Abstract
In the previous study, the rhizome mixture of Anemarrhena asphodeloides and Coptis chinensis (DW2007), improved TNBS-, oxazolone-, or DSS-induced colitis in mice by regulating macrophage activation. Therefore, to understand the effect of DW2007 on the T cell differentiation involved in the adaptive immunity, we measured its effect on both Th17 and Treg cell differentiation in splenocytes, in the lamina propria of mice with DSS-induced colitis (DIC), and in the spleens of mice with collagen-induced arthritis (CIA). Results showed that DW2007 potently inhibited the differentiation of splenocytes into Th17 cells, but increased Treg cell differentiation in vitro. In the colon of wild type and TLR4−/− mice with DIC, DW2007 potently suppressed DSS-induced colon shortening and myeloperoxidase activity. DW2007 also suppressed collagen-induced paw thickening, clinical index, and myeloperoxidase activity in CIA mice. Overall, DW2007 potently suppressed Th17 cell differentiation in mice with CIA and DIC, but increased Treg cell differentiation. Moreover, DW2007 strongly inhibited the expression of TNF-α and IL-1β, as well as the activation of NF-κB. Based on these findings, DW2007 may ameliorate inflammatory diseases by regulating the innate immunity via the inhibition of macrophage activation and the adaptive immunity via the correction of disturbed Th17/Treg cells.
Keywords: DW2007, Colitis, Arthritis, Th17 Cell, Treg cell
INTRODUCTION
A persistent and excessive inflammation causes progressive damages to the body, resulting in various inflammatory diseases such as colitis and rheumatoid arthritis (RA) (Ebert and Hagspiel, 2011; Wallace et al., 2014). T cells and macrophages in the inflamed sites increase the expression of inflammatory mediators such as tumor necrosis factor (TNF)-α and interleukin (IL)-17, which play a pivotal role in the initiation, evolution/progression, and persistence of chronic inflammation (Szekanecz and Koch, 2007; Qu et al., 2013; Mellado et al., 2015). These inflammatory mediators linked to chronic inflammation trigger the differentiation of T cells. Of these T cells, Th17 cells, which secrete IL-17 and IL-22, perpetuate chronic inflammatory diseases such as colitis and RA. IL-17, which is a cytokine that recruits monocytes and neutrophils to the site of inflammation, upregulates IL-1β and TNF-α expression in the immune cells of inflammatory sites (Sarra et al., 2010; Burska et al., 2014). IL-17 acts synergistically with proinflammatory cytokines, IL-1β and TNF-α (Shen et al., 2005; Yang et al., 2014). Th17 is antagonized by Treg cells, which produces anti-inflammatory cytokines such as IL-10 and transforming growth factor beta (TGFβ) (Noack and Miossec, 2014). The population of Th17 cells is higher in patients with RA and colitis than in healthy controls, suggesting that a Th17/Treg imbalance may contribute to the pathogenesis and progression of inflammatory and autoimmune diseases such as colitis and RA. Therefore, a therapeutic approach to suppress Th17 cell differentiation can be effective for chronic inflammatory diseases colitis and RA (Yan et al., 2014).
A rhizome mixture of Anemarrhena asphodeloides (AA, family Liliaceae) and the rhizome of Coptis chinensis (CC, family Ranunculaceae) exhibits anti-inflammatory effects in mice with TNBS-, DSS-, or oxazolone-induced colitis by inhibiting NF-κB and MAPKs signaling pathways (Jang et al., 2013; Jeong et al., 2014). However, its effect against the adaptive immune response has been not investigated.
In the preliminary study, DW2007 inhibited the differentiation of splenocytes into Th17 cells. Therefore, we investigated the effect of DW2007 on Th cell differentiation in mice with dextran sulfate sodium (DSS)-induced colitis (DIC) or with collagen-induced arthritis (CIA).
MATERIALS AND METHODS
Reagents
DSS, fetal bovine serum (FBS), hexadecyl trimethyl ammonium bromide radio-immunoprecipitation assay (RIPA) lysis buffer, and RPMI1640 were purchased from Sigma (St Louis, MO, USA). Bovine type II collagen and complete Freund’s adjuvant (FCA) were purchased from Chondrex (Redmond, WA, USA). The protease inhibitor cocktail was purchased from Roche Applied Science (Roche, Mannheim, Germany). Enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, MN, USA). Antibodies were purchased from Cell Signaling (Cell Signaling Technology, Inc., Danvers, MA, USA). The enhanced chemiluminescence (ECL) immunoblot system was purchased from Pierce Co. (Rockford, IL, USA).
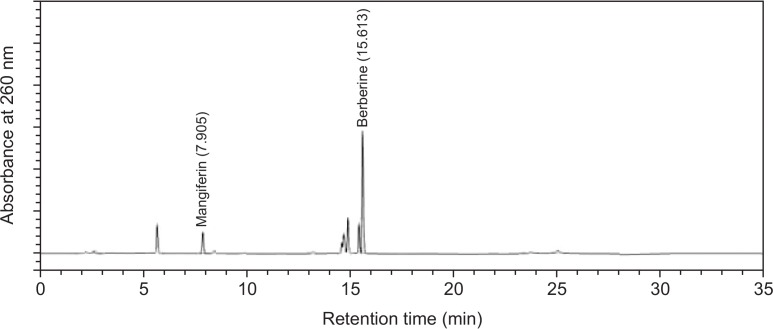
DW2007 (AC), an 80% ethanol extract mixture of AA and CC (3:1), was donated from DongWha Pharm Co. (Seoul, Republic of Korea). The contents of mangiferin and berberine in DW2007 were 1.069% and 4.383%, respectively (Fig. 1).
Fig. 1.
HPLC chromatogram of DW2007. HPLC (Waters, MA, USA) system with a diode array detector was equipped with a quaternary pump, a vacuum degasser, a column oven and an autosampler (Waters). The chromatographic separation was performed on a Kromasil column (KR100-5-C18-4.6X250) with the column temperature set at 25°C. The elution solvent was used with the linear gradient of A (water with 0.1% formic acid) and B (acetonitrile with 0.1% formic acid): 0 to 5 min, 90% A and 10% B; 5 to 9 min, 80% A and 20% B; 9 to 13 min, 58% A and 42% B; 13 to 21 min, 45% A and 55% B; 21 to 25 min, 10% A and 90% B. The flow rate was 1.0 mL/min, the injection volume was 5 μL, and the detection absorbance was 260 nm.
