Abstract
Quercetin, a flavonol, has been reported to exhibit a wide range of biological properties including anti-oxidant and anti-inflammatory activities. However, pharmacological properties of quercetin-3-O-β-D-glucuronide (QG), a glycoside derivative of quercetin, have not been extensively examined. The objective of this study is to elucidate the anti-inflammatory property and underlying mechanism of QG in lipopolysaccharide (LPS)-challenged RAW264.7 macrophage cells in comparison with quercetin. QG significantly suppressed LPS-induced extracellular secretion of pro-inflammatory mediators such as nitric oxide (NO) and PGE2, and pro-inflammatory protein expressions of iNOS and COX-2. To elucidate the underlying mechanism of the anti-inflammatory property of QG, involvement of MAPK signaling pathways was examined. QG significantly attenuated LPS-induced activation of JNK and ERK in concentration-dependent manners with a negligible effect on p38. In conclusion, the present study demonstrates QG exerts anti-inflammatory activity through the suppression of JNK and ERK signaling pathways in LPS-challenged RAW264.7 macrophage cells.
Keywords: Quercetin-3-O-β-D-glucuronide, Quercetin, RAW264.7 cells, Lipopolysaccharide, JNK, ERK
INTRODUCTION
Quercetin is a typical antioxidant dietary flavonoid that is ubiquitous in may plant foods such as buckwheat, onion, and tea, and medicinal plants (Hertog et al., 1993; Wang et al., 2009). Although quercetin, the aglycone form of the flavonoid, has been reported to exhibit a wide range of biological properties including anticancer, anti-oxidant, and anti-inflammatory activities (Jagtap et al., 2009; Pocernich et al., 2011), quercetin is mainly present in the form of a glycoside. Pharmacological properties of quercetin glycosides have not been extensively examined. Recently, it has been reported that avicularin, quercetin-3-α-L-arabinofuranoside, exerts antioxidant and anti-inflammatory activity (Vo et al., 2012). Quercetin-3-O-β-D-glucuronide (QG) has been reported to anti-cancer (Yamazaki et al., 2014), anti-aging (Yang et al., 2014), and anti-oxidant properties (Messer et al., 2015). However, the underlying mechanism by which QG exerts its pharmacological activity has not been clearly demonstrated. QG has been reported to be present in various medicinal herbs including Polygonum perfoliatum (Fan et al., 2014) and Polygonum aviculare (Yang et al., 2014). QG, used in the present study, was isolated from the stem of Persicaria thunbergii (Kim et al., 2014).
Macrophages play essential roles in the mobilization of the host defense against bacterial infection (Rehman et al., 2012). However, aberrant activation of macrophages has been reported to play detrimental roles in many inflammatory disorders including sepsis (Kim et al., 2012). In pathogenic conditions, abnormally activated macrophages produce excessive amount of a variety of pro-inflammatory mediators and cytokines that eventually aggravates the inflammatory conditions (Itharat and Hiransai, 2012). Bacterial activation of macrophages has been reported to cause a wide range of pro-inflammatory responses including secretion of pro-inflammatory mediators, expression of adhesion molecules and coagulation factors, phagocytosis, and cytoskeletal rearrangement (Sweet and Hume, 1996). Therefore, the inhibition of aberrant macrophage activation might be a valuable therapeutic target for the treatment of inflammatory disorders.
Mitogen-activated protein (MAP) kinase signaling pathway have been reported to be implicated in a wide range of inflammatory responses (Guha and Mackman, 2001; Rushworth et al., 2005). c-Jun N-terminal kinase (JNK) of MAP kinases has been reported to be implicated in the pathogenesis of various inflammatory disorders including sepsis (Ip and Davis, 1998; Supinski et al., 2009). JNK activation contributes to the progression of inflammation-induced cell dysfunction and activation of caspases in several organs through the transcription factor c-Jun (Cho and Choi, 2002; Wang et al., 2004). Recently, it has been reported that suppression of JNK activation with aromadendrin and N-(p-coumaryol)-tryptamine significantly attenuates lipopolysaccharide (LPS)-induced inflammatory responses in RAW264.7 cells (Lee et al., 2013; Vo et al., 2014). It has been also demonstrated that avicularin significantly suppressed LPS-induced ERK phosphorylation in RAW264.7 cells (Vo et al., 2012).
The present study was aimed to examine the possibility that QG possesses the anti-inflammatory activity in LPS-challenged RAW264.7 cells and to understand its underlying mechanism by which QG exerts the anti-inflammatory property in order to provide a valuable pharmacological agent that suppresses aberrantly activated macrophages in inflammatory conditions.
MATERIALS AND METHODS
Reagents and cell culture
Bacterial lipopolysaccharide (LPS) from Escherichia coli serotype 055:B5 was purchased from Sigma-Aldrich (St. Louis, MO, USA). Quercetin-3-O-β-D-glucuronide (QG) (Fig. 1) was isolated and identified from Persicaria thunbergii (Fig. 1) (Kim et al., 2014). QG was dissolved in ethanol and added to the cell culture at the desired concentrations. The macrophage RAW264.7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, Grand Island, NY, USA) containing 5% heat-inactivated fetal bovine serum and penicillin-streptomycin (Gibco BRL) at 37°C, 5% CO2. In all experiments, cells were incubated in the presence of the indicated concentrations of QG before the addition of LPS (200 ng/ml).
