Abstract
Lonchocarpine is a phenylpropanoid compound isolated from Abrus precatorius that has anti-bacterial, anti-inflammatory, antiproliferative, and antiepileptic activities. In the present study, we investigated the antioxidant effects of lonchocarpine in brain glial cells and analyzed its molecular mechanisms. We found that lonchocarpine suppressed reactive oxygen species (ROS) production and cell death in hydrogen peroxide-treated primary astrocytes. In addition, lonchocarpine increased the expression of antioxidant enzymes, such as heme oxygenase-1 (HO-1), NAD(P)H:quinone oxidoreductase 1 (NQO1), and manganese superoxide dismutase (MnSOD), which are all under the control of Nrf2/antioxidant response element (ARE) signaling. Further, mechanistic studies showed that lonchocarpine increases the nuclear translocation and DNA binding of Nrf2 to ARE as well as ARE-mediated transcriptional activities. Moreover, lonchocarpine increased the phosphorylation of AMP-activated protein kinase (AMPK) and three types of mitogen-activated protein kinases (MAPKs). By treating astrocytes with each signaling pathway-specific inhibitor, AMPK, c-jun N-terminal protein kinase (JNK), and p38 MAPK were identified to be involved in lonchocarpine-induced HO-1 expression and ARE-mediated transcriptional activities. Therefore, lonchocarpine may be a potential therapeutic agent for neurodegenerative diseases that are associated with oxidative stress.
Keywords: Lonchocarpine, Astrocytes, Antioxidant enzymes, AMPK, MAPK, Nrf2/ARE signaling
INTRODUCTION
Astrocytes are the most abundant cell types in the brain and provide structural, trophic, and metabolic support to neurons in addition to modulating synaptic activity (Sofroniew and Vinters, 2010; Finsterwald et al., 2015). In particular, astrocytes are enriched with antioxidant enzymes and maintain a homeostatic environment for neurons by controlling oxidative stress (Drukarch et al., 1998; Vargas and Johnson, 2009). Reactive oxygen species (ROS), such as hydrogen peroxide, superoxide, and highly reactive hydroxyl radicals, contribute to DNA/protein/tissue damage, inflammation, and neuronal apoptosis (de Vries et al., 2008). In particular, ROS production is known to be associated with the generation of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis (Barnham et al., 2004; de Vries et al., 2008). Therefore, controlling oxidative stress is an important therapeutic strategy for treating neurodegenerative diseases.
Lonchocarpine is a phenylpropanoid compound isolated from Abrus precatorius, which is a broadly distributed tropical medicinal plant (Gul et al., 2013). Abrus precatorius have been shown to have anti-inflammatory, anti-bacterial, antiproliferative, and antiepileptic activities (Georgewill and Georgewill, 2009; Premanand and Ganesh, 2010; Gul et al., 2013). The extract of Abrus precatorius produces an anti-inflammatory response following topical application to the ear of the rat after inducing inflammation by treatment of croton oil. The amount of reduction of the inflammatory response by the extract was similar to that by acetylsalicylic acid (Georgewill and Georgewill, 2009). Moreover, the petroleum ether extract of Abrus precatorius exhibits neuroprotection against hypoxic neurotoxicity. The extract recovered decreased levels of dopamine while reducing glutamate levels and acetylcholinesterase activity in hypoxia-induced rat brains (Premanand and Ganesh, 2010). Lonchocarpine is one of the major components of Abrus precatorius. Recently, the biological effects of lonchocarpine were reported in several papers. Lonchocarpine showed gastroprotective effects by inhibiting H+,K+-ATPase activity (Reyes-Chilpa et al., 2006). It also showed acute anti-inflammatory and anti-oedematogenic properties in a paw oedema model (Fontenele et al., 2009). In addition, lonchocarpine has anti-bacterial activity on Staphylococcus aureus (Lima et al, 2013). A recent study reported that lonchocarpine inhibited nitric oxide production in lipopolysaccharidestimulated BV2 microglial cells (Li et al., 2015). However, the antioxidant effect of lonchocarpine in astrocytes has not been reported thus far.
Therefore, in the present study, we investigated the effects of lonchocarpine on ROS production and expression of antioxidant enzymes in primary cultured astrocytes. Lonchocarpine inhibited ROS production induced by hydrogen peroxide and suppressed astroglial cell death. In addition, lonchocarpine increased the expression of antioxidant enzymes by upregulating Nrf2/ARE signaling. Further, mechanistic studies showed that AMPK and MAPK pathways are involved in HO-1 expression by modulating Nrf2/ARE signaling. The data collectively suggest a therapeutic potential for lonchocarpine in various neurodegenerative diseases.