Animals
Male DBA/1J mice (18–21 g, 6 weeks-old) obtained from Harlan (Indianapolis, IN, USA), and male wild type and TLR4−/− C57BL/6 (20–23 g, 6 weeks old) supplied from RaonBio (Seoul, Korea) were provided with water and food ad libitum, and maintained in a ventilated room at an ambient temperature of 22°C ± 1°C with 50% ± 10% humidity and a 12-h diurnal light cycle (lights on 07:00–19:00) for 2 weeks before the experiment.
All animal experiments were performed according to the Kyung Hee University (Seoul, Republic of Korea) guideline for Laboratory Animals Care and Use. The animal study was approved by the committee for the Care and Use of Laboratory Animals in Kyung Hee University (IRB No. KHP-2014-04-02).
Preparation of splenocytes
Spleens were aseptically isolated from C57BL/6 mice, minced, washed with tris-buffered ammonium chloride, and suspended in RPMI 1640 medium containing 10% FBS (Lee et al., 2015a). T cells were isolated using Pan T Cell Isolation Kit II and plated. Isolated T cells were stimulated with recombinant TGFβ (1 ng/mL), recombinant IL-6 (20 ng/mL), anti-CD3 (1 μg/mL), and anti-CD28 (1 μg/mL) in the absence or presence of DW2007 for 5 days, and analyzed by flow cytometry (C6 Flow Cytometer® System, San Jose, CA, USA).
Preparation of DSS-induced colitis (DIC) in mice
DIC was induced in wild type and TLR4−/− mice with 3% DSS (dissolved in drinking water) for 7 days (Jeong et al., 2014). Normal control mice were given with drinking water alone instead of 3% DSS. Test agents (NOR, vehicle alone in normal control mice; DSS, vehicle alone in DIC mice; AC10, 10 mg/kg DW2007 in DIC mice; AC20, 20 mg/kg DW2007 in DIC mice; SS50, 50 mg/kg sulfasalazine in DIC mice) were orally administered once a day for 3 days after the final DSS administration. The mice were killed 18 h after the final administration of test agents. The colons were quickly removed, opened longitudinally, and gently washed with PBS for immunoblotting, qRT-PCR, ELISA, and histologic exam (Jeong et al., 2014). Macroscopic assessment of the disease grade was scored according to a previously reported scoring system (Jeong et al., 2014).
Collagen-induced arthritis (CIA)
Male DBA/1J mice (8-week old) were divided into four groups of seven mice. For the CIA induction, mice were immunized by intradermal injection at the base of the tail with 100 μg of bovine type II collagen dissolved in 0.05 M acetic acid and emulsified in CFA (Arii et al., 2008; Lee et al., 2015b). Twenty-one days after the first immunization, mice were again immunized with collagen II emulsified in complete Freund’s adjuvant (CFA) again. Test agents (NOR, vehicle saline alone in normal control mice; CIA, vehicle alone in mice with CIA; CD, 20 mg/kg DW2007 in CIA mice; CI, 50 mg/kg ibuprofen in CIA mice) suspended in 0.1 ml of vehicle were orally administered daily after the second immunization. From day 1 after the second immunization, arthritis incidence, disease severity, paw thickness, and body weight were determined. Arthritis severity in each paw was scored on scale of 0–4 (Arii et al., 2008; Lee et al., 2015b). On the last day, animals were sacrificed, and paw joint tissues were collected and immediately frozen (−80°C) for immunoblotting, qRT-PCR, ELISA, and histologic exam.
Histologic examination
Colons and paw tissues were fixed in 4% paraformaldehyde, dehydrated by exposure to increasing ethanol concentrations, embedded in paraffin, sectioned with 20 μM thickness, then stained with either haematoxylin-eosin, toluidine blue, or safranin O. Microscopic assessment was then performed.
Tissue preparation
Colons and paw joint tissues of mice were excised, perfused with ice-cold perfusion solution (0.15 M KCl, 2 mM EDTA, pH 7.4), like the previously reported (Jeong et al., 2014; Lee et al., 2015a), homogenized in 50 mM Tris-HCl buffer (pH 7.4), and centrifuged at 10,000×g and 4°C for 30 min. Supernatants were used for the determination of myeloperoxidase activity, ELISA, and immunoblotting.
Assay of myeloperoxidase activity
The supernatant of the homogenate (50 μL) was added to a reaction mixture containing 1.6 mM tetramethyl benzidine and 0.1 mM H2O2, incubated at 37°C, and then the absorbance at 650 nm was monitored over time (Jeong et al., 2014; Lee et al., 2015a). Myeloperoxidase activity was defined according the method of Jeong et al. (2014).
ELISA and immunoblotting
For the ELISA of cytokines, the supernatant of colon and joint tissue homogenates was transferred to 96-well ELISA plates. TNF-α, IL-1β, IL-6, IL-10, and IL-17 concentrations were assayed using commercial ELISA kits (Pierce Biotechnology, Inc., Rockford, IL, USA) (Jeong et al., 2014; Lee et al., 2015a).
For the immunoblot analyses of proteins, the supernatant was used for the immunobloting. The supernatants were subjected to electrophoresis on 8–10% sodium dodecyl sulfatepolyacrylamide gel and then transferred to nitrocellulose membrane as previously described (Jeong et al., 2014; Lee et al., 2015a). Immunodetection was performed using an enhanced chemiluminescence detection kit (Millipore, Billerica, MA, USA).
Flow cytometry of Th17 and Treg cells in the colon lamina propria
For the flow cytometric analysis of Th cells in the lamina propria, colons were squashed and sequentially incubated with 2.5 mM EDTA at 37°C with shaking for 20 min, and 1 mg/mL collagenase type VIII (Sigma, St. Louis MO, USA) for 20 min at 37°C (Lee et al., 2015b). The reaction mixture were filtered and T cells were isolated using a Pan T cell Isolation Kit II, fixed, stained with anti-Foxp3 or anti-IL-17A antibodies (Biogems, CA, USA) and analyzed by flow cytometry.
For the flow cytometric analysis of Th17 and Treg cells in the spleens, spleens were crushed into a single-cell suspension, lysed with Tris-buffered ammonium chloride, suspended in RPMI 1640 medium, and then filtered, and the T cells were isolated using a Pan T cell Isolation Kit II (Lee et al., 2015a). Isolated T cells were fixed and stained with anti-FoxP3 or anti-IL-17A antibodies and then analyzed by flow cytometry.
