Abstract
The mechanisms contributing to persistent eosinophil activation and poor eosinopenic response to glucocorticoids in severe asthma are poorly defined. We examined the effect of cytokines typically overexpressed in the asthmatic airways on glucocorticoid signaling in in vitro activated eosinophils. An Annexin V assay used to measure eosinophil apoptosis showed that cytokine combinations of IL-2 plus IL-4 as well as TNFα plus IFNγ, or IL-3, GM-CSF and IL-5 alone significantly diminished the proapoptotic response to dexamethasone. We found that IL-2 plus IL-4 resulted in impaired phosphorylation and function of the nuclear glucocorticoid receptor (GCR). Proteomic analysis of steroid sensitive and resistant eosinophils identified several differentially expressed proteins, namely Protein Phosphatase 5 (PP5), Formyl Peptide Receptor (FPR2) and Annexin 1. Furthermore, an increased phosphatase activity of PP5 correlated with the impaired phosphorylation of the GCR. Importantly, suppression of PP5 expression with siRNA restored proper phosphorylation and the proapoptotic function of the GCR. We also examined the effect of lipoxin A4 on PP5 activation by IL-2 plus IL-4. Similar to PP5 siRNA inhibition, pretreatment of eosinophils with lipoxin A4 restored GCR phosphorylation and the proaptoptotic function of GC. Taken together, our results showed 1) a critical role for PP5 in cytokine-induced resistance to GC-mediated eosinophil death, 2) supported the dependence of GCR phosphorylation on PP5 activity and 3) revealed that PP5 is a target of the lipoxin A4-induced pathway countering cytokine-induced resistance to GCs in eosinophils.
Keywords: Eosinophils, Cytokines, Glucocorticoid Receptor, Protein Phosphatase 5, Steroid Resistance and Asthma
Introduction
Glucocorticoids are among the most potent anti-inflammatory drugs used in clinical medicine and remain the cornerstone of antiallergic and immunosuppressive therapy in bronchial asthma (1). While the majority of patients with asthma respond favorably to inhaled and systemic steroid therapy, the inflammation in bronchial asthma cannot always be fully controlled by steroid treatment (2, 3). Up to 10% of patients with difficult to control asthma have poor clinical responses even to high doses of glucocorticoids and these patients are at highest risk for asthma mortality (4). The absence of an effective therapeutic alternative for these patients poses both a health challenge as well as a financial burden since they account for more than 50% of asthma-related healthcare costs (5). Moreover, treatment of patients with GC’s who are resistant to glucocorticoid therapy also poses health risks as well.
The molecular mechanism of steroid resistance in asthma remains unclear was indicated to be multifactorial (6). Some patients with steroid-resistant asthma are characterized with persistent inflammation, overexpression of proinflammatory cytokines in airways, and a reduced eosinopenic response to GC treatment (4, 7). Except for non-compliance to GC treatment, abnormal steroid pharmacokinetics, and rare genetic defects in the GC receptors, we hypothesize that the majority of GC insensitivity in asthma can be linked to GC receptor signaling. Reports by others have implicated abnormalities in the glucocorticoid receptor phosphorylation, ligand binding, as well as interactions with nuclear transcription factors (8–10). Additionally, a reduction in inflammatory gene expression (e.g. histone deacetylase 2, and HDAC2) or overexpression of pro-inflammatory transcription factors (e.g. NF-κB) and overexpression of the decoy glucocorticoid receptor (GCRβ) have been reported to contribute to steroid-resistance in chronic inflammation (11).
The anti-inflammatory effects of GCs are mediated through the GC receptor (GCR), which acts mainly via genomic mechanisms (12). Before nuclear ligand binding, the GCR is predominantly cytoplasmic and bound to a chaperone protein complex containing several proteins, including heat shock protein 90 (HSP90), p23, and PP5. This complex was reported to inactivate transcriptional regulatory functions (13). GCs interact with the GCR in the cytoplasm resulting in phosphorylation and translocation of the hormone–receptor complex into the cell nucleus. The GCR is a phosphoprotein in the ligand-free form but additional phosphorylation events occur in conjunction with ligand binding. Three amino acid residues thought to be involved in the transcriptional activity of the GCR, include S203, S211, and S226, all of which are substrates for Cdk2 (S203, S211), p38 (S211) and JNK (S226) (14). Importantly, GCR dephosphorylation of S203 and S226 is reported to be regulated by PP5, whereas S211 phosphatase has not yet been identified (13–15). PP5 also binds to HSP90 and acts as a co-chaperone with HSP90 participating in GCR nucleocytoplasmic shuttling. Suppression of PP5 with antisense oligonucleotides stimulated the activity of GC-responsive genes without affecting the binding of GC to the GCR suggesting a critical role for a serine/threonine protein phosphatase in the regulation of GCR functionality (16). Transfection studies using a reporter construct containing GC responsive elements showed that the specific small interfering RNA-induced mRNA knockdown of PP5, partially reversed impairment of GCR phosphorylation and transactivation in bronchial smoot muscle cells suggesting a novel role of PP5 in mediating GC resistance in airway inflammatory cells (17).
To explore the possible mechanism of steroid resistance in allergic inflammation, we have examined the effect of GCs on eosinophil viability after cytokine stimulation. While GCs affect virtually all primary and secondary immune cells during treatment of bronchial asthma, a major therapeutic activity of GCs strongly correlates with the reduction of circulating and tissue eosinophils (18). Induction of eosinophil apoptosis, inhibition of β2-integrin mediated eosinophil adhesion and activation and stimulation of non-inflammatory phagocytosis of apoptotic cells are thought to play the predominant role in GC-induced reduction of eosinophilia in patients sensitive to glucocorticoid therapy (19). However, some patients with severe asthma are characterized with a decreased eosinopenic response suggesting abnormalities in eosinophil responsiveness to glucocorticoids. Thus, herein we have investigated GC signaling in eosinophils stimulated with cytokines previously reported to be overexpressed in the airways of severe asthmatics, including IL-2, IL-3, IL-4, IL-5, GM-CSF, INFγ, and TNFα. Since IL-2 plus IL-4 were indicated in patient studies to potentially be factors in steroid resistance (20) we sought to further inquire into the relationship of eosinophil activation and steroid resistance in a broader context. Further, comparative proteomic analysis of steroid sensitive versus resistant eosinophils identified a number of differentially expressed protein, notably PP5, FPR2 (Formyl Peptide Receptor 2), GILZ (Glucocorticoid-Induced Leucine Zipper protein) and Annexin 1. Since these proteins are known to be active components of GCR signaling, we subsequently investigated their role in the diminished responsiveness of eosinophils to GCs. We found that PP5 protein expression and activation were synergistically upregulated by treatment of eosinophils with IL-2 plus IL4 that correlated with decreased phosphorylation of the GCR protein expression of FPR2, GILZ and Annexin 1 and diminished eosinophil apoptosis in response to GC. Inhibiting PP5 in activated eosinophils with specific siRNA restored GC-inducible phosphorylation of the GCR and induction of apoptosis. A similar effect was observed upon treatment of eosinophils with lipoxin A4 that also suppressed activation of PP5 suggesting crosstalk between the lipoxin A4 and FPR2 counterregulatory pathway with GCR signaling is mediated by PP5. Taken together, we show for the first time that proinflammatory cytokines may protect eosinophils from GC-induced death through activation of PP5 phosphatase that in turn modifies phosphorylation and function of GR. This mechanism may explain the phenomenon of increased airway eosinophilia and diminished phosphorylation of GR in a group of severe asthmatics. This effect can be interfered with by inhibition of PP5 expression or by the use of lipoxin A4 that inhibits PP5 phosphatase activity.
Materials and Methods
Reagents/materials
Recombinant, human GM-CSF, IFNγ, IL-2, IL-3, IL-4, IL-5, and TNFα were newly purchased from Peprotech (Rocky Hill, NJ). Polyclonal antibodies against phosphorylated GCR (S203, S211, S226) were from Abcam (Cambridge, MA); and antibodies against GCR, FPR2, Annexin 1, PP5, GILZ, phospho-Raf-1 and MKP1 were from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal anti-phospho-ERK antibodies and the secondary HRP-conjugated anti-rabbit antibody were from Cell Signaling Technology (Danvers, MA). PP5 siRNA and an appropriately scrambled control and transfecting reagent were purchased from Santa Cruz Biotechnology. PE-conjugated anti-αMβ2 monoclonal antibody and isotype-specific control were also from Santa Cruz Biotechnology. Dexamethasone was purchased from Calbiochem. For experiments using DMSO, all control cells were identically treated with DMSO and the final concentration of DMSO never exceeded 0.1% of the culture volume. The chemiluminescence reagent employed was purchased from Millipore Corporation (Bedford, MA).
Eosinophil isolation from peripheral human blood
Peripheral blood (120 ml) was drawn from healthy subjects as we previously reported under a research protocol approved by the IRB committee at the University of Texas Medical Branch (IRB#04-371)(21). Eosinophils were obtained by sedimentation in 4–6% dextran for 50 min at RT, followed by centrifugation in a Ficoll-Hypaque gradient as described previously. Following centrifugation at 500×g, upper layers of plasma and mononuclear cells were removed and saved for further analysis. Eosinophil isolation used hypotomic lysis for elimination of erythrocytes and negative selection using a combination of anti-CD16, anti-CD3, anti-CD235, and anti-CD14 microbeads to remove neutrophils and other contaminating cells using the MACS system 9 (Miltenyi Biotec, Sunnyvale, CA). The final eosinophil homogeneity was assayed by microscopic examination using a Wright-stained cytospin preparation was ≥ 98% with activation levels typically ≤ 1% as evaluated by CD69 flow cytometry. Purified preparations were utilized immediately.