MATERIALS AND METHODS
Isolation of lonchocarpine from Abrus precatorius
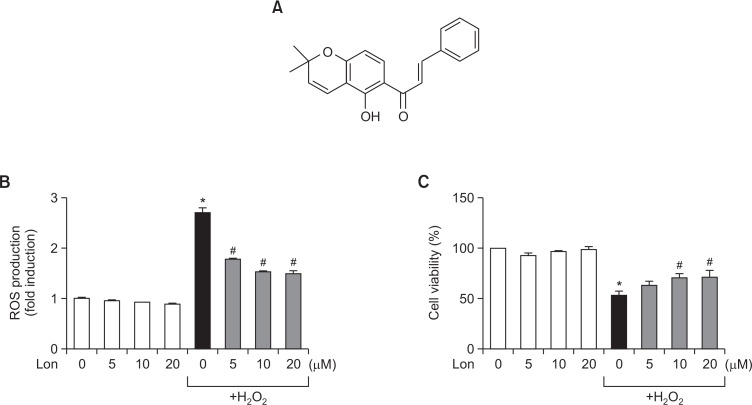
The root of Abrus precatorius (3 kg) was extracted three times with 80% MeOH under reflux at boiling temperature for 3 hours, filtered, and concentrated to obtain a MeOH extract (600 g). The MeOH extract was suspended in hot water and then partitioned with EtOAc (2.0 L×3) and BuOH (2.0 L×3) to generate EtOAc- (80 g) and BuOH- (350 g) soluble fractions. The BuOH fraction was chromatographed using a silica-gel column (40×15 cm) and eluted with a gradient of chloroform and MeOH (100:1 to 5:1) and then chloroform, MeOH, and water (65:35:15 to 0:100:0) to generate 12 fractions (B1-B12). Fraction B4 was used to generate four subfractions (B4.1-4.4) using a silica-gel column (30×7 cm) eluted with a gradient of chloroform and EtOAc (20:1-5:1), then chloroform and acetone (7:1-3:1), and finally chloroform and MeOH (5:1-0:100). Compound 1 (1.2 g) was isolated from these subfractions. This compound was identified as lonchocarpine by instrumental analysis and compared to an authentic standard described in the literature (Lima et al., 2013). The purity of lonchocarpine was more than 95%. The structure of lonchocarpine is shown in Fig. 1A.
Fig. 1.
Effects of lonchocarpine on ROS production in rat primary astrocytes. (A) Structure of lonchocarpine ((E)-1-(5-hydroxy-2,2-dimethyl-2H-chromen-6-yl)-3-phenylprop-2-en-1-one). (B) Rat primary astrocyte cells were treated with 5 to 20 μM lonchocarpine for 1 h, followed by treatment of H2O2 (50 μM) for 30 min. The intracellular ROS levels were then measured by the DCF-DA method. (C) Rat primary astrocyte viability was determined by the MTT assay after 24 h of H2O2 (50 μM) treatment. The data are expressed as the mean ± S.E.M. of three independent experiments. *p<0.05, significantly different from control samples; #p<0.05, significantly different from H2O2-treated samples.
Reagents
Antibodies against antioxidant enzymes (HO-1, NQO1, and MnSOD) and oligonucleotides for electrophoretic mobility shift assays (EMSAs) were purchased from Santa Cruz (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Antibodies against MAPKs and phosphorylated forms of AMPK were purchased from Cell Signaling Technology (Danvers, MA, USA). All reagents used for RT-PCR were purchased from Promega (Madison, WI, USA). Compound C (an AMPK inhibitor) and MAPK inhibitors (SB203580, PD98059, SP600125) were purchased from Merck-Millipore (Billerica, MA, USA). All other chemicals were obtained from Sigma Chemical Corporation (St. Louis, MO, USA) unless stated otherwise.
Rat primary astrocyte culture
Primary astrocyte cultures were prepared from mixed glial cultures by modifying a previously published method (Park et al., 2011). In brief, after cortices were dissected from 1-day-old rats, cells were dissociated by pipetting through pores of different sizes and resuspended in minimum essential medium (MEM) containing 10% fetal bovine serum, streptomycin (10 μg/mL), penicillin (10 U/mL), 2 mM glutamine, and 10 mM HEPES. Cell suspensions were plated on poly-D-lysine (1 μg/mL)-coated T75 flasks and incubated for 7 days. After the primary culture reached confluence, the culture flasks were shaken at 280 rev/min for 16 h to remove microglia and oligodendrocytes. Astrocyte-enriched culture purity (>95%) were confirmed by staining with antibodies against the astrocyte-specific marker glial fibrillary acidic protein (GFAP).
Measurement of intracellular ROS levels
Intracellular accumulation of ROS was measured using a modification of a previously described method (Park et al., 2011). In brief, astrocytes were stimulated with hydrogen peroxide (H2O2) for 30 min and then stained with 50 μM dichlorodi-hydrofluorescein diacetate (H2DCFDA) in phosphate-buffered saline (PBS) for 30 min at 37°C. DCF fluorescence intensities were measured at excitation and emission wavelengths of 485 and 535 nm, respectively, using a fluorescence plate reader (Molecular Devices, Palo Alto, CA, USA).