Quantitative real time-polymerase chain reaction (qPCR)
Total RNA (2 μg) was isolated from colons and joint tissues, and then qPCR for IL-10, IL-17, RORγt, Foxp3, and β-actin was performed as described previously (Lee et al., 2015a, 2015b), utilizing Takara thermal cycler, which used SYBER premix agents, as per the instructions from Takara biology incorporation: activation of DNA polymerase at 95°C for 5 min and 38 cycles of amplification at 95°C for 10 s and at 60°C for 30 s. The normalized expression of the assayed genes, with respect to β-actin, was computed for all samples by using the Microsoft Excel data spreadsheet.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD), with statistical significance analyzed using one-way ANOVA followed by a Student-Newman-Keuls test.
RESULTS
Effect of DW2007 on the differentiation of splenocytes into Th17 and Treg cell differentiation
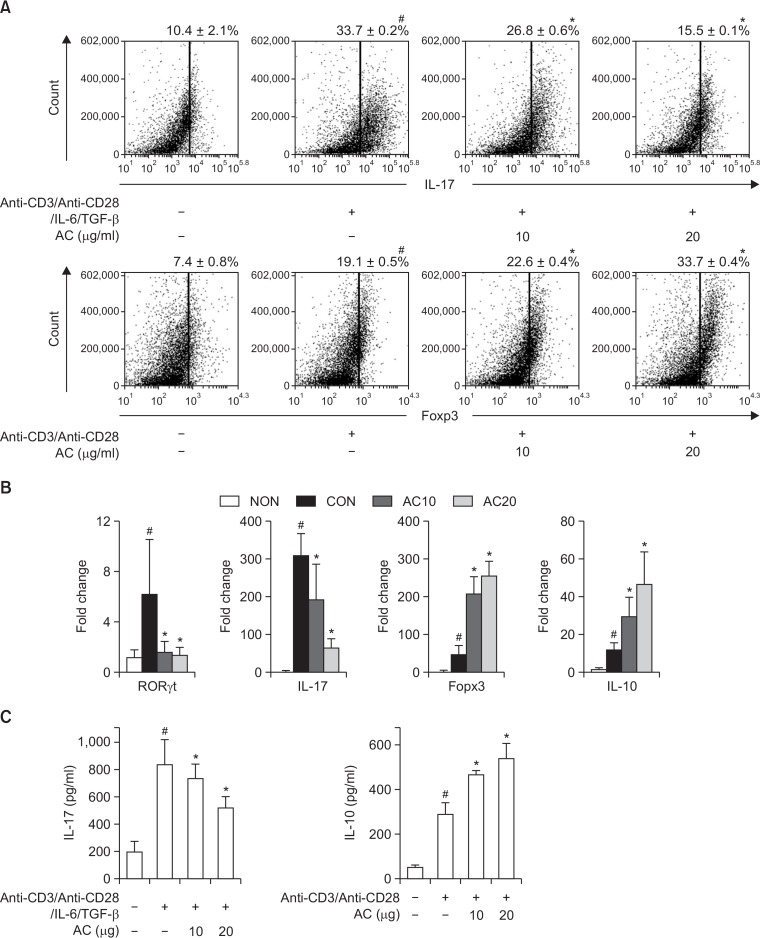
To investigate the effect of DW2007 on T cell differentiation involved in the adaptive immune response, we measured its effect on the differentiation of splenocytes into Th17 and Treg cells. The stimulation of anti-CD3/anti-CD28/IL-6/TGF-β significantly differentiated splenocytes into Th17 cells (33.7%) (Fig. 2). However, treatment with DW2007 significantly suppressed the Th17 cell population compared with non-treated cells. DW2007 inhibited RORγt and IL-17 expression regulating Th17 cell differentiation. However, DW2007 increased the differentiation of splenocytes into Treg cells and expression of Foxp3 and IL-10.
Fig. 2.
Effect of DW2007 on Th17 and Treg cell differentiation. (A) Effect on Th17 and Treg cell differentiation. Splenocytes were stimulated with anti-CD3 (1 μg/mL) and anti-CD28 (1 μg/mL) with or without IL-6 (20 ng/mL) plus TGF-β (1 ng/mL) in the presence of DW2007 (AC) for 5 days. Staining of cell surface CD4 antigen and intracellular IL-17 was performed and analyzed using flow cytometry. (B) Effect on RORγt, Foxp3, IL-17, and IL-10 expression by qRT-PCR. (C) Effect on IL-17 and IL-10 expression by ELISA. All data indicate mean ± SD (n=6). #p<0.05 vs. normal control. *p<0.05 vs. cells stimulated with anti-CD3/anti-CD28 and IL-6/TGFβ.