Fig. 1.
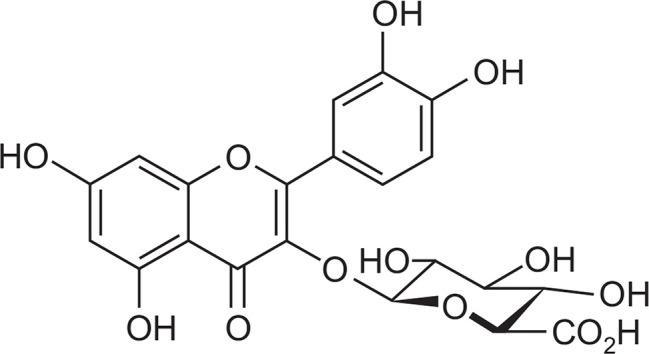
Chemical structure of quercetin-3-O-β-D-glucuronide.
Cell viability
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. RAW264.7 macrophage cells were seeded at 5×105 cells per well and incubated with CT at various concentrations for 24 hr at 37°C. After incubation, MTT (0.5 mg/ml in PBS) was added to each well, and the cells were incubated for 3 hr at 37°C and 5% CO2. The resulting formazan crystals were dissolved in dimethyl sulfoxide (DMSO). Absorbance was determined at 540 nm. The results were expressed as a percentage of surviving cells over control cells.
Nitrite quantification assay
The production of NO was estimated by measuring the amount of nitrite, a stable metabolite of NO, using the Griess reagent as described (Lee et al., 2012). After QG-pretreated RAW264.7 macrophage cells were stimulated with LPS in 12-well plates for 24 hr, 100 μL of the cell supernatant was mixed with an equal volume of Griess reagent. Light absorbance was read at 540 nm. The results were expressed as a percentage of released NO from LPS-stimulated RAW264.7 cells. To prepare a standard curve, sodium nitrite was used to prepare a standard curve.
ELISA assay for cytokines
The RAW264.7 macrophage cells were treated with QG in the absence or presence of LPS. After 24 hr incubation, TNF-α and IL-1β levels in culture media were quantified using monoclonal anti-TNF-α or IL-1β antibodies according to the manufacturer’s instruction (R&D Systems, Minneapolis, MN, USA).
Western blot analysis
The RAW264.7 macrophage cells were incubated with QG for 1 hr prior to LPS treatment. Cells were washed with PBS and lysed in PRO-PREP lysis buffer (iNtRON Biotechnology, Seongnam, Republic of Korea). Equal amounts of protein were separated on 10% SDS-polyacrylamide gel. Proteins were transferred to Hypond PVDF membrane (Amersham Biosciences, Piscataway, NJ, USA) and blocked in 5% skim milk in TBST for 1 hr at room temperature. Specific antibodies against inducible NO synthase (iNOS), COX-2, extracellular signal-regulated kinase (ERK), phosphorylated (p)-ERK, p38, p-p38 (1:1,000; Cell signaling Technology, Danvers, MA, USA), and β-actin (1:2,500; Sigma-Aldrich) were diluted in 5% skim milk. After thoroughly washing with TBST, horseradish peroxidase-conjugated secondary antibodies were applied. The blots were developed by the enhanced chemiluminescence detection (Amersham Biosciences).
Statistical analysis
All values shown in the figures are expressed as the mean ± SD obtained from at least three independent experiments. Statistical significance was analyzed by two-tailed Student’s t-test. Data with values of p<0.05 were considered as statistically significant. Single (* and #) and double (** and ##) marks represent statistical significance in p<0.05 and p<0.01, respectively.