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared from astrocytes as previously described (Lee et al., 2015). Double-stranded DNA oligonucleotides containing the ARE consensus sequences (Santa Cruz) were end-labeled with [γ-32P]ATP. Thirty micrograms of the nuclear proteins were incubated with 32P-labeled ARE probes on ice for 30 min and resolved on a 5% acrylamide gel as previously described (Lee et al., 2015).
Western blot analysis
Cells were appropriately treated, and total cell lysates and nuclear extracts were prepared as described previously (Park et al., 2009; Lee et al., 2015). The proteins (20–50 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (GE Healthcare, Little Chalfont, UK). The membranes were blocked with 5% bovine serum albumin in 10 mM Tris-HCl containing 150 mM NaCl and 0.5% Tween-20 (TBST), then incubated with primary antibodies (1:1000) that recognize HO-1, NQO1, MnSOD, Nrf2, β-actin, or lamin A. We also used antibodies that recognize the phospho-forms of AMPK or MAPKs. After thoroughly washing with TBST, horseradish peroxidase-conjugated secondary antibodies (1:2000 dilution in TBST; BioRad, Hercules, CA, USA) were applied, and the blots were developed using an enhanced chemiluminescence detection kit (Thermo Fisher Scientific, Waltham, MA, USA).
RT-PCR
Primary astrocytes were treated with lonchocarpine, and total RNA was isolated with TRI reagent (Ambion, Austin, TX, USA). For RT-PCR, total RNA (1 μg) was reverse transcribed in a reaction mixture containing 1 U RNase inhibitor, 500 ng random primers, 3 mM MgCl2, 0.5 mM dNTP, 1× RT buffer, and 10 U reverse transcriptase (Promega, Madison, WI, USA). The synthesized cDNA was used as a template for PCR reaction using GoTaq polymerase (Promega) and primers as follows: 5′-TGTCACCCTGTGCTTGACCT-3′ (sense) and 5′-GCCATGAAGGAGGCTGCT GT-3′ (antisense) for HO-1; 5′-ATCACCAGGTCTGCAGCTTC-3′ (sense) and 5′-GAACCTTGGACTCCCACAGA-3′ (antisense) for NQO1; 5′-GGCCAAGGGAG ATGTTACAA-3′ (sense) and 5′-GAACCTTGGACTCCCACAGA-3′ (antisense) for MnSOD; 5′-GTGCTGAGTATGTCGTGGAGTC-3′ (sense) and 5′-ACAGTCTTCTG AGTGGCAGTCA-3′ (antisense) for GAPDH.
Transient transfection and luciferase assay
Rat primary astrocytes were plated on a 12-well plate at a density of 2.5×105 cells/ml, and transfected with 0.5 μg of plasmid DNA using Convoy™ Platinum transfection reagent (ACTGene, Inc., Kendall Park, NJ, USA). To determine the effect of lonchocarpine on ARE promoter activity, cells were treated with lonchocarpine and incubated for 16 h prior to harvesting cells, after which a luciferase assay was performed as previously described (Park et al., 2011).
Statistical analysis
Unless otherwise stated, all of the experiments were performed with triplicate samples and repeated at least three times. The data are presented as the mean ± S.E.M., and statistical comparisons between groups were performed using one-way analysis of variance followed by the Newman-Keuls test. A p-value of less than 0.05 was considered to be statistically significant.
RESULTS
Lonchocarpine inhibited ROS production in H2O2-treated primary astrocytes
To determine the antioxidant capacity of lonchocarpine, we examined the effects of lonchocarpine on ROS production in H2O2-treated astrocytes. We found that lonchocarpine significantly inhibited H2O2-induced ROS production (Fig. 1B). In addition, lonchocarpine attenuated astroglial cell death induced by H2O2 (Fig. 1C). The data suggest that lonchocarpine may have cytoprotective effects by inhibiting ROS production.