Human eosinophil cell culture
Although most experiments relating to eosinophil activation were performed on freshly isolated cells in the presence of 2% FBS, in certain experiments involving inhibition of PP5 with siRNA, eosinophils were cultured for up to 48 h in the presence of 5% FBS (33). Briefly, eosinophils were suspended in RPMI 1640 medium (GIBCO BRL, Life Technologies, Grand Island, NY) supplemented with 2–5% FBS (High Clone Laboratories, Inc., Logan, UT), 100 U/ml penicillin G, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B (GIBCO BRL/Life Technologies, Grand Island, NY). The effect of GM-CSF on eosinophils survival was tested by incubation of cells with GM-CSF at 1 ng/ml in the presence of 2% FBS. Cells were cultured at a density of 1 × 106/ml in a humidified atmosphere containing 95% air and 5% CO2. The cultures were maintained in 12-well sterile, flat bottom plates (Costar Corp., Cambridge, MA) previously coated with 1% human serum albumin.
Western blot analysis
For protein identification following 1D gel electrophoresis, proteins were transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MS). After transfer, membranes were blocked with 5% milk Tris-buffered saline (100 mM Tris-HCl, 150 mM NaCl, pH 7.5) containing 0.1% (v/v) Tween 20 for 1 h and then incubated with the appropriate antibody (1:10000 dilution) overnight at 4°C. Membranes were washed 4× in Tris-buffered saline with 0.1% (v/v) Tween 20 and then incubated with horseradish peroxidase-conjugated secondary antibody. After washing, immune complexes were detected by reaction with an enhanced-chemiluminescence assay according to the manufacturer’s protocol.
Densitometry
Densitometry analysis was performed on Western blots from time-course experiments of protein expression and phosphorylation. Autoradiograms of the immunoblots were scanned using Adobe Photoshop (Adobe System, Inc., San Jose, CA). The blot’s contrast was adjusted for minimum background and the mean density for each band was analyzed using the ImageJ program (NIH, Bethesda, MD). The comparative results were calculated as “fold change” compared with control for each treatment time.
In-Gel protein phosphatase assay
Whole cell lysates were immunoprecipiated with anti-PP5 antibody and the resulting PP5 immunoprecipitates were fractionted by SDS-PAGE followed by a denaturation/renaturation process before detection of phosphatase activity using a fluorogenic substrates (22). After electrophoresis, SDS was removed by washing the gel with two changes of 100 ml each of 20% (v/v) 2-propanol in 50 mM Tris–HCl (pH 7.5) for 1 h at RT. Subsequently, the gel was treated with two changes of 50 ml of a denaturation buffer containing 50 mM Tris–HCl (pH 7.5), 5 mM 2-mercaptoethanol, and 8 M urea for 1 h at RT, to denature the fractionated proteins in the gel. Then the gel was put into 100 ml of a renaturation buffer containing 50 mM Tris–HCl, 0.02% (v/v) Tween 20, 20 mM 2-mercaptoethanol, and 1 mM MgCl2, pH 7.5, and gently shaken at 4 °C for 16–20 h with 5 changes. After treatment, the gels were incubated with a reaction mixture containing 50 mM Tris–HCl, pH 8.0, 0.1 mM EGTA, 0.01% (v/v) Tween 20, 2 mM dithiothreitol, 20 mM MnCl2, and 0.5 mM DiFMUP. The gels were then incubated at 37 °C for 15 min and the fluorescent bands were observed with a transilluminator with an excitation wavelength of 365 nm. Results were subsequently adjusted from the relative expresssion of PP5 based on densitometry results from western blotting detecting PP5 in whole cell lysates.
PP5 siRNA transfection
Freshly isolated eosinophils were resuspended (106 cells/ml) in antibiotic-free siRNA transfection medium (sc-36868, Santa Cruz) without FBS. One ml aliquots of eosinophils in transfection medium were added to each well of a 12-well culture plate. An aliquot of 3.6 µl of PP5 siRNA was added to 40 µl of siRNA Transfection Medium (sc-36868, Santa Cruz) and kept at RT for 5 min. In a separate tube, 2.4 µl of siRNA Transfection Reagent (sc-29528) was mixed with 40 µl of siRNA Transfection Medium and then kept at RT for 5 min. Subsequently, the contents from both tubes were mixed and incubated at RT for 20 min to form an siRNA complex. After 20 min of incubation, 0.32 ml of siRNA Transfection Medium was added to each tube containing the siRNA transfection complex. Subsequently, cells were resuspended in 0.4 ml of resulting siRNA/siRNA transfection complex and incubated for 8 h at 37°C. After incubation, cells were washed and resuspended in 1 ml of RPMI 1640 containing 10% of FBS followed by an additional incubation of 36 h. Transfection efficiency was evaluated by Western blotting for each experiment performed on eosinophil viability. Control siRNA (sc-370007) was a scrambled sequence that did not cause degradation of any known cellular mRNA.
Eosinophil viability
An Annexin VPE Apoptosis Detection kit (BD Biosciences) was used to quantify eosinophils undergoing apoptosis by virtue of their ability to bind Annexin V and exclude 7-aminoactinomycin (7-AAD). This assay detects viable cells (Annexin V negative/7-AAD negative), including cells undergoing early apoptosis (Annexin V positive/7-AAD negative), and dead cells (Annexin V positive/7-AAD positive). Eosinophils (2 × 105 cells) were stained with Annexin 5 and 7-ADD according to the manufacturer’s instructions. Data were acquired on a FACScan instrument (BD Biosciences) and analyzed using CellQuest software (BD Biosciences); 10,000 events per sample were acquired.
Flow cytometry
Flow cytometric analysis for the expression of Annexin1 and FPR2 on the surface of eosinophils was performed after staining cells with PE-conjugated monoclonal antibodies (Santa Cruz) according to the manufacture’s protocol. For each population, a matched isotype control mAb was used as a negative marker. Eosinophils were analyzed using a Becton Dickinson (San Jose, CA) FACSAria machine and at least 30,000 events were collected for each sample. The results were analyzed using CELL Quest software (Becton Dickinson).
Statistical analysis
The results of eosinophil viability, phosphatase assay and densitometry measurements were analyzed using one-way ANOVA with Tukey’s post hoc test for multiple comparisons of the control and treatment groups using SigmaPlot 11.0 Software (Systat Software). All results are reported as the mean ± SE (n = 3–8) and difference was considered statistically significant when p < 0.05.
Results
Proapoptotic effect of dexamethasone is diminished in activated eosinophils
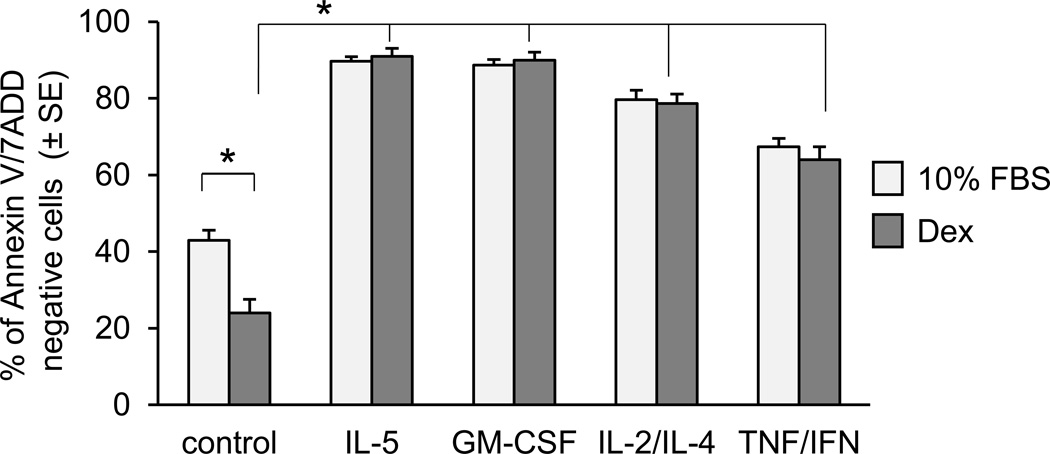
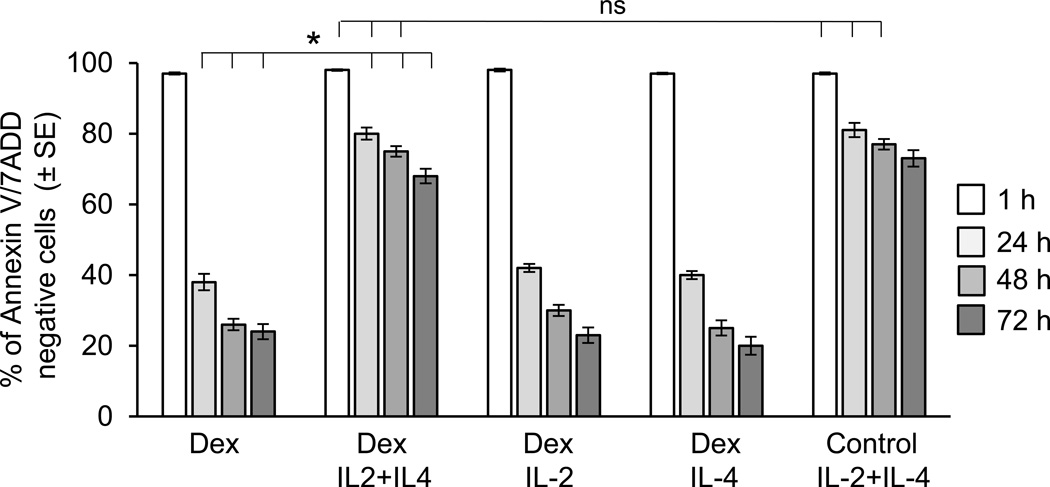
To test the effect of proinflammatory cytokines on the eosinophil response to GCs, we first assessed the time-course of Dex-induced apoptosis of eosinophil in vitro. We found that non-activated eosinophils are extremely sensitive to treatment with Dex, entering apoptosis within hours of stimulation as assessed by induction of Annexin V surface expression and loss of nuclear membrane integrity. Preincubation of eosinophils with cytokines with known antiapoptotic effect and elevated expression in the asthmatic airways not only extended cell viability in vitro but also significantly inhibited the proapoptotic effect of Dex as assessed at 48 h of in vitro culture (Fig. 1). Since cytokines IL-2 and IL-4 were reported to affect GC signaling in human monocytes and lymphocytes and were indicated to be factors in clinical studies of steroid resistance in asthma, we decided to initially focus on the antiapoptotic effect of IL-2 and IL-4 on eosinophils with in vitro studies (Fig. 2). Combination of IL-2 plus IL-4 significantly delayed eosinophil apoptosis and protected cells from Dex-induced apoptosis. Interestingly, neither IL-2 nor IL-4 administered alone showed any protective effect on the GC response suggesting a synergistic action of IL-2 plus IL-4 signaling in the propagation of eosinophil resistance to GC-induced cell death. Furthermore, in time-course experiments we found that the protective effect of IL-2/IL-4 required at least 8 h incubation of eosinophils with cytokines; shorter times of 2, 4 and 6 h did not elicit the same response (data not shown). These in vitro results combined with previous clinical reports linking IL-2 and IL-4 to steroid resistance in bronchial asthma led us to further explore the mechanism of IL-2 plus IL-4-inducible insensitivity to GC-induced inhibition of eosinophil activation and survival.
