Abstract
Lindera obtusiloba has been used in traditional herbal medicine for the treatment of blood stasis and inflammation. The leaves of Lindera obtusiloba have been reported to exhibit various physiological activities. However, there is little information available on their antiplatelet and antithrombotic activities. Thus, the present study aimed to evaluate the effect of Lindera obtusiloba leaf extract (LLE) on platelet activities, coagulation and thromboembolism. In a platelet aggregation study, LLE significantly inhibited various agonist-induced platelet aggregations in vitro and ex vivo. Furthermore, LLE significantly inhibited collagen-induced thromboxane A2 (TXA2) production in rat platelets. In addition, oral administration of LLE was protective in a mouse model of pulmonary thromboembolism induced by intravenous injection of a mixture of collagen and epinephrine. Interestingly, LLE did not significantly alter prothrombin time (PT) and activated partial thromboplastin time (aPTT). This study indicates that the antithrombotic effects of LLE might be due to its antiplatelet activities rather than anticoagulation. Taken together, these results suggest that LLE may be a candidate preventive and therapeutic agent in cardiovascular diseases associated with platelet hyperactivity.
Keywords: Lindera obtusiloba, Platelet aggregation, Thrombosis, Thromboxane
INTRODUCTION
Platelet adhesion, activation and aggregation on the exposed subendothelial extracellular matrix are essential for hemostasis and central events in the blood clotting process (Jennings, 2009). Platelets are activated by various endogenous factors (e.g., collagen, serotonin and TXA2), and a platelet-rich thrombus is subsequently formed in the lumen of the injured vessel (Jennings, 2009). Platelet deposition by aggregation at these sites appears to be an important event leading to thrombotic disorders. Thus, antiplatelet therapy features prominently in the treatment or prevention of thrombotic diseases (Gross and Weitz, 2009; Rondina et al., 2013).
Antithrombotic agents, such as aspirin, cilostazol and clopidogrel, have adverse effects, including internal bleeding, prolonged bleeding time, gastrointestinal bleeding, palpitation (CAPRIE Steering Committee, 1996; Johnson, 2008; Francescone and Halperin, 2008) and headache (Birk et al., 2006). For these reasons, a number of studies have been conducted to identify antithrombotic agents from medicinal plants with fewer adverse effects. Many reports have indicated that plant-based materials have the potential to be novel antithrombotic agents (Ballabeni et al., 2007; Vilahur and Badimon, 2013). Additionally, several of these compounds exhibit excellent pharmacological activities and are free from adverse effects (Ballabeni et al., 2007; Ryu et al., 2009; Fuentes et al., 2014). Moreover, natural flavonoids and polyphenols have been shown to effectively inhibit platelet aggregation (Bucki et al., 2003). Therefore, development of antithrombotic agents from medicinal plants has attracted much interest.
Lindera obtusiloba, which is found in many provinces of Korea and China, is a known medicinal herb traditionally used for the treatment of blood stasis and inflammation (Yook, 1989). The leaves of Lindera obtusiloba in particular are traditionally consumed in both food and tea. We previously demonstrated that a twig extract from Lindera obtusiloba inhibited rat platelet aggregation in vitro and decreased mortality in a mouse model of thromboembolism (Lee et al., 2010). Furthermore, a relevant patent application was published that describes preliminary data on the antithrombotic effects of Lindera obtusiloba twigs and leaves, which improve blood circulation (Oak et al., 2014). Many researchers have reported strong antioxidant effects and various physiological activities of Lindera obtusiloba leaf extracts (Hong et al., 2013a; Hong et al., 2013b; Hong, 2013). Despite the results of these studies, the antiplatelet and antithrombotic properties of the leaves of Lindera obtusiloba have not been studied.
In this study, we examined the effects of the leaves of Lindera obtusiloba on platelet function and coagulant activities using blood from rats and the antithrombotic effects using a mouse thromboembolism model.
MATERIALS AND METHODS
Reagents
Collagen, adenosine diphosphate (ADP), arachidonic acid (AA) and thrombin were purchased from Chrono-Log (Harvertown, PA, USA). Aspirin and heparin were purchased from Sigma (St. Louis, MO, USA). Quercitrin was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Afzelin was purchased from BioBioPha (Kunming, China).
