DSC on phosphorylation of β-Catenin, STAT3 and STAT6 in HDPCs
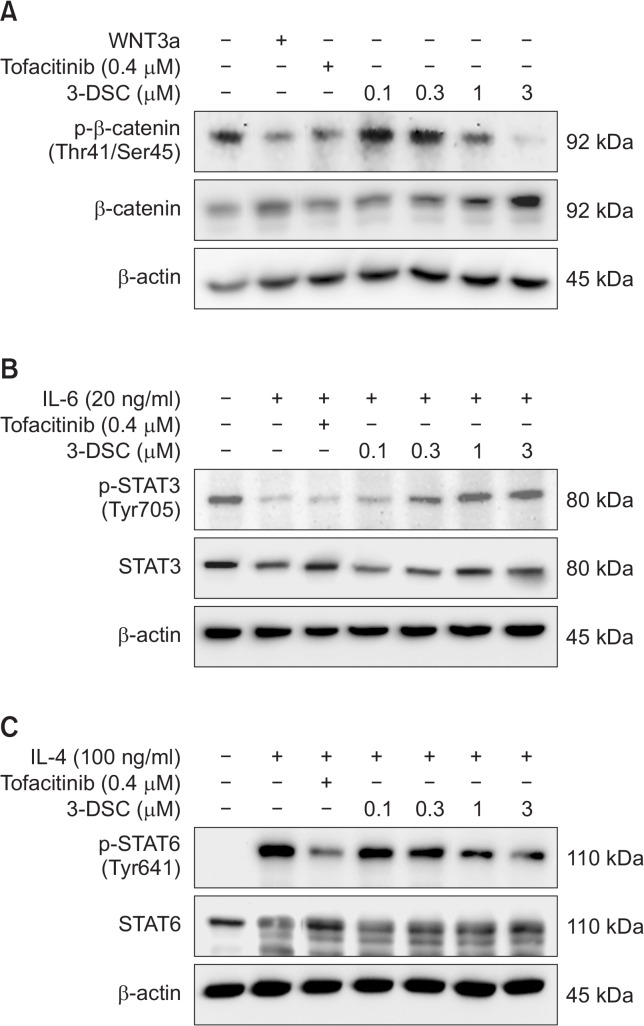
Based on the effects of 3-DSC on the mRNA expression of certain hair growth regulatory genes, we examined whether 3-DSC can modulate the phosphorylation of several key regulators of hair growth. As shown in Fig. 4A, 3-DSC decreased constitutive phosphorylation of β-catenin (Thr41 and Ser45). Treatment of HDPCs with WNT3a, which stabilizes β-catenin, also abolished constitutive β-catenin phosphorylation. Similar inhibition of β-catenin phosphorylation was noted when HDPCs were exposed to the standard drug tofacitinib. To examine the effects of 3-DSC on STAT3 and STAT6 phosphorylation, HDPCs were stimulated with IL6 and IL4, respectively, because STATs are not constitutively phosphorylated in HDPCs. As a result, 3-DSC exhibited inhibitory effects on IL6-induced STAT3 phosphorylation at the Tyr705 residue at lower concentrations (0.1 and 0.3 μM), but STAT3 phosphorylation was unchanged at a higher concentration of the compound (Fig. 4B). However, 3-DSC attenuated IL4-induced STAT6 phosphorylation at the Tyr641 residue in a concentration-dependent manner and it was comparable to tofacitinib (Fig. 4C).
Fig. 4.
Effects of 3-DSC on hair growth regulation protein expression. HDPCs incubated with various concentrations of 3-DSC (0.1–3 μM) or tofacitinib (0.4 μM) in serum free medium for 48 hr. STAT3 and STAT6 were stimulated by IL-6 (20 ng/ml) or IL-4 (100 ng/ml), respectively, 30 min prior to cell harvest. (A) The level of p-β-catenin and β-catenin was detected by western blotting using specific antibodies in HDPCs. (B) The level of p-STAT3 and STAT3 was detected by western blotting using specific antibodies in IL-6-induced HDPCs. (C) The level of p-STAT6 and STAT6 was detected by western blotting using specific antibodies in IL-4-induced HDPCs. β-Actin protein was used as an internal control. Immunoblot assay was performed as described in the Materials and Methods. Each blot is representative for 3 experiments.