RESULTS
Quercetin-3-O-β-D-glucuronide (QG) inhibits NO and PGE2 release in LPS-stimulated RAW264.7 macrophage cells
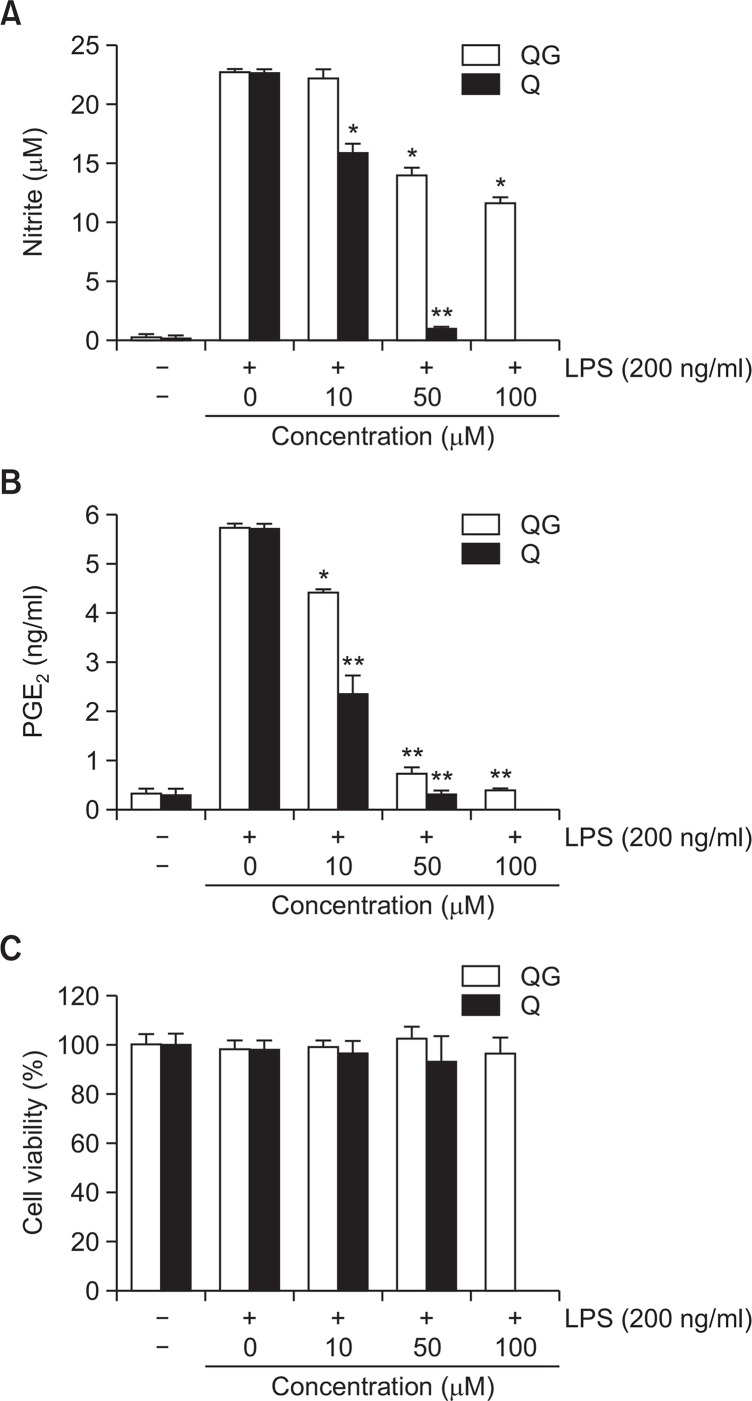
Pro-inflammatory mediators such as NO and PGE2 have been known to play key roles in the progression of inflammation (Ock et al., 2009; Lee et al., 2012), the effects of QG on the extracellular secretion of NO and PGE2 were examined in LPS-challenged RAW264.7 macrophages. Cells were incubated with QG or with a reference compound, quercetin, for 1 hr prior to LPS treatment (200 ng/ml). LPS treatment exhibited significant extracellular release of NO and PGE2 in RAW264.7 cells. However, QG significantly attenuated extracellular release of NO and PGE2 in LPS-stimulated RAW264.7 cells in concentration-dependent manners, although qeurcetin, the aglycone compound of QG, exhibited potentiated effects compared to QG (Fig. 2A, 2B). In addition, noticeable cytotoxicity was not observed with QG in concentration ranges used in the study (Fig. 2C).
Fig. 2.
Effects of QG on LPS-induced extracellular release of NO (A) and PGE2. (B) in RAW264.7 macrophage cells. RAW264.7 cells were pretreated with indicated concentrations of QG for 1 hr before incubation with LPS (200 ng/ml) for 24 hrs. The level of nitrite and PGE2 were measured using Griess reagent and ELISA assay, respectively. QG significantly suppressed LPS-induced extracellular secretion of NO and PGE2. (C) Effect of QG on the viability of RAW264.7 cells. No significant cell death was observed with QG concentrations used in the present study. The data were obtained from three independent experiments and expressed as mean ± SD (n=3). *p<0.05 and **p<0.01 indicate statistically significant differences from treatment with LPS alone. QC and Q stand for quercetin-3-O-β-D-glucuronide and quercetin, respectively. Quercetin was used as a reference. Examination of quercetin at 100 μM concentration was not done due to the decreased cell viability.