Plant materials and extract preparation
The dried leaves of Lindera obtusiloba were collected in the vicinity of Hongcheon and were purchased from Yakcho119 (Hongcheon, Republic of Korea). The dried leaves were extracted with 70% ethanol (v/v) at 70°C for 4 h, and the extracted solution was then filtered and evaporated. The extract was then freeze-dried to obtain powder and used as the Lindera obtusiloba leaf extract (LLE). The yield of LLE was 21.8%
Animals
Male Sprague-Dawley (SD) rats (7 weeks, 200–240 g) and ICR mice (7 weeks, 30–33 g) were purchased from Orient Bio (Sungnam, Republic of Korea). All animals were housed in colony cages, under standard laboratory conditions (12/12 h light/dark cycle), and had free access to food and water. All animal study protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and were approved by the Institutional Animal Care and Utilization Committee for Gyeonggi BioCenter (Suwon, Republic of Korea).
Preparation of blood samples
SD rats weighing 200–240 g were lightly anesthetized with diethyl ether. Blood samples were collected from the abdominal aorta into a syringe containing 3.8% sodium citrate. The ratio of blood to 3.8% sodium citrate was adjusted to 1:9 v/v. After centrifugation at 150×g for 10 min at room temperature, supernatants (platelet-rich plasma, PRP) were used for the aggregation study. The platelet count in PRP was finally adjusted to approximately 2×108 cells/ml with Tyrode’s solution (pH 7.4, 134 mM NaCl, 3 mM KCl, 2 mM MgCl2, 0.3 mM NaH2 PO4, 12 mM NaHCO3, 12 mM glucose). Platelet-poor plasma (PPP) was obtained by centrifuging the samples at 2000×g for 20 min for the coagulation assay.
In vitro platelet aggregation study
Platelet aggregation was determined by the turbidimetric method using an aggregometer (Chrono-Log, Havertown, PA, USA). PRP was diluted with Tyrode’s solution containing 0.3% BSA and adjusted to 400 μl to obtain a platelet count of 2×108 cells/ml. PRP was stimulated to aggregate using different aggregating agents at the following final concentrations: collagen, 2 μg/ml; ADP, 10 μM; AA, 100 μM; and thrombin, 0.2 U/ml. Platelet aggregation was recorded for 5 min after stimulation. Aggregations were measured and expressed as percent changes in light transmission with respect to PPP. In the in vitro study, PRP was preincubated with LLE (0.1, 0.3, 1 mg/ml) or aspirin at 37°C for 5 min before stimulation with the aggregating agents.
Ex vivo platelet aggregation study
SD rats were orally administered 70% polyethylene glycol (PEG)-saline, LLE (100, 200, 400 mg/kg), or aspirin (50 mg/kg) once a day for 5 days. Two hours after the last administration, rat blood samples were collected, and the platelet aggregation study was performed as described above.
Measurement of thromboxane A2 (TXA2) formation
PRP (2×108 cells/ml) was preincubated with LLE (0.1, 0.3, 1 mg/ml) or aspirin at 37°C for 5 min, and 2 mg/ml collagen was added. After incubation at 37°C for 5 min with stirring, 10 mM ethylenediaminetetraacetic acid (EDTA) was added to stop TXA2 formation. The amount of TXA2 was determined by measuring thromboxane B2 (TXB2, the stable metabolite of TXA2). After centrifugation at 12,000 g for 3 min, the amount of TXB2 in the supernatant was measured using a commercial TXB2 enzyme immunoassay (EIA) kit (Cayman, Ann Arbor, MI, USA).
Pulmonary embolism model
The antithrombotic effect of LLE was investigated using a mouse pulmonary thromboembolism test as previously described (Lee et al., 2010). In brief, LLE (100, 200, 400 mg/kg) and aspirin (50 mg/kg) as a positive control were orally administered once per day for 5 days to ICR mice weighing 30–33 g. One hour after the final oral administration, a mixture of collagen (20 μg/mouse) plus epinephrine (2 μg/mouse) was injected into the tail vein to induce pulmonary thromboembolism. A collagen solution containing native collagen fibrils from equine tendons was used (385, Chrono-Log). The number of dead mice was determined after 15 min, and the survival rate (%) was calculated using the following equation: [1−(dead or paralyzed mice)/total mice tested]×100.