Figure 1.
Effect of stimulation with cytokines and Dex on eosinophil viability in vitro. Eosinophil apoptosis was assessed by staining with PE labelled Annexin V and 7 AAD; viable, non-apoptotic cells are represented as Annexin V/7AAD-negative cells. Freshly isolated eosinophils were suspended in RPMI 1640 supplemented with 10% FBS and stimulated with different cytokines (IL-5 at 10 ng/ml, GM-CSF at 1 ng/ml, combination of IL-4 and IL-2 at 10 ng/ml and 10 ng/ml respectively, and combination of TNFα and INFγ at 10 ng/l and 20 ng/ml) for 16 h followed by treatment with Dex (1 µM) for an additional 48 h. The one way ANOVA was used to compare cytokine/Dex treated samples with Dex/control and Dex/control vs non-treated control as indicated. The asterisks are for p <0.05. The error bars represent the standard error of n=4–8 experiments.
Figure 2.
Effect of stimulation of IL-2 together with IL-4 on Dex-induced eosinophil apoptosis. Eosinophil apoptosis was assessed by staining with PE labelled Annexin V and 7 AAD; viable, non-apoptotic cells are represented as Annexin V/7AAD-negative cells. Eosinophil viability was tested for 72 h after exposure to IL-2 and IL-4 separately or in combination for 16 h followed by treatment to Dex at concentration of 1 µM. The error bars represent the standard error of n=3–8 experiments. The asterisk are for p values <0.05 by one-way ANOVA.
IL-2 plus IL-4 inhibited upregulation of FPR2, GILZ and Annexin 1 in response to GC
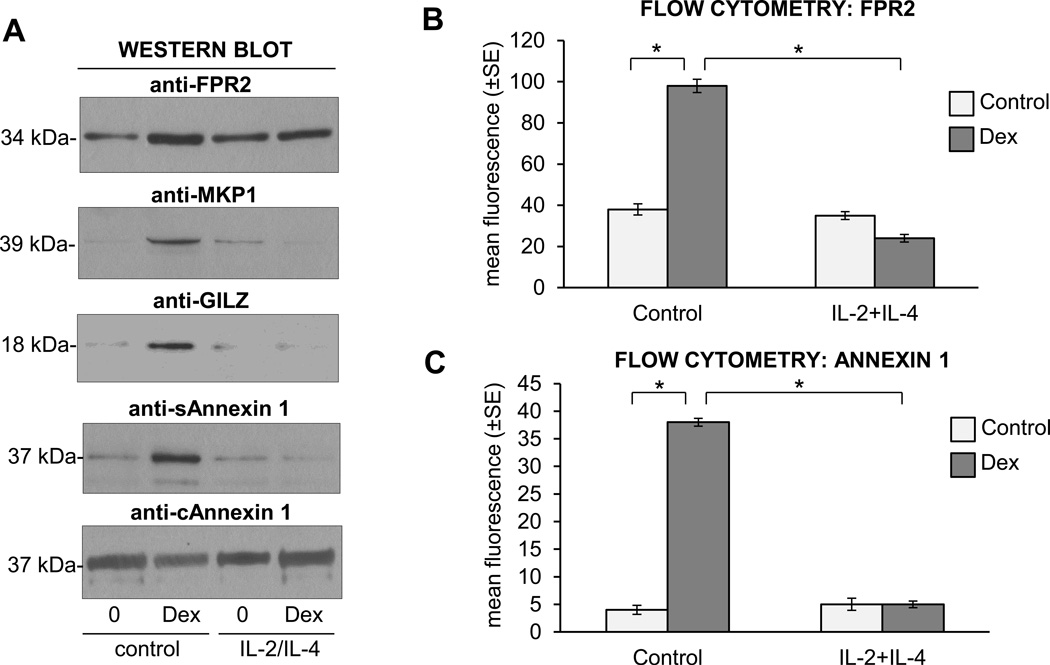
We examined the effect of the IL-2 plus IL-4 cytokine combination on GC-induced upregulation of MKP 1 phosphatase, FPR2 receptor, GILZ and Annexin 1 (Fig. 3). Western blot analysis of MKP1 expression showed the upregulation of GRE-dependent MKP1 in Dex-stimulated eosinophils while preincubation of cells with IL-2 plus IL-4 inhibited the Dex-induced increase of MKP1 (Fig. 3A). Similar effects were observed on upregulation of GILZ that was completely suppressed in IL-2/IL-4 activated eosinophils. We also examined the secretion of Annexin1, known to mediate the antiinflammatory effects of GC via the inhibition of phospholipase 2 (cPLA2), COX-2 and NF-κB. Glucocorticoids induced the extracellular release of Annexin 1 as detected by Western blot analysis in concentrated culture medium; however, IL-2 plus IL-4 significantly blocked this effect (Fig. 3A). Since some of the effects of the anti-inflammatory actions of Annexin 1 are mediated by the FPR2 receptor, we tested its expression on eosinophils cells surface. Flow cytometric analysis (Fig. 3B) showed the upregulation of FPR2 on the surface of eosinophils exposed to Dex and also the upregulation of Annexin 1 (Fig. 3C). Pretreatment of eosinophils with an IL-2 plus IL-4 combination significantly inhibited upregulation of FPR2 and Annexin 1 expression. Since MKP1, GILZ and Annexin1 were reported to be regulated in a GRE-dependent manner, our results suggested that IL-2 plus IL-4 affected the GCR-mediated transactivation processes.
Figure 3.
Effect of Dex on expression of GC-regulated proteins FPR2, MKP1 and secreted (S) and cytosolic (C) Annexin 1 in eosinophils stimulated with IL-2 plus IL-4. A. Western blot analysis of FPR2, MKP1, GILZ and Annexin 1. Eosinophils were stimulated with IL-2/IL-4 or control medium overnight (16 h) followed by treatment with Dex for 8 h. Cytosolic fraction of eosinophil and collected culture medium were analyzed for expression of cytosolic and secreted Annexin 1, respectively. B. Flow cytometric analysis of the surface expression of FPR2 showing FPR modulation in response to Dex treatment and effect of IL-2 and IL-4 stimulation. The one-way ANOVA test was used to compare indicated means with * p<0.05 considered significant. The bars represent the SEM for n=3 experiments. C. Flow cytometric analysis of surface expression of Annexin 1. These results are representative of 4 experiments and expressed as standard error of the mean. * p<0.05 was considered significant for intergroup comparisons..
Stimulation of eosinophils with IL-2 plus IL-4 caused dephosphorylation of the nuclear GC receptor
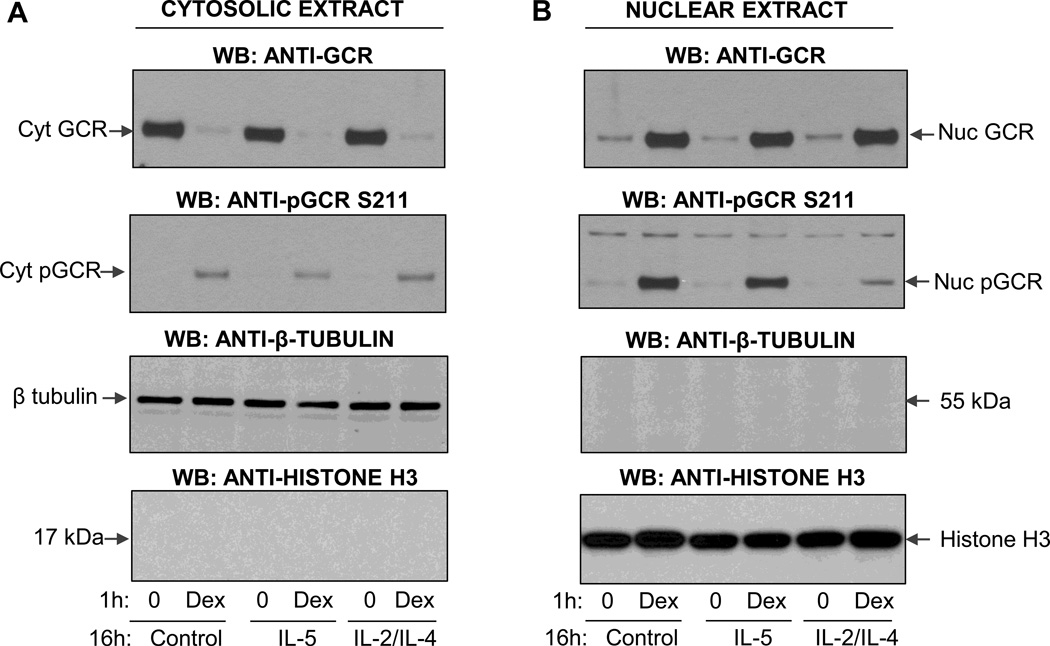
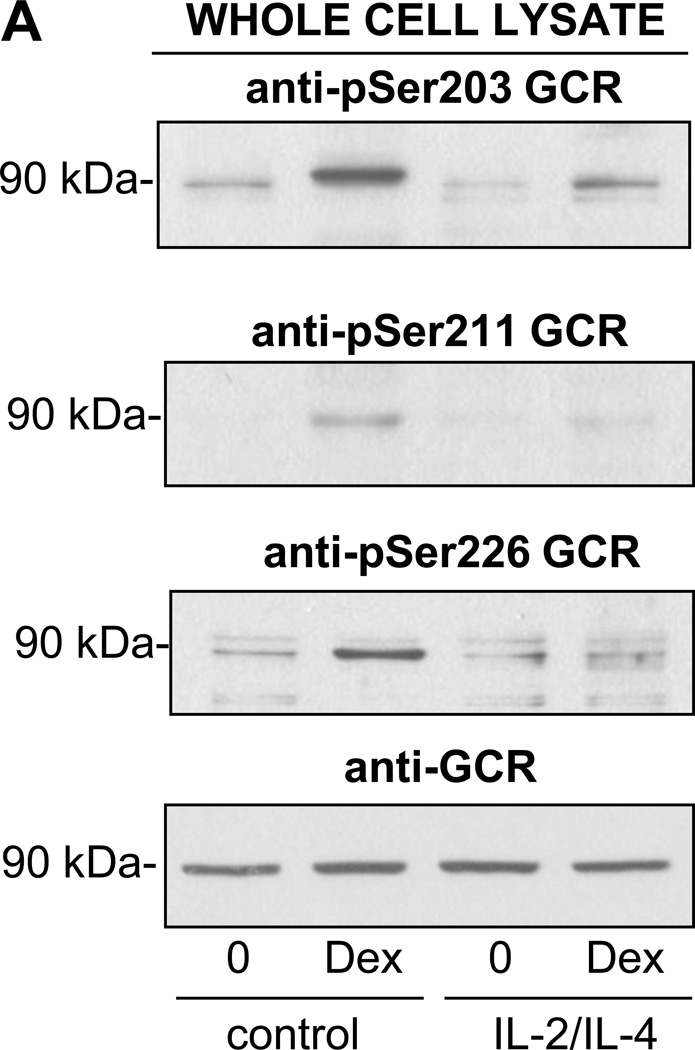
Apoptosis by GCs requires binding to the GCR. Although various GCR isoforms can be generated due to alternative splicing and different initiation sites, the proapototic activity is mainly attributed to the full-length 94 kDa GCRα (23). At least six seryl residues are phosphorylated on the human GCR and studies have shown that phosphorylation-deficient GCR mutants are compromised in their ability to activate reporter genes in a promoter-dependent and cell-specific fashion (24). Interestingly, only phosphorylation of GCR on Ser211 correlated with transcriptional activity of GCR while the phosphorylation on Ser203 was found to be cell-type and agonist dependent. Given the availability of antibodies against GCR phosphorylated on three different residues (S203, S211, S226), we tested the subcellular expression and phosphorylation state in eosinophils at different states of activation. Western blot analysis detection of the GCR in the cytoplasmic and nuclear fractions of eosinophils (Fig. 4A) showed an abundant cytosolic expression of the ~90 kDA GCR in quiescent cells and