Anti-inflammatory effect of DW2007 in DIC mice
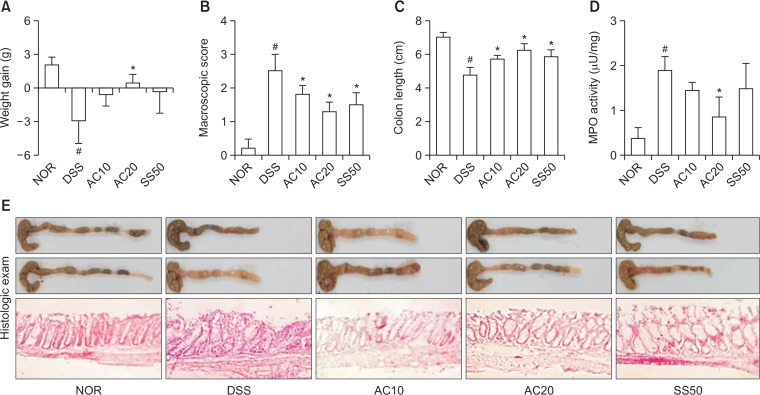
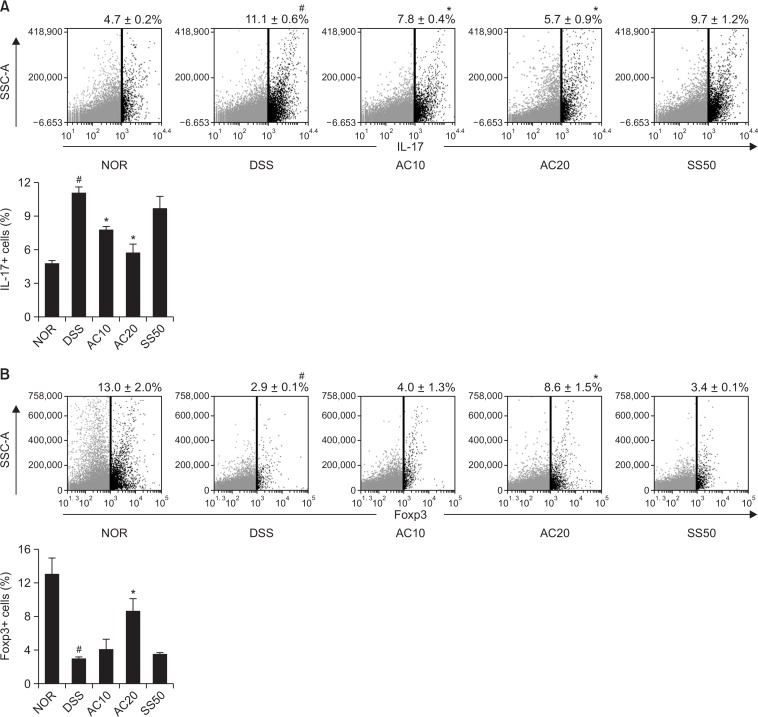
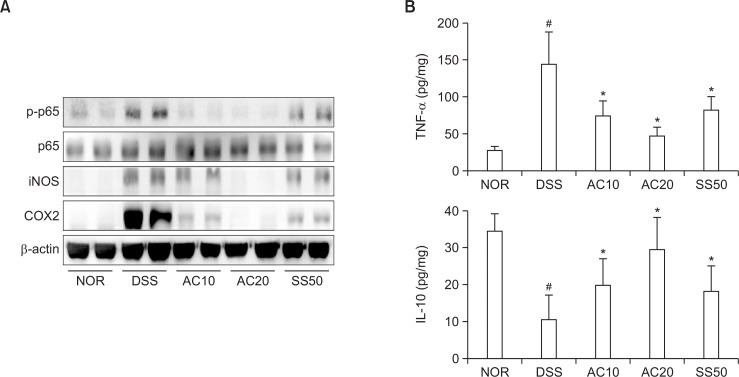
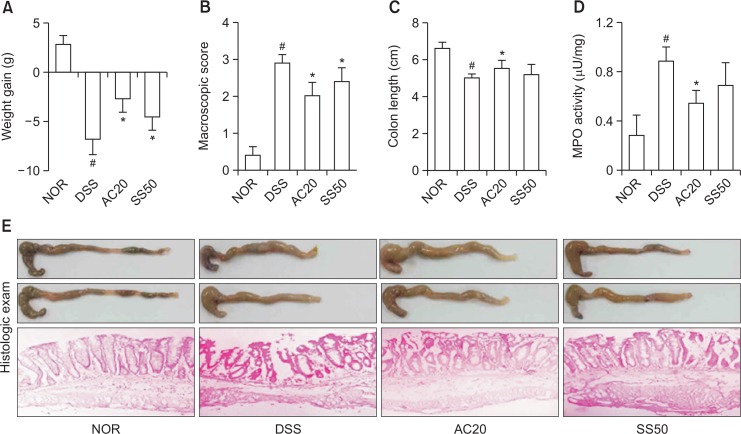
Next, we measured anti-inflammatory effect of DW2007 in mice with DIC. Treatment with DSS caused colon shortening and increased colonic macroscopic score and myeloperoxidase activity (Fig. 3). Treatment with DW2007 suppressed DSS-induced colon shortening and myeloperoxidase activity. Treatment with DSS in mice significantly increased Th17 cell differentiation and RORγt and IL-17 expression and suppressed Treg cell differentiation and Foxp3 and IL-10 expression (Fig. 4). However, treatment with DW2007 in DIC mice suppressed the differentiation of Th17 cells and expression of IL-17. However, oral administration of DW2007 restored DSS-suppressed differentiation of Treg cells and expression of IL-10. Treatment with DSS also increased iNOS and COX-2 expression (Fig. 5). However, treatment with DW2007 in DIC mice suppressed iNOS and COX-2 expression, as well as NF-κB activation. DW2007 also suppressed DSS-induced expression of TNF-α, however, increased DSS-suppressed expression of IL-10.
Fig. 3.
Effect of DW2007 on body weight (A), macroscopic disease (B), colon length (C), colonic myeloperoxidase (MPO) activity (D), and histological exam (E) in wild-type mice with DSS-induced colitis. DSS, except in the control group, was orally administered to mice treated with saline, DW2007 (AC), or sulfasalazine (SS). Test agents (AC10, 10 mg/kg DW2007; AC20, 20 mg/kg DW2007; or SS50, 50 mg/kg SS) or saline were orally administered for 3 days after DSS treatment. The mice were killed 18 h after the final administration of test agents. All data are mean ± SD (n=6). #p<0.05 vs. control group. *p<0.05 vs. DSS group.
Fig. 4.
Effect of DW2007 on the differentiation to Th17 and Treg cells in wild-type mice with DSS-induced colitis. (A) Effect on Th17 (CD4+IL17+) cell differentiation. (B) Effect on Treg (CD4+Foxp3+) cell differentiation. DSS, except in the control group, was orally administered to mice treated with saline, DW2007 (AC), or sulfasalazine (SS). Test agents (AC10, 10 mg/kg DW2007; AC20, 20 mg/kg DW2007; or SS50, 50 mg/kg SS) or saline were orally administered for 3 days after DSS treatment. The mice were killed 18 h after the final administration of test agents. The CD4+ T cells were isolated from the lamina propria of the colon using Pan T cell isolation kit. So, we performed single staining for flow cytometry (Th17 cells: IL-17, Treg cells: Foxp3). Th17 and Treg cells were then analyzed by flow cytometry. All values are mean ± SD (n=6). #p<0.05 vs. control group. *p<0.05 vs. DSS group.
Fig. 5.