Coagulation assay
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were automatically measured using an Automated Coagulation Laboratory 100 (Instrumentation Laboratory, Bedford, MA, USA) according to the manufacturer’s instruction. PPP was prepared as described above. In brief, PPP was incubated with LLE for 3 min at 37°C, and the aPTT reagent was then added to the mixture and incubated for 3 min at 37°C. Then, 20 μM CaCl2 was added, and the clotting time was recorded. For the PT assay, PPP was incubated with LLE for 3 min at 37°C. PT reagent was then added, and the clotting time was recorded. Saline was used as a control.
High-performance liquid chromatography (HPLC) analysis
The analytical HPLC profile was obtained using an Alliance® HPLC system (e2695, Waters, Milford, MA, USA). Chromatographic separation was performed on a Capcell Pak UG120 column (4.6×250 mm, 5 μm, Shiseido, Tokyo, Japan) at 30°C. The mobile phase was 20% acetonitrile in water containing 0.08% trifluoroacetic acid. The flow rate was 1.0 ml/min with an injection volume of 10 μl, and UV detection was performed at 254 nm.
Statistical analysis
In pulmonary thromboembolism experiment, the chi-square test was used to determine significant differences between the vehicle and treated groups. In the other experiments, data are expressed as the mean ± SE. The statistical comparisons were performed using one-way analysis of variance (ANOVA) followed by Dunnett’s test or one-way ANOVA followed by Tukey’s test. A significant difference was defined as p<0.05.
RESULTS
Effect of LLE on platelet aggregation in vitro
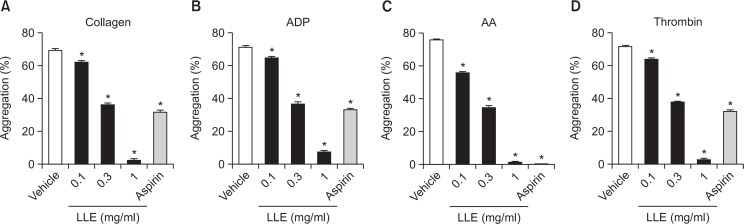
We evaluated the antiplatelet effect of LLE using rat PRP. Aspirin (1 mg/ml) was used as a positive control. Our results showed that LLE had a maximum inhibitory effect on collagen (2 μg/ml, Fig. 1A)-, ADP (10 μM, Fig. 1B)-, AA (100 μM, Fig. 1C)-, and thrombin (0.2 U/ml, Fig. 1D)-induced platelet aggregation (96.9 ± 0.8%, 89.4 ± 0.8%, 97.9 ± 0.5% and 96.1 ± 1.0% inhibition respectively) at a concentration of 1 mg/ml. The half maximal inhibitory concentration (IC50) values were 0.48 ± 0.0, 0.48 ± 0.0, 0.44 ± 0.0 and 0.49 ± 0.0 mg/ml, respectively.
Fig. 1.
Inhibitory effect of the extract of Lindera obtusiloba leaves (LLE) on platelet aggregation in vitro. After preincubation with LLE (0.1, 0.3, 1 mg/ml) or aspirin (1 mg/ml) for 5 min at 37°C, platelets were stimulated with 2 μg/ml collagen (A), 10 μM adenosine diphosphate (ADP) (B), 100 μM arachidonic acid (AA) (C), and 0.2 U/ml thrombin (D). Data are expressed as the mean ± SEM. n=5, *p<0.05 compared with the vehicle.
Effect of LLE on platelet aggregation ex vivo
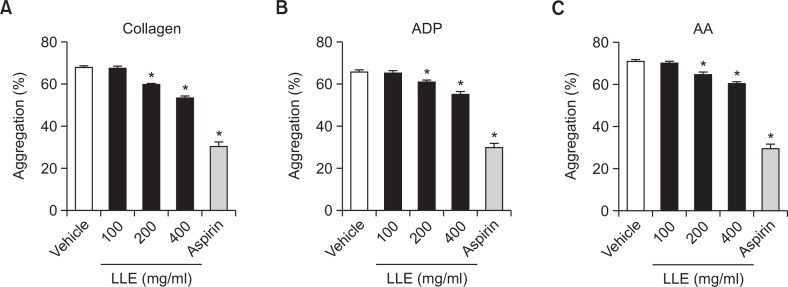
The inhibitory effect of LLE on ex vivo platelet aggregation is shown in Fig. 2. Aspirin (50 mg/kg) was used as a positive control. Isolated platelets from LLE (200 and 400 mg/kg)-treated rats showed significant inhibition in collagen (2 μg/ml)-, ADP (10 μM)-, and AA (100 μM)-induced platelet aggregation, which concurred with the results obtained when PRP was directly incubated with LLE.