significant nuclear redistribution of GCR after 1 h of stimulation with Dex. Nuclear translocation was not affected by pretreatment of eosinophils with IL-5 or the combination of IL-2 plus IL-4. Interestingly, Western blot analysis with an antibody that detected GCR phosphorylated on S211 showed intact phosphorylation of GCR remaining in the cytosol in both quiescent and cytokine-activated cells exposed to Dex (Fig. 4B). However, phosphorylation of the nuclear GCR was significantly diminished in cells pretreated with IL-2 plus IL-4 suggesting loss of GCR phosphorylation and phosphatases actively occurring in the nucleus. IL-5 effect on GCR at S211 was much less pronounced. Additionally, we found that IL-2 plus IL-4 affected Dex-inducible phosphorylation on other serine residues as well, including phosphorylation of S203 and S226 (Fig. 5).
Figure 4.
Western blot analysis of subcellular localization of the total and phosphorylated form of GR. Eosinophils were preincubated wil IL-5 or combination of IL-2 and IL-4 for 16 h followed by stimulation with Dex for 1 h. Cytosolic and nuclear extracts were analyzed for nuclear translocation of the GR and purity was assessed by expression of cytosolic and nuclear proteins, β-tubulin and histone-3, respectively. A. Effect of cytokines on Dex-induced GCR translocation from the cytosolic to the nuclear compartment. B. Phosphorylation of GCR in cytosolic and nuclear compartments upon Dex and cytokine exposure. These results are representative of two independent experiments
Figure 5.
Western blot analysis of Dex-induced GR phosphorylation in IL-2 plus IL-4 activated eosinophils. Freshly isolated eosinophils were cultured for 16 h in the presence of IL-2 plus IL-4 or control carrier followed by 1 h treatment with Dex at the concentration of 1 µM. Whole cell lysates were analyzed for the phosphorylation of the GCR using phospho-specific antibodies as indicated. These results are representative of three independent experiments.
Upregulation of PP5 expression and activity in IL-2 plus IL-4 stimulated eosinophils
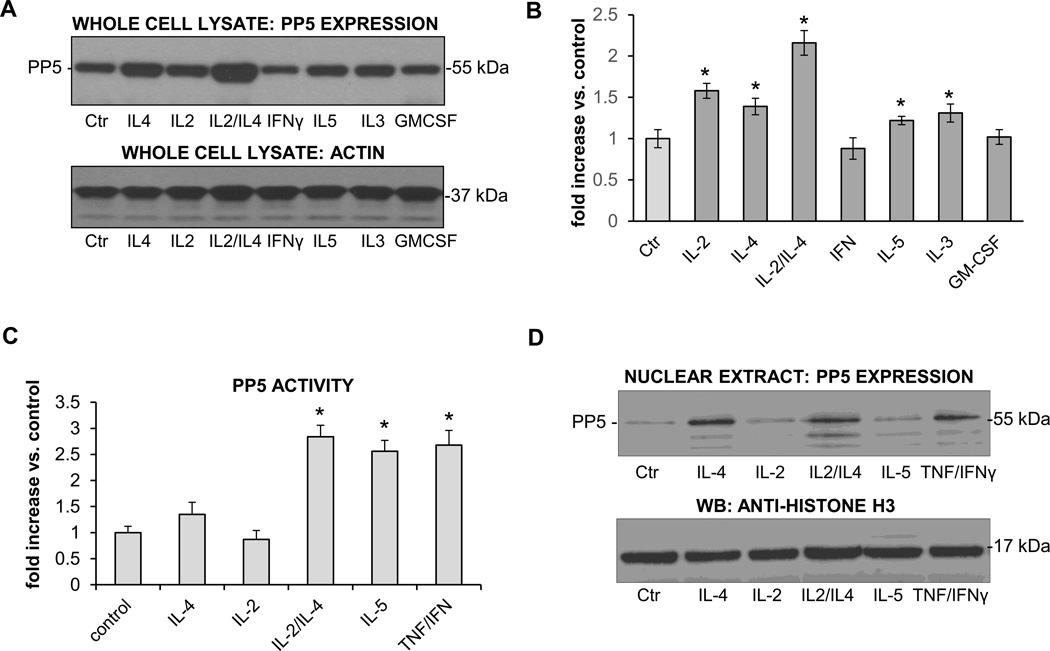
Dephosphorylation of the GCR in nuclei of steroid resistant eosinophils suggested the activation of a GCR-targeted protein phosphatase(s) and therefore we examined the expression and phosphatase activity of several protein phosphatases involved in signaling relating to eosinophil activation and its GCR involvement. We found no significant change in the expression of PP1 or PP2A protein phosphatases in cytokine stimulated eosinophils (results not shown); however, we observed an upregulation of PP5 expression in cells stimulated with IL-4, IL-2 plus IL-4, IL-5, and IL-3 (Fig. 6A). Furthermore, we identified increased PP5 in the nuclei of eosinophils stimulated with cytokines (Fig. 6D). To evaluate if these cytokines regulated phosphatase activity, we immunoprecipitated PP5 from activated cells and performed an in-gel phosphatase activity assay in vitro using a fluorogenic substrate (4-methylumbeliferyl phosphate, MUP) (22). We found a significant upregulation of PP5 activity by cytokines that rendered eosinophils steroid insensitive (Fig. 6C). Although IL-5 and IL-2/IL-4 showed a similar increase in PP5 activity as compared with similar amounts of immunoprecipitated PP5, the gross PP5 activity in IL-2/IL-4 eosinophils was significantly higher, about at least double amount of detectable PP5 in IL-2/IL-4 stimulated eosinophils. Furthermore, we identified increased PP5 in the nuclei of eosinophils stimulated with IL-4, IL-2/IL-4 and TNFα/IFNγ (Fig. 6D), surprisingly, IL-5 did not induce significant nuclear translocation of PP5. Overall, these experiments demonstrated increased expression and activation of PP5 in the proximity of the nuclear GCR suggesting the involvement of the cytokine-upregulated nuclear protein PP5 in the modulation of phosphorylation and function of the GCR in steroid-insensitive eosinophils.
Figure 6.
Expression and activation of PP5 in activated eosinophils. Eosinophils were cultured in the presence of different proinflammatory cytokines for 16 h followed by analysis of the PP5 expression and activity and nuclear translocation. A. Western blot analysis of the whole cell lysates showing PP5 expression in cells stimulated with IL-4, IL-2 plus IL-4, IL-5 and GM-CSF. Equal protein content was confirmed by detection of F-actin. B. Densitometry analysis of the PP5 expression in whole cell lysates. Values are representative of 3 independent experiments and adjusted to actin expression. One way ANOVA was used to compare cytokine-treated groups with control, * p<0.05 was considered significant. The error bars represent the standard error of the mean. C. PP5 phosphatase activity in whole cell lysates from activated eosinophils. Equal amounts of PP5 immunoprecipiatted with anti-PP5 antibody were assayed for phosphatase activity using MUP fluorogenic substrates. Values are presented for the same amount of PP5 protein. One way ANOVA was used to compare cytokine-treated groups with control, * p<0.05 was considered significant. The error bars represent the standard error of the mean, n=5 D. Nuclear expression of PP5 in activated eosinophils. The results are representative of three independent experiments.