Effects of DW2007 on the expression of iNOS and COX-2, activation of NF-κB (A), and expression of inflammatory cytokines (B) and in wild-type mice with DSS-induced colitis. DSS, except in the control group, was orally administered to mice treated with saline, DW2007 (AC), or sulfasalazine (SS). Test agents (AC10, 10 mg/kg DW2007; AC20, 20 mg/kg AC; or SS50, 50 mg/kg SS) or saline were orally administered for 3 days after DSS treatment. iNOS, COX-2, and NF-κB were determined by immunoblotting. TNF-α and IL-10 were determined by ELISA. All values are mean ± SD (n=6). #p<0.05 vs. control group. *p<0.05 vs. DSS group.
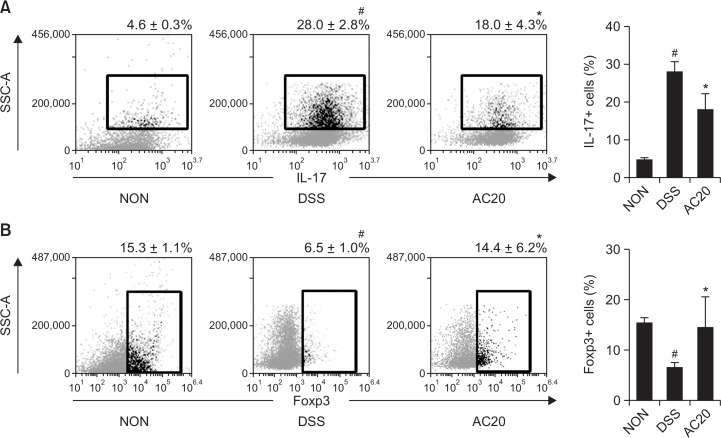
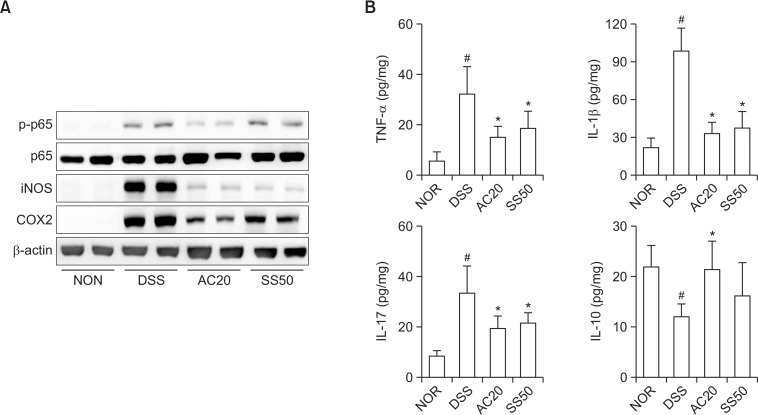
We also investigated the anti-colitic effect of DW2007 in TLR4−/− mice with DIC. Treatment with DSS in TLR4−/− mice caused severe colon shortening with severe edema and increased myeloperoxidase activity (Fig. 6). Treatment with DW2007 significantly inhibited DSS-induced colon shortening and myeloperoxidase activity compared with those of mice with DIC. Treatment with DSS in TLR4−/− mice also significantly increased Th17 cell differentiation (Fig. 7). Treatment with DW2007 suppressed DSS-induced differentiation of Th17 cells, but restored DSS-suppressed differentiation of Treg cells. Furthermore, treatment with DW2007 inhibited DSS-induced iNOS and COX-2 expression and NF-κB activation (Fig. 8). DW2007 treatment also suppressed DSS-induced TNF-α, IL-1β and IL-17 expression, but increased IL-10 expression suppressed by DSS.
Fig. 6.
Effect of DW2007 on body weight (A), macroscopic disease (B), colon length (C), colonic myeloperoxidase (MPO) activity (D), and histologic exam (E) in TLR4−/− mice with DSS-induced colitis. DSS, except in the control group, was orally administered to mice treated with saline, DW2007 (AC), or sulfasalazine (SS). Test agents (AC10, 10 mg/kg DW2007; AC20, 20 mg/kg AC; or SS50, 50 mg/kg SS) or saline were orally administered for 3 days after DSS treatment. The mice were killed 18 h after the final administration of test agents. All data are mean ± SD (n=6). #p<0.05 vs. control group. *p<0.05 vs. DSS group.
Fig. 7.
Effect of AC on the differentiation to Th17 and Treg cells in TLR4−/− mice with DSS-induced colitis. (A) Effect on Th17 cell differentiation. (B) Effect on Treg cell differentiation. DSS, except in the normal control group (NOR), was orally administered to mice treated with saline (DSS) or DW2007 (AC). Test agents (AC10, 10 mg/kg DW2007; AC20, 20 mg/kg DW2007) or saline were orally administered for 3 days after DSS treatment. The mice were killed 18 h after the final administration of test agents. The T cells were isolated from the lamina propria and Th17 and Treg cells were then analyzed by flow cytometry. All values are mean ± SD (n=6). #p<0.05 vs. control group. *p<0.05 vs. DSS group.
Fig. 8.
Effects of DW2007 on the expression of iNOS and COX-2, activation of NF-κB (A), and expression of inflammatory cytokines (B) and in TLR4−/− mice with DSS-induced colitis. DSS, except in the normal control group (NOR), was orally administered to mice treated with saline (DSS), DW2007 (AC), or sulfasalazine (SS). Test agents (AC10, 10 mg/kg DW2007; AC20, 20 mg/kg AC; or SS50, 50 mg/kg SS) or saline were orally administered for 3 days after DSS treatment. iNOS, COX-2, and NF-κB were determined by immunoblotting. TNF-α, IL-1β, IL-6, IL-10, and IL-17 were determined by ELISA. All values are mean ± SD (n=6). #p<0.05 vs. control group. *p<0.05 vs. DSS group.