Fig. 2.
Inhibitory effect of the extract of Lindera obtusiloba leaves (LLE) on platelet aggregation ex vivo. After rats were treated with with LLE (100, 200, 400 mg/kg) or aspirin (50 mg/kg), blood samples were collected, and platelets were stimulated with 2 μg/ml collagen (A), 10 μM adenosine diphosphate (ADP) (B), 100 μM arachidonic acid (AA) (C). Data are expressed as the mean ± SEM. n=6, *p<0.05 compared with the vehicle.
Effect of LLE on TXA2 formation
TXA2 is an important mediator of collagen-induced platelet activation and aggregation. Therefore, we evaluated the effect of LLE on TXA2 formation. However, TXA2 is unstable and is quickly converted to TXB2, the stable metabolite of TXA2. Thus, a TXB2 EIA kit was used to determine the TXA2 concentrations. As shown in Fig. 3, the TXB2 level in the control group was 77.1 ± 1.6 pg/ml, and this increased to 2327.8 ± 61.0 pg/ml after stimulation with collagen (2 μg/ml). As shown in Fig. 3, LLE (0.1, 0.3, 1 mg/ml) significantly inhibited collagen-induced TXB2 formation (1676.6 ± 82.5, 987.1 ± 26.2, 930.1 ± 9.8 pg/ml, respectively). Aspirin, the positive control, almost completely blocked TXB2 formation (63.5 ± 3.3 pg/ml) at a concentration of 0.1 mg/ml.
Fig. 3.
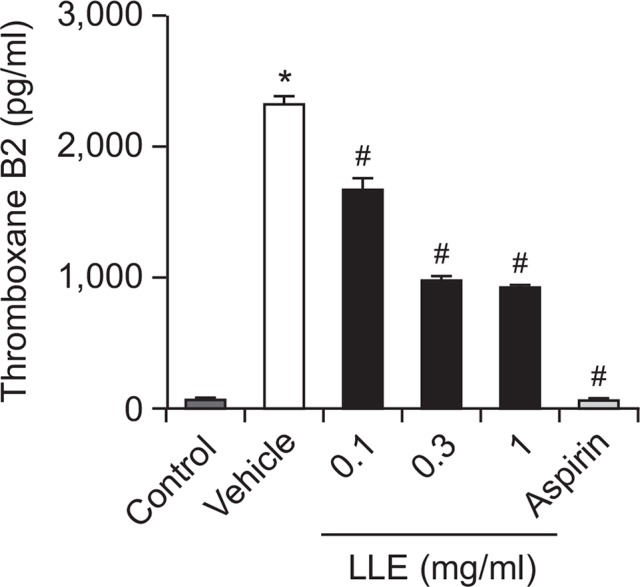
Inhibitory effect of the extract of Lindera obtusiloba leaves (LLE) on thromboxane A2 (TXA2) formation. Platelets were preincubated with LLE (0.1, 0.3, 1 mg/ml) or aspirin (0.1 mg/ml) for 5 min and then stimulated with collagen (2 μg/ml) for 5 min. The TXA2 content in the supernatant was determined using a commercial EIA kit. Data are expressed as the mean ± SEM. n=3, *p<0.05 compared with the control. #p<0.05 compared with the vehicle.
Effect of LLE on pulmonary thromboembolism in mice
To investigate the effect of LLE on occlusive thrombus formation, we tested the antithrombotic effect using a pulmonary thromboembolism model. An intravenous injection of collagen (20 μg/mouse) and epinephrine (2 μg/mouse) in mice induced massive pulmonary thrombotic occlusion, causing acute paralysis and death. As shown in Table 1, oral administration of LLE (400 mg/kg) to mice resulted in significant protection from the death due to thrombosis. Aspirin (50 mg/kg) was used as a positive control.
Table 1.