Inhibition of PP5 restored GCR phosphorylation and functionality
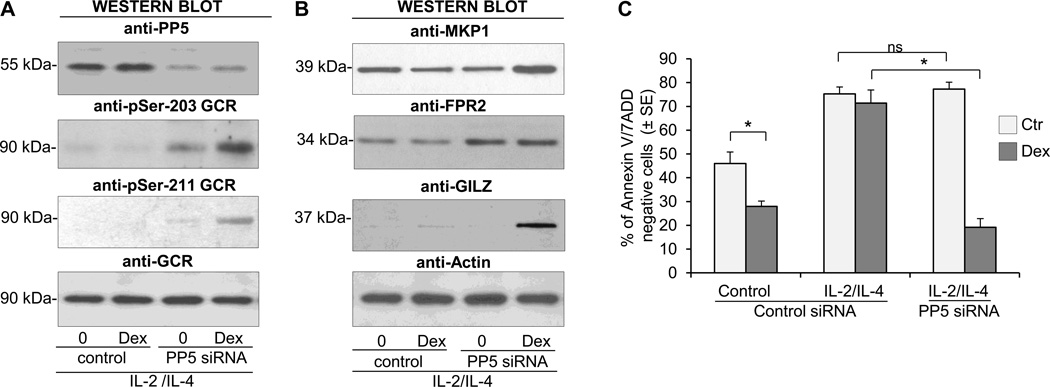
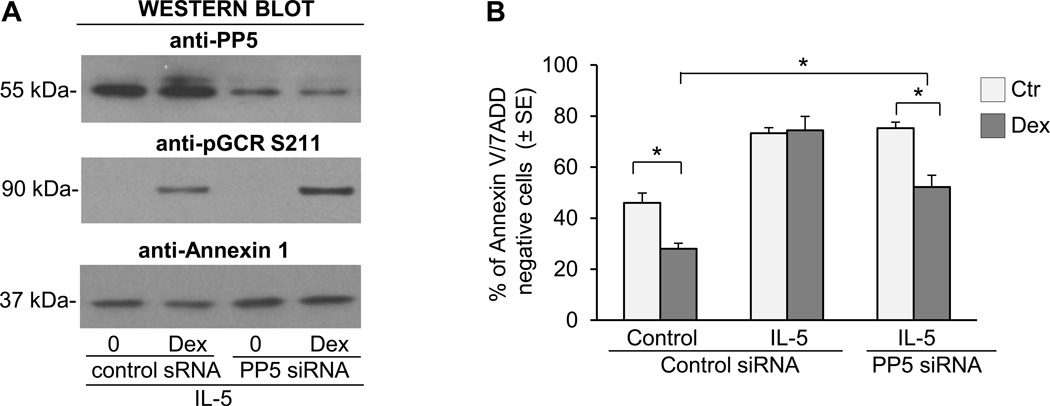
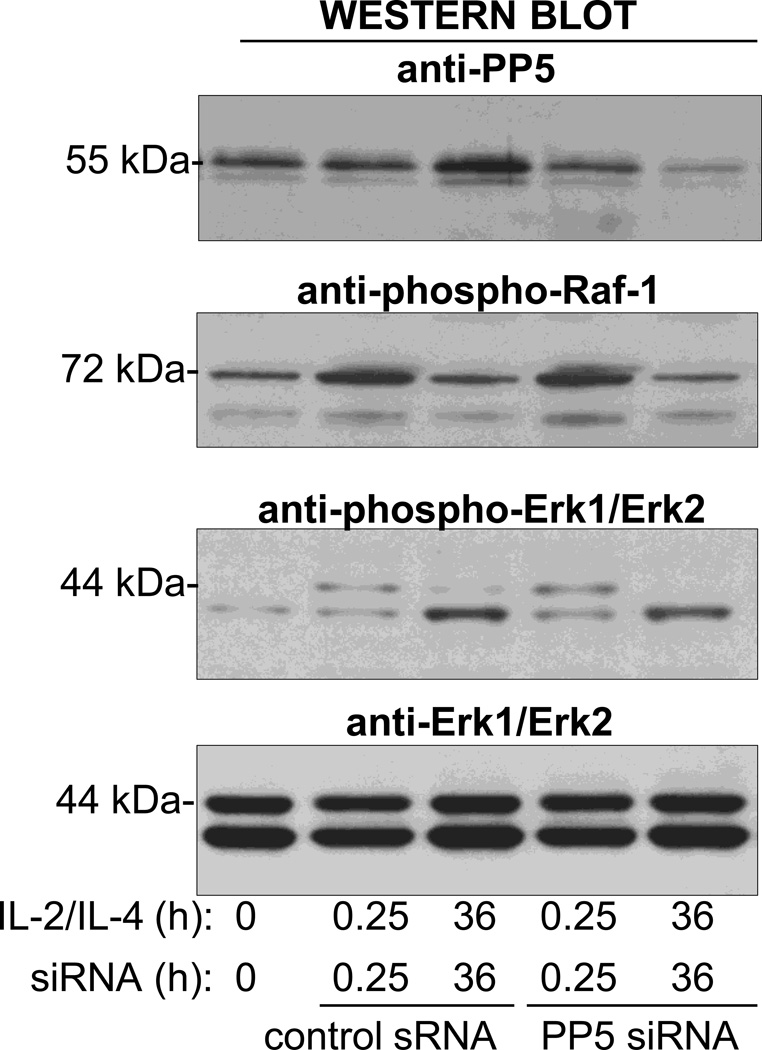
The short-lived nature of PP5 expression allowed us to use siRNA to decrease the expression of PP5 using small interfering RNA (siRNA) after concurrent cytokine activation. We found that inhibition of PP5 with siRNA increased the phosphorylation of GCR on S203 and restored the Dex-inducible GCR phosphorylation on S203 and S211 (Fig. 7A). Moreover, the diminished expression of PP5 restored the upregulation of MKP1 and GILZ by Dex and increased the constitutive expression of FPR2 (Fig. 7B). The inhibition of PP5 in eosinophils did not affect their extended viability typically induced by cytokines as eosinophil survival rates remained identical in PP5 siRNA treated and the nonspecific siRNA control treated group. However, the inhibition of PP5 expression had a significant effect on eosinophil’s response to Dex as eosinophils with diminished PP5 showed an apoptotic response to Dex comparable to the response observed in control, steroid-sensitive cells (Fig. 7C). We also examined the effect of PP5 inhibition on IL-5-induced resistance to GCs. Inhibition of PP5 increased Dex-inducible-phosphorylation of the GCR on S211 (Fig. 8A) and decreased eosinophil viability in response to Dex (Fig. 8B); however, this effect was less pronounced than with IL-2 plus IL-IL-4 pretreated cells suggesting the involvement of other, perhaps IL-5 pathways specific components mediating resistance of eosinophils to GC (25). In additional experiments (Fig. 9) we addressed the effect of PP5 inhibition on the activation of Raf-1-MEK-1-Erk1 kinase since 1) previous studies on cancer cells suggested the role of PP5 as a key dephosphorylation regulator of Raf-1 (26), 2) possible role of Erk-fos in steroid resistance (27) and 3) the role of Raf-1 in inhibition of apoptosis in IL-5-stimulated eosinophils (28). We found that IL-2/IL-4 stimulation did induce phosphorylation of Raf-1 on Ser338 that is responsible for its enzymatic activation and concomitant phosphorylation of Erk1 and Erk 2 kinases. However, the suppression of PP5 by siRNA did not increase phosphorylation and hence the activation of the Raf-1/Erk1 kinase pathways in eosinophils activated with IL-2/IL-4 for 36 h argues against the effect of PP5 inhibition on Raf-1/Erk pathway in the late stage of cells activation.
Figure 7.
Effect of PP5 inhibition on GCR signaling and apoptotic response to GC treatment in IL-2 plus IL-4 stimulated eosinophils. Eosinophils were incubated in the presence of PP5 specific siRNA or scrambled RNA nonspecific control for 24 h followed by 16 h stimulation with IL-2 plus IL-4 and treatment with Dex at the concentration of 1 µM for time dependent assays. A. Effect of PP5 inhibition (top Western blot) on phosphorylation of the GCR receptor on S203 and S211 in response to 1 h stimulation with Dex. B. Effect of PP5 inhibition on expression of MKP1, GILZ, FPR2 and Annexin 1 in response to 8 h stimulation with Dex. C. Effect of PP5 inhibition on proapototic response to Dex as assessed by Annexin V/7-AAD flow cytometric analysis after 24 Dex treatment. The ANOVA test was used to compare indicated means with *p<0.05 considered significant. The bars represent the standard error of the mean for n=4.
Figure 8.
Effect of PP5 inhibition on GCR signaling and apoptotic response to GC treatment in IL-5 stimulated eosinophils. Eosinophils were incubated in the presence of PP5 specific siRNA or scrambled RNA nonspecific control for 24 h followed by 16 h stimulation with IL-5 and treatment with Dex at the concentration of 1 µM for time dependent assays. A. Effect of PP5 inhibition (top Western blot) on phosphorylation of GCR on Ser-211 in response to 1 h stimulation with Dex. B. Effect of PP5 inhibition of PP5 on apoptosis in IL-5 stimulated cells exposed to Dex. The * p value <0.05 was considered significant by ANOVA. The bars represent the standard error of the mean for n=4.
Figure 9.
Effect of PP5 inhibition on IL-2/IL-4 phosphorylation of Raf-1 (Ser338) and Erk1 and Erk 2 kinases (Thr202 and Tyr204, respectively). Whole cell lysates from eosinophils were cultured in the presence of IL-2/IL-4 and control or PP5 siRNA for 15 min and 36 h were analyzed for phosphorylation of Raf-1 and Erk1 and Erk2 kinases.