Effects of 3-DSC on hair growth in mice
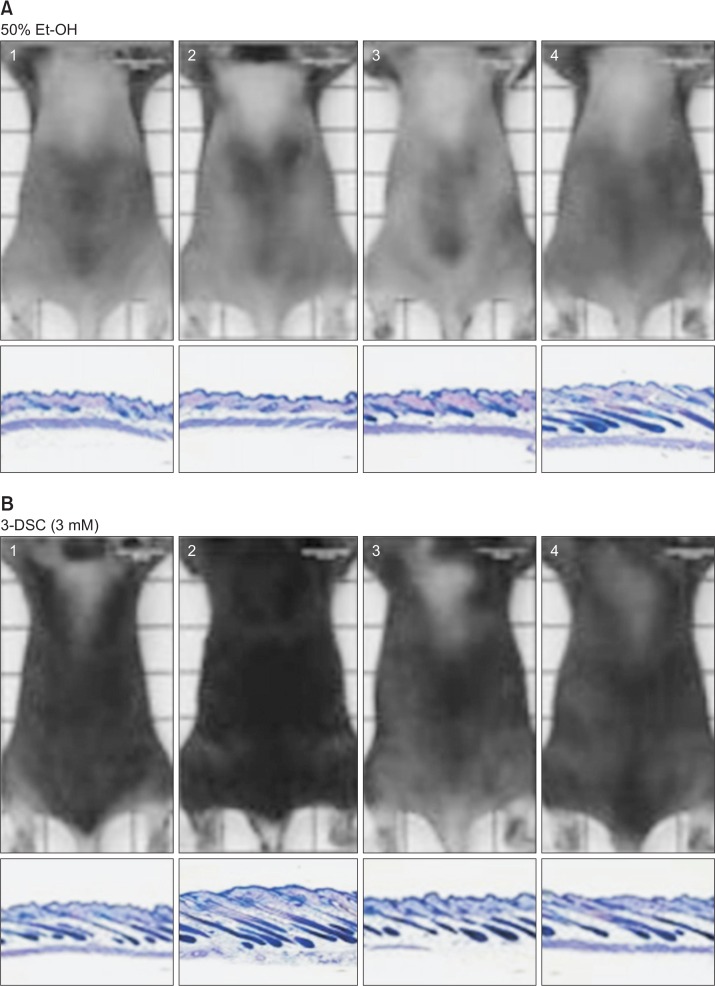
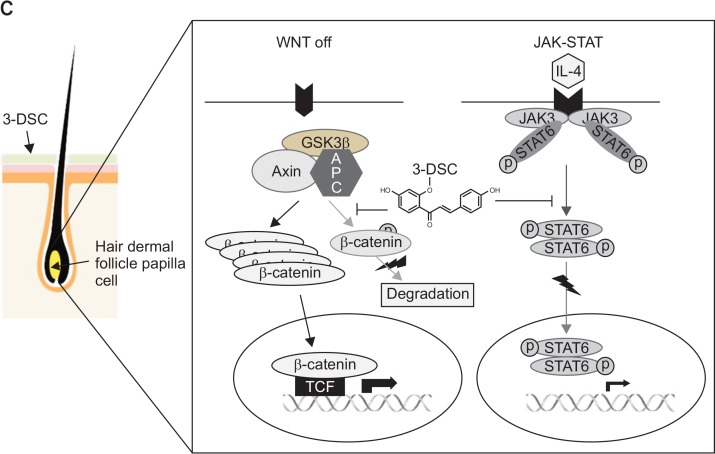
Finally, we attempted to examine the effects of 3-DSC on hair growth in mice. The back of C57BL/6 mice was shaved and topically treated with a vehicle (ethanol) or 3-DSC for 15 days. Compared to the vehicle control, 3-DSC promoted rapid and intense hair growth in mice (Fig. 5A, 5B). Histopathological analysis of mouse skin including the follicular and dermal layers at autopsy showed that the diameter and depth of the hair follicles were remarkably higher in mice that were administered with 3-DSC. A representative scheme of 3-DSC regulation of WNT/β-catenin and JAK-STAT pathway in human hair dermal follicle papilla cells (Fig. 5C).
Fig. 5.
Effect of 3-DSC on hair growth in C57BL/6 mice. After synchronizing the telogen phase, shaved backs of C57BL/6 mice were topically treated with 3-DSC or ethanol for 15 days. (A) Control, ethanol (50%). (B) 3-DSC (3 mM). Typical photos of dorsal skin (upper panel), histopathological analysis (lower panel). (C) A representative scheme of 3-DSC regulation of WNT/ β-catenin and JAK-STAT pathway in human hair dermal follicle papilla cells. The treatment of 3-DSC not only increased β-catenin and TCF/LEF, a cell growth regulatory transcriptional factor in nucleus, but also interfered with IL-4-JAK3-STAT6 pathway, which activated STAT6 transcription factor.