Lonchocarpine increased the expression of HO-1, NQO1, and MnSOD in astrocytes
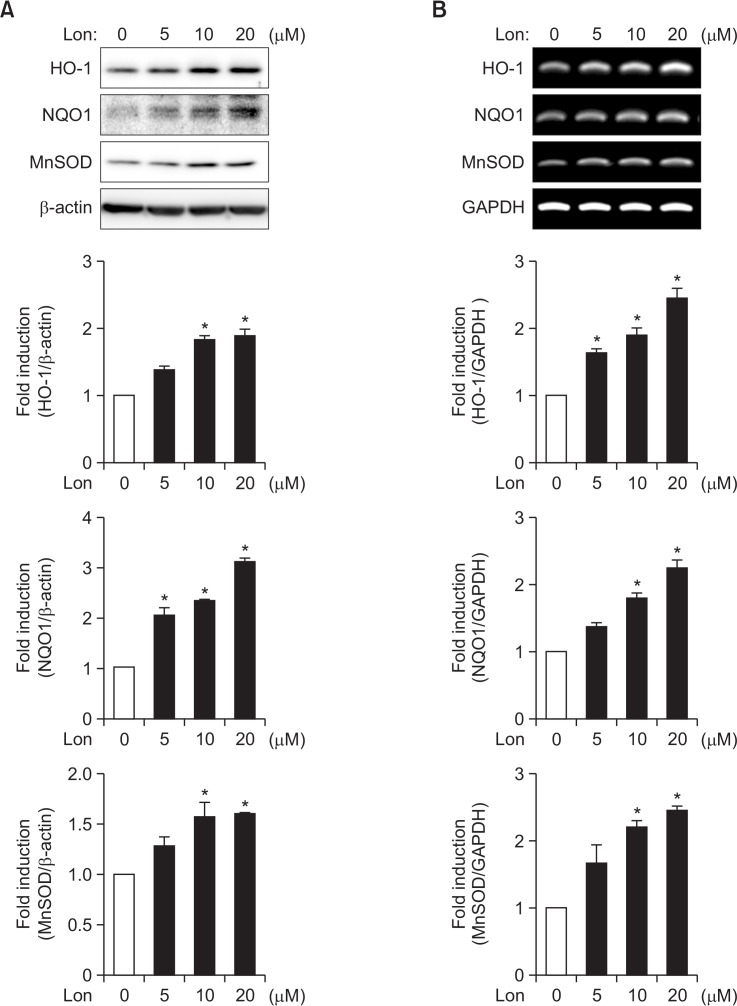
Next, we examined whether lonchocarpine induces the expression of antioxidant enzymes that play a pivotal role in cellular defense mechanisms against oxidative stress (Sypin, 2008; Vargas and Johnson, 2009). Using western blot analysis we found that lonchocarpine increased the protein expression of antioxidant enzymes such as HO-1, NQO1, and MnSOD in rat primary astrocytes (Fig. 2A). In addition, lonchocarpine upregulated the expression of those enzymes at the mRNA level as shown by RT-PCR analysis (Fig. 2B). The data suggest that lonchocarpine regulates antioxidant enzyme genes at a transcriptional level.
Fig. 2.
Effect of lonchocarpine on anti-oxidant enzyme expression in rat primary astrocytes. (A) Cells were incubated with lonchocarpine for 16 h, after which protein expression of antioxidant enzymes was determined by Western blotting. (B) Cells were treated with various concentrations of lonchocarpine for 6 h and total RNA was isolated. The HO-1, NQO1, and MnSOD mRNA levels were determined by RT-PCR analysis. The data shown are representative of three independent experiments. Quantification data are shown at the bottom of each panel. *p<0.05, significantly different from control sample.
Lonchocarpine increased nuclear protein binding to ARE and ARE-mediated transcriptional activities in astrocytes
To further investigate the molecular mechanisms underlying upregulation of antioxidant enzymes by lonchocarpine, we examined the effects of lonchocarpine on Nrf2/ARE signaling, which plays a key role in the gene expression of antioxidant enzymes such as HO-1, NQO1, and MnSOD (Vargas and Johnson, 2009). In particular, Nrf2 is known to bind to the ARE sequence on the promoter of antioxidant enzyme genes and regulate their expression (Jaiswal, 2004). In the present study, we found that lonchocarpine increased nuclear protein binding to ARE (Fig. 3A). Transient transfection analysis showed that lonchocarpine also increased ARE-mediated transcriptional activities in astrocytes (Fig. 3B). In support of this, lonchocarpine increased translocation of Nrf2 into the nuclei of astrocytes (Fig. 3C). The data suggest that lonchocarpine increases antioxidant enzyme gene expression by enhancing Nrf2/ARE signaling.
Fig. 3.