Lipoxin A4 reversed the effect of IL-2 plus IL-4 on the eosinophilic response to GC
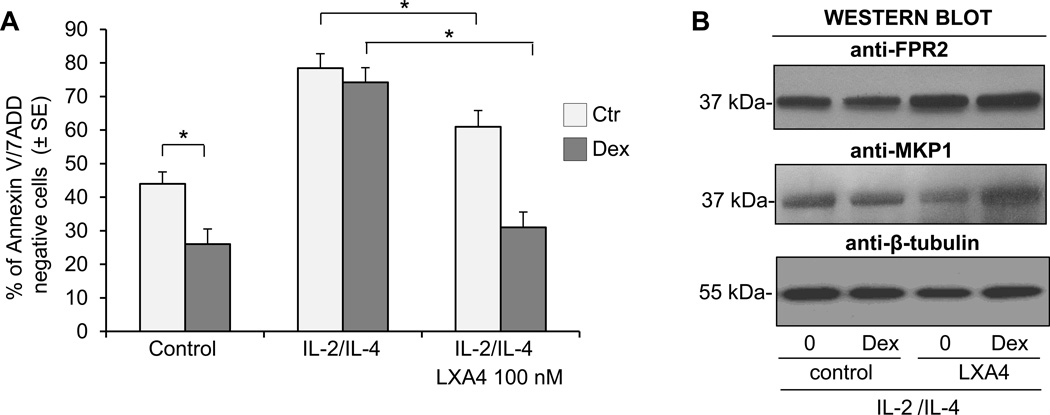
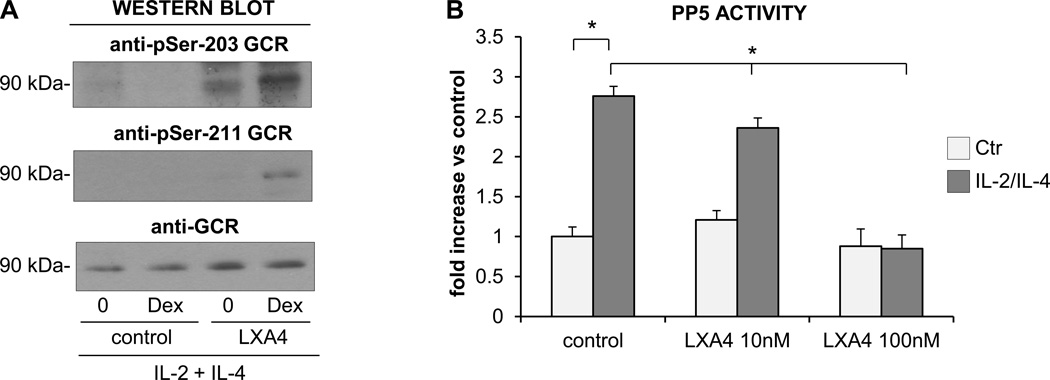
Lipoxin A4 is a natural anti-inflammatory eicosanoid that is reported to have a potent inhibitory effect on the function of activated eosinophils. We have reported that lipoxin A4 counter-regulates the prosurvival effects of GM-CSF and inhibits the expression of integrins and the release of cytokines (29). The lipoxin A4 effect on eosinophils is thought to be mediated by the FPR2-receptor, which also transduces the anti-inflammatory effect of Annexin 1. Thus, we examined the effect of lipoxin A4 on eosinophil apoptotic response to GC after exposure to a combination of IL-2 plus IL-4 cytokines. We found no effect of lipoxin A4 on the survival of the control, nonstimulated eosinophils; however, in IL-2 plus IL-4 stimulated cells there was significant inhibition of antiapoptotic signaling in response to lipoxin A4 (Fig. 10A). For this reason, we examined the effect of lipoxin A4 added after 24 h of exposure to IL-2 plus IL-4 and 1 h before treatment with GC. In these experiments we found that IL-2 plus IL-4 stimulated eosinophils responded to GC with increased apoptosis when exposed to lipoxin A4 suggesting the reversal of cytokine-induced insensitivity to GC. This apoptosis-restoring effect of lipoxin A4 was highest when the eicosanoid was added before GC treatment; however, significant apoptosis was also observed when eosinophils were stimulated with lipoxin A4 2 h after adding GC. We also examined the phosphorylation of the GCR and expression of PP5 and FPR2 in lipoxin A4 treated cells to evaluate for possible targets and the mechanisms of lipoxin A4-driven restoration of apoptotic response to GC and found increased expression of FPR2 in lipoxin A4-treated eosinophils (Fig. 10B). Western blot analysis showed phosphorylation of the GCR on S203, S211 and S226 residues in cytokine stimulated cells after exposure to lipoxin A4 and Dex indicating a reversal effect of the applied eicosanoid on impaired phosphorylation of GCR in activated cells (Fig. 11A). In addition, PP5 expression and phosphorylation, had no lipoxin A4 effect on the expression of PP5 as tested by Western blot analysis within 24 h of lipoxin A4 stimulation (not shown). However, lipoxin A4 had a significant effect on PP5 activity and this effect was detectable as early as 2 h of stimulation with eicosanoid (Fig. 11B). There was no significant effect on the expression of Annexin 1 observed in cytokine-stimulated eosinophils treated with lipoxin A4 for 24 h. These results showed a suppressive effect of lipoxin A4 on PP5 activation that restored GCR phosphorylation and supported the dependence of GCR phosphorylation on PP5 which suggested that PP5 was one of the targets of the lipoxin A4-induced pathway involved in the reversal of cytokine-induced resistance to GC in eosinophils.
Figure 10.
Effect of lipoxin A4 on eosinophil response to Dex. Freshly isolated eosinophils were stimulated with IL-2 plus IL-4 for 16 h followed by stimulation with lipoxin A4 (100 µM) for 4 h and treatment with Dex for 24 h. A. Eosinophil apoptosis assessment using flow cytometric analysis of Annexin V/7 AAD. The asterisk for p<0.05 indicated significance by one-way ANOVA. The bars represent the standard error of the mean for n=3. B. Effect of lipoxin A4 stimulation on the expression of FPR2 in control and Dex treated eosinophils. The results are representative of 3 independent experiments.
Figure 11.
Effect of lipoxin A4 on GCR phosphorylation and PP5 activity. A. Eosinophils were stimulated with IL-2 plus IL-4 for 16 h followed by stimulation with lipoxin A4 (100 µM) for 4 h and treatment with Dex for 1 h and tested for GCR phosphorylation and expression by Western blotting. B. PP5 activity in PP5 immunoprecipiates obtained from eosinophils treated with lipoxin A4 for 4 h with or without prior stimulation with IL-2 plus IL-4. PP5 activity was assessed with a fluorogenic substrate MUP. The one-way ANOVA test was used to compare indicated groups with *p<0.05 considered significant for n=3–5. The bars represent the standard error of the mean.
Discussion
This comprehensive molecular signaling in vitro study has shown that human blood eosinophils activated by cytokines known to be important factors in disease pathogenesis, e.g. asthma, are relatively steroid resistant through a PP5 related mechanism involving phosphorylation of the GCR. Specific inflammatory conditions are believed to underlie insensitivity to therapeutic effects of GCs; however, the mechanism of steroid resistance in airway inflammatory cells has been elusive. Seven well studied cytokines have been found to inhibit proapoptotic effect of GC on eosinophils in our study, including IL-2, IL-3, IL-4, IL-5, GM-CSF, TNFα, and IFNγ all of which are known to activate eosinophils. We have found that eosinophil resistance to GC-induced apoptosis correlated with the activation of protein phosphatase 5 (PP5) and dephosphorylation of the GR. In this paper we also present evidence for diminished expression of FPR2 receptor in activated eosinophils suggesting impairment of lipoxin A4 anti-inflammatory signaling transduced by FPR2 and the ability of Lipoxin A4 to inhibit PP5 activity and restore function of GR in eosinophils. Thus we identified a possible biological basis for the resistance to GC therapy seen in certain types of asthma with elevated cytokines IL-2, IL-4 and IL-5 and increased number of activated eosinophils.
We and others have reported the inhibition of IL-5-activated eosinophils to GC-induced apoptosis (25, 30, 31) which in the present study we have extended to cytokines previously reported to play a role in modulating GCR function in airway inflammatory cells. Of note, Kam and others showed that a combination of IL-2 plus IL-4 inhibited GC-induced apoptosis and cell proliferation in lymphocytes and macrophages and this effect correlated with decreased nuclear translocation, affinity binding, and impaired transactivational function of the GCR (32). Although we observed an increased expression of PP5 that effected the GCR in the cytoplasm, we did not observe any change in GCR nuclear translocation in activated eosinophils. Surprisingly, we did observe the nuclear translocation of PP5 in cells exposed to GC supporting the possibility of a direct interaction of PP5 and phosphorylated GCR occurring in the nucleus. The effect of PP5 on GCR function with a potential for targeting it for therapeutic purposes was previously reported in leukemic cells; this effect was believed to rely on the physical cytoplasmic retention of the GCR interacting with PP5 that served as a scaffold function in GCR-HSP90 complexes. Although we cannot fully exclude the mechanism of restoring GCR function upon inhibition of PP5 expression, there are several lines of evidence supporting the possibility of PP5 dephosphorylating the GCR in the nucleus. First, GC-induced phosphorylation of the GCR and changes in the interaction of the GCR with PP5 in the cytoplasm and nuclear translocation of the GCR remains similar in steroid sensitive and steroid resistant eosinophils. Second, the increased expression of PP5 and decreased phosphorylation of the GCR are detectable in the nuclei from steroid resistant eosinophils. Third, resistance-inducing cytokines upregulated PP5 activity while the lipoxin A4 restoring effect on the GCR phosphorylation correlated with decreased activity of PP5. Furthermore, our study indicated the importance of phosphorylation of the GCR for the antiinflammatory function of GC as dephosphorylation of GCR and its restoration positively correlated with the expression of MKP1, FPR2, and Annexin1 which are molecules known to transduce the antiinflammatory effects of GCs. Six serine residues (S113, S141, S203, S141, S226, and S404) are reported to be phosphorylated on the human GCR (14). Kinases identified as phosphorylating the GCR include MAPKs, cyclin-dependent kinases, and GSK-3 (glycogen synthase kinase-3). A previous report on T lymphocytes showed that GC-induced apoptosis required GCR-induced transactivation and is dependent on phosphorylation of the GCR on S211 (33, 34). Our studies showed a constitutive phosphorylation of the GCRα on S203 and GC stimulation also increased phosphorylation on S203, S211 and S226. However, preincubating eosinophils with cytokines IL-2 together with IL-4 abolished phosphorylation on residues S203, S211, and S226, which correlated with the loss of GC’s ability to induce eosinophil apoptosis. Whether PP5 is directly responsible for the loss of phosphorylation on three seryl residues examined is not known since the possibility of the involvement of other phosphatases in GCR function has been reported, e.g. PP2A (35). Interestingly, PP5 is known to associate with a number of protein kinases, including Raf1, ASK1, ATM, ATR/Chk1, DNA-PKcs, eIF2α kinase, and IKKβ although the role of PP5 in the regulation of these kinases is unclear. We addressed the possible effect of PP5 inhibition of Raf-1-MEK-Erk pathways and found no effect of diminished expression of PP5 on late phosphorylation state of these kinases. While our findings are consistent with the transient nature of Raf-1 activation in the propagation of early (within 15 min) cytokine signal for cell activation that may be not affected by relatively late (requiring at least 8 h) upregulation of PP5 phosphatase, we could not assess the effect of PP5 inhibition on early Raf-1 signaling given requirement for concurrent cytokine activation of eosinophils to achieve a meaningful suppression of PP5 by siRNA. Nonetheless, many signaling proteins that associate with PP5 interact with HSP90 (eg. GCR or Raf-1) and these interactions may occur on separate subcellular sites. Our findings suggest nuclear interaction between activated PP5 and phosphorylated GCR thus affecting GCR function. Whether dephosphorylation of GCR by PP5 modulates interaction of GCR with other signaling molecules (e.g. Med14) or affects transrepression or transactivation remains to be investigated in an eosinophil- and cytokine-specific context.