QG suppresses LPS-induced expressions of iNOS and COX-2
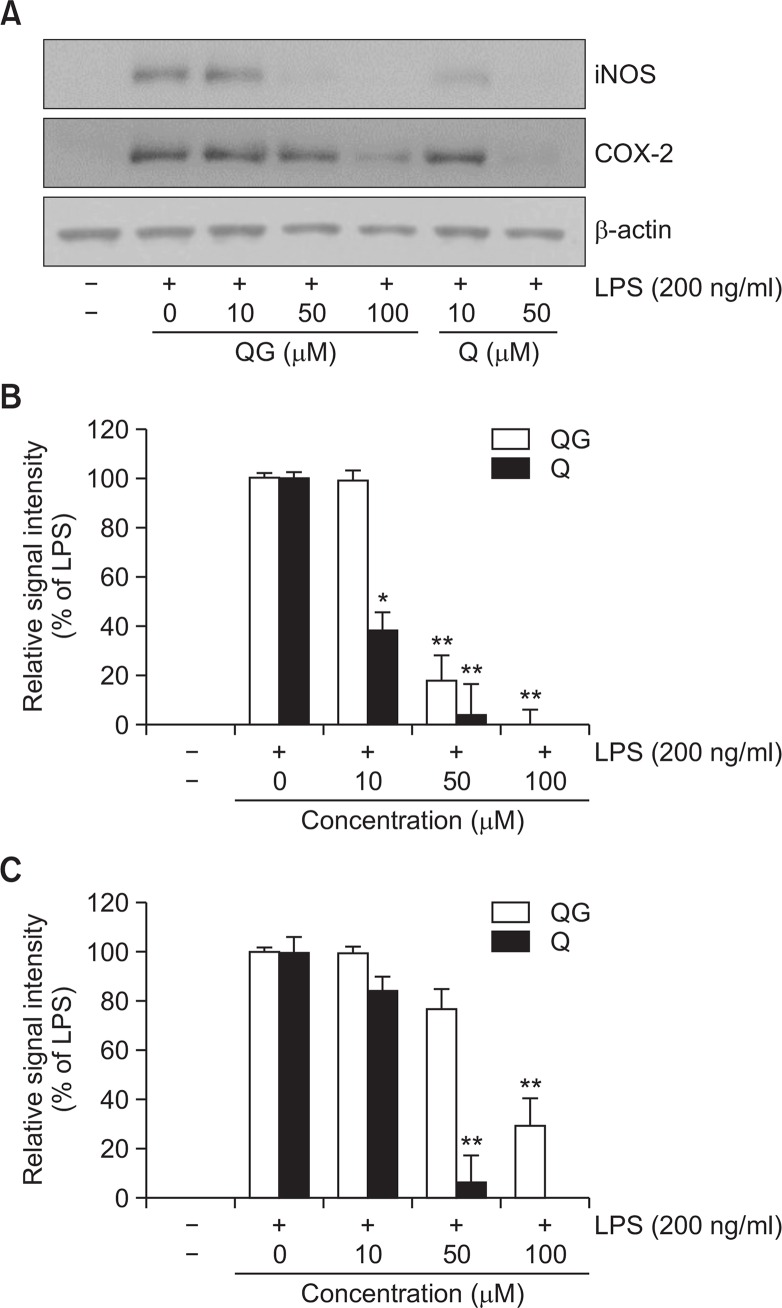
As QG inhibited LPS-induced extracellular release of NO and PGE2 in RAW264.7 cells (Fig. 2), whether the decreased production of NO and PGE2 was due to the attenuated expression of iNOS and COX-2 proteins was examine. LPS treatment showed the significantly increased expression of iNOS and COX-2 proteins in RAW264.7 cells. Pretreatment of QG resulted in the significant suppression in LPS-induced iNOS and COX-2 expressions in concentration-dependent manners (Fig. 3), indicating that decreased release of NO and PGE2 is due to the suppressed expression of their responsible genes by QG.
Fig. 3.
Effects of QG on LPS-induced expression of iNOS and COX-2 in RAW264.7 cells. (A) The cell lysates were subjected to SDSPAGE, and then protein levels of iNOS and COX-2 were determined by Western blot analysis. QG significantly attenuated LPS-induced overexpression of iNOS and COX-2. Images are representative of three independent experiments that shows reproducible results. (B, C) Quantitative analyses of immunoblots of iNOS and COX-2. QG significantly suppressed LPS-induced iNOS and COX-2 expression. The data were obtained from three independent experiments and expressed as mean ± SD (n=3). *p<0.05 and **p<0.01 indicate statistically significant differences from treatment with LPS alone. QC and Q stand for quercetin-3-O-β -D-glucuronide and quercetin, respectively. Quercetin was used as a reference. Examination of quercetin at 100 μM concentration was not done due to the decreased cell viability.
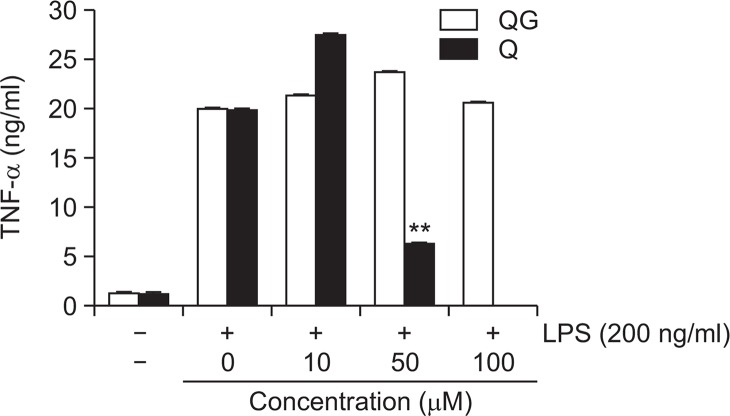
Quercetin but not QG attenuates LPS-induced release of a pro-inflammatory cytokine TNF-α
To examine the effects of QG on the extracellular release of pro-inflammatory cytokines such as IL-1β and TNF-α, secretion of these cytokines was measured using ELISA assay in LPS-stimulated RAW264.7 cells. LPS treatment resulted in excessive extracellular release of TNF-α in RWA 264.7 cells. Quercetin significantly attenuated LPS-induced extracellular release of TNF-α in a concentration-dependent manner. However, QG exhibited negligible effect in LPS-induced extracellular release of TNF-α (Fig. 4). In addition, QG did not suppress the LPS-induced extracellular release of IL-1β (data not shown).