DISCUSSION
Caesalpinia sappan L. has been reported to have various beneficial pharmacological activities such as immune function modulation, depression of the central nervous system, anti-inflammation, and vasorelaxation. The present study was designed to investigate the effect of 3-DSC on hair growth and it revealed that 3-DSC promoted in vivo hair growth and modulated intracellular signaling pathways, implicated in hair cycle regulation. The remodeling of hair follicles involves cyclical periods of growth (anagen), regression (catagen), rest (telogen) and shedding (exogen) (Paus and Cotsarelis, 1999). Many follicles undergo programmed cell death during catagen that leads to reduced hair size at the beginning of the telogen phase (Cotsarelis, 1997). Follicular regeneration at the onset of the subsequent anagen phase requires activation of rarely cycling epithelial stem cells located in the permanent, bulge region of the follicle (Cotsarelis et al., 1990). This stem cell progeny forms a new follicular matrix during early anagen, and the hair shaft and inner root sheath are derived from these relatively undifferentiated matrix cells (Oshima et al., 2001). The size and length of the hair shaft correspond to the size of the hair follicle and to the duration of anagen, respectively. It is well established that STAT3 is one of the factors required for anagen onset (Sano et al., 2000). Activation of another intracellular signal pathway mediated via WNT-β-catenin is also required for the proliferation and differentiation of the hair shaft (Millar et al., 1999; Kishimoto et al., 2000; Cotsarelis and Millar, 2001). In fact, the volume of the dermal papilla reflects the number of matrix cells and it determines the size of the resulting hair shaft (Hardy, 1992). Because the size of the follicle is determined during the early stages of anagen, this could be a critical time for hair follicles undergoing miniaturization in androgenetic alopecia. Some factors (e.g. hormones, drugs, morphogens) might act by enhancing or preventing miniaturization only during this span of time at anagen onset, thereby requiring prolonged periods of time to alter a significant number of follicles. This might partially explain why the process of miniaturization takes years to both develop and treat.
During the progression of anagen stage, the maintenance of follicular epithelium requires transduction of signals from the dermal papilla to the follicular epithelium. Interestingly, dermal papilla cells cultured in the presence of β-catenin protein maintain their inductive abilities over many rounds of culture, suggesting that the epithelial signal is comprised of one or more WNT/β-catenin family members (Kishimoto et al., 2000; Cotsarelis and Millar, 2001). Thus, the activation of STAT3 and β-catenin appears to be the underlying mechanism of hair growth stimulation by 3-DSC. Besides STAT3, several other members of the STAT family and their upstream JAK kinase have been known to regulate hair growth. Several studies have reported that suppression of JAK signaling in mice activates a pro-growth/anti-quiescence signal during telogen (Plikus et al., 2008; Festa et al., 2011; Jahoda and Christiano, 2011), thereby allowing entry into anagen. In particular, Harel et al. (2015) reported that inhibition of JAK-STAT signaling promotes hair growth by stimulating the activation and/or proliferation of hair follicular stem cells, highlighting the role of this pathway in maintenance of hair follicular quiescence. Increased proliferation or differentiation of stem/progenitor cells upon inhibition of JAK-STAT signals is not unique only for hair follicular cells, but it has also been observed in other types of progenitor cells to the hair follicles. For example, the loss of STAT5 in hematopoietic stem cells induces exit from a quiescent state, leading to increased bone marrow-repopulating capacity after irradiation (Wang et al., 2009). Likewise, inhibition of JAK-STAT signaling improves skeletal muscle regeneration in aged mice by promoting expansion of symmetric satellite cells in culture and their engraftment in vivo (Price et al., 2014). These findings are consistent with the involvement of the JAK-STAT pathway in the maintenance of quiescence in the hair follicular cells (Lin et al., 2004) and the role of Stat3 in progression of the normal hair cycle in adult mice (Sano et al., 2000). Moreover, recent studies have shown that increased JAK-STAT signaling in aged mice inhibits hair follicular stem cell function in vitro (Doles et al., 2012). Goldstein et al. (2014) reported that quiescence of hair growth during pregnancy and lactation was partly mediated through prolactin-induced phosphorylation of Stat5. Therefore, JAK-STAT signaling appears to play a generalized role in promoting quiescence in adult stem cell populations.