Anti-inflammatory effect of DW2007 in mice with CIA
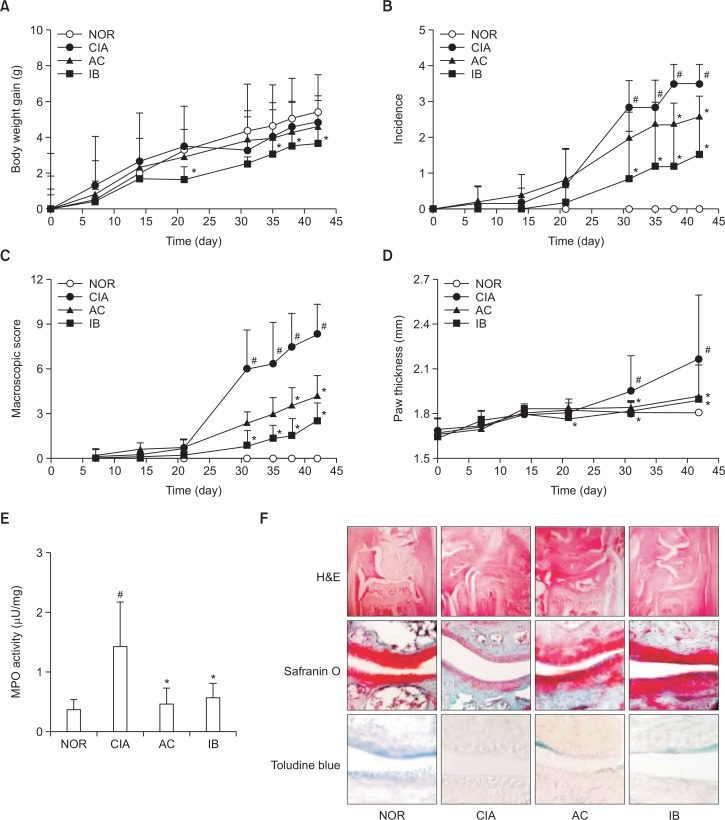
Next, we investigated the anti-inflammatory effect of DW2007 in DBA/1J mice with CIA. Treatment with collagen in mice gradually caused severe CIA from day 22 (the second injection of collagen II), including the increase of macroscopic score, volume, and thickness of inflamed paws. However, treatment with DW2007 significantly suppressed collagen-induced macroscopic score, arthritis incidence, volume, and thickness in the paws compared with those in CIA mice (Fig. 9). Furthermore, treatment with DW2007 significantly inhibited myeloperoxidase activity in the joint tissues. Histologic exam revealed that DW2007 also suppressed collagen-induced synovial hyperplasia and adjacent cartilage and bone erosion.
Fig. 9.
Anti-inflammatory effect of DW2007 in CIA mice. (A) Effect on body weight gain. (B) Effect on arthritis incidence. (C) Effect on macroscopic score (the number and severity of inflamed paws). (D) Effect on paw thickness. (E) Effect on myeloperoxidase (MPO) activity of the paw joint tissues. (F) Effect on histology. Arthritis was induced by the first intradermal injection of bovine type II collagen and the second intradermal injection of collagen with CFA at the base of the tail of DBA/1 J mice except in the normal control group (NOR). Normal control group was treated with saline instead of collagen. Test agents (AC, 20 mg/kg DW2007; IB, 50 mg/kg ibuprofen; or CIA, vehicle alone) were orally administrated daily for 20 days from day 1 after the second injection. All data indicate mean ± SD (n=7). #p<0.05 vs. control group. *p<0.05 vs. CIA group.
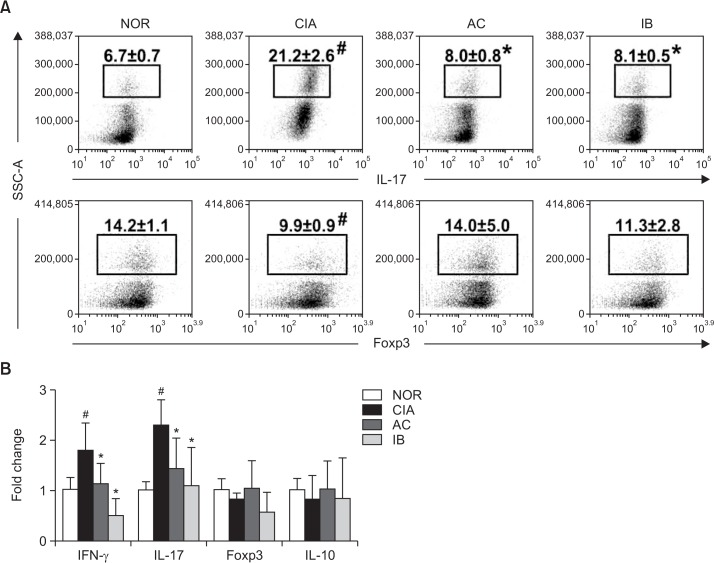
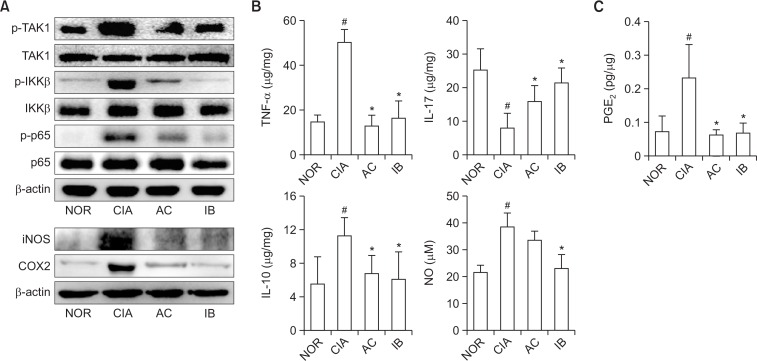
We investigated the effect of DW2007 on the differentiation of Th17 and Treg cells in spleen (Fig. 10). Treatment with collagen in mice significantly increased Th17 cell differentiation and RORγt and IL-17 expression, while collagen treatment weakly, but not significantly, Treg cell differentiation and Foxp3 and IL-10 expression. However, oral administration of DW2007 in CIA mice suppressed the differentiation of Th17 cells and expression of IL-17 and its transcription factor RORγt and weakly, but not significantly, increased Treg cell differentiation and IL-10 and FoxP3 expression. Next, we examined the inhibitory effect of DW2007 on the levels of NF-kB signaling molecules and inflammatory mediators in the paw joint tissue of CIA mice. Treatment with DW2007 inhibited collagen-induced TAK-1 and IKKβ phosphorylation and NF-κB activation. Furthermore, DW2007 treatment reduced collagen-induced TNF-α, IL-17, PGE2, and NO levels, but increased collagen-suppressed IL-10 expression (Fig. 11).
Fig. 10.