Fig. 4.
Effect of QG on LPS-induced extracellular secretion of TNFalpha in RAW264.7 macrophage cells. RAW264.7 cells were pretreated with indicated concentrations of QG for 1 hr, then incubated with LPS (200 ng/ml) for 24 hrs. The concentration of TNFalpha in collected cell culture media was measured by ELISA assay as described in the methods. Although quercetin significantly suppressed LPS-induced TNFalpha cytokine in a concentration-dependent manner, QG showed a negligible effect in LPS-stimulated TNFalpha production. The values are expressed as mean ± SD for three independent experiments. **p<0.01 indicate statistically significant differences from treatments with LPS alone. QC and Q stand for quercetin-3-O-β-D-glucuronide and quercetin, respectively. Quercetin was used as a reference. Examination of quercetin at 100 μM concentration was not done due to the decreased cell viability.
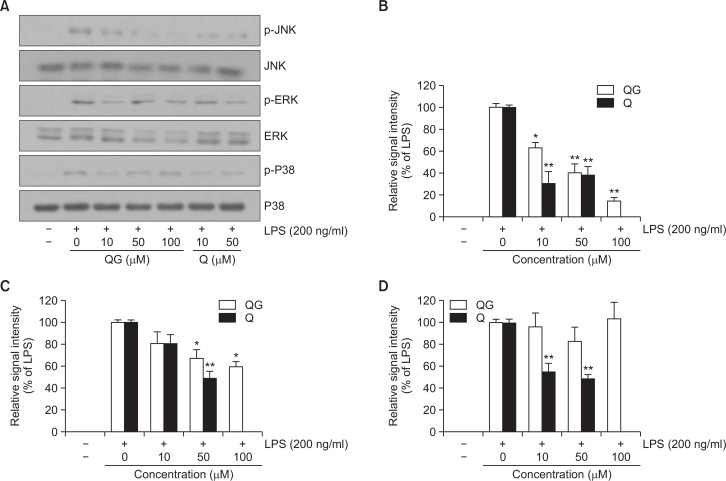
QG inhibits the phosphorylation of JNK and ERK in LPS-stimulated RAW264.7 cells
Signaling pathways of MAP kinases have been reported to be involved in the LPS-induced inflammatory responses (Guha and Mackman, 2001; Rushworth et al., 2005). In the present study, to understand the underlying signaling mechanism by which QG exhibits its anti-inflammatory action, the effect of QG on LPS-stimulated phosphorylation of JNK, ERK, and p38 kinase in RAW264.7 cells was examined. Cells were pretreated with QG or quercetin at indicated concentrations and then were treated with LPS (200 ng/ml) for 30 min. LPS treatment resulted in the activation of all three MAP kinases and quercetin attenuated LPS-induced phosphorylation of all three MAP kinases (Fig. 5). However, QG significantly attenuated only JNK and ERK phosphorylation in concentration-dependent manners in LPS-challenged RAW264.7 cells with no significant effect on p38 phosphorylation (Fig. 5), suggesting that QG exerts its anti-inflammatory activity through the suppression of JNK and ERK signaling pathways in RAW264.7 cells.
Fig. 5.
Effect of QG on LPS-induced activation of MAPK signaling pathway in RAW264.7 macrophage cells. (A) Representative immunoblots; (B, C, D) quantitative analyses of immunoblots. Cells were challenged with 200 ng/ml LPS in the absence or presence of QG. LPS-induced phosphorylation of JNK (B) and ERK (C) was significantly attenuated with QG treatment with a minor effect on p38 (D) phosphorylation. Images are representative of three independent experiments that shows reproducible results. The values are expressed as mean ± SD for three independent experiments. *p<0.05 and **p<0.01 indicate statistically significant differences from treatments with LPS alone. QC and Q stand for quercetin-3-O-β-D-glucuronide and quercetin, respectively. Quercetin was used as a reference. Examination of quercetin at 100 μM concentration was not done due to the decreased cell viability.