As a first report, our study revealed that 3-DSC attenuated mRNA expression, and IL-4-induced phosphorylation and reporter gene activity of STAT6, which is a signaling molecule downstream of JAK3 in cultured HDPCs. Although our study showed that tofacitinib, a known JAK inhibitor, suppressed mRNA expression and IL4-induced phosphorylation of STAT6, whether 3-DSC, which has a similar effect, can attenuate JAK activation is yet to be examined. However, the present study delineates the effects of 3-DSC on STATs, which are effector signaling molecules downstream of JAK, thus indicating the key molecular aspect of hair follicle stimulation by 3-DSC via modulation of STATs. Histopathological analysis of mouse dermis also revealed that 3-DSC increased the diameter and depth of the hair follicle in the dermis. In conclusion, our study suggests that 3-DSC promotes proliferation of dermal papillae and stimulates hair growth partly via activation of Wnt/β-catenin signaling and inhibition of STAT6-mediated quiescence of hair follicular cells.
Acknowledgments
This research was financially supported by the Ministry of Trade, Industry and Energy (MOTIE, R0004663) and Korea Institute for Advancement of Technology (KIAT) through the Promoting Regional specialized Industry.
Footnotes
CONFLICT OF INTEREST
Authors declare no competing financial interests.
REFERENCES
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev. Cell. 2002;2:643–653. doi: 10.1016/S1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc. 2003;8:46–55. doi: 10.1046/j.1523-1747.2003.12171.x. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G. The hair follicle: dying for attention. Am J Pathol. 1997;151:1505–1509. [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Millar SE. Towards a molecular understanding of hair loss and its treatment. Trends Mol Med. 2001;7:293–301. doi: 10.1016/S1471-4914(01)02027-5. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-C. [DOI] [PubMed] [Google Scholar]
- Doles J, Storer M, Cozzuto L, Roma G, Keyes WM. Age-associated inflammation inhibits epidermal stem cell function. Genes Dev. 2012;26:2144–2153. doi: 10.1101/gad.192294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/S0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Goldstein J, Fletcher S, Roth E, Wu C, Chun A, Horsley V. Calcineurin/Nfatc1 signaling links skin stem cell quiescence to hormonal signaling during pregnancy and lactation. Genes Dev. 2014;28:983–994. doi: 10.1101/gad.236554.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-D. [DOI] [PubMed] [Google Scholar]
- Harel S, Higgins CA, Cerise JE, Dai Z, Chen JC, Clynes R, Christiano AM. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:e1500973. doi: 10.1126/sciadv.1500973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/S0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Jahoda CA, Christiano AM. Niche crosstalk: intercellular signals at the hair follicle. Cell. 2011;146:678–681. doi: 10.1016/j.cell.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Kang BM, Shin SH, Kwack MH, Shin H, Oh JW, Kim J, Moon C, Moon C, Kim JC, Kim MK, Sung YK. Erythropoietin promotes hair shaft growth in cultured human hair follicles and modulates hair growth in mice. J Dermatol Sci. 2010;59:86–90. doi: 10.1016/j.jdermsci.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Ke N, Wang X, Xu X, Abassi YA. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol Biol. 2011;740:33–43. doi: 10.1007/978-1-61779-108-6_6. [DOI] [PubMed] [Google Scholar]
- Kim JH, Choo YY, Tae N, Min BS, Lee JH. The anti-inflammatory effect of 3-deoxysappanchalcone is mediated by inducing heme oxygenase-1 via activating the AKT/mTOR pathway in murine macrophages. Int Immunopharmacol. 2014;22:420–426. doi: 10.1016/j.intimp.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- Kitagawa T, Matsuda K, Inui S, Takenaka H, Katoh N, Itami S, Kishimoto S, Kawata M. Keratinocyte growth inhibition through the modification of Wnt signaling by androgen in balding dermal papilla cells. J Clin Endocrinol Metab. 2009;94:1288–1294. doi: 10.1210/jc.2008-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotanides H, Reich NC. Interleukin-4-induced STAT6 recognizes and activates a target site in the promoter of the interleukin-4 receptor gene. J Biol Chem. 1996;271:25555–25561. doi: 10.1074/jbc.271.41.25555. [DOI] [PubMed] [Google Scholar]