Effect of DW2007 on the expression of T cell differentiation (A) and its cytokines (B) in CIA mice. Arthritis was induced by the first intradermal injection of bovine type II collagen and the second intradermal injection of collagen with complete Freund’s adjuvant at the base of the tail of DBA/1 J mice. Test agents (AC, 20 mg/kg DW2007; IB, 50 mg/kg ibuprofen; or CIA, vehicle alone) were orally administrated daily for 20 days from day 1 after the second injection. T cells were isolated from the spleen. Th17 and Treg cells were then analyzed by a flow cytometer. Cytokines were determined in the paw joint tissues by real-time PCR (for IL-10, IL-17a, IFN-γ, Foxp3, and β-actin). All data indicate mean ± SD (n=7). #p<0.05 vs. control group. *p<0.05 vs. CIA group.
Fig. 11.
Effect of DW2007 on the expression of iNOS and COX-2, activation of NF-κB (A), expression of inflammatory cytokines (B), and production of PGE2 and NO (C) in CIA mice. Arthritis was induced by the first intradermal injection of bovine type II collagen and the second intradermal injection of collagen with complete Freund’s adjuvant at the base of the tail of DBA/1 J mice. Test agents (AC, 20 mg/kg DW2007; IB, 50 mg/kg ibuprofen; or CIA, vehicle alone) were orally administrated daily for 20 days from day 1 after the second injection. Proteins were determined in the paw joint tissues by immunoblotting. Cytokines were determined in the paw joint tissues by ELISA (IL-1β, IL-10, IL-17a, TNF-α, and PGE2). NO was measured using Griess reagent. All data indicate mean ± SD (n=7). #p<0.05 vs. control group. *p<0.05 vs. CIA group.
DISCUSSION
Chronic inflammation is regulated by cytokine secretion and transmigration of neutrophils, dendritic cells, and monocytes/macrophages involved in the innate immune system, and lymphocytes in the adaptive immune system (Parker et al., 2007; Artis and Spits, 2015). Of these immune cells, macrophages and dendritic cells involoved in the innate immune response are activated by various stresses such as pathogens and produces inflammatory mediators such as TNF-α, IL-1β, IL-6, and PGE2 that collectively play a critical role in the initiation, evolution/progression, and persistence of chronic inflammation (Li and Verma, 2002; Perkins and Gilmore, 2006; Arango Duque and Descoteaux, 2014; Isailovic et al., 2015). Furthermore, these mediators, such as TNF-α, IL-6, and IL-10, stimulate the adaptive immune response such as T cell differentiation. T lymphocytes, including Th17 and Treg cells, are involved in the pathogenesis of chronic inflammatory diseases (Yang et al., 2014). Of these T cells, Th17 cells produce IL-17, IL-21, and IL-22, and then facilitate the recruitment of monocytes and neutrophils to the inflammation sites (Monteleone et al., 2012). Moreover, IL-17 with proinflammatory cytokines such as TNF-α synergistically accelerates chronic inflammation, as well as suppresses Treg cell differentiation (Granet and Miossec, 2004; Kalim et al., 2012; Isailovic et al., 2015). Treg cells produce IL-10 and TGFβ, which are key regulators of the immune system by limiting the inflammatory response which could otherwise cause tissue damage.
In the previous study of Jeong et al. (2014) and Jang et al. (2013), DW2007 has been shown to ameliorate TNBS-induced colitis or DIC in mice by inhibiting NF-κB signaling pathway in macrophages, which are involved in an innate immune response (Li and Verma, 2002; Artis and Spits, 2015). DW2007 also has showed its ability to inhibit the expression of TNF-α and IL-6, which regulate T cell differentiation (Artis and Spits, 2015). In our results, we found that DW2007 significantly inhibited Th17 cell differentiation in vitro. In addition to this, DW2007 suppressed collagen-induced Th17 cell differentiation and IL-17 and RORγt expression in mice with CIA. However, treatment with collagen to mice weakly, but not significantly, decreased Treg cell differentiation and Foxp3 and IL-10 mRNA expression, while treatment with DW2007 weakly, but not significantly, increased Foxp3 and IL-10 mRNA expression and Treg cell differentiation in mice with CIA. These results suggest that DW2007 may ameliorate RA by suppressing Th17 cells differentiation in the adaptive immune response. Furthermore, to understand the effect of DW2007 in the adaptive immune response, we investigated its anticolitic effect in wild type and TLR−/− mice, which are regressive in the adaptive immune response compared to wild-type mice. Moreover, DW2007 potently inhibited Th17 cell differentiation in both mice and increased Treg cell differentiation. This supported the suggestion that DW2007 down-regulated Th17 cell differentiation and up-regulated Treg cell differentiation. Thus, DW2007 may improve RA and colitis by correcting the disturbance of Th17/Treg balance.
TNF-α antibodies, including infliximab, are prominently used to control RA and colitis by suppressing the inflammatory responses of macrophages and Th1 cells rather than those of Th17 cells (Lopez and Peyrin-Biroulet, 2013). Methotrexate suppresses chronic inflammatory diseases by upregulating IL-10 expression and downregulating TNF-α expression (Robinson, 1998). Some natural products, including ocotillol, icariin, andrographolide, and oleanolic acid regulate T cell differentiation (Tao et al., 2013; Liu et al., 2014; Kang et al., 2015; Lee et al., 2015a). These findings suggest that inflammatory mediators such as TNF-α, IL-10, and IL-17 secreted by immune cells (neutrophils, monocytes, and T lymphocytes) regulate chronic inflammation. Excessive stimulation by TNF-α and IL-17 deteriorates chronic inflammatory diseases, however, IL-10 attenuates these diseases (Liu et al., 2011). IL-10 deficiency stimulates Th1 and Th17 cell differentiation and suppresses Treg cell differentiation, leading to the promtion of chronic inflammatory diseases such as RA and colitis (Chaudhry et al., 2011). Therefore, the distruption of the Th17/Treg balance, including Th17 cell inhibitors and Treg cell activators, plays a pivotal role in the initiation and development of these chronic inflammatory diseases. The correction of a Th17/Treg imbalance may be beneficial as a potential therapeutic target for chronic inflammatory diseases such as colitis and RA.
Therefore, we concluded that DW2007 may ameliorate inflammatory diseases by inhibiting NF-κB signaling pathway and correcting the disturbance of Th17/Treg cells.
Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C1020).