DISCUSSION
The present study demonstrated that Quercetin-3-O-β-D-glucuronide (QG) suppresses LPS-induced inflammatory responses through the suppression of JNK and ERK signaling pathways in LPS-challenged RAW264.7 macrophage cells. QG significantly attenuated the LPS-induced extracellular release of pro-inflammatory mediators, and the LPS-induced expressions of iNOS and COX-2 proteins. Previously, anti-inflammatory activity of QG was reported in LPS-stimulated BV2 microglia cells (Yoon et al., 2014). However, anti-inflammatory properties and its underlying mechanism of QG were not examined in RAW264.7 cells.
Given the previous studies that quercetin and its glycoside derivatives have pharmacological properties such as anticancer, anti-inflammatory, and anti-oxidant actions (Jagtap et al., 2009; Pocernich et al., 2011; Vo et al., 2012; Yang et al., 2014), the present study demonstrated that QG, a glucuronic acid glycoside of quercetin, possesses anti-inflammatory properties in LPS-induced RAW264.7 macrophage cells. In addition, the underlying mechanism by which QG exert the anti-inflammatory activity has not been clearly demonstrated. Therefore, the present study elucidated that QG exerts the anti-inflammatory action through the suppression of LPS-induced activation JNK and ERK signaling pathways.
Although macrophages play essential roles in innate immunity for the host defense against bacterial infection (Rehman et al., 2012), aberrant activation of macrophages also plays pathogenic roles in inflammation-related conditions by producing a wide range of pro-inflammatory mediators and cytokines (Rietschel and Brade, 1992). Activated macrophages initiate bacterial-mediated pro-inflammatory gene transcription, which leads to the phosphorylation of multiple kinases, which subsequently activates various pro-inflammatory transcription factors including NF-κB and AP-1 (O’Connell et al., 1998; Guha and Mackman, 2001). We previously demonstrated that bacterial LPS results in the expression of pro-inflammatory mediators and the increased degradation IκB (Vo et al., 2012). In the present study, quercetin but not QG significantly attenuated LPS-induced extracellular release of pro-inflammatory cytokines such as TNF-α. However, QG-induced suppression of extracellular release of TNF-α was reported in BV2 microglia cells (Yoon et al., 2014), suggesting that although BV2 microglia are also immune cells and have anti-bacterial characteristics in common with RAW264.7 cells, different immune cells could show altered anti-bacterial responses. Although TNF-α has been reported to play key roles in the progression of inflammatory responses, it has been known that other cytokines and transcription factors also play essential roles. Therefore, further studies are necessary to definitely determine the effect of QG on other cytokines and transcription factors in LPS-mediated inflammatory conditions.
It has been extensively reported that LPS activates all three MAP kinases such as ERK, JNK, and p38 in macrophages (Sweet and Hume, 1996) and that many of the downstream targets of MAPK pathways are transcription factors, which regulate various genes encoding inflammatory mediators (Zhang et al., 2006; Lee et al., 2009; Zhang et al., 2011). In the present study, LPS treatment exhibited increased phosphorylation of all three MAPKs in RAW264.7 cells. However, QG selectively attenuated LPS-induced JNK and ERK phosphorylation in concentration-dependent manners with concurrent significant attenuation of LPS-induced pro-inflammatory responses. The JNK signaling pathway has been implicated in the development of inflammatory responses in various conditions leading to cellular dysfunction and organ failures (Ip and Davis, 1998; Supinski et al., 2009). It has been also reported that inhibition of JNK activation significantly attenuates LPS-induced inflammatory responses in RAW264.7 cells (Lee et al., 2013). In addition, we recently demonstrated that N- (p-coumaryol)-tryptamine significantly inhibited LPS-induced inflammatory responses through the suppression of JNK activation in RAW264.7 cells (Vo et al., 2014). Furthermore, we also observed that that ERK signaling pathway was activated with LPS treatment and its activation and abnormal inflammatory responses were significantly attenuated with avicularin in LPS-challenged RAW264.7 cells (Vo et al., 2012). No noticeable changes were observed in phosphorylation p38 with QG, indicating that QG exerts its anti-inflammatory action through the selective inhibition of JNK and ERK signaling pathways in RAW264.7 cells.
It has been previously reported that aglycones of flavonoids exhibited more potent activities such as free radical scavenging activity and suppression of nitric oxide production than their glycosides (Hou et al., 2004; Kwon et al., 2004). However, the effects of glycosidation in terms of the position and degree of sugar substitution were not clearly elucidated. In the present study, QG exhibited attenuated anti-inflammatory properties compared to quercetin. The attenuated biological activities of QG might be attributed to the decreased intracellular transport of QG due to the increased hydrophilicity by the glucuronic acid residue. At the same time, aglycones exhibited potentiated cytotoxicity compared to their glycosides. Recently, it has been suggested that aglycones and glycosides exhibit different pharmacokinetic profile resulting in altered biological efficacy (Guo et al., 2015). Therefore, it is necessary to compare the biological properties of glycosides compared to their aglycones.
In conclusion, the present study clearly demonstrates that QG exerts anti-inflammatory activity through the inhibition of JNK and ERK signaling pathways in LPS-challenged RAW264.7 macrophage cells, suggesting that QG might be a valuable therapeutic agent for the treatment of inflammation-related pathogenic conditions.