- Lin KK, Chudova D, Hatfield GW, Smyth P, Andersen B. Identification of hair cycle-associated genes from timecourse gene expression profile data by using replicate variance. Proc Natl Acad Sci USA. 2004;101:15955–15960. doi: 10.1073/pnas.0407114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AL, Shu SH, Qin HL, Lee SM, Wang YT, Du GH. In vitro anti-influenza viral activities of constituents from Caesalpinia sappan. Planta Med. 2009;75:337–339. doi: 10.1055/s-0028-1112208. [DOI] [PubMed] [Google Scholar]
- Millar SE, Willert K, Salinas PC, Roelink H, Nusse R, Sussman DJ, Barsh GS. WNT signaling in the control of hair growth and structure. Dev Biol. 1999;207:133–149. doi: 10.1006/dbio.1998.9140. [DOI] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/S0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- Paus R, Foitzik K. In search of the “hair cycle clock”: a guided tour. Differentiation. 2004;72:489–511. doi: 10.1111/j.1432-0436.2004.07209004.x. [DOI] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price FD, von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J, Rudnicki MA. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat Med. 2014;20:1174–1181. doi: 10.1038/nm.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/S0925-4773(01)00452-X. [DOI] [PubMed] [Google Scholar]
- Sano S, Kira M, Takagi S, Yoshikawa K, Takeda J, Itami S. Two distinct signaling pathways in hair cycle induction: Stat3-dependent and -independent pathways. Proc Natl Acad Sci USA. 2000;97:13824–13829. doi: 10.1073/pnas.240303097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Zhang H, Lin H, Su H, Xing D, Du L. Brazilein protects the brain against focal cerebral ischemia reperfusion injury correlating to inflammatory response suppression. Eur J Pharmacol. 2007;558:88–95. doi: 10.1016/j.ejphar.2006.11.059. [DOI] [PubMed] [Google Scholar]
- Soma T, Fujiwara S, Shirakata Y, Hashimoto K, Kishimoto J. Hair-inducing ability of human dermal papilla cells cultured under Wnt/β-catenin signalling activation. Exp Dermatol. 2012;21:307–309. doi: 10.1111/j.1600-0625.2012.01458.x. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Sennett R, Rezza A, Clavel C, Grisanti L, Zemla R, Najam S, Rendl M. Wnt/β-catenin signaling in dermal condensates is required for hair follicle formation. Dev Biol. 2014;385:179–188. doi: 10.1016/j.ydbio.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li G, Tse W, Bunting KD. Conditional deletion of STAT5 in adult mouse hematopoietic stem cells causes loss of quiescence and permits efficient nonablative stem cell replacement. BZlood. 2009;113:4856–4865. doi: 10.1182/blood-2008-09-181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, de Jong A, Harel S, DeStefano GM, Rothman L, Singh P, Petukhova L, Mackay-Wiggan J, Christiano AM, Clynes R. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Zhou WL, Liu AL, Qin HL, Lee SM, Wang YT, Du GH. The protective effect of 3-deoxysappanchalcone on in vitro influenza virus-induced apoptosis and inflammation. Planta Med. 2012;78:968–973. doi: 10.1055/s-0031-1298620. [DOI] [PubMed] [Google Scholar]
- Yodsaoue O, Cheenpracha S, Karalai C, Ponglimanont C, Tewtrakul S. Anti-allergic activity of principles from the roots and heartwood of Caesalpinia sappan on antigen-induced β-hexosaminidase release. Phytother Res. 2009;23:1028–1031. doi: 10.1002/ptr.2670. [DOI] [PubMed] [Google Scholar]
- Youn UJ, Nam KW, Kim HS, Choi G, Jeong WS, Lee MY, Chae S. 3-Deoxysappanchalcone inhibits tumor necrosis factor-α-induced matrix metalloproteinase-9 expression in human keratinocytes through activated protein-1 inhibition and nuclear factor-kappa B DNA binding activity. Biol Pharm Bull. 2011;34:890–893. doi: 10.1248/bpb.34.890. [DOI] [PubMed] [Google Scholar]
- Yu BD, Mukhopadhyay A, Wong C. Skin and hair: models for exploring organ regeneration. Hum Mol Genet. 2008;17:R54–R59. doi: 10.1093/hmg/ddn086. [DOI] [PubMed] [Google Scholar]
- Zeidler C, von Kockritz A, Luger TA, Stander S, Husstedt IW, Tsianakas A. Tofacitinib, a novel JAK3 inhibitor, as a potential cause of distal symmetric polyneuropathy. J Eur Acad Dermatol Venereol. 2016;30:1066–1067. doi: 10.1111/jdv.13114. [DOI] [PubMed] [Google Scholar]