Further mechanistic studies leading to increased PP5 activity in cytokine-activated eosinophils are ongoing. To date, the only reported activators of PP5 activity include the S100 proteins (A1, B, P), suramin, caveolin-1 and arachidonic acid (35–38). Our proteomic characterization of peripheral blood eosinophils identified expressed S100 proteins and caveolin in quiescent cells (39); however, whether the proteins are upregulated in activated eosinophils and play a role in the activation of PP5 remains to be investigated. Importantly, we also report here that lipoxin A4 inhibited PP5 activity and restored phosphorylation and function of the GCR receptor. Lipoxin A4 is a natural antiinflammatory eicosanoid with potent inhibitory effect on the function of activated eosinophils (29, 40). We have previously reported that lipoxin A4 counterregulates the prosurvival effects of GM-CSF, and inhibits the expression of integrins and the release of cytokines (29). The proposed mechanisms underlying pathological airway responses in severe asthma include diminished production of lipoxin A4 and decreased expression of the lipoxin A4 receptor(s) (40, 41). Lipoxin A4’s effect on eosinophils is thought to be mediated by the FPR2-receptor, which also transduces the antiinflammatory effect of Annexin 1 (42). Both Annexin 1 and FPR2 are upregulated by GC’s and lead to the inhibition of arachidonic acid (AA) production in a cytosolic phospholipase A2 (cPLA2)-dependent manner (43, 44). While the increased production of AA is directly associated with synthesis of proinflammatory leukotrienes, AA may also directly activate the phosphatase activity of PP5 (45). Our screening of steroid-resistant eosinophils showed the downregulation of Annexin 1 and FPR2, indicating that some of the mechanism of steroid resistance may be characterized by defective FPR2-mediated counter-regulatory circuits. Most importantly, we found that exposure of steroid-resistant eosinophils to lipoxin A4 inhibited the activity of PP5 and restored the phosphorylation and function of the GCR. This finding links the beneficial effect of lipoxin A4 reported in previous studies on severe asthma with the activation of PP5 and impaired function of GCR pathway.
Of note, a recent study on children with severe asthma has reported a decreased level of lipoxin A4 that correlated with the diminished expression of FPR2 on peripheral blood granulocytes and decreased phosphorylation of the GCR (46). Interestingly, in vitro stimulation of granulocytes from asthmatic children with lipoxin A4 increased expression of FPR2 and restored phosphorylation of GR. These clinical observations complement our mechanistic in vitro study linking impaired phosphorylation of the GCR with the diminished expression of lipoxin A4 indicating that a critical role of PP5 mediated this crosstalk.
Although our in vitro model of steroid resistance in activated eosinophils has number of limitations, the implications of our findings are intriguing. For example, synergistic effect of IL-2 and IL-4 combination as opposed to effect of IL-2 or IL-4 applied alone on activation of PP5 phosphatase and induction of steroid resistance imply that inhibiting either IL-2 or IL-4 alone may abrogate synergy between these signals and exert therapeutic effect. In this regard, the best response of anti-cytokine therapy in severe asthma that is by definition steroid resistant date was observed in a selected group of patients characterized by high airway eosinophilia in spite of treatment with high doses of GCs (47, 48). Promising trials of anti-IL-4 and anti-IL-5 therapies in patients with high eosinophilia and inadequate GC therapy in these patients are consistent with the in vitro conditions tested in the present study. Of importance was our observation that inhibition of PP5 had limited effect on IL-5-activated eosinophils as compared with activation by IL-2 plus IL-4. The basis for this difference is not known, and may involve a differential effect of IL-5 on PP5 expression and nuclear translocation as compared with IL-2 and IL-4. It is also possible that IL-5-specific, other than PP5 mediated mechanism may protect eosinophils from GC-induced apoptosis even with inhibited PP5 phosphatase. In this regard, we recently reported the role of NFIL3 in mediating resistance of IL-5-activated eosinophils (25). Interestingly, although NFIL3 was reported to be upregulated in IL-4-stimulated macrophages, we did not observe upregulation of NFIL3 in IL-2 and IL-4 stimulated eosinophils suggesting specificity of cytokine signaling mediating resistance of eosinophils to GC-induced apoptosis.
This study is the first to examine at the molecular level the network of signaling pathways from inflammatory stimuli that modulate the phosphorylation and function of the GCR in eosinophils that gives rise to an impaired eosinopenic response to steroid therapy. Our findings have identified and characterized mechanisms and cellular compartments of crosstalk between inflammatory signals and the GCR; e.g. activation of protein phosphatases and dephosphorylation of the GCR within the cytoplasm, with a subsequent disconnection of proapoptotic signaling. Of particular interest this study also demonstrated a role of lipoxin A4 and the FPR2 receptor in the reversal of eosinophil effector functions and the inhibition of survival. We expect that these findings will lead to future studies concerning the molecular mechanisms underpinning steroid resistance in activated eosinophils and asthma. All cytokines tested in our study were potent activators of eosinophil function suggesting that measurable scale of eosinophil activity (e.g. PP5 expression and activation) could be used for selecting or excluding asthmatic patients with persistent eosinophilia for GC therapy.
Acknowledgments
We thank Mark Griffin for technical assistance in conducting flow cytometry analyses.
This research was supported by the NIH/NHLBI Proteomics Initiative N01-HV-00245 (HHSN26820100-0037-C-001) (to AK), NIH/NCRR KL2RR029875 (to KP), NIH/NIEHS Center Grant P30-ES006676 (to C. Elferink/AK) and a MedImmune LLC grant MA-409325.
Abbreviations used in this article
- αMβ2
CD11b/CD18 integrin
- AAD
aminoactinomycin
- DSP
dithio-bis(succinimydl)propionate
- Co-IP
co-immunoprecipitation
- cPLA2
cytosolic phospholipase 2
- CP
eosinophil cationic protein
- EPX
eosinophil peroxidase
- GM-CSF
Granulocyte-Macrophage Colony Stimulating Factor
- GC
glucocorticoid
- GCR
glucocorticoid receptor
- GILZ
gluccocorticoid-induced leucine zipper
- HDAC2
histone deacetylase 2
- Syk
spleen tyrosine kinase
- 1DE
one-dimensional electrophoresis
- 2DGE
two-dimensional gel electrophoresis
- MS
mass spectrometry
- RT
room temperature
- PP5
protein phosphatase 5
- FPR2
formyl receptor 2
- HSP90
heat shock protein 90
- DMSO
dimethylsulfoxide
- GRE
glucocorticoid response element
- Dex
dexamethasone
- siRNA
small interfering RNA
References
- 1.Barnes PJ. Mechanisms of action of glucocorticoids in asthma. Am J Respir Crit Care Med. 1996;154:S21–S26. doi: 10.1164/ajrccm/154.2_Pt_2.S21. discussion S26–27. [DOI] [PubMed] [Google Scholar]
- 2.Bel EH, Sousa A, Fleming L, Bush A, Chung KF, Versnel J, Wagener AH, Wagers SS, Sterk PJ, Compton CH C. G. Unbiased Biomarkers for the Prediction of Respiratory Disease Outcome Consortium. Diagnosis and definition of severe refractory asthma: an international consensus statement from the Innovative Medicine Initiative (IMI) Thorax. 2011;66:910–917. doi: 10.1136/thx.2010.153643. [DOI] [PubMed] [Google Scholar]
- 3.Kupczyk M, Wenzel S. U.S. and European severe asthma cohorts: what can they teach us about severe asthma? J Intern Med. 2012;272:121–132. doi: 10.1111/j.1365-2796.2012.02558.x. [DOI] [PubMed] [Google Scholar]
- 4.Wenzel SE, Busse WW L. National Heart, and P. Blood Institute's Severe Asthma Research. Severe asthma: lessons from the Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:14–21. doi: 10.1016/j.jaci.2006.10.025. quiz 22-13. [DOI] [PubMed] [Google Scholar]
- 5.Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J. 2002;19:61–67. doi: 10.1183/09031936.02.00232001. [DOI] [PubMed] [Google Scholar]
- 6.Durham A, Adcock IM, Tliba O. Steroid resistance in severe asthma: current mechanisms and future treatment. Curr Pharm Des. 2011;17:674–684. doi: 10.2174/138161211795428984. [DOI] [PubMed] [Google Scholar]
- 7.Corren J. Cytokine inhibition in severe asthma: current knowledge and future directions. Curr Opin Pulm Med. 2011;17:29–33. doi: 10.1097/MCP.0b013e3283413105. [DOI] [PubMed] [Google Scholar]
- 8.Adcock IM, Lane SJ, Brown CR, Peters MJ, Lee TH, Barnes PJ. Differences in binding of glucocorticoid receptor to DNA in steroid-resistant asthma. J Immunol. 1995;154:3500–3505. [PubMed] [Google Scholar]
- 9.Ito K, Chung KF, Adcock IM. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2006;117:522–543. doi: 10.1016/j.jaci.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Matthews JG, Ito K, Barnes PJ, Adcock IM. Defective glucocorticoid receptor nuclear translocation and altered histone acetylation patterns in glucocorticoid-resistant patients. J Allergy Clin Immunol. 2004;113:1100–1108. doi: 10.1016/j.jaci.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Butler CA, McQuaid S, Taggart CC, Weldon S, Carter R, Skibinski G, Warke TJ, Choy DF, McGarvey LP, Bradding P, Arron JR, Heaney LG. Glucocorticoid receptor beta and histone deacetylase 1 and 2 expression in the airways of severe asthma. Thorax. 2012;67:392–398. doi: 10.1136/thoraxjnl-2011-200760. [DOI] [PubMed] [Google Scholar]