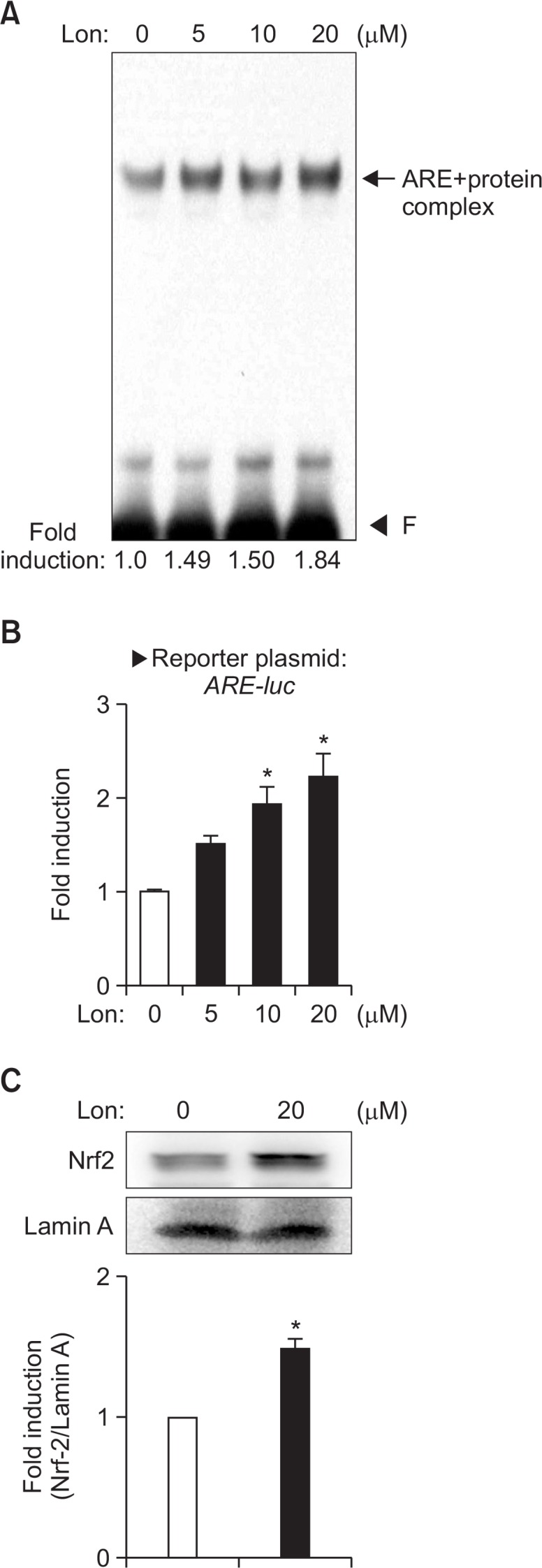
Effect of lonchocarpine on nuclear protein binding to ARE and ARE-mediated transcriptional activities. The nuclear extracts were isolated from cells treated with 5 to 20 μM lonchocarpine for 3 h. (A) The extracts were incubated with a 32P-labeled ARE probe. The arrow indicates the ARE-nuclear protein complex. ‘F’ indicates free probe. (B) Primary astrocytes were transfected with ARE-luc reporter plasmids and treated with lonchocarpine. After 16 h, cells were harvested and luciferase assays were performed. (C) The nuclear extracts were assessed by Western blot using antibodies against Nrf2. The data shown are representative of three independent experiments. Values correspond to the mean ± S.E.M. of three independent experiments. *p<0.05, compared with control cells.
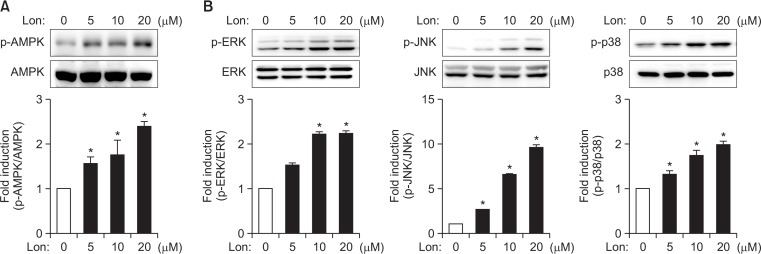
AMPK and MAPK signaling are involved in antioxidant enzyme expression by modulating the Nrf2/ARE axis in lonchocarpine-treated astrocytes
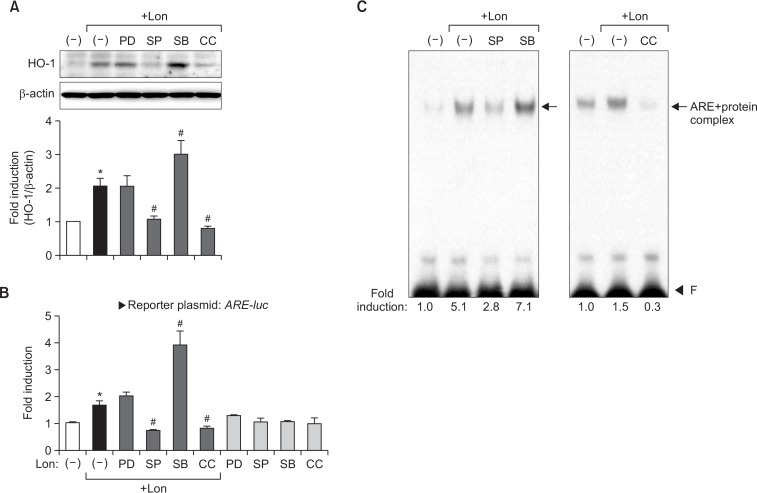
Previous studies have reported that phase II antioxidant enzyme expression is under the control of AMPK, PI3K, MAPK, or protein kinase C signaling pathways in various cell types (Jaiswal, 2004; Niture et al, 2010). Here, we performed western blot analysis to determine the signaling pathways involved in antioxidant enzyme gene expression in lonchocarpine-treated cells. As shown in Fig. 4, lonchocarpine increased the phosphorylation of AMPK and three types of MAPKs. To determine whether AMPK and MAPKs mediate the expression of HO-1 as a representative of antioxidant enzymes, cells were treated with each signaling pathway-specific inhibitor before stimulation with lonchocarpine. We found that treatment with AMPK or a JNK inhibitor (compound C, SP600125) suppressed HO-1 expression in lonchocarpine-treated astrocytes (Fig. 5A). However, an ERK inhibitor (PD98059) did not affect the HO-1 expression. Intriguingly, treatment with a p38 inhibitor (SB203580) increased HO-1 expression. When we examined the effects of these inhibitors on Nrf2/ARE, similar patterns of changes were observed; Nrf2 DNA-binding and ARE reporter gene activities induced by lonchocarpine were decreased by compound C and SP600125, increased by SB203580, and unaltered by PD98059 (Fig. 5B, 5C). We observed that the inhibitor-only condition did not show any change in the basal level of HO-1 expression or ARE-mediated transcription (data not shown & Fig. 5B). The data suggest that lonchocarpine-induced HO-1 expression is positively regulated by JNK and AMPK, while negatively regulated by p38 MAPK via Nrf2/ARE signaling.