Effect of the extract of Lindera obtusiloba leaves (LLE) on pulmonary thrombosis in mice
| Dose (mg/kg) | Dead or paralyzed mice | Survival rate (%) | |
|---|---|---|---|
| Vehicle | 15 | 0 | |
| LLE | 100 | 13 | 13.3 |
| 200 | 12 | 20.0 | |
| 400 | 6 | 60.0* | |
| Aspirin | 50 | 7 | 53.3* |
LLE (100, 200, 400 mg/kg) or aspirin (50 mg/kg) was orally administered to mice 2 h before the experiments. The chi-square test used to examine the difference between the vehicle and treated group. n=15.
p<0.05 compared with vehicle.
Effect of LLE on coagulation in vitro
To determine whether LLE has anticoagulant properties, PT and aPTT were evaluated using rat plasma. As shown in Table 2, LLE did not affect the rat plasma coagulation times. Heparin was used as a positive control and significantly prolonged PT and aPTT.
Table 2.
Effect of the extract of Lindera obtusiloba leaves (LLE) on rat plasma coagulation time in vitro
| Concentration | PT (sec) | aPTT (sec) | |
|---|---|---|---|
| Vehicle | 8.55 ± 0.00 | 15.93 ± 0.93 | |
| LLE | 0.1 mg/ml | 8.45 ± 0.17 | 15.20 ± 1.07 |
| 0.3 mg/ml | 8.50 ± 0.09 | 15.13 ± 2.50 | |
| 1 mg/ml | 8.95 ± 0.09 | 16.70 ± 0.46 | |
| Heparin | 10 IU/ml | 37.05 ± 10.82* | >106* |
Data are expressed as means ± SEM. n=3.
p<0.05 compared with vehicle. PT: prothrombin time, aPTT: activated partial thromboplastin time.
HPLC analysis
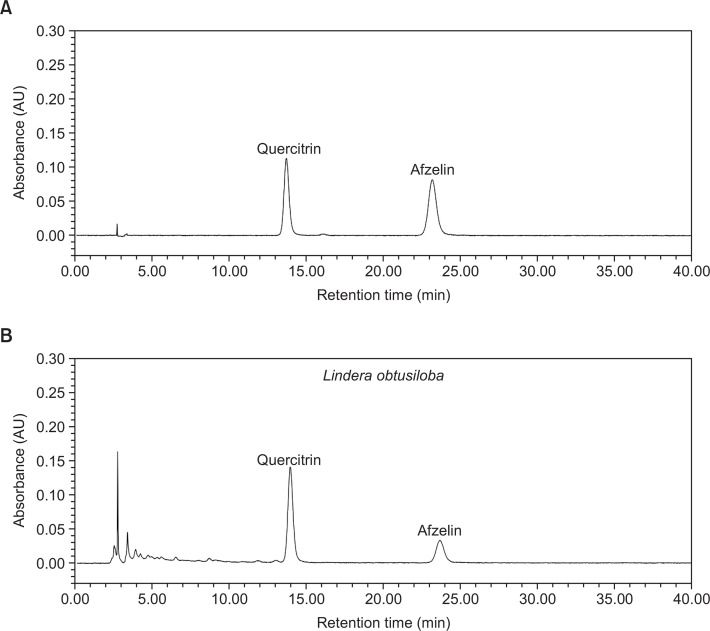
Several peaks were identified in the HPLC chromatogram of LLE (Fig. 4). Comparing the chromatographic peaks of LLE with reference chromatographic peaks, quercitrin and afzelin were identified.
Fig. 4.
HPLC chromatogram of the extract of Lindera obtusiloba leaves (B). Quercitrin and afzelin were identified based on the retention times of each reference compound (A).
DISCUSSION
In the in vitro platelet aggregation study, LLE significantly inhibited platelet aggregation induced by thrombin, collagen, ADP, and AA in a concentration-dependent manner. Additionally, collagen-induced TXA2 production was significantly inhibited by preincubation with LLE. However, the mechanism underlying the antiplatelet effect of LLE is unknown. It has been reported that several plant flavonoids inhibit platelet function by antagonistically binding to the TXA2 receptor (Guerrero et al., 2005). However, based on the similar IC50 values against various agonists, we hypothesize that LLE may regulate common signaling pathways, such as phospholipase (PLC)-associated signaling (Li et al., 2010), rather than bind to specific receptors. Further studies are needed to elucidate the detailed mechanisms of LLE antiplatelet activity. The anti-aggregatory effect was also confirmed by an ex vivo study in rats. Oral administration of LLE resulted in dose-dependent inhibition of the platelet aggregation induced by collagen, ADP and AA. Disappointingly, however, the inhibitory effect of LLE was much less potent in the ex vivo assay compared to that of the in vitro assay. We speculate that oral LLE bioavailability and persistency in rat plasma may be factors in the difference between the ex vivo and in vitro results. Further investigations to determine the pharmacokinetic and pharmacodynamic properties will be helpful for understanding the effects of LLE. In the thromboembolism model, oral administration of LLE significantly increased the survival rate of mice with acute pulmonary thrombosis. These results indirectly demonstrate that the LLE antithrombotic effect was correlated with inhibition of platelet activity. Notably, LLE did not prolong PT and aPTT in the in vitro coagulation assay. These results suggest that LLE intake can prevent platelet hyperactivity with few side effects.