Acknowledgments
This study was supported by 2015 Research Grant from Kangwon National University (520150350).
REFERENCES
- Cho SG, Choi EJ. Apoptotic signaling pathways: caspases and stress-activated protein kinases. J Biochem Mol Biol. 2002;35:24–27. doi: 10.5483/bmbrep.2002.35.1.024. [DOI] [PubMed] [Google Scholar]
- Fan D, Zhao Y, Zhou X, Gong X, Zhao C. Simultaneous determination of esculetin, quercetin-3-O-β-D-glucuronide, quercetin-3-O-β-D-glucuronopyranside methyl ester and quercetin in effective part of Polygonum perfoliatum L. using high performace liquid chromatography. Pharmacogn Mag. 2014;10:359–366. doi: 10.4103/0973-1296.137379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/S0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Guo J, Du L, Shang E, Li T, Liu Y, Qian D, Tang Y, Duan J. Conjugated metabolites represent the major circulating forms of Abelmoschus manihot in vivo and show an altered pharmacokinetic profile in renal pathology. Pharm Biol. 2015;54:595–603. doi: 10.3109/13880209.2015.1068337. [DOI] [PubMed] [Google Scholar]
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- Hou L, Zhou B, Yang L, Liu ZL. Inhibition of free radical initiated peroxidation of human erythrocyte ghosts by flavonols and their glycosides. Org Biomol Chem. 2004;2:1419–1423. doi: 10.1039/b401550a. [DOI] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun Nterminal kinase (JNK)--from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/S0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Itharat A, Hiransai P. Dioscoreanone suppresses LPSinduced nitric oxide production and inflammatory cytokine expression in RAW264.7 macrophages by NF-kappaB and ERK1/2 signaling transduction. J Cell Biochem. 2012;113:3427–3435. doi: 10.1002/jcb.24219. [DOI] [PubMed] [Google Scholar]
- Jagtap S, Meganathan K, Wagh V, Winkler J, Hescheler J, Sachinidis A. Chemoprotective mechanism of the natural compounds, epigallocatechin-3-O-gallate, quercetin and curcumin against cancer and cardiovascular diseases. Curr Med Chem. 2009;16:1451–1462. doi: 10.2174/092986709787909578. [DOI] [PubMed] [Google Scholar]
- Kim SY, Park JY, Park PS, Bang SH, Lee KM, Lee YR, Jang YH, Kim MJ, Chun W, Heo MY, Kwon YS. Flavonoid Glycosides as Acetylcholinesterase Inhibitors from the Whole Plants of. Persicaria thunbergii Nat Prod Sci. 2014;20:191–195. [Google Scholar]
- Kim YJ, Shin Y, Lee KH, Kim TJ. Anethum graveloens flower extracts inhibited a lipopolysaccharide-induced inflammatory response by blocking iNOS expression and NF-κB activity in macrophages. Biosci Biotechnol Biochem. 2012;76:1122–1127. doi: 10.1271/bbb.110950. [DOI] [PubMed] [Google Scholar]
- Kwon YS, Kim SS, Sohn SJ, Kong PJ, Cheong IY, Kim CM, Chun W. Modulation of suppressive activity of lipopolysaccharide-induced nitric oxide production by glycosidation of flavonoids. Arch Pharm Res. 2004;27:751–756. doi: 10.1007/BF02980144. [DOI] [PubMed] [Google Scholar]
- Lee JW, Bae CJ, Choi YJ, Kim SI, Kim NH, Lee HJ, Kim SS, Kwon YS, Chun W. 3,4,5-Trihydroxycinnamic Acid Inhibits LPS-Induced iNOS Expression by Suppressing NF-kappaB Activation in BV2 Microglial Cells. Korean J Physiol Pharmacol. 2012;16:107–112. doi: 10.4196/kjpp.2012.16.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Kim NH, Kim JY, Park JH, Shin SY, Kwon YS, Lee HJ, Kim SS, Chun W. Aromadendrin Inhibits Lipopolysaccharide-Induced Nuclear Translocation of NF-κB and Phosphorylation of JNK in RAW264.7 Macrophage Cells. Biomol. Ther. (Seoul) 2013;21:216–221. doi: 10.4062/biomolther.2013.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Kim S, Lee SJ, Ham I, Whang WK. Antioxidant activities of new flavonoids from Cudrania tricuspidata root bark. Arch Pharm Res. 2009;32:195–200. doi: 10.1007/s12272-009-1135-z. [DOI] [PubMed] [Google Scholar]
- Messer JG, Hopkins RG, Kipp DE. Quercetin Metabolites Up-Regulate the Antioxidant Response in Osteoblasts Isolated From Fetal Rat Calvaria. J Cell Biochem. 2015;116:1857–1866. doi: 10.1002/jcb.25141. [DOI] [PubMed] [Google Scholar]