Fig. 4.
Effects of lonchocarpine on AMPK and MAPK phosphorylation. Cell extracts were prepared from astrocytes treated with lonchocarpine for 1 h and they were subjected to immunoblot analysis using antibodies against phospho-or total forms of AMPK (A) or three types of MAP kinases (ERK1/2, JNK, p38 MAPK) (B). The blots shown are representative of three independent experiments. Phosphorylation levels of AMPK and MAP kinases were quantified by densitometry and expressed as fold inductions (bottom panel). *p<0.05, compared with control cells.
Fig. 5.
Effects of AMPK or MAPK inhibitors on ARE-mediated HO-1 expression in lonchocarpine-treated astrocytes. (A) Effects of AMPK and MAPK inhibitors on HO-1 protein expression. Cells were treated with the inhibitors, followed by treatment with lonchocarpine (20 μM) for 16 h, after which western blot analyses were performed. The blots shown are representative of three independent experiments. Quantification data are shown at the bottom panel. (B) Effects of AMPK and MAPK inhibitors on ARE-mediated transcriptional activity. Primary astrocytes were transfected with ARE-luc and treated with lonchocarpine (20 μM) in the presence or absence of inhibitors. (C) Effect of inhibitors on nuclear protein binding to ARE. CC; compound C (AMPK inhibitor, 10 μM), SP; SP600125 (JNK inhibitor, 20 μM), SB; SB203580 (p38 inhibitor, 20 μM), PD; PD98059 (ERK inhibitor, 20 μM). *p<0.05, compared with control samples. #p<0.05, compared with lonchocarpine-treated samples.
DISCUSSION
In the present study, we demonstrated that lonchocarpine inhibited ROS production in H2O2-treated rat primary astrocytes. Lonchocarpine increased anti-oxidant enzymes such as HO-1, NQO1, and MnSOD by enhancing Nrf2/ARE signaling. In addition, AMPK, JNK and p38 signaling pathways were identified to be involved in HO-1 expression by modulating Nrf2 binding and ARE-mediated transcriptional activities in lonchocarpine-treated astrocyte cells.
Several studies have reported that the Nrf2/ARE signaling pathway is a promising therapeutic target for neuroinflammation and brain disorders as it plays a key role as a regulator of cellular redox status (Syapin, 2008; Johnson and Johnson, 2015). Nrf2/ARE signaling governs phase II antioxidant gene expression such as HO-1 and NQO1, and Nrf2 activation has shown neuroprotective effects in animal models of cerebral ischemia, Parkinson’s disease, Alzheimer’s disease, and amyotrophic lateral sclerosis (Vargas et al., 2008; Calkins et al., 2009; Johnson and Johnson, 2015). Thus, the upregulation of Nrf2/ARE signaling by lonchocarpine may contribute to neuroprotection against oxidative stress.
Recently, AMPK activation has been considered a potential therapeutic target against oxidative stress-induced neuronal damage (de Vries et al., 2008; Carling et al., 2012). AMPK is a serine/threonine kinase that plays a pivotal role in redox signaling and cellular energy homeostasis (Zang et al., 2004; Zimmermann et al., 2015). It has been shown that AMPK has neuroprotective effects by reducing oxidative damage and recovering normal brain function in cerebral ischemia (Ronnett et al., 2009). In addition, AMPK protects against cholesterol-induced and amyloid β-accumulated neurotoxicity (Martinez de Morentin et al., 2010; Vingtdeux et al., 2010). Several papers have demonstrated the interaction of AMPK and Nrf2 signaling. AMPK has been shown to induce HO-1 expression via Nrf2/ARE signaling in mouse embryonic fibroblasts and endothelial cells (Liu et al., 2011; Zimmermann et al., 2015). AMPK also plays an important role in the anti-inflammatory effects of berberine by modulating Nrf2 in macrophages (Mo et al., 2014). In accordance with these results, our present study showed that AMPK plays a critical role in HO-1 expression by modulating Nrf2 signaling in lonchocarpine-treated astrocyte cells.