The leaves and twigs of Lindera obtusiloba were reported to contain polyphenols and flavonoids (Hong, 2013). Daily consumption of these compounds was shown to inhibit platelet function (Murphy et al., 2003). In this study, we observed that quercitrin and afzelin were the most abundant compounds in LLE. Quercitrin inhibited AA-induced platelet aggregation in rat platelets (Kim and Yun-Choi, 2008). In addition, quercetin, the aglycone of quercitrin, inhibited collagen-induced platelet aggregation by blocking hydrogen peroxidase production, calcium mobilization, and 1,3,4-inositol triphosphate formation in human platelets (Pignatelli et al., 2000). Furthermore, kaempferol, the aglycon of afzelin, inhibited collagen-induced platelet aggregation and adhesion in vitro by suppressing the tyrosine phosphorylation of key components involved in the collagen receptor signaling cascade, such as spleen tyrosine kinase (Syk), Bruton’s tyrosine kinase (Btk) and PLCγ2 (Wang et al., 2015). Although we confirmed that quercitrin and afzelin are the major constituents of LLE, the pharmacological effects of these compounds were not investigated. Therefore, further studies are necessary to determine the effect of these compounds on platelets and thrombosis. Additionally, the mechanism of action should be clarified.
In summary, our present results demonstrated that LLE has antiplatelet and antithrombotic effects in vitro and in vivo. These findings suggest that LLE may have therapeutic potential for the prevention or treatment of platelet-associated cardiovascular diseases.
Acknowledgments
This research was financially supported by the Ministry of Trade, Industry and Energy (MOTIE) and the Korea Institute for Advancement of Technology (KIAT) through Promoting Regional Specialized Industry (R0002418).
REFERENCES
- Ballabeni V, Tognolini M, Bertoni S, Bruni R, Guerrini A, Rueda GM, Barocelli E. Antiplatelet and antithrombotic activities of essential oil from wild Ocotea quixos (Lam.) Kosterm. (Lauraceae) calices from Amazonian Ecuador. Pharmacol Res. 2007;55:23–30. doi: 10.1016/j.phrs.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Birk S, Kruuse C, Petersen KA, Tfelt-Hansen P, Olesen J. The headache-inducing effect of cilostazol in human volunteers. Cephalalgia. 2006;26:1304–1309. doi: 10.1111/j.1468-2982.2006.01218.x. [DOI] [PubMed] [Google Scholar]
- Bucki R, Pastore JJ, Giraud F, Sulpice J-C, Janmey PA. Flavonoid inhibition of platelet procoagulant activity and phosphoinositide synthesis. J Thromb Haemost. 2003;1:1820–1828. doi: 10.1046/j.1538-7836.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. doi: 10.1016/S0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- Francescone S, Halperin JL. “Triple therapy” or triple threat? Balancing the risks of antithrombotic therapy for patients with atrial fibrillation and coronary stents. J Am Coll Cardiol. 2008;51:826–827. doi: 10.1016/j.jacc.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Fuentes E, Alarcón M, Fuentes M, Carrasco G, Palomo I. A novel role of Eruca sativa Mill. (rocket) extract: antiplatelet (NF-κB inhibition) and antithrombotic activities. Nutrients. 2014;6:5839–5852. doi: 10.3390/nu6125839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross PL, Weitz JI. New antithrombotic drugs. Clin Pharmacol Ther. 2009;86:139–146. doi: 10.1038/clpt.2009.98. [DOI] [PubMed] [Google Scholar]
- Guerrero JA, Lozano ML, Castillo J, Benavente-García O, Vicente V, Rivera J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J Thromb Haemost. 2005;3:369–376. doi: 10.1111/j.1538-7836.2004.01099.x. [DOI] [PubMed] [Google Scholar]