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES
- Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arii K, Kumon Y, Sugahara K, Nakatani K, Ikeda Y, Suehiro T, Hashimoto K. Edaravone inhibits collagen-induced arthritis possibly through suppression of nuclear factor-kappa B. Mol Immunol. 2008;45:463–469. doi: 10.1016/j.molimm.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- Burska A, Boissinot M, Ponchel F. Cytokines as biomarkers in rheumatoid arthritis. Mediators Inflamm. 2014;2014:545493. doi: 10.1155/2014/545493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Brüning JC, Müller W, Rudensky AY. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert EC, Hagspiel KD. Gastrointestinal and hepatic manifestations of rheumatoid arthritis. Dig Dis Sci. 2011;56:295–302. doi: 10.1007/s10620-010-1508-7. [DOI] [PubMed] [Google Scholar]
- Granet C, Miossec P. Combination of the pro-inflammatory cytokines IL-1, TNF-α and IL-17 leads to enhanced expression and additional recruitment of AP-1 family members, Egr-1 and NFκB in osteoblast-like cells. Cytokine. 2004;26:169–177. doi: 10.1016/j.cyto.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Isailovic N, Daigo K, Mantovani A, Selmi C. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1–11. doi: 10.1016/j.jaut.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Jang SE, Jeong JJ, Hyam SR, Han MJ, Kim DH. Anticolitic Effect of the rhizome mixture of Anemarrhena asphodeloides and Coptidis chinensis (AC-mix) in mice. Biomol. Ther. (Seoul) 2013;21:398–404. doi: 10.4062/biomolther.2013.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JJ, Jang SE, Hyam SR, Han MJ, Kim DH. The rhizome mixture of Anemarrhena asphodeloides and Coptidis chinensis ameliorates acute and chronic colitis in mice by inhibiting the binding of lipopolysaccharide to TLR4 and IRAK1 phosphorylation. Evid Based Complement Alternat Med. 2014;2014:809083. doi: 10.1155/2014/809083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalim KW, Basler M, Kirk CJ, Groettrup M. Immunoproteasome subunit LMP7 deficiency and inhibition suppresses Th1 and Th17 but enhances regulatory T cell differentiation. J Immunol. 2012;189:4182–4193. doi: 10.4049/jimmunol.1201183. [DOI] [PubMed] [Google Scholar]
- Kang GD, Lim S, Kim DH. Oleanolic acid ameliorates dextran sodium sulfate-induced colitis in mice by restoring the balance of Th17/Treg cells and inhibiting NF-κB signaling pathway. Int Immunopharmacol. 2015;29:393–400. doi: 10.1016/j.intimp.2015.10.024. [DOI] [PubMed] [Google Scholar]
- Lee SY, Jeong JJ, Le TH, Eun SH, Nguyen MD, Park JH, Kim DH. Ocotillol, a majonoside R2 metabolite, ameliorates 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice by restoring the balance of Th17/Treg cells. J Agric Food Chem. 2015a;63:7024–7031. doi: 10.1021/acs.jafc.5b02183. [DOI] [PubMed] [Google Scholar]
- Lee SY, Jeong JJ, Kim KA, Kim DH. Lactobacillus sakei OK67 ameliorates collagen-induced arthritis in mice by inhibiting NF-κB activation and restoring Th17/Treg cell balance. J. Funct. Foods. 2015b;18, Part A:501–511. doi: 10.1016/j.jff.2015.08.006. [DOI] [Google Scholar]
- Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Liu B, Tonkonogy SL, Sartor RB. Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice. Gastroenterology. 2011;141:653–662. doi: 10.1053/j.gastro.2011.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Guo W, Guo L, Gu Y, Cai P, Xie N, Yang X, Shu Y, Wu X, Sun Y, Xu Q. Andrographolide sulfonate ameliorates experimental colitis in mice by inhibiting Th1/Th17 response. Int Immunopharmacol. 2014;20:337–345. doi: 10.1016/j.intimp.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Lopez A, Peyrin-Biroulet L. 5-Aminosalicylic acid and chemoprevention: does it work? Dig Dis. 2013;31:248–253. doi: 10.1159/000353806. [DOI] [PubMed] [Google Scholar]
- Mellado M, Martínez-Muñoz L, Cascio G, Lucas P, Pablos JL, Rodríguez-Frade JM. T cell migration in rheumatoid arthritis. Front Immunol. 2015;6:384. doi: 10.3389/fimmu.2015.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone I, Sarra M, Pallone F, Monteleone G. Th17-related cytokines in inflammatory bowel diseases: friends or foes? Curr Mol Med. 2012;12:592–597. doi: 10.2174/156652412800620066. [DOI] [PubMed] [Google Scholar]
- Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13:668–677. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Parker LC, Prince LR, Sabroe I. Translational mini-review series on Toll-like receptors: networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clin Exp Immunol. 2007;147:199–207. doi: 10.1111/j.1365-2249.2006.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-κB. Cell Death Differ. 2006;13:759–771. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- Qu N, Xu M, Mizoguchi I, Furusawa J, Kaneko K, Watanabe K, Mizuguchi J, Itoh M, Kawakami Y, Yoshimoto T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin Dev Immunol. 2013;2013:968549. doi: 10.1155/2013/968549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. Medical therapy of inflammatory bowel disease for the 21st century. Eur J Surg Suppl. 1998;582:90–98. doi: 10.1080/11024159850191517. [DOI] [PubMed] [Google Scholar]
- Sarra M, Pallone F, Macdonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16:1808–1813. doi: 10.1002/ibd.21248. [DOI] [PubMed] [Google Scholar]
- Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- Tao F, Qian C, Guo W, Luo Q, Xu Q, Sun Y. Inhibition of Th1/Th17 responses via suppression of STAT1 and STAT3 activation contributes to the amelioration of murine experimental colitis by a natural flavonoid glucoside icariin. Biochem Pharmacol. 2013;85:798–807. doi: 10.1016/j.bcp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan JW, Wang YJ, Peng WJ, Tao JH, Wan YN, Li BZ, Mei B, Chen B, Yao H, Yang GJ, Li XP, Ye DQ, Wang J. Therapeutic potential of interleukin-17 in inflammation and autoimmune diseases. Expert Opin. Ther. Targets. 2014;18:29–41. doi: 10.1517/14728222.2013.843669. [DOI] [PubMed] [Google Scholar]
- Yang J, Sundrud MS, Skepner J, Yamagata T. Targeting Th17 cells in autoimmune diseases. Trends Pharmacol Sci. 2014;35:493–500. doi: 10.1016/j.tips.2014.07.006. [DOI] [PubMed] [Google Scholar]