MAPK signaling pathways are also related to the upregulation of phase II antioxidant enzymes (Jaiswal, 2004; Niture et al., 2010). For example, JNK induces Nrf2 binding to ARE and improves endogenous antioxidant responses in murine macrophages (Xu et al., 2006; Vari et al., 2011). The ERK and p38 MAPK have been implicated in HO-1 activation by arsenite in hepatocytes (Elbirt et al., 1998; Ryter et al., 2002). In addition, many studies implied a combined role of more than one MAPK pathway in gaining optimal gene expression (Chen and Maines, 2000; Ryter et al., 2006). Furthermore, the studies indicate that there is considerable variation in the type of MAPK(s) involved in HO-1 activation in an inducer-specific, cell type-specific and species-specific manner (Ryter et al., 2006). In the present study, we found that lonchocarpine induced phosphorylation of three types of MAPKs. By treating astrocytes with specific inhibitors, we found that JNK is responsible for HO-1 upregulation via Nrf2/ARE. In contrast, p38 MAPK appears to be involved in HO-1 expression in the opposite manner, and ERK is not involved in this regulation. Therefore, the coordinated regulation by AMPK, MAPKs, and other signaling pathways may result in the final outcome of upregulation of antioxidant enzyme expression in lonchocarpine-treated astrocytes. Further study is necessary to clarify the mechanisms underlying this differential regulation of HO-1 by MAPKs and other signaling pathways.
In conclusion, the present study has demonstrated that lonchocarpine has antioxidant effects and that it upregulates antioxidant enzyme expression by modulating AMPK, MAPKs, and Nrf2 signaling pathways. The antioxidant effects of lonchocarpine may have potential therapeutic utility for various neurodegenerative diseases.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (Grant #2010-0027945).
REFERENCES
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Calkins MJ, Johnson DA, Townsend JA, Vargas MR, Dowell JA, Williamson TP, Kraft AD, Lee JM, Li J, Johnson JA. The Nrf2/ARE pathway as a potential therapeutic target in neurodengenerative disease. Antioxid Redox Signal. 2009;11:497–508. doi: 10.1089/ars.2008.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D, Thornton C, Woods A, Sanders MJ. AMP-activated protein kinase: new regulation, new roles? Biochem J. 2012;445:11–27. doi: 10.1042/BJ20120546. [DOI] [PubMed] [Google Scholar]
- Chen K, Maines MD. Nitric oxide induces heme oxygenase-1 via mitogen-activated protein kinases ERK and p38. Cell Mol Biol. 2000;46:609–617. [PubMed] [Google Scholar]
- de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Rad Biol Med. 2008;45:1375–1383. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Drukarch B, Schepens E, Stoof JC, Langeveld CH, Van Muiswinkel FL. Astrocyte-enhanced neuronal survival is mediated by scavenging of extracellular reactive oxygen species. Free Rad Biol Med. 1998;25:217–220. doi: 10.1016/S0891-5849(98)00050-1. [DOI] [PubMed] [Google Scholar]
- Elbirt KK, Whitmarsh AJ, Davis RJ, Bonkosky HL. Mechanism of sodium arsenite-mediated induction of heme oxygenase-1 in hepatoma cells. Role of mitogen-activated protein kinases. J Biol Chem. 1998;273:8922–8931. doi: 10.1074/jbc.273.15.8922. [DOI] [PubMed] [Google Scholar]
- Finsterwald C, Magistretti PJ, Lengacher S. Astrocytes: New targets for the treatment of neurodegenerative diseases. Curr Pharm Des. 2015;21:3570–3581. doi: 10.2174/1381612821666150710144502. [DOI] [PubMed] [Google Scholar]
- Fontenele JB, Leal LK, Felix FH, Silveira ER, Viana GS. Studies on the anti-oedematogenic properties of a fraction rich in lonchocarpin and derricin isolated from Lonchocarpus sericeus. Nat Prod Res. 2009;23:1677–1688. doi: 10.1080/14786410802181745. [DOI] [PubMed] [Google Scholar]
- Georgewill OA, Georgewill UO. Evaluation of the antiinflammatory activity of extract of Abrus precatorious. East J Med. 2009;14:23–25. [Google Scholar]