- Hong C-O, Lee HA, Rhee CH, Choung S-Y, Lee K-W. Separation of the antioxidant compound quercitrin from Lindera obtusiloba Blume and its antimelanogenic effect on B16F10 melanoma cells. Biosci Biotechnol Biochem. 2013a;77:58–64. doi: 10.1271/bbb.120562. [DOI] [PubMed] [Google Scholar]
- Hong C-O, Rhee CH, Won N-H, Choi H-D, Lee K-W. Protective effect of 70% ethanolic extract of Lindera obtusiloba Blume on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food Chem Toxicol. 2013b;53:214–220. doi: 10.1016/j.fct.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Hong J-H. Physiological activities of leaf and twig extracts from Lindera obtusiloba Blume. Korean J Food Cookery Sci. 2013;29:573–580. doi: 10.9724/kfcs.2013.29.5.573. [DOI] [Google Scholar]
- Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009;102:248–257. doi: 10.1160/TH09-03-0192. [DOI] [PubMed] [Google Scholar]
- Johnson S. Known knowns and known unknowns: risks associated with combination antithrombotic therapy. Thromb. Res. 2008;123(Suppl 1):S7–S11. doi: 10.1016/j.thromres.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Kim JM, Yun-Choi HS. Anti-platelet effects of flavonoids and flavonoid-glycosides from Sophora japonica. Arch Pharm Res. 2008;31:886–890. doi: 10.1007/s12272-001-1242-1. [DOI] [PubMed] [Google Scholar]
- Lee J-O, Kim C-Y, Lee S-W, Oak M-H. Antiplatelet and Antithrombotic Activities of Lindera obtusiloba Extract in vitro and in vivo. Biomol. Ther (Seoul) 2010;18:205–210. doi: 10.4062/biomolther.2010.18.2.205. [DOI] [Google Scholar]
- Li Z, Delaney MK, O’Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KJ, Chronopoulos AK, Singh I, Francis MA, Moriarty H, Pike MJ, Turner AH, Mann NJ, Sinclair AJ. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am J Clin Nutr. 2003;77:1466–1473. doi: 10.1093/ajcn/77.6.1466. [DOI] [PubMed] [Google Scholar]
- Oak M-H, Lee J-O, Kang S-H, Sohn J-D, Kim J-H, Lim JW, Na Y, Oh Y, Lee S-W. Han Wha Pharma Co., Ltd. Yang Ji Chemical Co., Ltd. Method for preventing and treating thrombotic disorders using a pharmaceutical composition comprising an extract of Lindera obtusiloba. 2014. May 20, p. A1. inventors; assignees. United States patent US20140255529.
- Pignatelli P, Pulcinelli FM, Celestini A, Lenti L, Ghiselli A, Gazzaniga PP, Violi F. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am J Clin Nutr. 2000;72:1150–1155. doi: 10.1093/ajcn/72.5.1150. [DOI] [PubMed] [Google Scholar]
- Rondina MT, Weyrich AS, Zimmerman GA. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res. 2013;112:1506–1519. doi: 10.1161/CIRCRESAHA.113.300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu KH, Han HY, Lee SY, Jeon SD, Im G-J, Lee BY, Kim K, Lim K-M, Chung J-H. Ginkgo biloba extract enhances antiplatelet and antithrombotic effects of cilostazol without prolongation of bleeding time. Thromb Res. 2009;124:328–334. doi: 10.1016/j.thromres.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Vilahur G, Badimon L. Antiplatelet properties of natural products. Vascul Pharmacol. 2013;59:67–75. doi: 10.1016/j.vph.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Wang SB, Jang JY, Chae YH, Min JH, Baek JY, Kim M, Park Y, Hwang GS, Ryu J-S, Chang T-S. Kaempferol suppresses collagen-induced platelet activation by inhibiting NADPH oxidase and protecting SHP-2 from oxidative inactivation. Free Radic Biol Med. 2015;83:41–53. doi: 10.1016/j.freeradbiomed.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Yook C. Medicinal plants of Korea. Academy Publishing; Seoul: 1989. p. 184. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.