- 12.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 13.Silverstein AM, Galigniana MD, Chen MS, Owens-Grillo JK, Chinkers M, Pratt WB. Protein phosphatase 5 is a major component of glucocorticoid receptor. hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem. 1997;272:16224–16230. doi: 10.1074/jbc.272.26.16224. [DOI] [PubMed] [Google Scholar]
- 14.Ismaili N, Garabedian MJ. Modulation of glucocorticoid receptor function via phosphorylation. Ann N Y Acad Sci. 2004;1024:86–101. doi: 10.1196/annals.1321.007. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Chen W, Kono E, Dang T, Garabedian MJ. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal-associated protein phosphatase. Mol Endocrinol. 2007;21:625–634. doi: 10.1210/me.2005-0338. [DOI] [PubMed] [Google Scholar]
- 16.Zuo Z, Urban G, Scammell JG, Dean NM, McLean TK, Aragon I, Honkanen RE. Ser/Thr protein phosphatase type 5 (PP5) is a negative regulator of glucocorticoid receptor-mediated growth arrest. Biochemistry. 1999;38:8849–8857. doi: 10.1021/bi990842e. [DOI] [PubMed] [Google Scholar]
- 17.Bouazza B, Krytska K, Debba-Pavard M, Amrani Y, Honkanen RE, Tran J, Tliba O. Cytokines alter glucocorticoid receptor phosphorylation in airway cells: role of phosphatases. Am J Respir Cell Mol Biol. 2012;47:464–473. doi: 10.1165/rcmb.2011-0364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deykin A, Lazarus SC, Fahy JV, Wechsler ME, Boushey HA, Chinchilli VM, Craig TJ, Dimango E, Kraft M, Leone F, Lemanske RF, Martin RJ, Pesola GR, Peters SP, Sorkness CA, Szefler SJ, Israel E N. H. L. Asthma Clinical Research Network, and N. I. H. Blood Institute. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J Allergy Clin Immunol. 2005;115:720–727. doi: 10.1016/j.jaci.2004.12.1129. [DOI] [PubMed] [Google Scholar]
- 19.Schleimer RP, Bochner BS. The effects of glucocorticoids on human eosinophils. J Allergy Clin Immunol. 1994;94:1202–1213. doi: 10.1016/0091-6749(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 20.Leung DY, Martin RJ, Szefler SJ, Sher ER, Ying S, Kay AB, Hamid Q. Dysregulation of interleukin 4, interleukin 5, and interferon gamma gene expression in steroid-resistant asthma. J Exp Med. 1995;181:33–40. doi: 10.1084/jem.181.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pazdrak K, Young TW, Straub C, Stafford S, Kurosky A. Priming of eosinophils by GM-CSF is mediated by protein kinase CbetaII-phosphorylated L-plastin. J Immunol. 2011;186:6485–6496. doi: 10.4049/jimmunol.1001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kameshita I, Baba H, Umeda Y, Sueyoshi N. In-gel protein phosphatase assay using fluorogenic substrates. Anal Biochem. 2010;400:118–122. doi: 10.1016/j.ab.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 23.Yudt MR, Cidlowski JA. The glucocorticoid receptor: coding a diversity of proteins and responses through a single gene. Mol Endocrinol. 2002;16:1719–1726. doi: 10.1210/me.2002-0106. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Frederick J, Garabedian MJ. Deciphering the phosphorylation "code" of the glucocorticoid receptor in vivo. J Biol Chem. 2002;277:26573–26580. doi: 10.1074/jbc.M110530200. [DOI] [PubMed] [Google Scholar]
- 25.Pazdrak K, Moon Y, Straub C, Stafford S, Kurosky A. Eosinophil resistance to glucocorticoid-induced apoptosis is mediated by the transcription factor NFIL3. Apoptosis. 2016;21:421–431. doi: 10.1007/s10495-016-1226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah BH, Catt KJ. Protein phosphatase 5 as a negative key regulator of Raf-1 activation. Trends Endocrinol Metab. 2006;17:382–384. doi: 10.1016/j.tem.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Tsitoura DC, Rothman PB. Enhancement of MEK/ERK signaling promotes glucocorticoid resistance in CD4+ T cells. J Clin Invest. 2004;113:619–627. doi: 10.1172/JCI18975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pazdrak K, Olszewska-Pazdrak B, Stafford S, Garofalo RP, Alam R. Lyn, Jak2, and Raf-1 kinases are critical for the antiapoptotic effect of interleukin 5, whereas only Raf-1 kinase is essential for eosinophil activation and degranulation. J Exp Med. 1998;188:421–429. doi: 10.1084/jem.188.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starosta V, Pazdrak K, Boldogh I, Svider T, Kurosky A. Lipoxin A4 counterregulates GM-CSF signaling in eosinophilic granulocytes. J Immunol. 2008;181:8688–8699. doi: 10.4049/jimmunol.181.12.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brode S, Farahi N, Cowburn AS, Juss JK, Condliffe AM, Chilvers ER. Interleukin-5 inhibits glucocorticoid-mediated apoptosis in human eosinophils. Thorax. 2010;65:1116–1117. doi: 10.1136/thx.2009.124909. [DOI] [PubMed] [Google Scholar]
- 31.Hagan JB, Kita H, Gleich GJ. Inhibition of interleukin-5 mediated eosinophil viability by fluticasone 17-propionate: comparison with other glucocorticoids. Clin Exp Allergy. 1998;28:999–1006. doi: 10.1046/j.1365-2222.1998.00363.x. [DOI] [PubMed] [Google Scholar]
- 32.Kam JC, Szefler SJ, Surs W, Sher ER, Leung DY. Combination IL-2 and IL-4 reduces glucocorticoid receptor-binding affinity and T cell response to glucocorticoids. J Immunol. 1993;151:3460–3466. [PubMed] [Google Scholar]
- 33.Miller AL, Webb MS, Copik AJ, Wang Y, Johnson BH, Kumar R, Thompson EB. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol. 2005;19:1569–1583. doi: 10.1210/me.2004-0528. [DOI] [PubMed] [Google Scholar]
- 34.Planey SL, Litwack G. Glucocorticoid-induced apoptosis in lymphocytes. Biochem Biophys Res Commun. 2000;279:307–312. doi: 10.1006/bbrc.2000.3922. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi Y, Mercado N, Barnes PJ, Ito K. Defects of protein phosphatase 2A causes corticosteroid insensitivity in severe asthma. PLoS One. 2011;6:e27627. doi: 10.1371/journal.pone.0027627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taira J, Higashimoto Y. Caveolin-1 interacts with protein phosphatase 5 and modulates its activity in prostate cancer cells. Biochem Biophys Res Commun. 2013;431:724–728. doi: 10.1016/j.bbrc.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi F, Umeda Y, Shimamoto S, Tsuchiya M, Tokumitsu H, Tokuda M, Kobayashi R. S100 proteins modulate protein phosphatase 5 function: a link between CA2+ signal transduction and protein dephosphorylation. J Biol Chem. 2012;287:13787–13798. doi: 10.1074/jbc.M111.329771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi F, Yamamura S, Shimamoto S, Tokumitsu H, Tokuda M, Kobayashi R. Suramin is a novel activator of PP5 and biphasically modulates S100-activated PP5 activity. Appl Biochem Biotechnol. 2014;172:237–247. doi: 10.1007/s12010-013-0522-6. [DOI] [PubMed] [Google Scholar]
- 39.Straub C, Pazdrak K, Young TW, Stafford SJ, Wu Z, Wiktorowicz JE, Haag AM, English RD, Soman KV, Kurosky A. Toward the Proteome of the Human Peripheral Blood Eosinophil. Proteomics Clin Appl. 2009;3:1151–1173. doi: 10.1002/prca.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Planaguma A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, Bleecker ER, Curran-Everett D, Erzurum SC, Calhoun WJ, Castro M, Chung KF, Gaston B, Jarjour NN, Busse WW, Wenzel SE, Levy BD. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178:574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E N. H. L. Severe Asthma Research Program, and I. Blood. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brancaleone V, Dalli J, Bena S, Flower RJ, Cirino G, Perretti M. Evidence for an anti-inflammatory loop centered on polymorphonuclear leukocyte formyl peptide receptor 2/lipoxin A4 receptor and operative in the inflamed microvasculature. J Immunol. 2011;186:4905–4914. doi: 10.4049/jimmunol.1003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sano A, Munoz NM, Sano H, Choi J, Zhu X, Jacobs B, Leff AR. Inhibition of cPLA2 translocation and leukotriene C4 secretion by fluticasone propionate in exogenously activated human eosinophils. Am J Respir Crit Care Med. 1999;159:1903–1909. doi: 10.1164/ajrccm.159.6.9810005. [DOI] [PubMed] [Google Scholar]
- 44.Zhu X, Munoz NM, Kim KP, Sano H, Cho W, Leff AR. Cytosolic phospholipase A2 activation is essential for beta 1 and beta 2 integrin-dependent adhesion of human eosinophils. J Immunol. 1999;163:3423–3429. [PubMed] [Google Scholar]
- 45.Ramsey AJ, Chinkers M. Identification of potential physiological activators of protein phosphatase 5. Biochemistry. 2002;41:5625–5632. doi: 10.1021/bi016090h. [DOI] [PubMed] [Google Scholar]
- 46.Gagliardo R, Gras D, La Grutta S, Chanez P, Di Sano C, Albano GD, Vachier I, Montalbano AM, Anzalone G, Bonanno A, Riccobono L, Gjomarkaj M, Profita M. Airway lipoxin A/formyl peptide receptor 2-lipoxin receptor levels in pediatric patients with severe asthma. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 47.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O'Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]