- Gul MZ, Ahmad F, Kondapi AK, Qureshi IA, Ghazi IA. Antioxidant and antiproliferative activities of Abrus precatorius leaf extracts--an in vitro study. BMC Complement Altern Med. 2013;13:53. doi: 10.1186/1472-6882-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Rad Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Johnson JA. Nrf2-a therapeutic target for the treatment of neurodegenerative diseases. Free Rad Biol Med. 2015;88:253–267. doi: 10.1016/j.freeradbiomed.2015.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Ko HM, Jeong YH, Park EM, Kim HS. β-Lapachone suppresses neuroinflammation by modulating the expression of cytokines and matrix metalloproteinases in activated microglia. J. Neuroinflammation. 2015;12:133. doi: 10.1186/s12974-015-0355-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Jiang Z, Li X, Hou Y, Liu F, Li N, Liu X, Yang L, Chen G. Natural therapeutic agents for neurodegenerative diseases from a traditional herbal medicine Pongamia pinnata (L.) Pierre. Bioorg Med Chem Lett. 2015;25:53–58. doi: 10.1016/j.bmcl.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Lima NM, Andrade JI, Lima KC, dos Santos FN, Barison A, Salome KS, Matsuura T, Nunez CV. Chemical profile and biological activities of Deguelia duckeana A.M.G. Azevedo (Fabaceae) Nat Prod Res. 2013;27:425–432. doi: 10.1080/14786419.2012.733387. [DOI] [PubMed] [Google Scholar]
- Liu XM, Peyton KJ, Shebib AR, Wang H, Korthuis RJ, Durante W. Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am J Physiol Heart Circ Physiol. 2011;300:H84–93. doi: 10.1152/ajpheart.00749.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez de Morentin PB, González CR, López M. AMP-activated protein kinase: ‘a cup of tea’ against cholesterolinduced neurotoxicity. J Pathol. 2010;222:329–334. doi: 10.1002/path.2778. [DOI] [PubMed] [Google Scholar]
- Mo C, Wang L, Zhang J, Numazawa S, Tang H, Tang X, Han X, Li J, Yang M, Wang Z, Wei D, Xiao H. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014;20:574–588. doi: 10.1089/ars.2012.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niture SK, Kaspar JW, Shen J, Jaiswal AK. Nrf2 signaling and cell survival. Toxicol App Pharmacol. 2010;244:37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Jung JS, Jeong YH, Hyun JW, Le TK, Kim DH, Choi EC, Kim HS. Antioxidant mechanism of isoflavone metabolites in hydrogen peroxide-stimulated rat primary astrocytes: critical role of hemeoxygenase-1 and NQO1 expression. J Neurochem. 2011;119:909–919. doi: 10.1111/j.1471-4159.2011.07395.x. [DOI] [PubMed] [Google Scholar]
- Park JS, Park EM, Kim DH, Jung K, Jung JS, Lee EJ, Hyun JW, Kang JL, Kim HS. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J Neuroimmunol. 2009;209:40–49. doi: 10.1016/j.jneuroim.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Premanand R, Ganesh T. Neuroprotective effects of Abrus precatorius Linn. aerial extract on hypoxic neurotoxicity induced rats. Int J Chem Pharmac Sci. 2010;1:9–15. [Google Scholar]
- Reyes-Chilpa R, Baggio CH, Alavez-Solano D, Estrada-Muniz E, Kauffman FC, Sanchez RI, Mesia-Vela S. Inhibition of gastric H+,K+-ATPase activity by flavonoids, coumarins and xanthones isolated from Mexican medicinal plants. J Ethnopharmacol. 2006;105:167–172. doi: 10.1016/j.jep.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J. Neurochem. 2009;109 Suppl(1):17–23. doi: 10.1111/j.1471-4159.2009.05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Xi S, Hartsfield CL, Choi AM. Mitogen activated protein kinase (MAPK) pathway regulates heme oxygenase-1 gene expression by hypoxia in vascular cells. Antioxid Redox Signal. 2002;4:587–592. doi: 10.1089/15230860260220085. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syapin P. Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Brit J Pharmacol. 2008;155:623–640. doi: 10.1038/bjp.2008.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vari R, D’Archivio M, Filesi C, Carotenuto S, Scazzocchio B, Santangelo C, Giovannini C, Masella R. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J Nutr Biochem. 2011;22:409–417. doi: 10.1016/j.jnutbio.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Yuan X, Pan Z, Shen G, Kim JH, Yu S, Khor TO, Li W, Ma J, Kong AN. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol Cancer Ther. 2006;5:1918–1926. doi: 10.1158/1535-7163.MCT-05-0497. [DOI] [PubMed] [Google Scholar]
- Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA. AMPactivated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004;279:47898–47905. doi: 10.1074/jbc.M408149200. [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Baldinger J, Mayerhofer B, Atanasov AG, Dirsch VM, Heiss EH. Activated AMPK boosts the Nrf2/HO-1 signaling axis-A role for the unfolded protein response. Free Rad Biol Med. 2015;88:417–426. doi: 10.1016/j.freeradbiomed.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]