- O’Connell MA, Bennett BL, Mercurio F, Manning AM, Mackman N. Role of IKK1 and IKK2 in lipopolysaccharide signaling in human monocytic cells. J Biol Chem. 1998;273:30410–30414. doi: 10.1074/jbc.273.46.30410. [DOI] [PubMed] [Google Scholar]
- Ock J, Kim S, Suk K. Anti-inflammatory effects of a fluorovinyloxyacetamide compound KT-15087 in microglia cells. Pharmacol Res. 2009;59:414–422. doi: 10.1016/j.phrs.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Lange ML, Sultana R, Butterfield DA. Nutritional approaches to modulate oxidative stress in Alzheimer’s disease. Curr. Alzheimer Res. 2011;8:452–469. doi: 10.2174/156720511796391908. [DOI] [PubMed] [Google Scholar]
- Rehman MU, Yoshihisa Y, Miyamoto Y, Shimizu T. The anti-inflammatory effects of platinum nanoparticles on the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages. Inflamm Res. 2012;61:1177–1185. doi: 10.1007/s00011-012-0512-0. [DOI] [PubMed] [Google Scholar]
- Rietschel ET, Brade H. Bacterial endotoxins. Sci Am. 1992;267:54–61. doi: 10.1038/scientificamerican0892-54. [DOI] [PubMed] [Google Scholar]
- Rushworth SA, Chen XL, Mackman N, Ogborne RM, O’Connell MA. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J Immunol. 2005;175:4408–4415. doi: 10.4049/jimmunol.175.7.4408. [DOI] [PubMed] [Google Scholar]
- Supinski GS, Ji X, Callahan LA. The JNK MAP kinase pathway contributes to the development of endotoxin-induced diaphragm caspase activation. Am J Physiol Regul Integr Comp Physiol. 2009;297:R825–R834. doi: 10.1152/ajpregu.90849.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- Vo VA, Lee JW, Chang JE, Kim JY, Kim NH, Lee HJ, Kim SS, Chun W, Kwon YS. Avicularin Inhibits Lipopolysaccharide-Induced Inflammatory Response by Suppressing ERK Phosphorylation in RAW264.7 Macrophages. Biomol. Ther. (Seoul) 2012;20:532–537. doi: 10.4062/biomolther.2012.20.6.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo VA, Lee JW, Park JH, Kwon JH, Lee HJ, Kim SS, Kwon YS, Chun W. N-(p-Coumaryol)-Tryptamine Suppresses the Activation of JNK/c-Jun Signaling Pathway in LPSChallenged RAW264.7 Cells. Biomol. Ther. (Seoul) 2014;22:200–206. doi: 10.4062/biomolther.2014.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Liu JR, Gao JM, Parry JW, Wei YM. Antioxidant activity of Tartary buckwheat bran extract and its effect on the lipid profile of hyperlipidemic rats. J Agric Food Chem. 2009;57:5106–5112. doi: 10.1021/jf900194s. [DOI] [PubMed] [Google Scholar]
- Wang WH, Gregori G, Hullinger RL, Andrisani OM. Sustained activation of p38 mitogen-activated protein kinase and c-Jun N-terminal kinase pathways by hepatitis B virus X protein mediates apoptosis via induction of Fas/FasL and tumor necrosis factor (TNF) receptor 1/TNF-alpha expression. Mol Cell Biol. 2004;24:10352–10365. doi: 10.1128/MCB.24.23.10352-10365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Miyoshi N, Kawabata K, Yasuda M, Shimoi K. Quercetin-3-O-glucuronide inhibits noradrenaline-promoted invasion of MDA-MB-231 human breast cancer cells by blocking β2-adrenergic signaling. Arch Biochem Biophys. 2014;557:18–27. doi: 10.1016/j.abb.2014.05.030. [DOI] [PubMed] [Google Scholar]
- Yang HH, Hwangbo K, Zheng MS, Cho JH, Son JK, Kim HY, Baek SH, Choi HC, Park SY, Kim JR. Quercetin-3-O-β-D-glucuronide isolated from Polygonum aviculare inhibits cellular senescence in human primary cells. Arch Pharm Res. 2014;37:1219–1233. doi: 10.1007/s12272-014-0344-2. [DOI] [PubMed] [Google Scholar]
- Yoon CS, Kim DC, Ko WM, Kim KS, Lee DS, Kim DS, Cho HK, Seo J, Kim SY, Oh HC, Kim YC. Antineuroinflammatory Effects of Quercetin-3-O-glucuronide Isolated from the Leaf of Vitis labruscana on LPS-induced Neuroinflammation in BV2 Cells. Kor J Pharmacogn. 2014;45:17–22. [Google Scholar]
- Zhang WY, Lee JJ, Kim IS, Kim Y, Myung CS. Stimulation of glucose uptake and improvement of insulin resistance by aromadendrin. Pharmacology. 2011;88:266–274. doi: 10.1159/000331862. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hung TM, Phuong PT, Ngoc TM, Min BS, Song KS, Seong YH, Bae K. Anti-inflammatory activity of flavonoids from Populus davidiana. Arch Pharm Res. 2006;29:1102–1108. doi: 10.1007/BF02969299. [DOI] [PubMed] [Google Scholar]